Introduction
The evidence that transplantation of adult stem and progenitor cells can reconstitute the infarcted myocardium has ushered in an era of cell therapy for cardiac regeneration. Although the precise mechanisms underlying cardiac functional and structural benefits of cell therapy remain controversial, enhanced differentiation of transplanted cells into cardiac lineages can only augment this reparative process. Accordingly, intense research efforts have been focused on the identification of molecular triggers that may direct the most plastic cells toward a cardiomyocytic fate. Although several families of signaling molecules, including FGF, TGFβ, and Notch, have been implicated in cardiac development (Zaffran and Frasch 2002, Olson and Schneider 2003, Brand 2003), identification of a lone signal capable of supporting cardiomyocyte differentiation has proven difficult. In this regard, members of the Wnt (related to wingless [wg] in Drosophila) gene family have drawn considerable attention for two reasons: i) utilizing complex and mechanistically divergent intracellular signaling pathways, Wnt proteins have been shown to play a critical role in cardiac specification and morphogenesis in flies, birds, fishes, and amphibians; and ii) these cardiogenic effects have been faithfully recapitulated in vitro in embryonic as well as adult stem/progenitor cells. However, a critical appraisal of the literature regarding the involvement of Wnt signaling in cardiac specification reveals discordant evidence as to the relative importance of signaling via the canonical vs. noncanonical Wnt pathways. Following a discussion of Wnt signaling, this review will focus on Wnt11/noncanonical pathways with an emphasis on its role in embryonic cardiogenesis and cardiomyocytic commitment of adult cells.
The Wnt Family
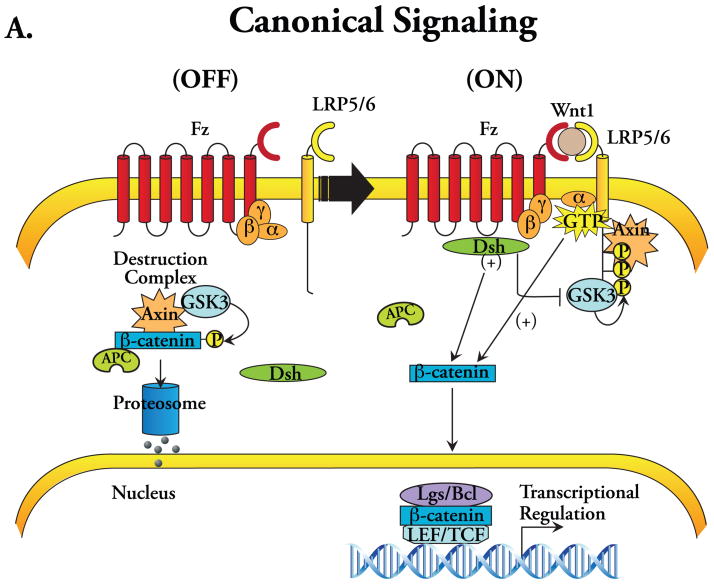
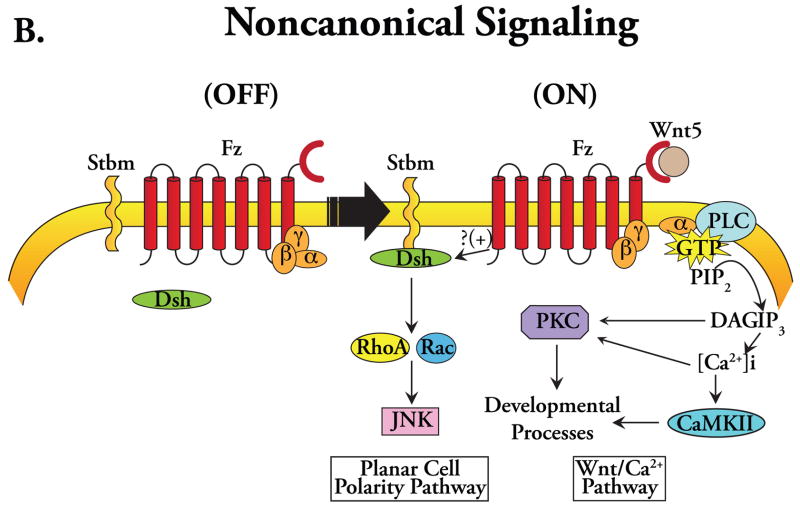
The evolutionarily conserved Wnt family of glycoproteins consists of 19 known members in mammals. These secreted signaling proteins serve as the molecular cues that provoke as well as orchestrate a diverse array of biological activities that includes: (i) determination of cellular polarity, proliferation, and migration; (ii) craniofacial and neural development; (iii) cardiac development; (iv) tumorigenesis and metastasis; and (v) stem cell differentiation and renewal (Logan and Nusse 2004, Eisenberg and Eisenberg 2006, Miller et al. 1999). In vertebrates, the expression of Wnt genes begins in the early stages of embryonic development at gastrulation, before any mesodermal subdivision. During the process of organogenesis, the expression of different Wnts, each with distinct functional roles, results in an elaborate symphony of time- and context-dependent signaling that orchestrates with great precision a variety of cellular processes, including morphogenesis and remodeling. Although Wnt pathways are primarily triggered by Wnt binding to a seven-span transmembrane Frizzled (Fz) family of cell surface receptors, these disparate signaling activities require that Wnt ligands be classified into two distinct groups based on the subcellular signaling elements. The first group, referred to as the Wnt1 group, predominantly uses the ‘canonical’ pathway for intracellular signaling and includes Wnt1, Wnt2a, Wnt3a, and Wnt8 (Figure 1A). The second group, the Wnt5a group, includes Wnt4, Wnt5a, and Wnt11 and utilizes a very different subcellular signaling network, the ‘noncanonical’ pathway (Figure 1B).
