Abstract
Rationale
Reinforcing abstinence from drug use with alternative nondrug reinforcers (e.g., contingency management) is one of the most effective interventions for drug abuse. While nonhuman studies have also shown that access to alternative nondrug reinforcers reduces drug self-administration, this effect has not been examined in nicotine self-administration models. Moreover, abstinence contingencies per se under free-operant conditions have not been examined.
Objective
The objective of this experiment was to begin development of a model of contingency management interventions by employing a differential-reinforcement-of-alternative-behavior (DRA) schedule of alternative nondrug reinforcement in rats self-administering nicotine.
Methods
Two groups of rats were trained to self-administer nicotine under a multiple schedule of nicotine and sucrose delivery. The DRA-group was then exposed to an interlocking FR3 nicotine DRA t-sec sucrose schedule. Under this schedule, nicotine continued to be available under the FR schedule while a sucrose pellet was made available contingent upon every pause in self-administration responding (DRA interval) of 40, 80, or 160 sec. The FT-group was exposed to noncontingent delivery of sucrose under fixed time (FT) schedules at an average rate equal to that obtained under the DRA schedule in the DRA-group.
Results
The DRA schedule significantly reduced NSA by 73, 69, and 59% at the DRA 40, 80, and 160 sec intervals, respectively, compared to baseline, while noncontingent sucrose had no significant effect. The effect of the DRA schedule was apparent throughout the NSA sessions.
Conclusions
The present assay approximates the abstinence contingencies arranged in contingency management interventions for drug abuse and provides a preliminary nonhuman model of such interventions.
Introduction
Several types of behavioral treatments have been examined for their efficacy in facilitating cessation of drug use in humans. Of these, Contingency Management (CM) interventions are one of the most effective (Carroll and Rounsaville 2007; Higgins et al. 2008; Lussier et al. 2006). CM involves delivering alternative nondrug reinforcers (e.g., money, vouchers, employment, housing) contingent upon biochemically-verified abstinence from drug use. CM interventions are effective at increasing abstinence rates for a wide range of drugs (e.g., cocaine, nicotine, heroin) in a wide range of populations (e.g., pregnant women, adolescents, schizophrenics) (Higgins et al. 2008; Lussier et al. 2006; Prendergast et al. 2006). The well established efficacy of CM interventions and the fact that they are based on basic principles of operant conditioning that have continuity across species makes them well suited for translation into a nonhuman model, in contrast to other types of behavioral treatments (e.g., 12-step programs, cognitive therapy, psychoanalysis). Development of a nonhuman model of CM interventions for drug abuse is important for several reasons.
First, while the primary focus on neural mechanisms in nonhuman drug self-administration models has been very useful for discovery of neurobiological substrates of drug reinforcement and potential targets for medication development (Lerman et al. 2007), it has resulted in much less emphasis on the important role behavioral variables play in controlling drug self-administration (Carroll 1994; Johanson 1975; LeSage et al. 1999; Poling and Appel 1979; Spealman and Goldberg 1978; Woolverton 1992). Given that combining medications with behavioral interventions is the most effective clinical approach to treating drug abuse (Carroll et al. 2004; Stitzer and Walsh 1997), one could argue that nonhuman research on behavioral mechanisms of drug reinforcement is at least as important as that focused on neuropharmacological mechanisms (LeSage et al. 1999; Woolverton 1992). Second, parametric research on the effects of variations in alternative reinforcement contingencies could be conducted that may be impractical in humans, due to the substantial cost of CM interventions, the time required, and the relative lack of experimental control over potentially important variables (e.g., learning and drug history, polydrug use, concurrent psychological disorders). Third, studies in nonhumans allow examining the effects of combining alternative reinforcement with novel medications not yet approved for used in humans. Fourth, the applicability of alternative reinforcement to some drug abuse phenomena, such as acquisition and provoked relapse, can be experimentally examined more readily in nonhumans. Finally, neurobiological mechanisms underlying the efficacy of combining alternative reinforcement with novel medications can be more easily studied in nonhumans. Such work may be helpful in identifying medications that might best compliment behavioral interventions.
Numerous studies have shown that availability of alternative nondrug reinforcers can reduce drug self-administration in nonhumans under free-operant concurrent schedules or discrete-trials choice assays (Carroll et al. 1989; Carroll and Lac 1993; Carroll 1985; Nader and Woolverton 1991, 1992b; Anderson and Woolverton 2002). However, these studies have not arranged abstinence contingencies per se that are analogous to those in CM interventions. Discrete-trials procedures (e.g., Nader and Woolverton 1991; Foltin 1999; Negus 2003) have involved a mutually-exclusive choice between drug and food. Although subjects have had to refrain from responding for drug in order to receive food on a given trial under such procedures, availability of food on that trial has not depended upon whether subjects abstained from drug intake on previous trials (i.e., no abstinence period was specified). Moreover, discrete-trials procedures are not analogous to the free-operant environment of a drug abuser (i.e., drug is essentially always available during a cessation attempt in outpatient settings). When free-operant procedures have been employed (e.g., Carroll et al. 1989; Lenoir and Ahmed 2007), subjects have been allowed free access to an alternative reinforcer, rather than arrange access to the alternative reinforcer contingent on abstaining from self-administration.
