Abstract
Sodium butyrate (NaBu) can enhance the expression of foreign genes in recombinant Chinese hamster ovary (rCHO) cells, but it can also inhibit cell growth and induce cellular apoptosis. In this study, the potential role of calnexin (Cnx) expression in rCHO cells treated with 5 mM NaBu was investigated for rCHO cells producing tumor necrosis factor receptor FC. To regulate the Cnx expression level, a tetracycline-inducible system was used. Clones with different Cnx expression levels were selected and investigated. With regard to productivity per cell (qp), NaBu enhanced the qp by over twofold. Under NaBu treatment, Cnx overexpression further enhanced the qp by about 1.7-fold. However, under NaBu stress, the cells overexpressing Cnx showed a poorer viability profile with a consistent difference of over 25% in the viability when compared to the Cnx-repressed condition. This drop in the viability was attributed to increased apoptosis seen in these cells as evidenced by enhanced poly (ADP-ribose) polymerase cleavage and cytochrome C release. Ca2+ localization staining and subsequent confocal imaging revealed elevated cytosolic Ca2+ ([Ca2+]c) in the Cnx-overexpressing cells when compared to the Cnx-repressed condition, thus endorsing the increased apoptosis observed in these cells. Taken together, Cnx overexpression not only improved the qp of cells treated with NaBu, but it also sensitized cells to apoptosis.
Keywords: Calnexin, Chinese hamster ovary (CHO) cells, Apoptosis
Introduction
Cnx was first reported as a 90-kDa endoplasmic reticulum (ER) protein, which shared several sequence motifs with another major calcium-binding intraluminal resident ER protein, calreticulin (Crt; Wada et al. 1991). In addition, Cnx was also found to be expressed on the cell surface, regardless of the cell type, lineage, or maturation stage of the cell (Okazaki et al. 2000).
Cnx interacts with newly synthesized glycoproteins during folding and maturation (Ou et al. 1993; Hammond and Helenius 1994; Hammond et al. 1994; Jackson et al. 1994; Pind et al. 1994; Hebert et al. 1996). Specifically, Cnx promotes proper folding, prevents premature oligomerization, inhibits degradation, and mediates quality control for a variety of glycoproteins (David et al. 1993; Ou et al. 1993; Hammond and Helenius 1994; Jackson et al. 1994; Kearse et al. 1994; Le et al. 1994; Loo and Clarke, 1994; Pind et al. 1994; Tector and Salter, 1995; Hebert et al. 1996; Vassilakos et al. 1996). As a molecular chaperone, Cnx is also found to have a determining role in the folding, assembly, and production of proteins (Bass et al. 1998; Fayadat et al. 2000; Siffroi-Fernandez et al. 2002; Chung et al. 2004).
An important aspect of Cnx’s function is its calcium-binding capacity and its influence on calcium homeostasis. It was found that the depletion of ER Ca2+ ([Ca2+]ER) and the subsequent elevation of cytosolic Ca2+ ([Ca2+]c) or mitochondrial Ca2+ ([Ca2+]m) are involved in the execution of apoptosis (Martikainen et al. 1991; Kruman et al. 1998; Zirpel et al. 1998; Tombal et al. 1999; Foyouzi-Youssefi et al. 2000; Lynch et al. 2000; Nakamura et al. 2000; Rizzuto et al. 2003). Given the fact that Cnx, along with Crt, is a key Ca2+-binding chaperone and that it also serves as a Ca2+ buffer in the ER, it is pertinent that the expression levels of Cnx have some bearing on Ca2+ handling by the ER and in Ca2+ signal dissemination from the ER to the mitochondria (Meldolesi and Pozzan 1998; Michalak et al. 2002; Hajnoczky et al. 2003, Walter and Hajnóczky 2005). Therefore, the effect of Cnx expression on cellular apoptosis is one facet of this study.
CHO cells have been most widely used for the production of therapeutic glycoproteins, probably because they can perform complex post-translational modifications, including glycosylation, in a manner resembling human proteins (Parekh 1991; Gramer et al. 1995; Wurm et al. 1996). During the large-scale cultures of these cells for the commercial production of therapeutic proteins, it is seen that apoptosis, which can be triggered by unavailability of nutrients or serum/growth factors and accumulation of toxic metabolites during cultures, is the major cause of cell death and deterioration of the product quality (Singh et al. 1994; Moore et al. 1995; Goswami et al. 1999). Chemicals such as NaBu can also induce apoptosis.
NaBu has been widely used in rCHO cell cultures for the high-level expression of recombinant proteins such as antibody (Kim and Lee 2001, 2002; Yoon et al. 2004), erythropoietin (Chung et al. 2001), tissue plasminogen activator (Hendrick et al. 2001), follicle-stimulating hormone (Chotigeat et al. 1994), thrombopoietin (Sung and Lee 2005), and nitric oxide synthase (Laubach et al. 1996). It can not only enhance the expression of specific genes controlled by some of the mammalian promoters, including cytomegalovirus (Cockett et al. 1990; Laubach et al. 1996; Chang et al. 1999) and simian virus 40 (Gorman et al. 1983; Palermo et al. 1991; Oster et al. 1993), but it can also inhibit cell growth and induce cellular apoptosis (Mimura et al. 2001; Kim and Lee 2002). Thus, the beneficial effect of using a high concentration of NaBu on recombinant glycoprotein expression is often compromised by its detrimental effect on cell growth and quality of the glycoprotein.
When recombinant protein expression level is elevated under NaBu stress, there might exist post-translational limitation on the protein production. This limitation can be relieved by the expression of chaperones, like Cnx, thus affecting the qp positively. Hence, we attempted to investigate the potential role of Cnx expression under the stressful conditions of apoptosis induced by NaBu treatment in rCHO cell cultures. To study the effect of Cnx expression level on these cells, we used the Tet-Off System, a tetracycline or doxycycline (dox) controlled expression system (Gossen and Bujard 1992). By employing this type of controlled expression system, we exclude the possibility of clonal variability usually encountered in constitutive overexpression experiments.
To elucidate the function of Cnx under NaBu stress, experiments are carried out with various cell lines, each possessing a noticeably distinct Cnx expression level. Batch cultures, Western blotting for apoptotic markers, and immunostaining to determine the Cnx and Ca2+ levels were performed, analyzed, and compared among the clones. The conclusions drawn from these analyses help in delineating a role for Cnx in sensitizing cells to NaBu-induced apoptosis.
Materials and methods
Construction of the Cnx expression plasmid
pT7 Blue(R)T-Cnx vector was generated as described previously (Chung et al. 2004). The CHO Cnx gene was isolated and sequenced by an automated DNA sequencer (ABI prism model 377, Perkin Elmer, Foster City, CA, USA). Double digestion using XbaI and EcoRI was performed, and the Cnx fragment was inserted into a pTRE vector (Clontech, Palo Alto, CA, USA), yielding pTRE-Cnx.
pTet-Off-Zeo vector was also constructed by inserting a zeocin-resistance gene sequence from pcDNA 3.1(+) vector (Invitrogen, Carlsbad, CA, USA) into a pTet-Off-Neo vector (Mohan et al. 2007) for use in Tet-Off-TNF cell line construction.
Generation of double-stable cell lines
The rCHO cell line expressing tumor necrosis factor receptor FC (TNFR-Fc) (CHO-TNF) was a generous gift from Aprogen (Daejon). To regulate the Cnx expression level, the Tet-Off system was introduced in CHO-TNF cells, and stable Tet-Off CHO-TNF cells (Tet-Off-TNF) were screened by the luciferase assay, as described previously (Hwang et al. 2003).
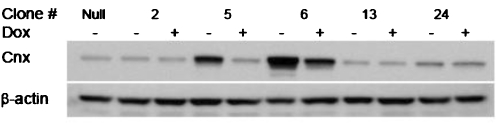
The double stable Tet-Off-TNF cell line expressing Cnx (Tet-TNF-Cnx) was established by co-transfecting pTRE-Cnx with pTk-Hyg vector (Clontech) into Tet-Off-TNF cells. Upon reaching 90% confluency, Tet-Off-TNF cells were transfected with 3.6 μg pTRE-Cnx and 0.4 μg pTk-Hyg using 10 μl Lipofectamine™ 2000 transfection reagent (Invitrogen) according to the manufacturer’s protocol. To select stably transfected cells, drug selection was carried out for 2 weeks by seeding 1 × 103 cells per well in 96-well tissue-culture plates containing Iscove’s modified Dulbecco’s medium (IMDM; Invitrogen) containing the selection antibiotic, 250 μg/ml hygromycin (Sigma, St. Louis, MO, USA). Among hygromycin-resistant clones, 19 clones were selected randomly and transferred into six-well tissue culture plates for expansion. For a negative control, Tet-Off-TNF cells transfected with a null pTRE vector (Tet-TNF-Null) was also established. The final Tet-TNF-Cnx clone was selected based on the Western blot analysis of intracellular Cnx showing the maximum overexpression (with respect to the null cells) and tight regulation of the Cnx level by dox (Fig. 1).
Fig. 1.
Selection of dox-regulated Tet-TNF-Cnx clones. Western blot analysis of Cnx in the selected overexpressing Tet-TNF-Cnx clones (2, 5, 6, 13, and 24) and a negative control cell (Tet-TNF-Null) to choose highly dox-regulatable clones. Cells were cultivated in the maintenance medium containing 1 μg/ml dox to regulate Cnx expression to a basal level. Two days after seeding, cells were cultivated for 24 h with 2 μg/ml dox or without dox after which they were sampled. Cell lysates were loaded at 5 × 104 cells/10 μl concentration. Relative band intensity was calculated using TINA 2.0 software. Regulation folds were calculated by dividing band intensity of 0 μg/ml dox condition by that of 2 μg/ml dox condition
Culture medium and maintenance
The medium for culture maintenance of Tet-TNF-Cnx and Tet-TNF-Null cells was IMDM supplemented with 10% dialyzed fetal bovine serum (Gibco, Grand Island, NY, USA), 250 μg/ml hygromycin, 350 μg/ml zeocin, 1 μg/ml dox (Clontech), and 0.02 μM methotrexate (MTX, Sigma). Dox was added to the medium every 2 days keeping in view of its short life during culture (Tet Systems User Manual, PT3001-1). The cells were maintained as monolayer cultures in 25-cm2 T-flasks (Nunc, Roskilde, Denmark) in a humidified 5% CO2 incubator at 37°C. The cells were passed every 3 to 4 days upon reaching confluency.
Cell culture with NaBu treatment
Cell culture was performed with four Tet-TNF-Cnx clones in addition to the null cells. Exponentially growing cells were inoculated into 25-cm2 T-flasks (Nunc) with 5 ml of the maintenance medium. The initial cell concentration was approximately 0.1 × 106 cells/ml. After 2 days of cultivation, the spent medium was replaced with 5 ml of fresh media with (+) or without (−) 5 mM NaBu (Sigma) or with (+) or without (−) dox. The cells were grown in a humidified 5% CO2 incubator at 37°C. T-flasks were sacrificed periodically to determine cell concentration and viability. Culture supernatants were aliquoted and kept at −70°C for further analysis. Cell pellets, for Western blot analysis, were collected after centrifuging the cells and subsequent PBS wash.
Cell concentration, viability, and TNFR-Fc assay
Cell concentration was estimated using a hemacytometer. Viable cells were distinguished from dead cells using the trypan blue dye exclusion method. Trypan blue is actively excluded from the live cells having an intact cell membrane, while the dead cells take up the dye. The percent ratio of the viable cells to the total cells gives the percent viability of the culture on the specified sampling time. Secreted TNF was quantified using an enzyme-linked immunosorbent assay (ELISA) according to the protocol provided by R&D Systems (Minneapolis, MN, USA).
Evaluation of productivity per cell, qp
The qp, productivity per cell, was based on the data collected during 4-day culture after the medium exchange with dox. When the TNFR-Fc concentration is plotted against the time integral values of the growth curve, the slope represents qp.
Western blot analysis
To determine the intracellular Cnx expression level and to assess the apoptotic markers, Western blot analysis was performed. Cell pellets collected at periodic time intervals were incubated in a triple-detergent lysis buffer containing 50 mM Tris (pH 8.0), 150 mM NaCl, 0.02% NaN3, 0.1% sodium dodecyl sulfate (SDS), 1.0% NP-40, and a tablet of protease inhibitor cocktail (Roche, Mannheim, Germany) for 30 min at 1 × 107 cells/ml. Cell lysate was centrifuged at 12,000×g for 5 min at 4°C, and the supernatant was collected. The cell lysate was boiled with SDS sample buffer for 10 min before being run on a 4–20% Tris-glycine SDS-polyacrylamide pre-cast gel (Invitrogen). The lysate from 1 × 105 or 2 × 105 cells as well as prestained protein molecular weight markers (Bio-Rad, Hercules, CA, USA) were loaded into each lane. Gels were transferred to Immuno-Blot™ polyvinylidene difluoride membranes (Bio-Rad) for 1 h at 100 V. Blots were then soaked in 5% skimmed milk for 2 h at room temperature. Antibodies for immunoblotting included anti-Cnx (Stressgen, Victoria, Canada), anti-PARP (Cell Signaling Technology), anti-cytochrome C (Biolegend, San Diego, CA, USA), anti-LC3 (Clone 51-11, Medical and Biological Laboratories, Japan), and anti-β-actin (AC-74, Sigma). Bands were then visualized by the ECL Western blot analysis system (Amersham Pharmacia Biotech, Piscataway, NJ, USA).
Live cell immunostaining and confocal imaging
Cells were seeded at 0.1 × 106 cells/ml in a 60-mm dish with 5 ml of media or on gelatin-coated cover slips. After 24 h of seeding, the media were exchanged with the test media (+ NaBu, and +/− dox). At the designated imaging time, the cells were prepared for immunocytochemical analysis of Cnx and ER by blocking with 5% donkey serum (Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA) in phosphate-buffered saline (PBS) and subsequent incubation with anti-Cnx rabbit polyclonal primary antibody for 2 h. After washing with PBS + Tween 20, cells were incubated with flourescein isothiocyanate (FITC)-conjugated AffiniPure Goat Anti-Rabbit secondary antibody (Jackson ImmunoResearch Laboratories) and 1 μM ER-tracker™ dye (Molecular Probes) for 1 h. The cover slips were then mounted and viewed. For the ER and Ca2+ staining, 1 μM Fluo-4 AM (Molecular Probes) and 1 μM ER-tracker™ dye in Hanks’ balanced salt solution (HBSS; Sigma) were added to the cells seeded in the culture plates and incubated for 30 min at 37°C. Cells were washed with HBSS twice and incubated in HBSS at 37°C for 30 min for complete de-esterification of the Fluo-4 AM stain. 4′,6-Diamino-2-phenylinole (DAPI, Hoechst 1:1,000 dilution) was used for nucleus staining. The cells were then viewed under an LSM 510 confocal laser scanning microscope (Zeiss, Nussloch, Germany), and the pictures were processed using Adobe Photoshop software.