CM interventions represent a type of differential-reinforcement schedule, under which reinforcement is contingent upon the non-occurrence of a target behavior (drug use) and occurrence of other behavior. Such differential-reinforcement schedules have been used extensively as a response-deceleration technique in both basic and clinical research (Poling and Ryan, 1982). For example, in one of the first studies of differential-reinforcement-of-other-behavior (DRO) schedules (Zeiler 1979), food-deprived pigeons previously trained to key-peck under a fixed-ratio (FR) schedule of food delivery received food following a specified interval of not responding, in addition to the food earned under the FR schedules. In other words, pigeons were reinforced for abstaining from key-pecking (i.e., engaging in other behavior). Exposure to the DRO schedule reduced key-pecking to low levels comparable to that achieved by extinction. Applying similar differential reinforcement schedules in nonhuman models of drug abuse has been suggested as a way of modeling CM interventions (Hughes and Bickel 1997; LeSage and Glowa 2000; LeSage et al. 1999). One recent study reported that a DRO schedule of food delivery hastened the rate of extinction of cocaine self-administration in rats in a novel context, and this history subsequently decreased reinstatement of cocaine self-administration when rats were returned to the original context in which cocaine self-administration was trained (Kearns and Weiss 2007). However, cocaine was never available under the DRO schedule or during the reinstatement test. The purpose of the present experiment was to examine the direct effects of a differential-reinforcement-of-alternative-behavior (DRA) schedule of sucrose delivery on nicotine self-administration in rats, as a preliminary step in developing a nonhuman model of CM interventions. A DRA schedule is identical to a DRO schedule, except that an alternative response is required to obtain the alternative reinforcer, whereas no such response is required under a DRO schedule. A DRA schedule was chosen instead of a DRO schedule because it involves quantification of a specified alternative behavior, which is more analogous to the clinical approach of reinforcing specific alternative behaviors that are consistent with treatment goals (e.g., attending therapy sessions, Higgins et al. 2008). Though not the focus of the present study, a DRA schedule would also allow for detecting nonspecific effects of pharmacological treatments when combined with alternative reinforcement. Nicotine was chosen because the effects of alternative reinforcement have not yet been studied with this drug, while numerous studies in humans have demonstrated the efficacy of CM interventions in facilitating smoking cessation (Prendergast et al. 2006).
Materials and Methods
Subjects
Sixteen Male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing 350–400 g were maintained under a restricted feeding regimen (18–20 g/day) to prevent excess weight gain and maintain good health. Rats were housed individually in a temperature-and humidity-controlled environment with unlimited access to water on a reversed 12 hr light/dark cycle (lights off at 10:00 am) and allowed to recover from shipment for approximately two weeks prior to being placed in an experiment. Animal husbandry and experimental protocols were approved by the Institutional Animal Care and Use Committee of the Minneapolis Medical Research Foundation and were in accordance with the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council 2003).
Apparatus
Experimental sessions occurred in eight operant-conditioning chambers (MED Associates, St. Albans, VT). The front panel contained two response levers, one on the left side of the panel that served as the sucrose lever that activated the sucrose pellet dispenser and one on the right side of the panel (inactive lever) to measure nonreinforced responding, and a receptacle mounted between the levers to allow delivery of 45 mg sucrose pellets (Test Diet, Richmond, IN). Four of the chambers were equipped with a photocell on the receptacle that allowed measurement of nose pokes into the receptacle. The back panel of the chamber contained the drug lever that activated the infusion pump for nicotine delivery. The chambers were also equipped with white stimulus lights over each response lever and a tone module to present an auditory stimulus. Masking of extraneous noise was provided by a continuous white noise generator and operation of exhaust fans built into the sound-attenuating box in which each chamber was housed. Infusion pumps (Model RHSY, Fluid Metering, Syosset, NY) placed outside of each chamber, but within the sound-attenuating box, delivered infusions through PE 90 tubing connected to a fluid swivel mounted above the chamber and from the swivel through a stainless steel spring tether connected to a guide cannula mounted in a harness assembly on the animal’s back. MED-PC IV software (MED Associates, St. Albans, VT) was used for chamber operation and data acquisition.
Drugs
Nicotine bitartrate (Sigma Chemical, St. Louis, MO) was dissolved in sterile hepranized saline (30 U/ml). The pH of the solution was adjusted to 7.4 with dilute NaOH. All nicotine doses are expressed as the base.
Surgery
After recovery from shipment (1 wk), each rat was implanted with a chronic indwelling jugular catheter under droperidol (2 mg/kg) and fentanyl (0.04 mg/kg) anesthesia. Materials and methods were similar to those used routinely in our laboratory (e.g., (LeSage et al. 2004, 2003). The catheter (0.20 mm o.d. x 0.037 mm i.d., silicone tubing) was inserted into the right jugular vein and pushed down toward the heart until it terminated outside the right atrium (approximately 30 mm). The catheter was anchored to tissue in the area and continued subcutaneously to the back where it exited between the scapulae and connected to a 22-gauge guide cannula mounted in a silicone-strap harness assembly on the back of the rat (Covance Infusion Harness, Instech Laboratories, Plymouth Meeting, PA). Following surgery, each rat received a daily i.v. infusion of antibiotic (enrofloxacin, 1.1 mg) dissolved in heparinized saline for three consecutive days. Rats were allowed a minimum of four days to recover from surgery before beginning an experiment. To maintain patency, catheters were flushed daily with heparinized saline (30 units/ml). In addition, the flushing solution contained 0.67 mg/ml streptokinase twice weekly. The swivel and tether assembly were counter-balanced to permit relatively unrestrained movement of the animal. At the end of each session, the tether was disconnected, the catheter flushed, and a Tygon cap placed on the guide cannula before the rats were returned to their home cages. The daily allotment of food was provided in the home cage no sooner than 30 min after each session.
Self-Administration Training Procedures
Procedures were similar to those that have proven effective in training and maintaining nicotine self-administration (NSA) in previous studies using limited access conditions (e.g., Corrigall and Coen 1989; Donny et al. 1995; LeSage et al. 2004). Rats were not trained to press the drug lever with food prior to NSA training. During each 1-hr session, the stimulus light above the drug lever was illuminated and an FR 1 schedule of NSA was arranged. Under this schedule, each press of the drug response lever produced an infusion of nicotine (0.03 mg/kg/inf) dissolved in the heparinized saline vehicle. Based on published studies, this unit dose of nicotine maintains stable self-administration and lies near the peak of the unit dose-response curve under small FR schedules (e.g., Corrigall and Coen, 1989; Donny et al., 1998; LeSage et al., 2004). Infusions were delivered in a volume of 0.1 ml/kg in 1 sec. Responses on the other two levers were recorded but had no programmed consequences. Infusions were signaled by offset of the stimulus light over the drug lever which remained off for the duration of the infusion and a subsequent 5-sec timeout (TO), during which responses on all levers were recorded but had no programmed consequences. Once robust responding developed under the FR 1 schedule, the FR value was gradually increased to FR 3 across several sessions. Rats were considered to have acquired self-administration when, under the FR 3 schedule, they earned at least 8 nicotine infusions per session and the ratio of drug to inactive lever presses was at least 2:1 for three consecutive sessions. These criteria are common in studies of NSA under limited access conditions. NSA was judged stable when there was no trend in the number of infusions per session across three consecutive sessions.
Multiple Schedule of Drug and Sucrose Delivery Training
The purpose of this phase of training was to engender sucrose-maintained behavior. After stable self-administration was obtained under the FR 3 schedule, rats were placed on one of two versions of a multiple schedule of drug and sucrose delivery. For rats to be exposed to abstinence-contingent sucrose delivery (DRA-group), each self-administration session began with a 1-hr component for NSA. During this component an FR 3 schedule of drug delivery was in effect identical to that during the training sessions described above. After completion of the drug component, a 5-min timeout occurred during which all stimulus lights were extinguished and responses on all levers were recorded but had no programmed consequence. Then, a 30-min component of sucrose delivery occurred, during which a discrete-trial FR 1 schedule of sucrose delivery was arranged. Under this schedule, the stimulus light above the sucrose lever was illuminated and a sucrose pellet became available after a variable inter-trial interval (ITI) averaging 1 min. Sucrose availability was signaled by onset of a tone, at which point a single lever press on the sucrose lever produced a sucrose pellet, 0.25-sec offset of the stimulus light, offset of the tone, and initiation of the next ITI. Responses on the other two levers (drug and inactive lever) were recorded but had no programmed consequences. For rats to be exposed to noncontingent sucrose delivery (FT-group), a similar multiple schedule as that just described was used, but no lever press was required for delivery of sucrose pellets. That is, a variable-time (VT) 1-min schedule of sucrose delivery was arranged. When the VT timer elapsed, a 1-sec tone was presented followed by 0.25-sec offset of the stimulus light above the sucrose lever, delivery of a sucrose pellet, and initiation of the next VT interval. Under both versions of the multiple schedule, sessions ended after completion of the 30-min sucrose component. After drug self-administration and sucrose maintained behavior stabilized under the multiple schedule (no trend in the number of drug infusions and a mean lever-press latency under the FR 1 sucrose schedule or mean latency to procure pellets under the VT sucrose schedule of less than 5 sec over three consecutive sessions), rats were exposed to one of two conjoint schedules of nicotine and sucrose delivery. The multiple schedule served as the baseline schedule throughout the experiment in order to ensure that responding for sucrose was well maintained.
Interlocking Schedule of Nicotine and Sucrose Delivery
The DRA-group was exposed to an interlocking FR 3 nicotine DRA t -sec sucrose schedule. Interlocking schedules involve two concurrent schedules under which a parameter of one schedule changes as a function of performance under the other schedule (Lattal 1991). Under this schedule in the present study, responses on the nicotine lever continued to produce nicotine infusions under the FR 3 schedule, while, at the same time, pauses in responding on the nicotine lever that were t-sec in duration resulted in onset of a tone signaling availability of a sucrose pellet. A single response on the sucrose lever then produced a single sucrose pellet, 0.25-sec offset of the stimulus light above the sucrose lever, offset of the tone, and initiation of the next DRA interval. Responses on the nicotine lever before the DRA interval elapsed (before onset of the tone) reset the DRA interval. Responses on the nicotine lever after the DRA interval elapsed (i.e., during the tone), turned off the tone and reset the DRA interval timer. Responses on the sucrose lever before the DRA interval elapsed were recorded but had no programmed consequence. DRA intervals of 40, 80, and 160 sec were examined in each rat, arranged in a randomized order across subjects. These DRA values were based on pilot data and were sufficiently short to allow contact with the DRA contingency, which is essential to the efficacy such contingencies (Lamb et al. 2004; Poling and Ryan 1982). All DRA intervals were tested in each rat, with each interval in effect for at least 5 sessions and until responding was stable (no trend in number of infusions per session across three consecutive sessions). The baseline multiple schedule was reinstated between each DRA schedule phase until NSA stabilized.
The FT-group was exposed to noncontingent delivery of sucrose pellets under a concurrent FR 3 nicotine FT t-sec sucrose schedule. Under this schedule, nicotine was available under the FR 3 schedule while sucrose pellets were delivered regardless of pauses in NSA. The FT values to which each rat in the FT-group was exposed was yoked to the mean inter-pellet interval of sucrose delivery obtained by a master rat from the DRA-group that exhibited a similar baseline rate of self-administration. Thus, in rats with baseline infusion rates of <15 infusions/session, FT values of 42, 83, and 172 sec were used as the noncontingent control for the DRA 40, 80, and 160 sec schedules, respectively, while values of 42, 88, and 256 sec were used for rats with baseline infusion rates of ≥15 infusions/session. This served to match rates of sucrose delivery between the DRA and FT schedules. All FT values were tested in each rat, with each interval in effect for at least 5 sessions and until responding was stable (no trend in number of infusions per session across three consecutive sessions). The baseline multiple schedule was reinstated between each FT schedule phase until NSA stabilized.