Statistical analysis
The results are expressed as mean ± standard deviation (SD), n = 2. Data were analyzed by double-tail Student’s t test. The differences were considered significant at p < 0.005.
Results
Establishment of double-stable rCHO cell lines with Cnx expression
To establish the stable rCHO cell lines with Tet-regulated Cnx expression, Tet-Off-TNF cells co-transfected with the pTRE-Cnx vector encoding the Cnx gene and the pTk-Hyg vector harboring a hygromycin-resistance gene were selected for hygromycin resistance. The overexpressed Cnx is turned on (induced) in the absence of dox and turned off (repressed) in the presence of dox. Nineteen hygromycin-resistant Tet-TNF-Cnx clones were randomly selected and cultivated in the absence of dox to select Cnx overexpressing clones. As a negative control, Tet-TNF-Null cells were also cultivated in the absence of dox. After 3 days of cultivation, cells and culture supernatants were sampled for determination of Cnx expression levels by Western blot analysis and for the determination of TNFR-Fc by ELISA.
Considering both Cnx and TNFR-Fc expression levels, relatively high (over twofold) Cnx overexpressing clones were selected (data not shown) and subjected to regulation study. To select highly dox-regulatable Cnx-expressing clones, the overexpressing clones having high titer were cultivated in the absence and presence of 2 μg/ml dox. As a negative control, the null cell line was also cultivated in the absence of dox. Figure 1 shows the result of Western blot analysis of 5 Tet-TNF-Cnx clones and a negative control cell line. Among the five clones, clones 5 and 6, having dissimilar Cnx basal levels, showed relatively higher regulation fold compared to the other clones (5- and 2.5-fold, respectively). These clones were selected, in addition to the null cells and the dox-non-responsive clone 2, for further experiments.
Culture of Tet-TNF-Cnx clones with regulated Cnx expression
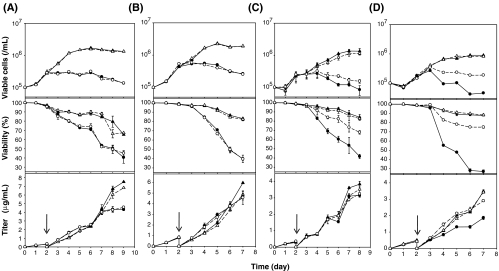
To analyze the effect of Cnx expression on cell growth, viability, and TNFR-Fc production, Tet-TNF-Cnx clones were cultivated initially in the maintenance medium containing 1 μg/ml dox. Cells were seeded at 0.1 × 106 cells/ml in T-25 flasks for culture. On the second day after seeding, medium was changed to the test medium (+/− NaBu, and +/− dox). Subsequently, dox was added to the flasks every 2 days. It has been previously observed that dox, even at 2 μg/ml, does not inhibit cell growth and can be used for experimental purposes (Hwang et al. 2003; Chung et al. 2004; Mohan et al. 2007). Figure 2 shows the cell growth, viability, and the TNFR-Fc production profiles of Tet-TNF-Cnx clones during cultures.
Fig. 2.
Culture of Tet-TNF-Cnx clones with regulated Cnx expression. A Culture profile of Tet-TNF-Null (Null cells). B Culture profile of Tet-TNF-Cnx 2 (Clone 2, dox-non-responsive clone). C Culture profile of Tet-TNF-Cnx 5 (Clone 5 with low basal level Cnx). D Culture profile of Tet-TNF-Cnx 6 (clone 6 with high basal level Cnx). Spent medium was replaced with fresh medium containing control without dox (filled triangle), control with dox (empty triangle), 5 mM NaBu without dox (filled circle), and 5 mM NaBu with dox (empty circle) on day 2. Culture supernatant was collected everyday and the cells on the designated days for Western blot analysis. Cell number and viability percent was determined using the trypan blue dye exclusion method. Protein titer was quantitated by ELISA. Arrows indicate the time of NaBu addition. The error bars represent the standard deviation calculated from the data obtained in duplicate experiments
The top panel in Fig. 2A shows the details of the null cells during culture. The control cells in the absence of NaBu showed a better growth profile with the culture reaching maximum viable cell numbers of (1.71 ± 0.03) × 106 cells/ml (− dox) and (1.56 ± 0.02) × 106 cells/ml (+ dox). Cells with 5 mM NaBu succumbed to the stress imposed by NaBu treatment, and the maximum cell density was (0.28 ± 0.01) × 106 cells/ml (− dox) and 0.31 ± 0.01 × 106 cells/ml (+ dox). The top panel of Fig. 2B depicts the culture details for clone 2, the dox-non-responsive clone. This clone also showed a similar growth pattern as the null cells with the maximum viable cells reaching over 2.00 × 106 cells/ml for the 0 mM NaBu culture and 0.5 × 106 cells/ml for the cells treated with 5 mM NaBu. The top panel of Fig. 2C elucidates the culture profile of clone 5, the clone with a low basal level of Cnx. This clone showed a maximum cell number of 1.00 × 106 cells/ml for the control cells, whereas the 5 mM NaBu-treated cells were 0.26 × 106 cells/ml, irrespective of the addition or removal of doxycycline. The top panel of Fig. 2D illustrates the culture profile of clone 6, the dox responsive clone with a higher basal level of Cnx. The maximum viable cells in clone 6 was 0.87 × 106 cells/ml for the control cells and 0.33 × 106 cells/ml for the 5 mM NaBu treated cells, irrespective of the Cnx expression level.
It can be broadly concluded from these data that the control cells without NaBu addition always showed better growth, as expected. The cells treated with NaBu, however, were adversely affected.
The middle panels of Fig. 2A and B illustrate the viability profile of null cells and clone 2, respectively. The null cells and clone 2 showed similar viability profiles during cultures with the control cells maintaining a viability of over 65% on the last day of the culture. In the cells treated with 5 mM NaBu, the viability, however, dropped to about 40%. As these clones are either Cnx non-overexpresser (null) or not responsive to dox (clone 2), the viability was similar irrespective of the addition or removal of dox. Throughout the batch culture of clones 5 and 6 under NaBu treatment, it was seen that when the cells were overexpressing Cnx (− dox), they showed a poorer viability profile, with a steady difference of over 25%, when compared to the Cnx-repressed condition (+ dox). Clone 5, the clone with a low basal level of Cnx, showed a viability difference of about 26% between the − dox and + dox states, whereas (Fig. 2C, middle panel) clone 6, having a higher basal Cnx, displayed a greater difference of about 48% between the different Cnx expression states (Fig. 2D, middle panel). The maximum viable cell number and the viability percentage of the clones during culture are summarized in Table 1.
Table 1.