Data Analysis
The number of drug infusions per session served as the primary dependent measure for drug self-administration. The number of sucrose pellets delivered per session was the primary measure of sucrose-maintained behavior. Overall- and within-session assessment of nicotine infusion rates were conducted to examine both between- and within-session patterns of responding. Within-session analysis was conducted to examine whether satiation to sucrose at shorter DRA intervals resulted in reduced efficacy late in the NSA session (cf. Negus 2003). Each dependent measure was calculated as a percentage of baseline (mean of the three baseline sessions preceding each DRA interval phase, averaged across the three baseline determinations). Effects of the DRA and FT schedules of sucrose delivery on overall mean infusions were compared using a general linear model (GLM), with schedule type as a nonrepeated factor and schedule value as a repeated factor, followed by Bonferroni post-hoc tests. Effects of each type of sucrose schedule were also examined within each group using one-way repeated measures ANOVA, followed by Dunnet’s post hoc test comparing infusion rates under each sucrose schedule to baseline. Because analysis of overall infusions only showed a significant difference between groups at the longest DRA and FT schedules, comparison of within-session effects between groups was analyzed statistically only at this schedule value using a GLM analysis, with schedule type as a nonrepeated factor and session segment (10-min) as a repeated factor, followed by Bonferroni post-hoc tests. In addition, the frequency of sucrose-maintained behavior in the DRA-group (total sucrose lever presses plus nose pokes in the pellet trough) and FT-group (total nose pokes in the pellet trough) under the longest DRA and FT schedule were compared via t-test in a subset of rats for which nose-poke data were available. This subset of rats included seven from the original 16 (four from the DRA-group, three from the FT-group). Due to the small sample size in each group, data from an additional five rats were included (one from the DRA-group, four from the FT-group). These rats were exposed to the longest DRA or FT schedule, but were excluded from the analyses above because catheter failure prevented testing under all of the schedule values. Catheter patency and baseline performance was confirmed in these rats both before and after exposure to the DRA or FT schedule. Significance level for all statistical analyses was p < 0.05.
Results
Mean (±SEM) baseline infusions earned per session were 16.0 ± 2.6 and 13.7 ± 1.2 for rats exposed to the DRA and FT sucrose schedules, respectively. Mean baseline responses on the nicotine, inactive, and sucrose levers (during the sucrose component) were 47.4 ± 7.4, 5.8 ± 0.6, and 106.1 ± 21.5, respectively, for rats in the DRA-group. Mean baseline response rates on the nicotine and inactive levers were 41.7 ± 3.8 and 4.1 ± 0.8, respectively, for rats in the FT-group. Mean baseline infusions earned during each of 6 consecutive 10-min segments of baseline sessions for each group are shown in Table 1. In both groups, the highest NSA rates occurred in the first 10 min of the session, indicating a drug “loading” phase. No statistically significant differences in any of these baseline measures were observed between groups.
Table 1.
Mean (±SEM) infusions earned during each consecutive 10-min segment of baseline sessions.
| Session Segment | ||||||
|---|---|---|---|---|---|---|
| Group | 1 | 2 | 3 | 4 | 5 | 6 |
| DRA | 3.7 ± 0.41 | 2.3 ± 0.43 | 2.5 ± 0.39 | 2.2 ± 0.44 | 2.3 ±0.38 | 2.3 ± 0.39 |
| FT | 4.2 ± 0.31 | 2.1 ± 0.21 | 1.9 ± 0.25 | 1.9 ± 0.23 | 1.8 ± 0.23 | 1.9 ± 0.21 |
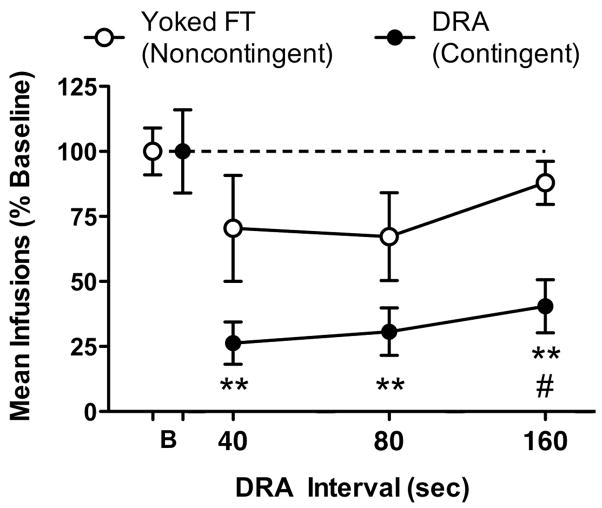
Figure 1 shows the effect of each sucrose schedule on mean nicotine infusions earned per session, expressed as a percentage of baseline. GLM analysis indicated a significant main effect of schedule type (F=10.9, p<0.01), and no main effect of schedule value or schedule type x value interaction. Post hoc tests indicated NSA was decreased to a greater extent in rats exposed to the DRA 160-sec sucrose schedule compared to rats exposed to the associated FT schedule (t=2.7, p<0.05). Within group analyses indicated that the DRA sucrose schedule significantly reduced NSA (F=16.2, p<0.0001) by 73, 69, and 59% (q=5.9, 6.1, 4.6, respectively, p<0.001) at the DRA 40, 80, and 160 sec intervals, respectively, compared to baseline. In contrast, no statistically significant reduction in NSA was observed at any FT interval compared to baseline. However, marked variability in NSA was observed at the two lowest FT intervals, attributable to marked suppression of NSA in two rats at these intervals. The mean number of sucrose pellets delivered under each DRA interval was 82.5 (±0.9), 39.9 (±1.2), and 18.3 (±1.0) at the DRA 40, 80, and 160 sec intervals, respectively; and 83 (± 0), 41.4 (±0.4), and 18.3 (±1.1) at each of the respective yoked FT intervals. No significant difference between groups was observed, indicating essentially identical overall rates of sucrose delivery under the two schedules.
Figure 1.
Mean (±SEM) nicotine infusions earned under an FR 3 schedule (expressed as percentage of baseline) during sessions when the indicated DRA or FT schedule of sucrose pellet delivery was conjointly in effect. The FT interval values were yoked to the average inter-pellet interval obtained under the DRA schedule (see text for further details). Each point is the mean of the last three sessions at each interval value (log scale) in seven (FT) or nine (DRA) rats. The dashed line indicates baseline level of performance. **Significantly different from baseline, p<0.01. #Significantly different from the FT group, p<0.05.
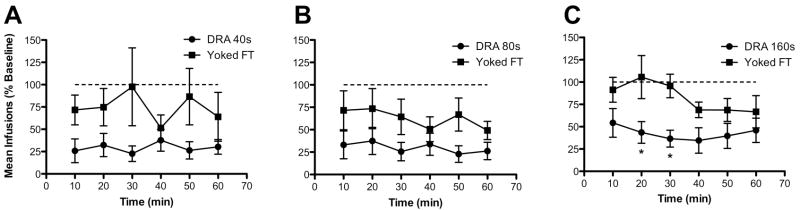
Figure 2 shows within session patterns of NSA as a percentage of baseline under each DRA and associated FT schedule of sucrose delivery. Rats exposed to the DRA schedule exhibited a decrease in NSA within the first 10-min of the session, and this suppression was maintained across the remainder of the session. Rats exposed to the FT schedule showed no significant reduction in NSA early in the session, and the patterns of NSA were variable both within and between each FT schedule. Statistical analysis of within session patterns of NSA under the DRA 160 sec schedule and associated FT schedule of sucrose delivery indicated a significant main effect of schedule type (F=4.5, p<0.05) and no significant main effect of session segment or schedule type x segment interaction. Post hoc tests indicated that NSA was decreased to a greater extent in rats exposed to the DRA schedule compared to the FT schedule at 20 and 30 min into the session (t=3.0 and 2.9, respectively, p<0.05).
Figure 2.
Mean (±SEM) nicotine infusions earned during consecutive 10-min segments of sessions when the DRA or FT sucrose schedules were in effect. Each panel shows data obtained under a different DRA or FT interval. See Figure 1 for further details. *Significantly different from the FT group, p<0.05.
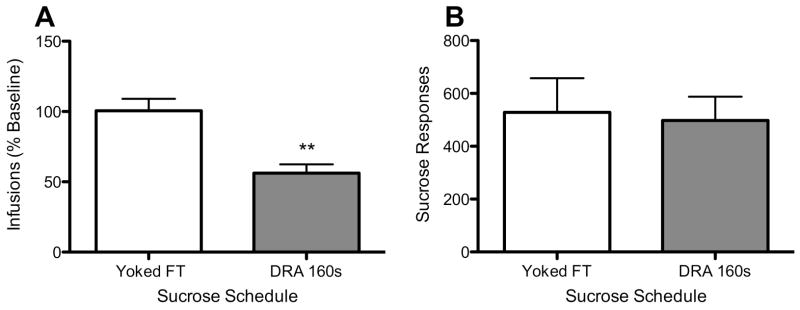
Figure 3 shows the mean number of infusions when the DRA 160-sec or associated FT sucrose schedule was in effect in rats for which nose poke data were available (Panel A). The DRA schedule significantly reduced NSA by 43.8% in this subset of rats (t=3.9, p<0.01), while the FT schedule had no effect. Also shown is the mean number of sucrose-maintained responses when the DRA 160-sec (lever presses plus nose pokes in the pellet receptacle) or associated FT sucrose schedule was in effect (nose pokes, Panel B). There was no difference in the frequency of sucrose-maintained responses between groups.
Figure 3.
Panel A: mean (±SEM) nicotine infusions earned when the DRA 160-sec or associated FT sucrose schedule was in effect. Panel B: mean number of sucrose responses emitted under each sucrose schedule (i.e., lever presses plus nose pokes in the pellet receptacle under the DRA schedule or number of nose pokes under the associated FT schedule). Data are the means of a subset of seven rats (DRA-group N=4, FT-group N=3) for which nose poke data were available, plus 5 additional rats for which data from these schedules was available (DRA-group N=1, FT-group, N=4), but that did not complete testing under all schedule values. See Figure 1 and text for further details. **Significantly different from the FT schedule, p<0.01.
Discussion
The main findings of the present study were that a) a DRA schedule of alternative nondrug reinforcement significantly decreased NSA, b) noncontingent delivery of a nondrug alternative reinforcer had no significant effect on NSA for subjects as a group, indicating that abstinence contingent reinforcement was more effective than noncontingent reinforcement in decreasing NSA, c) attenuation of NSA by the DRA schedule was apparent throughout the session, indicating that satiation to sucrose did not develop at shorter DRA intervals and result in reduced efficacy late in the session, d) some subjects showed significant decreases in NSA under the two shortest FT schedules, suggesting that relatively high rates of noncontingent alternative nondrug reinforcement may have the potential to reduce drug self-administration in some individuals.
Findings from the present study are consistent with numerous studies showing that access to alternative nondrug reinforcers decreases drug self-administration in nonhumans (e.g., Carroll 1993, 1998; Lenoir and Ahmed 2007; Nader and Woolverton 1992a; Negus 2003) and demonstrate that this effect extends to nicotine self-administration. They also extend previous work by directly comparing the efficacy of abstinence-contingent and noncontingent alternative reinforcement in suppressing drug-maintained responding. The finding of better efficacy with abstinence-contingent reinforcement is analogous to the differences in effect size that are apparent between some previous studies employing different schedules of alternative nondrug reinforcement. For example, larger decreases in cocaine self-administration in rhesus monkeys have been reported in studies using discrete trials choice procedures (e.g., Nader and Woolverton 1991) compared to those using concurrent free operant schedules (e.g., Comer et al. 1994; Rodefer et al. 1997). Under discrete trials procedures, choice between drug and food was mutually exclusive. Thus, delivery of food on a given trial required declining the drug choice on that trial, which is somewhat analogous to the abstinence contingency in the present study. Under concurrent schedules, the alternative reinforcer was freely available regardless of whether cocaine self-administration occurred, which is somewhat analogous to the noncontingent sucrose schedule in the present study. Although other variables may account for the differences between these studies (e.g., route of administration, type or magnitude of alternative reinforcer), the present findings support the notion that the greater efficacy observed under discrete trials procedures may also be due, in part, to the mutually exclusive choice arranged in those procedures. Taken together, these findings suggest that further study of factors that contribute to the efficacy of abstinence contingencies in reducing drug self-administration may be vital to continued development of valid and useful nonhuman models of CM interventions.