The maximum viable cell number and viability in culture of Tet-TNF-Cnx clones during batch culture
| Clone | NaBu (mM) | Dox/Cnx | Viable cells/ml (x106) | Viability (%) |
|---|---|---|---|---|
| Tet-TNF-Null | 0 | −/ON | 1.71 ± 0.03a | 65.35 ± 0.42 |
| 0 | +/OFF | 1.56 ± 0.02 | 67.25 ± 0.15 | |
| 5 | −/ON | 0.28 ± 0.01 | 40.51 ± 6.24 | |
| 5 | +/OFF | 0.31 ± 0.01 | 45.12 ± 2.89 | |
| Tet-TNF-Cnx 2 | 0 | −/ON | 2.31 ± 0.07 | 83.09 ± 0.76 |
| 0 | +/OFF | 2.22 ± 0.04 | 82.30 ± 1.63 | |
| 5 | −/ON | 0.55 ± 0.01 | 39.77 ± 4.01 | |
| 5 | +/OFF | 0.59 ± 0.02 | 39.53 ± 1.31 | |
| Tet-TNF-Cnx | 0 | −/ON | 1.32 ± 0.23 | 87.93 ± 2.26 |
| 0 | +/OFF | 1.07 ± 0.15 | 82.63 ± 1.75 | |
| 5 | −/ON | 0.27 ± 0.08 | 41.67 ± 2.36 | |
| 5 | +/OFF | 0.32 ± 0.12 | 67.81 ± 1.92 | |
| Tet-TNF-Cnx 6 | 0 | −/ON | 0.89 ± 0.01 | 87.74 ± 0.69 |
| 0 | +/OFF | 0.84 ± 0.01 | 88.25 ± 1.06 | |
| 5 | −/ON | 0.27 ± 0.01 | 27.06 ± 1.75 | |
| 5 | +/OFF | 0.38 ± 0.01 | 75.07 ± 1.24 |
Various clones under culture were treated with/without NaBu and with/without dox on the second day after seeding. Viability was measured using the trypan blue dye exclusion method. The difference in viability between the Cnx-overexpressed and Cnx-repressed states in clones 5 and 6 is highlighted in bold.
aMean ± SD; standard deviation between the duplicates
All the clones tested showed no effect of Cnx overexpression per se, but under NaBu treatment, even though the growth was suppressed, the qp was enhanced by over twofold, which is probably due to the alleviation of post-translational limitation. Under NaBu stress, null cells and clone 2 showed an enhanced titer by about fourfold (Fig. 2A,B, lower panels). The qp of clones 5 and 6 without the addition of NaBu was almost around 0.78 μg/106 cells/day. Under NaBu stress, however, it was enhanced by about threefold (clone 5) and twofold (clone 6). The effect of Cnx expression solely on the qp (by comparing the − dox and + dox conditions) was varied. Under the control condition, without NaBu treatment, Cnx overexpression had no significant effect on the qp, but under NaBu stress, the qp was further enhanced by 1.7-fold for clone 5, whose basal level Cnx is low, whereas in clone 6, Cnx expression did not help in enhancing the qp further. It should be noted that, under NaBu treatment, even though the qp was enhanced, the maximum titer was not detectably affected (Fig. 2C,D, lower panels).
It can be deduced that Cnx overexpression as such did not have any enhancing effect on the titer in this cell line. However, under NaBu treatment, the qp was enhanced. This finding too was variable depending on the basal level of Cnx, with a low basal level resulting in more enhancements and vice versa.
The results mentioned are statistically significant using the Student’s t test (n = 2, p < 0.005). Table 2 summarizes the details of TNFR-Fc production during culture for all the clones tested.
Table 2.
The maximum TNFR-Fc and qp in culture of Tet-TNF-Cnx clones during batch culture
| Clone | NaBu (mM) | Dox/Cnx | qpa (μg/106 cells/day) | Maximum titer (μg/ml) |
|---|---|---|---|---|
| Tet-TNF-Null | 0 | −/ON | 0.71 ± 0.1b | 7.52 ± 0.06 |
| 0 | +/OFF | 0.72 ± 0.09 | 6.83 ± 0.08 | |
| 5 | −/ON | 2.74 ± 0.41 | 4.34 ± 0.34 | |
| 5 | +/OFF | 2.80 ± 0.03 | 4.58 ± 0.14 | |
| Tet-TNF-Cnx 2 | 0 | −/ON | 0.46 ± 0.07 | 5.96 ± 0.05 |
| 0 | +/OFF | 0.40 ± 0.05 | 4.88 ± 0.30 | |
| 5 | −/ON | 1.81 ± 0.44 | 4.64 ± 0.28 | |
| 5 | +/OFF | 1.96 ± 0.28 | 4.58 ± 0.63 | |
| Tet-TNF-Cnx 5 | 0 | −/ON | 0.74 ± 0.01 | 3.84 ± 0.17 |
| 0 | +/OFF | 0.82 ± 0.05 | 3.52 ± 0.48 | |
| 5 | −/ON | 4.17 ± 0.12 | 3.15 ± 0.07 | |
| 5 | +/OFF | 2.44 ± 0.00 | 3.24 ± 0.10 | |
| Tet-TNF-Cnx 6 | 0 | −/ON | 0.77 ± 0.00 | 3.48 ± 0.09 |
| 0 | +/OFF | 0.81 ± 0.01 | 3.43 ± 0.06 | |
| 5 | −/ON | 1.59 ± 0.03 | 1.86 ± 0.10 | |
| 5 | +/OFF | 1.99 ± 0.10 | 2.91 ± 0.06 |
Samples from the culture were collected and analyzed using ELISA to determine the TNFR-Fc titer. The qp was affected positively by Cnx overexpression under NaBu stress. The maximum titer, however, was unaffected.
aThe qp was based on the data collected for 4 days of cultivation after medium exchange.
bMean ± SD; standard deviation between the duplicates.
Cnx overexpression sensitizes cells to apoptosis
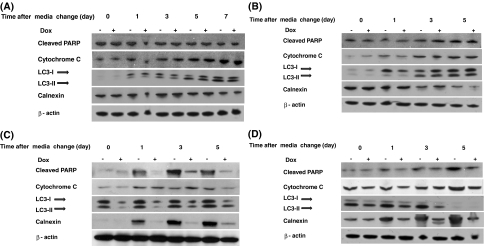
The cell pellets collected at periodic time intervals during the batch culture were subjected to Western blot analysis for the determination of the cellular marker proteins for cell death and to check the Cnx expression status. It was found that, for null cells and clone 2 (Fig. 3A,B, respectively), the apoptotic markers, cytochrome c release, and PARP cleavage were unaffected by the addition or removal of dox from the culture medium. The expression of Cnx was also unaffected by dox.
Fig. 3.
Western blot analysis of Tet-TNF-Cnx clones for cellular proteins from batch culture with NaBu treatment. A Western blot data for Tet-TNF-Null (null cells). B Western blot data for Tet-TNF-Cnx 2 (clone 2, dox-non-responsive clone). C Western blot data for Tet-TNF-Cnx 5 (Clone 5 with low basal level Cnx). D Western blot data for Tet-TNF-Cnx 6 (Clone 6 with high basal level Cnx). Cells were loaded at a concentration of 1 × 105 cells/10 μl and resolved using the 4–20% Tris-glycine gel. Note the elevated cytochrome C release and PARP cleavage for clones 5 and 6 under Cnx-overexpressed condition (− dox)
The reason for the difference in the viability observed for clones 5 and 6 was investigated, assuming it to be due to apoptosis. The cause for the lower viability of the Cnx overexpressing cells was indeed attributed to the increased incidence of apoptosis noted in these cells. Western blot analysis for clone 5 cultures with NaBu treatment (Fig. 3C) revealed that cytochrome C release and PARP cleavage were less (about 1.8-fold) in Cnx repressed cells in comparison to the Cnx up-regulated cells after days 1 and 5 of media change. Day 3, however, shows some discrepancy with respect to cytochrome C release. Another form of programmed cell death, autophagy, identified as a cell death mechanism undertaken by CHO cells during stress (Hwang and Lee 2008), was also observed by the conversion of the autophagic marker LC3-I to LC3-II. Both forms of programmed cell death, apoptosis and autophagy, were seen to be increased in the cells overexpressing Cnx suggesting that the overexpression of Cnx sensitized cells to apoptosis. The case with clone 6, the clone with a higher basal level of Cnx, was the same (Fig. 3D). Increased PARP cleavage and cytochrome C release for the Cnx-overexpressing condition was observed (over 1.9-fold) on all days of sampling.