The lack of effect of noncontingent sucrose delivery under the FT schedule is consistent with a prior study reporting no effect of an FT 1-min schedule of food delivery on the maintenance of nicotine self-administration in rhesus monkeys (Slifer 1983). Interestingly, this contrasts with laboratory studies in human smokers showing schedule-induced increases in smoking while performing an alternative intermittently-reinforced operant task (Cherek 1982; Wallace and Singer 1976). However, FT schedules of food delivery increase acquisition of NSA in nonhumans with relatively low unit doses that do not normally serve as positive reinforcers (Falk et al. 1994; Slifer 1983). Why schedule induction is not observed during maintenance of NSA in nonhumans but is observed in experienced smokers in unclear, but could be attributable to several procedural differences between studies, such as route of nicotine delivery (i.v. versus smoke inhalation), type of inducing stimulus (food versus money), or nutritional state of the subjects (e.g., level of food deprivation).
While there was no overall effect of noncontingent sucrose delivery, some subjects showed significant decreases in NSA under the two shortest FT schedules. This finding suggests that relatively high rates of noncontingent alternative nondrug reinforcement may have the potential to reduce drug self-administration in some individuals. The mechanism for this effect is unclear, but one possibility is that “superstitious” reinforcement of abstinence from NSA (i.e., engaging in other behaviors) occurred in these individuals. FT schedules of food delivery are known to increase the rate of a variety of behaviors as a result of their contiguity with food delivery, and that such behaviors are more likely with relatively short FT intervals (e.g., Skinner 1948; Staddon and Simmelhag 1971). Development of such behavior could have interfered with NSA. However, rates of inactive lever pressing were not affected by the FT schedule in the present study, and no other behavior patterns were measured to examine this mechanism further.
Suppression of NSA by abstinence-contingent alternative reinforcement in the present study extends the generality of findings from previous studies of DRO and DRA schedules in three ways. First, the majority of target behaviors in nonhuman research on DRO and DRA schedules have been maintained by nondrug reinforcers (e.g., food). Although Kearns and Weiss (2007) employed a DRO schedule to facilitate extinction of cocaine self-administration, they did not assess the effects of the DRO during maintenance of cocaine self-administration. The present study therefore extends this research by demonstrating suppression of a drug-maintained target behavior. Second, DRO and DRA schedules in the form of CM interventions have been shown to reduce smoking in both human laboratory and clinical studies (Prendergast et al. 2006; Roll and Higgins 2000). Thus, with respect to nicotine maintained-behavior, the present findings extend the generality of effects across species. Third, in CM studies that have directly compared the efficacy of abstinence-contingent versus noncontingent vouchers for facilitating cessation, noncontingent vouchers were less effective than contingent vouchers (Higgins et al. 2008; Lussier et al. 2006). The present study extends this finding to a nonhuman drug self-administration model.
There are a number of limitations to the present study. First, the range of DRA intervals studied was not wide enough to demonstrate interval-dependent efficacy of the schedule, as typically shown with other target behaviors in nonhumans (Zeiler 1979). Although a DRA 320-sec schedule was examined in a few of the present subjects after completion of this study (data not presented) and appeared to be much less effective, future studies are needed to characterize fully the interval-effect function for suppression of NSA under DRA schedules of alternative nondrug reinforcement. Second, the FT schedule provided only an average rate of sucrose delivery that was the same as that under the DRA schedule. Given that NSA rates varied during baseline sessions (i.e., the highest rates were observed in the first 10 min), within session variation in the rate of sucrose delivery may have also occurred under the DRA schedule (i.e., lower rates in the first 10 min). Future studies employing a more precise yoking of alternative reinforcement rates to the DRA schedule would provide a more ideal noncontingent control schedule. Third, each DRA interval was examined for a limited number of sessions (i.e., at least 5, but typically no more than 7–10). Because some studies have reported transient effects of alternative nondrug reinforcement in some subjects (see Carroll and Lac 1993), it will be important to study the durability of the DRA schedule’s effects during more chronic exposure. Alternatively, short phases in the present study may not have been long enough to allow maximal learning of the abstinence contingency. Thus, more chronic exposure to the DRA schedule may result in increased efficacy and a rightward shift in the DRA interval-effect function. Fourth, there are important features of CM interventions that were not incorporated into the DRA schedule used in the present study. For example, CM interventions typically employ a progressive increase in voucher value after each consecutive period of abstinence, and a reset to the initial voucher value if relapse occurs. Because these contingencies are known to contribute to the efficacy of CM interventions (Roll and Higgins 2000; Roll et al. 1996; Roll and Shoptaw 2006), it will be important to incorporate similar contingencies into the DRA procedures used in the present study in order to develop a more valid nonhuman model.
Another limitation concerns the use of a FT schedule for the control condition. Unlike the DRA schedule, this schedule did not require a lever press for sucrose delivery. This difference in response requirement may have contributed to differences in the effects of the two schedules, independent of the abstinence contingencies. The DRA schedule could be viewed as a signaled variable-interval schedule on the sucrose lever, in which the interval value was determined by response rates on the nicotine lever. Such a schedule might have engendered higher rates of sucrose responding that interfered with NSA not because of the abstinence contingency, but because more time was spent responding for sucrose under the DRA schedule and less time was available for NSA. However, the FT schedule also maintained high rates of nose poking for sucrose. In fact, the frequency of sucrose lever presses plus nose pokes in the DRA group was equal to the frequency of nose pokes alone in the FT-group. These data support the conclusion that the effects of the DRA schedule are due to the abstinence contingency, not differences in rates of sucrose-maintained behavior engendered by the two schedules. Nonetheless, future studies should employ a control schedule that specifies the same response requirement as the DRA schedule, as this would be more analogous to CM studies in which the same response requirement (e.g. clinic attendance) is required for both contingent and noncontingent vouchers.