As changes in intracellular Ca2+ homeostasis plays a role in modulation of apoptosis, the amount of [Ca2+]c was investigated by fluorescence-imaging methods. Confocal images, shown in Fig. 4, depict cells stained for ER, Ca2+, and Cnx. The right panel (pictures in the first three columns) represents cells stained for Ca2+ (green stain) and ER (red stain), and the left panel (pictures in the last three columns) shows Cnx expression level (green stain) and ER (red stain) in the cells. Null cells with and without dox (Fig. 4A,B) and clone 2 with and without dox (Fig. 4C,D) showed no difference with respect to Cnx expression and the Ca2+ levels. This is as expected, as these clones are not Cnx overexpressers and hence show low, dox-non-regulated Cnx levels. The Cnx overexpression in clones 5 and 6 was confirmed even with immunostaining in the absence of dox (Fig. 4E,G). The total [Ca2+]c was found to be higher when cells were overexpressing Cnx in the absence of dox (Fig. 4E,G). The increased [Ca2+]c levels for cells with overexpressing Cnx (− dox) actually correlates with increased PARP cleavage and cytochrome C release, indicating that apoptotic signal generation, transmission, and execution was elevated in these cells when compared to the Cnx repressed cells. This again reiterates that these cells are more sensitive to apoptosis, induced by NaBu, when overexpressing Cnx. Cnx expression was tightly regulated by dox throughout the culture period as seen in the Western blot data.
Fig. 4.
Confocal microscopic images of immunostaining of Tet-TNF-Cnx clones. Cell nucleus (blue stain), ER (red stain), Ca2+ (green stain in the first panel), and Cnx (Green stain in the second panel) were stained using DAPI, ER-Tracker™ dye, Fluo-4 AM, and anti-Cnx-FITC antibody, respectively, after 48 h of medium exchange with/without dox and with 5 mM NaBu. A Immunostaining of Tet-TNF-Null cells − dox. B Immunostaining of Tet-TNF-Null cells + dox. C Immunostaining of Tet-TNF-Cnx 2 cells − dox. D Immunostaining of Tet-TNF-Cnx 2 cells + dox. E Immunostaining of Tet-TNF-Cnx 5 cells − dox. F Immunostaining of Tet-TNF-Cnx 5 cells + dox. G Immunostaining of Tet-TNF-Cnx 6 cells − dox. H Immunostaining of Tet-TNF-Cnx 6 cells + dox. Cnx expression is found to be less in null and clone 2, whereas clones 5 and 6 showed an increased level of Cnx. Ca2+ levels were unchanged for null and clone 2 but were higher for clones 5 and 6 when cells were overexpressing Cnx (− dox). Scale bar = 50 µm
Discussion
Cnx, a glycoprotein-binding chaperone, has significant effects on intracellular Ca2+ homeostasis, affecting the uptake and release of Ca2+ from the ER in several ways, including direct regulation of the SERCA Ca2+ pump (Michalak et al. 1999; Roderick et al. 2000). These changes could either directly influence the apoptotic processes or lead to the changes in the expression of proteins involved in apoptosis (Nakamura et al. 2000; Pinton et al. 2000; Foyouzi-Youssefi et al. 2000).
In this study, the potential role of Cnx overexpression under the stressful conditions of NaBu-induced apoptosis and effect on productivity in rCHO cells was investigated. We subjected cells under culture to treatment with NaBu. NaBu, a sodium salt of butyric acid has been use in CHO cell cultures to enhance the productivity per cell of various target proteins. But the drawback of using NaBu is suppressed cell growth and, at a later stage, apoptosis induction (Palermo et al. 1991; Laubach et al. 1996; Chang et al. 1999; Kim and Lee 2001; Sung et al. 2004). In this study, under NaBu treatment, the response with regard to qp was positive. The qp was enhanced by over twofold (Table 2). It has been reported in Schizosaccharomyces pombe that protein secretion did not require the chaperoning action of Cnx (Maréchal et al. 2004). In rCHO cells producing thrombopoietin, controlled overexpression of Cnx and Crt enhanced the qp by about 1.9-fold under normal conditions, without stress (Chung et al. 2004), probably because the post-translational limitation in the cells with high qp was relieved by the overexpression of these chaperones. However, there are no known reports citing the relationship between Cnx expression and qp of a protein under the stressful condition of NaBu treatment in CHO cells. In this study, without NaBu treatment, Cnx overexpression had no significant effect on the qp. However, under NaBu stress, the qp was enhanced by over twofold. It is unlikely that there is a post-translational bottleneck under normal culture conditions, without NaBu treatment, where qp is not that high. This meant that, in this study, a post-translational limitation existed for cells under NaBu treatment, which was alleviated by Cnx overexpression, as hypothesized. More enhancements were observed in cells with a low basal level of Cnx (clone 5) than with cells having a higher basal level of Cnx (clone 6). This implies that NaBu creates a post-translational limitation, which was relieved by the simultaneous overexpression of Cnx. However, beyond a certain essential level of Cnx, overexpression of Cnx does not help in improving the qp. With regard to maximum TNFR-Fc titer, in none of the clones tested, the effect of Cnx overexpression was positive, although the qp was enhanced.
One interesting finding that led us to investigate the relationship between Cnx expression and apoptosis was the poorer viability profile seen with Cnx-overexpressing cells (clones 5 and 6 in the absence of dox) under NaBu stress. A steady difference of over 25% in viability was observed between the Cnx-repressed and Cnx-induced conditions during batch cultures. Western blotting for the apoptotic markers, PARP cleavage and cytochrome C release, reveals that cells when overexpressing Cnx are more sensitive to apoptosis induced by NaBu. Autophagy, another form of programmed cell death reported in CHO cells under nutrient deprivation (Hwang and Lee 2008), was also observed under NaBu treatment, as seen by the conversion of the autophagic marker from the LC3-I to the LC3-II form.
Ca2+ localization staining and confocal imaging demonstrate the elevated [Ca2+]c level in the Cnx-overexpressed cells, justifying the elevated apoptosis. Differential expression of Cnx affects the Ca2+ storage capacity of the ER, and most importantly, it modulates Ca2+ release from the ER. In this study, we found that overexpression of Cnx in the Tet-Off-inducible system led to a decrease in the amount of available Ca2+ in the ER and to an increased level of [Ca2+]c in response to NaBu (Fig. 4). The control and null cells without NaBu treatment, however, showed no difference in the cell growth, viability profile, and Ca2+ levels between the Cnx-overexpressed condition and the Cnx-repressed condition.
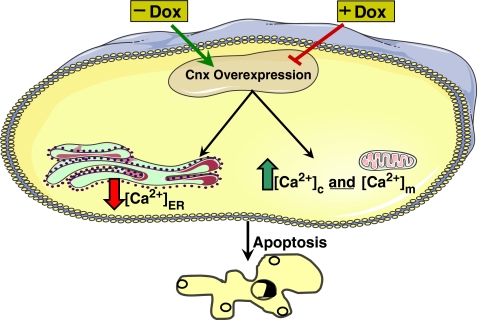
Our findings suggest that changes in the expression of Cnx, via Ca2+ homeostasis, may play an important role in the modulation of cell sensitivity to apoptosis induced by an external stimulus, such as NaBu. This is elucidated by the schematic diagram in Fig. 5.
Fig. 5.
Schematic diagram showing the effect differential expression of Cnx has on Ca2+ homeostasis and hence on apoptosis. Removal of dox induces the overexpression of Cnx, which in turn reduces the amount of available Ca2+ in the ER. The subsequent step of elevation of total [Ca2+]c and [Ca2+] m leads the cell to apoptosis
Contrasting reports on the effect of Cnx on apoptosis exist. Cnx-deficient cells were reported to be resistant to ER-stress induced apoptosis due to their resistance to Bap31 cleavage, but the ER to mitochondria communication was not affected as indicated by unchanged caspase-3, caspase-8, and cytochrome C release (Zuppini et al. 2002). Nakamura et al. (2000) observed that the overexpression of Crt, an ER luminal protein, resulted in an increased sensitivity of the cells to drug-induced apoptosis accompanied by increased release of cytochrome C and caspase activity. This substantiated the ER and the mitochondrial crosstalk, probably mediated by Ca2+. In the present study, the ER and mitochondrial crosstalk was affected by the differential expression of Cnx, as seen by the altered expression levels of PARP cleavage and cytochrome C release, a process probably mediated by Ca2+. On the other hand, overexpression of Cnx had no effect on drug-induced apoptosis (Nakamura et al. 2000). With a human breast cancer cell line, MCF-7, Cnx was found to protect the cells from tunicamycin-induced apoptosis by sequestration of Bap31. MCF-7 resistance to apoptosis was partially reverted by reducing Cnx expression levels (Delom et al. 2007). In addition, attenuation of Cnx expression significantly increased cell sensitivity to tunicamycin (Delom et al. 2006). The response of Cnx to apoptosis induced by various factors also varied. GD3 synthase-induced apoptosis was suppressed by Cnx where Bax-induced apoptosis was not (Tomassini et al. 2004). Serum deprivation is known to induce apoptosis in cells, and in this study, we also subjected Tet-TNF-Cnx cells to growth under serum deprivation. The effect of Cnx expression on apoptosis induced by serum deprivation was the same as that for NaBu treatment. Cnx-overexpressing cells were more sensitive to apoptosis whereas cells were resistant to apoptosis when Cnx was repressed (data not shown). The overexpression of Crt, a luminal counterpart of Cnx, which shares structural and functional similarities with it, in HEK cell line resulted in increased susceptibility of the cells to apoptotic stimuli (Arnaudeau et al. 2002). Hence, it can be generalized that the way Cnx responds to stressful conditions and apoptosis is varied depending on the cell type and the apoptosis inducer.
Not completely eliminating the contribution of the unfolded protein response (UPR) in our observed results, we speculate that because these cells are a stable cell line adapted to secrete high amounts of protein and because the molecular chaperone Cnx is being overexpressed (a regular response to the activation of UPR of cells under stress), the possibility of the activation of UPR giving rise to the results observed by us is less convincing.
In conclusion, in this study, the effect of Cnx expression under NaBu stress was evaluated on two aspects of CHO cell culture—the qp and apoptosis. It can be said that Cnx overexpression not only had a positive effect on the qp, but it also sensitized cells to apoptosis induced by NaBu. Hence, when chaperone engineering for the enhancement of recombinant protein production is considered, the variable response of a chaperone has to be taken into account, and a balance has to be struck between the positive effect and the negative effect of the chaperone on the cell line.
Acknowledgments
The authors wish to thank Dr. Min Soo Kim and Ms. Jane Koo for their help. This research was supported in part by grants from the Ministry of Commerce, Industry, and Energy, Daejeon city (Bio/RIS program) and the Ministry of Education (Brain Korea 21 Program).
References
- Arnaudeau S, Frieden M, Nakamura K, Castelbou C, Michalak M, Demaurex N. Calreticulin differentially modulates calcium uptake and release in the endoplasmic reticulum and mitochondria. J Biol Chem. 2002;277:46696–46705. doi: 10.1074/jbc.M202395200. [DOI] [PubMed] [Google Scholar]
- Bass J, Chiu G, Argon Y, Steiner DF. Folding of insulin receptor monomers is facilitated by the molecular chaperones calnexin and calreticulin and impaired by rapid dimerization. J Cell Biol. 1998;141:637–646. doi: 10.1083/jcb.141.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KH, Kim KS, Kim JH. N-Acetylcysteine increases the biosynthesis of recombinant EPO in apoptotic Chinese hamster ovary cells. Free Radic Res. 1999;30:85–91. doi: 10.1080/10715769900300091. [DOI] [PubMed] [Google Scholar]
- Chotigeat W, Watanapokasin Y, Mahler S, Gray PP. Role of environmental conditions on the expression levels, glycoform pattern and levels of sialyltransferase for hFSH produced by recombinant CHO cells. Cytotechnology. 1994;15:217–221. doi: 10.1007/BF00762396. [DOI] [PubMed] [Google Scholar]
- Chung BS, Jeong YT, Chang KH, Kim JS, Kim JH. Effect of sodium butyrate on glycosylation of recombinant erythropoietin. J Microbiol Biotechnol. 2001;11:1087–1092. [Google Scholar]
- Chung JY, Lim SW, Hong YJ, Hwang SO, Lee GM. Effect of doxycycline-regulated calnexin and calreticulin expression on specific thrombopoietin productivity of recombinant Chinese hamster ovary cells. Biotechnol Bioeng. 2004;85:539–546. doi: 10.1002/bit.10919. [DOI] [PubMed] [Google Scholar]
- Cockett MI, Bebbington CR, Yarranton GT. High level expression of tissue inhibitor of metalloproteinases in Chinese hamster ovary cells using glutamine synthetase gene amplification. Biotechnology. 1990;8:662–667. doi: 10.1038/nbt0790-662. [DOI] [PubMed] [Google Scholar]
- David V, Hochstenbach F, Rajagopalan S, Brenner MB. Interaction with newly synthesized and retained proteins in the endoplasmic reticulum suggests a chaperone function for human integral membrane protein IP90 (calnexin) J Biol Chem. 1993;268:9585–9592. [PubMed] [Google Scholar]
- Delom F, Emadali A, Cocolakis E, Lebrun JJ, Nantel A, Chevet E. Calnexin-dependent regulation of tunicamycin-induced apoptosis in breast carcinoma MCF-7 cells. Cell Death Differ. 2006;3:586–596. doi: 10.1038/sj.cdd.4402012. [DOI] [PubMed] [Google Scholar]
- Delom F, Fessart D, Chevet E. Regulation of calnexin sub-cellular localization modulates endoplasmic reticulum stress-induced apoptosis in MCF-7 cells. Apoptosis. 2007;12:293–305. doi: 10.1007/s10495-006-0625-4. [DOI] [PubMed] [Google Scholar]