Despite these limitations, the present study provides an initial model of CM interventions for drug abuse and a preliminary step toward investigation of behavioral and pharmacological variables that could enhance the efficacy of such interventions. Use of a nonhuman CM model may help to improve the validity of drug self-administration procedures for screening novel medications for drug abuse. Current drug self-administration screening procedures do not typically use alternative reinforcers to motivate “abstinence” (but see Comer et al. 1996; Negus 2003; Rodefer et al. 1997). As such, these standard models are more analogous to human studies of medications for reduction of drug use in people not motivated to quit (e.g., smoking reduction interventions, Hatsukami et al. 2007; LeSage et al. 2003) than for cessation of drug use in people who are motivated to quit via behavioral interventions (e.g., late-phase clinical trials). Thus, a nonhuman model of CM that motivates abstinence from drug self-administration would provide a behavioral platform for assessment of novel medications for drug abuse more analogous to human clinical trials for cessation. Such models may help advance translational research in medication development, where the predictive validity of nonhumans models has not been well established (Lerman et al. 2007).
Acknowledgments
The author thanks Danielle Burroughs and Erianne Gustaf for their technical assistance, as well as Drs. Paul Pentel, Marylin Carroll, Steven Higgins, Kenneth Silverman, and Dorothy Hatsukami for their support and helpful advice at various stages of this work. Supported by NIDA grant DA020136 and funding from the Minneapolis Medical Research Foundation.
References
- Anderson KG, Velkey AJ, Woolverton WL. The generalized matching law as a predictor of choice between cocaine and food in rhesus monkeys. Psychopharmacology (Berl) 2002;163:319–26. doi: 10.1007/s00213-002-1012-7. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Kosten TR, Rounsaville BJ. Choosing a behavioral therapy platform for pharmacotherapy of substance users. Drug Alcohol Depend. 2004;75:123–34. doi: 10.1016/j.drugalcdep.2004.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Rounsaville BJ. A perfect platform: combining contingency management with medications for drug abuse. Am J Drug Alcohol Abuse. 2007;33:343–65. doi: 10.1080/00952990701301319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME. Concurrent phencyclidine and saccharin access: presentation of an alternative reinforcer reduces drug intake. J Exp Anal Behav. 1985;43:131–44. doi: 10.1901/jeab.1985.43-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME. The economic context of drug and non-drug reinforcers affects acquisition and maintenance of drug-reinforced behavior and withdrawal effects. Drug Alcohol Depend. 1993;33:201–10. doi: 10.1016/0376-8716(93)90061-t. [DOI] [PubMed] [Google Scholar]
- Carroll ME. Pharmacological and behavioral treatment of cocaine addiction: animal models. NIDA Res Monogr. 1994;145:113–30. [PubMed] [Google Scholar]
- Carroll ME. Acquisition and reacquisition (relapse) of drug abuse: modulation by alternative reinforcers. NIDA Res Monogr. 1998;169:6–25. [PubMed] [Google Scholar]
- Carroll ME, Lac ST. Autoshaping i.v. cocaine self-administration in rats: effects of nondrug alternative reinforcers on acquisition. Psychopharmacology (Berl) 1993;110:5–12. doi: 10.1007/BF02246944. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lac ST, Nygaard SL. A concurrently available nondrug reinforcer prevents the acquisition or decreases the maintenance of cocaine-reinforced behavior. Psychopharmacology (Berl) 1989;97:23–9. doi: 10.1007/BF00443407. [DOI] [PubMed] [Google Scholar]
- Cherek DR. Schedule-induced cigarette self-administration. Pharmacol Biochem Behav. 1982;17:523–7. doi: 10.1016/0091-3057(82)90314-8. [DOI] [PubMed] [Google Scholar]
- Comer SD, Hunt VR, Carroll ME. Effects of concurrent saccharin availability and buprenorphine pretreatment on demand for smoked cocaine base in rhesus monkeys. Psychopharmacology (Berl) 1994;115:15–23. doi: 10.1007/BF02244746. [DOI] [PubMed] [Google Scholar]
- Comer SD, Lac ST, Wyvell CL, Carroll ME. Combined effects of buprenorphine and a nondrug alternative reinforcer on i.v. cocaine self-administration in rats maintained under FR schedules. Psychopharmacology (Berl) 1996;125:355–60. doi: 10.1007/BF02246018. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology. 1989;99:473–8. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Knopf S, Brown C. Nicotine self-administration in rats. Psychopharmacology (Berl) 1995;122:390–94. doi: 10.1007/BF02246272. [DOI] [PubMed] [Google Scholar]
- Falk JL, Zhang J, Chen R, Lau CE. A schedule induction probe technique for evaluating abuse potential: comparison of ethanol, nicotine and caffeine, and caffeine-midazolam interaction. Behav Pharmacol. 1994;5:513–520. doi: 10.1097/00008877-199408000-00012. [DOI] [PubMed] [Google Scholar]
- Foltin RW. Food and cocaine self-administration by baboons: effects of alternatives. J Exp Anal Behav. 1999;72:215–34. doi: 10.1901/jeab.1999.72-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami D, Mooney M, Murphy S, LeSage M, Babb D, Hecht S. Effects of high dose transdermal nicotine replacement in cigarette smokers. Pharmacol Biochem Behav. 2007;86:132–9. doi: 10.1016/j.pbb.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Silverman K, Heil SH. Contingency management in substance abuse treatment. New York, NY: The Guilford Press; 2008. [Google Scholar]
- Hughes JR, Bickel WK. Modeling drug dependence behaviors for animal and human studies. Pharmacol Biochem Behav. 1997;57:413–7. doi: 10.1016/s0091-3057(96)00443-1. [DOI] [PubMed] [Google Scholar]
- Johanson CE. Pharmacological and environmental variables affecting drug preference in rhesus monkeys. Pharmacol Rev. 1975;27:343–55. [PubMed] [Google Scholar]