- Fayadat L, Siffroi-Fernandez S, Lanet J, Franc JL. Calnexin and calreticulin binding to human thyroperoxidase is required for its first folding step(s) but is not sufficient to promote efficient cell surface expression. Endocrinology. 2000;141:959–966. doi: 10.1210/en.141.3.959. [DOI] [PubMed] [Google Scholar]
- Foyouzi-Youssefi R, Arnaudeau S, Borner C, Kelley WL, Tschopp J, Lew DP, Demaurex N, Krause KH. Bcl-2 decreases the free Ca2+ concentration within the endoplasmic reticulum. Proc Natl Acad Sci. 2000;97:5723–5728. doi: 10.1073/pnas.97.11.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman CM, Howard BH, Reeves R. Expression of recombinant plasmids in mammalian cells is enhanced by sodium butyrate. Nucleic Acids Res. 1983;11:7631–7648. doi: 10.1093/nar/11.21.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami J, Sinskey AJ, Steller H, Stephanopoulos GN. Apoptosis in batch cultures of Chinese hamster ovary cells. Biotechnol Bioeng. 1999;62:632–640. doi: 10.1002/(SICI)1097-0290(19990320)62:6<632::AID-BIT2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Gramer MJ, Goochee CF, Chock VY, Brousseau DT, Sliwkowski MB. Removal of sialic acid from a glycoprotein in CHO cell culture supernatant by action of an extracellular CHO cell sialidase. Bio/Technology. 1995;13:692–698. doi: 10.1038/nbt0795-692. [DOI] [PubMed] [Google Scholar]
- Hajnoczky G, Davies E, Madesh M. Calcium signaling and apoptosis. Biochem Biophys Res Commun. 2003;304:445–454. doi: 10.1016/S0006-291X(03)00616-8. [DOI] [PubMed] [Google Scholar]
- Hammond C, Helenius A. Folding of VSV G protein: sequential interaction with BiP and calnexin. Science. 1994;266:456–458. doi: 10.1126/science.7939687. [DOI] [PubMed] [Google Scholar]
- Hammond C, Braakman I, Helenius A. Role of N-linked oligosaccharide recognition, glucose trimming, and calnexin in glycoprotein folding and quality control. Proc Natl Acad Sci U S A. 1994;91:913–917. doi: 10.1073/pnas.91.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert DN, Foellmer B, Helenius A. Calnexin and calreticulin promote folding, delay oligomerization and suppress degradation of influenza hemagglutinin in microsomes. EMBO J. 1996;5:2961–2968. [PMC free article] [PubMed] [Google Scholar]
- Hendrick V, Winnepenninckx P, Abdelkafi C, Vandeputte O, Cherlet M, Marique T, Renemann G, Loa A, et al. Increased productivity of recombinant tissular plasminogen activator (t-PA) by butyrate and shift of temperature: a cell cycle phases analysis. Cytotechnology. 2001;36:71–83. doi: 10.1023/A:1014088919546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SO, Lee GM. Nutrient deprivation induces autophagy as well as apoptosis in Chinese hamster ovary cell culture. Biotechnol Bioeng. 2008;99:678–685. doi: 10.1002/bit.21589. [DOI] [PubMed] [Google Scholar]
- Hwang SO, Chung JY, Lee GM. Effect of doxycycline-regulated ERp57 expression on specific thrombopoietin productivity of recombinant CHO cells. Biotechnol Prog. 2003;19:179–184. doi: 10.1021/bp025578m. [DOI] [PubMed] [Google Scholar]
- Jackson MR, Cohen-Doyle MF, Peterson PA, Williams DB. Regulation of MHC class I transport by the molecular chaperone, Calnexin (p88, IP90) Science. 1994;263:384–386. doi: 10.1126/science.8278813. [DOI] [PubMed] [Google Scholar]
- Kearse KP, Williams DB, Singer A. Persistence of glucose residues on core oligosaccharides prevents association of TCRa and TCRb with calnexin and results specifically in accelerated degradation of nascent TCRa proteins within the endoplasmic reticulum. EMBO J. 1994;13:3678–3686. doi: 10.1002/j.1460-2075.1994.tb06677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim NS, Lee GM. Overexpression of bcl-2 inhibits sodium butyrate-induced apoptosis in Chinese hamster ovary cells resulting in enhanced humanized antibody production. Biotechnol Bioeng. 2001;71:184–193. doi: 10.1002/1097-0290(2000)71:3<184::AID-BIT1008>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Kim NS, Lee GM. Inhibition of sodium butyrate-induced apoptosis in recombinant Chinese hamster ovary cells by constitutively expressing antisense RNA of caspase-3. Biotechnol Bioeng. 2002;78:217–228. doi: 10.1002/bit.10191. [DOI] [PubMed] [Google Scholar]
- Kruman I, Guo Q, Mattson MP. Calcium and reactive oxygen species mediate staurosporine-induced mitochondrial dysfunction and apoptosis in PC12 cells. J Neurosci Res. 1998;51:293–308. doi: 10.1002/(SICI)1097-4547(19980201)51:3<293::AID-JNR3>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Laubach VE, Garvey EP, Sherman PA. High-level expression of human inducible nitric oxide synthase in Chinese hamster ovary cells and characterization of the purified enzyme. Biochem Biophys Res Commun. 1996;218:802–807. doi: 10.1006/bbrc.1996.0143. [DOI] [PubMed] [Google Scholar]
- Le A, Steiner JL, Ferrell GA, Shaker JC, Sifers RN. Association between calnexin and a secretion-incompetent variant of human a1-antitrypsin. J Biol Chem. 1994;269:7514–7519. [PubMed] [Google Scholar]
- Loo TW, Clarke DM. Prolonged association of temperature-sensitive mutants of human P-glycoprotein with calnexin during biogenesis. J Biol Chem. 1994;269:28683–28689. [PubMed] [Google Scholar]
- Lynch K, Fernandez G, Pappalardo A, Peluso JJ. Basic fibroblast growth factor inhibits apoptosis of spontaneously immortalized granulosa cells by regulating intracellular free calcium levels through a protein kinase Cdelta-dependent pathway. Endocrinology. 2000;141:4209–4217. doi: 10.1210/en.141.11.4209. [DOI] [PubMed] [Google Scholar]
- Maréchal A, Tanguay PL, Callejo M, Guérin R, Boileau G, Rokeach LA. Cell viability and secretion of active proteins in Schizosaccharomyces pombe do not require the chaperone function of calnexin. Biochem J. 2004;380(Pt 2):441–448. doi: 10.1042/BJ20031546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martikainen P, Kyprianou N, Tucker RW, Isaacs JT. Programmed death of nonproliferating androgen-independent prostatic cancer cells. Cancer Res. 1991;51:4693–4700. [PubMed] [Google Scholar]
- Meldolesi J, Pozzan T. The endoplasmic reticulum Ca2+ store: a view from the lumen. Trends Biochem Sci. 1998;23:10–14. doi: 10.1016/S0968-0004(97)01143-2. [DOI] [PubMed] [Google Scholar]
- Michalak M, Corbett EF, Mesaeli N, Nakamura K, Opas M. Calreticulin: one protein, one gene, many functions. Biochem J. 1999;344:281–292. doi: 10.1042/0264-6021:3440281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak M, Robert Parker JM, Opas M. Ca2+ signaling and calcium binding chaperones of the endoplasmic reticulum. Cell Calcium. 2002;32:269–278. doi: 10.1016/S0143416002001884. [DOI] [PubMed] [Google Scholar]
- Mimura Y, Lund J, Church S, Dong S, Li J, Goodall M, Jefferis R. Butyrate increases production of human chimeric IgG in CHO-K1 cells whilst maintaining function and glycoform profile. J Immunol Methods. 2001;247:205–216. doi: 10.1016/S0022-1759(00)00308-2. [DOI] [PubMed] [Google Scholar]
- Mohan C, Park SH, Chung JY, Lee GM. Effect of doxycycline-regulated protein disulfide isomerase expression on the specific productivity of recombinant CHO cells: Thrombopoietin and antibody. Biotechnol Bioeng. 2007;983:611–615. doi: 10.1002/bit.21453. [DOI] [PubMed] [Google Scholar]
- Moore A, Donahue CJ, Hooley J, Stocks DL, Bauer KD, Mather JP. Apoptosis in CHO cell batch cultures: examination by flow cytometry. Cytotechnology. 1995;17:1–11. doi: 10.1007/BF00749215. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Bossy-Wetzel E, Burns K, Fadel MP, Lozyk M, Goping IS, Opas M, Bleackley RC, et al. Changes in endoplasmic reticulum luminal environment affect cell sensitivity to apoptosis. J Cell Biol. 2000;150:731–740. doi: 10.1083/jcb.150.4.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki Y, Ohno H, Takase K, Ochiai T, Saito T. Cell surface expression of calnexin, a molecular chaperone in the endoplasmic reticulum. J Biol Chem. 2000;275:35751–35758. doi: 10.1074/jbc.M007476200. [DOI] [PubMed] [Google Scholar]
- Oster T, Thioudellet C, Chevalot I, Masson C, Wellman M, Marc A, Siest G. Induction of recombinant human gamma-glutamyl transferase by sodium butyrate in transfected V79 and CHO Chinese hamster cells. Biochem Biophys Res Commun. 1993;193:406–412. doi: 10.1006/bbrc.1993.1638. [DOI] [PubMed] [Google Scholar]
- Ou W-J, Cameron PH, Thomas DY, Bergeron JJM. Association of folding intermediates of glycoproteins with calnexin during protein maturation. Nature. 1993;364:771–776. doi: 10.1038/364771a0. [DOI] [PubMed] [Google Scholar]
- Palermo DP, DeGraaf ME, Marotti KR, Rehberg E, Post LE. Production of analytical quantities of recombinant proteins in Chinese hamster ovary cells using sodium butyrate to elevate gene expression. J Biotechnol. 1991;19:35–48. doi: 10.1016/0168-1656(91)90073-5. [DOI] [PubMed] [Google Scholar]
- Parekh RB. Mammalian cell gene expression: protein glycosylation. Curr Opin Biotechnol. 1991;2:730–734. doi: 10.1016/0958-1669(91)90043-5. [DOI] [PubMed] [Google Scholar]
- Pind S, Riordan JR, Williams DB. Participation of the endoplasmic reticulum chaperone calnexin (p88, IP90) in the biogenesis of the cystic fibrosis transmembrane conductance regulator. J Biol Chem. 1994;269:12784–12788. [PubMed] [Google Scholar]
- Pinton P, Ferrari D, Magalhães P, Schulze-Osthoff K, Virgilio F, Pozzan T, Rizzuto R. Reduced loading of intracellular Ca (2+) stores and downregulation of capacitative Ca (2+) influx in Bcl-2-overexpressing cells. J Cell Biol. 2000;148:857–862. doi: 10.1083/jcb.148.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, Pinton P, Ferrari D, Chami M, Szabadkai G, Magalhães PJ, Virgilio F, Pozzan T. Calcium and apoptosis: facts and hypotheses. Oncogene. 2003;22:8619–8627. doi: 10.1038/sj.onc.1207105. [DOI] [PubMed] [Google Scholar]
- Roderick HL, Lechleiter JD, Camacho P. Cytosolic phosphorylation of calnexin controls intracellular Ca(2+) oscillations via an interaction with SERCA2b. J Cell Biol. 2000;149:1235–1248. doi: 10.1083/jcb.149.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siffroi-Fernandez S, Giraud A, Lanet J, Franc JL. Association of the thyrotropin receptor with calnexin, calreticulin and BiPEffects on the maturation of the receptor. Eur J Biochem. 2002;269:4930–4937. doi: 10.1046/j.1432-1033.2002.03192.x. [DOI] [PubMed] [Google Scholar]
- Singh RP, Al-Rubeai M, Gregory CD, Emery AN. Cell death in bioreactors: a role for apoptosis. Biotechnol Bioeng. 1994;44:720–726. doi: 10.1002/bit.260440608. [DOI] [PubMed] [Google Scholar]
- Sung YH, Lee GM. Enhanced human thrombopoietin production by sodium butyrate addition to serum-free suspension culture of bcl-2-overexpressing CHO cells. Biotechnol Prog. 2005;21:50–57. doi: 10.1021/bp049892n. [DOI] [PubMed] [Google Scholar]
- Sung YH, Song YJ, Lim SW, Chung JY, Lee GM. Effect of sodium butyrate on the production, heterogeneity and biological activity of human thrombopoietin by recombinant Chinese hamster ovary cells. J Biotechnol. 2004;112:323–335. doi: 10.1016/j.jbiotec.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Tector M, Salter RD. Calnexin influences folding of human class I histocompatibility proteins but not their assembly with B2-microglobulin. J Biol Chem. 1995;270:19638–19642. doi: 10.1074/jbc.270.8.3944. [DOI] [PubMed] [Google Scholar]
- Tomassini B, Malisan F, Franchi L, Nicolò C, Calvo GB, Saito T, Testi R. Calnexin suppresses GD3 synthase-induced apoptosis. FASEB J. 2004;18:1553–1555. doi: 10.1096/fj.04-1675fje. [DOI] [PubMed] [Google Scholar]
- Tombal B, Denmeade SR, Isaacs JT. Assessment and validation of a microinjection method for kinetic analysis of [Ca2+]i in individual cells undergoing apoptosis. Cell Calcium. 1999;25:19–28. doi: 10.1054/ceca.1998.0005. [DOI] [PubMed] [Google Scholar]
- Vassilakos A, Cohen-Doyle MF, Peterson PA, Jackson MR, Williams DB. The molecular chaperone calnexin facilitates folding and assembly of class I histocompatibility molecules. EMBO J. 1996;15:1495–1506. [PMC free article] [PubMed] [Google Scholar]
- Wada I, Rindress D, Cameron PH, Ou W, Doherty JJ, Louvard D, Bell AW, Dignard D, et al. SSRa and associated calnexin are major calcium binding proteins of the endoplasmic reticulum membrane. J Biol Chem. 1991;226:19599–19610. [PubMed] [Google Scholar]
- Walter L, Hajnóczky G. Mitochondria and endoplasmic reticulum: the lethal interorganelle cross-talk. J Bioenerg Biomembr. 2005;37:191–206. doi: 10.1007/s10863-005-6600-x. [DOI] [PubMed] [Google Scholar]
- Wurm FM, Petropoulos CJ, O’Connor JV. Manufacture of proteins based on recombinant Chinese hamster ovary cells: assessment of genetic issues and assurance of consistency and quality. In: Schmidt ER, Hankeln T, editors. Transgenic organisms and biosafety. Berlin: Springer; 1996. p. 546. [Google Scholar]
- Yoon SK, Hong JK, Lee GM. Effect of simultaneous application of stressful culture conditions on specific productivity and heterogeneity of erythropoietin in Chinese hamster ovary cells. Biotechnol Prog. 2004;20:1293–1296. doi: 10.1021/bp034382z. [DOI] [PubMed] [Google Scholar]
- Zirpel L, Lippe WR, Rubel EW. Activity-dependent regulation of [Ca2+]i in avian cochlear nucleus neurons: roles of protein kinases A and C and relation to cell death. J Neurophysiol. 1998;79:2288–2302. doi: 10.1152/jn.1998.79.5.2288. [DOI] [PubMed] [Google Scholar]
- Zuppini A, Groenendyk J, Cormack LA, Shore G, Opas M, Bleackley RC, Michalak M. Calnexin Deficiency and Endoplasmic Reticulum Stress-Induced Apoptosis. Biochemistry. 2002;41:2850–2858. doi: 10.1021/bi015967+. [DOI] [PubMed] [Google Scholar]