- Kearns DN, Weiss SJ. Contextual renewal of cocaine seeking in rats and its attenuation by the conditioned effects of an alternative reinforcer. Drug Alcohol Depend. 2007;90:193–202. doi: 10.1016/j.drugalcdep.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Kirby KC, Morral AR, Galbicka G, Iguchi MY. Improving contingency management programs for addiction. Addict Behav. 2004;29:507–23. doi: 10.1016/j.addbeh.2003.08.021. [DOI] [PubMed] [Google Scholar]
- Lattal KA. Scheduling positive reinforcers. In: Iversen IH, Lattal KA, editors. Experimental Analysis of Behavior, Part 1. New York, NY: Elsevier; 1991. [Google Scholar]
- Lenoir M, Ahmed SH. Supply of a Nondrug Substitute Reduces Escalated Heroin Consumption. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301602. [DOI] [PubMed] [Google Scholar]
- Lerman C, LeSage MG, Perkins KA, O’Malley SS, Siegel SJ, Benowitz NL, Corrigall WA. Translational research in medication development for nicotine dependence. Nat Rev Drug Discov. 2007;6:746–62. doi: 10.1038/nrd2361. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Burroughs D, Dufek M, Keyler DE, Pentel PR. Reinstatement of nicotine self-administration in rats by presentation of nicotine-paired stimuli, but not nicotine priming. Pharmacol Biochem Behav. 2004;79:507–13. doi: 10.1016/j.pbb.2004.09.002. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Glowa JR. Effects of reinforcing abstinence in rats self-administering cocaine. Drug Alcohol Depend. 2000;60:S125. [Google Scholar]
- LeSage MG, Keyler DE, Collins G, Pentel PR. Effects of continuous nicotine infusion on nicotine self-administration in rats: relationship between continuously infused and self-administered nicotine doses and serum concentrations. Psychopharmacology (Berl) 2003;170:278–286. doi: 10.1007/s00213-003-1539-2. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Stafford D, Glowa JR. Preclinical research on cocaine self-administration: environmental determinants and their interaction with pharmacological treatment. Neurosci Biobehav Rev. 1999;23:717–41. doi: 10.1016/s0149-7634(99)00015-9. [DOI] [PubMed] [Google Scholar]
- Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101:192–203. doi: 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- Nader MA, Woolverton WL. Effects of increasing the magnitude of an alternative reinforcer on drug choice in a discrete-trials choice procedure. Psychopharmacology (Berl) 1991;105:169–74. doi: 10.1007/BF02244304. [DOI] [PubMed] [Google Scholar]
- Nader MA, Woolverton WL. Choice between cocaine and food by rhesus monkeys: effects of conditions of food availability. Behav Pharmacol. 1992a;3:635–638. [PubMed] [Google Scholar]
- Nader MA, Woolverton WL. Effects of increasing response requirement on choice between cocaine and food in rhesus monkeys. Psychopharmacology (Berl) 1992b;108:295–300. doi: 10.1007/BF02245115. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research. The National Academies Press; Washington, DC: 2003. [PubMed] [Google Scholar]
- Negus SS. Rapid assessment of choice between cocaine and food in rhesus monkeys: effects of environmental manipulations and treatment with d-amphetamine and flupenthixol. Neuropsychopharmacology. 2003;28:919–31. doi: 10.1038/sj.npp.1300096. [DOI] [PubMed] [Google Scholar]
- Poling A, Appel JB. Procedures for reducing drug intake: Nonhuman studies. In: Thompson T, Dews PB, editors. Advances in Behavioral Pharmacology. Academic Press; New York, NY: 1979. pp. 209–228. [Google Scholar]
- Poling A, Ryan C. Differential-reinforcement-of-other-behavior schedules. Behavior Modification. 1982;6:3–21. [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: a meta-analysis. Addiction. 2006;101:1546–60. doi: 10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- Rodefer JS, Mattox AJ, Thompson SS, Carroll ME. Effects of buprenorphine and an alternative nondrug reinforcer, alone and in combination on smoked cocaine self-administration in monkeys. Drug Alcohol Depend. 1997;45:21–9. doi: 10.1016/s0376-8716(97)01341-0. [DOI] [PubMed] [Google Scholar]
- Roll JM, Higgins ST. A within-subject comparison of three different schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. Drug Alcohol Depend. 2000;58:103–9. doi: 10.1016/s0376-8716(99)00073-3. [DOI] [PubMed] [Google Scholar]
- Roll JM, Higgins ST, Badger GJ. An experimental comparison of three different schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. J Appl Behav Anal. 1996;29:495–504. doi: 10.1901/jaba.1996.29-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll JM, Shoptaw S. Contingency management: schedule effects. Psychiatry Res. 2006;144:91–3. doi: 10.1016/j.psychres.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Slifer BL. Schedule-induction of nicotine self-administration. Pharmacol Biochem Behav. 1983;19:1005–9. doi: 10.1016/0091-3057(83)90407-0. [DOI] [PubMed] [Google Scholar]
- Skinner BF. “Superstition” in the pigeon. J Exp Anal Behav. 1948;38:168–172. doi: 10.1037/h0055873. [DOI] [PubMed] [Google Scholar]
- Spealman RD, Goldberg SR. Drug self-administration by laboratory animals: control by schedules of reinforcement. Annu Rev Pharmacol Toxicol. 1978;18:313–39. doi: 10.1146/annurev.pa.18.040178.001525. [DOI] [PubMed] [Google Scholar]
- Staddon JER, Simmelhag VL. The “Superstition” experiment: a reexamination of its implications for the principle of adaptive behavior. Psych Review. 1971;78:3–43. [Google Scholar]
- Stitzer ML, Walsh SL. Psychostimulant abuse: the case for combined behavioral and pharmacological treatments. Pharmacol Biochem Behav. 1997;57:457–70. doi: 10.1016/s0091-3057(96)00436-4. [DOI] [PubMed] [Google Scholar]
- Wallace M, Singer G. Adjunctive behavior and smoking induced by a maze solving schedule in humans. Physiol Behav. 1976;17:849–52. doi: 10.1016/0031-9384(76)90052-4. [DOI] [PubMed] [Google Scholar]
- Woolverton WL. Determinants of cocaine self-administration by laboratory animals. Ciba Found Symp. 1992;166:149–61. doi: 10.1002/9780470514245.ch9. [DOI] [PubMed] [Google Scholar]
- Zeiler MD. Reinforcing the absence of fixed-ratio performance. J Exp Anal Behav. 1979;31:321–332. doi: 10.1901/jeab.1979.31-321. [DOI] [PMC free article] [PubMed] [Google Scholar]