Figure 1.
Schematic diagrams depicting Wnt signal transduction pathways. (A) Canonical signaling: (OFF) in the absence of Wnt ligand binding to a receptor complex consisting of Frizzled (Fz) and LRP5/6 co-receptors, cytoplasmic dishevelled (Dsh) remains inactive, and β-catenin, sequestered within the destruction complex comprising APC and Axin, is phosphorylated by GSK-3 thereby targeting it for ubiquitination and proteosomal degradation. Note also the presence of heterotrimeric G-proteins interacting with the Fz receptor. (ON) Wnt ligand binding to the Fz-LRP5/6 receptor complex results in activation of Dsh, which results in direct and indirect inactivation of GSK-3, and hyperphosphorylation of the intracellular domain of LRP5/6 where it binds Axin with high affinity, sequestering it away from the destruction complex, thereby allowing cytoplasmic stabilization and nuclear accumulation of β-catenin where it complexes with Legs/Bcl-9 leading to the activation of TCF/LEF-dependent transcription. The Gα subunit has also been implicated in the disassembly of the β-catenin destruction complex, linking it to the canonical signaling. (B) Noncanonical signaling (represented as two distinct pathways: the Wnt/Ca2+ and PCP pathways): Ca2+/PKC signaling occurs via Wnt activation of Fz receptors causing heterotrimeric G-protein-dependent Ca2+ release thereby activating PKC and CaMKII; the Rho/JNK signaling occurs via the cooperative interaction between strabismus (Stbm) and Fz receptors, which activates Rho/Rac-mediated activation of JNK through Dsh.
The Biphasic Role of Canonical Wnt Signaling in Cardiogenesis
The canonical pathway is activated by Wnt binding to an Fz receptor and a low-density lipoprotein receptor-related protein (LRP, isoforms 5 or 6), which serves as a co-receptor. Apart from the requirement for the Wnt/Fz/LRP complex interaction for intracellular signaling, what truly defines canonical signaling is its dependence upon cytoplasmic stabilization and nuclear migration of β-catenin to activate target gene transcription (Figure 1A). Briefly, in the absence of Wnt ligand, cytosolic β-catenin is phosphorylated by glycogen synthase kinase-3 (GSK-3), the primary kinase of the destruction complex comprising the scaffolding protein Axin, adenomatosis polyposis coli (APC), and GSK-3, thereby targeting β-catenin for ubiquitination and proteosomal degradation (Logan and Nusse 2004, Miller et al. 1999). The ligation of Fz-LRP by Wnt1 members activates cytosolic Dishevelled (Dsh), resulting in direct and indirect inactivation of GSK-3. In addition, binding of Wnt ligand to the Fz-LRP5/6 receptor complex triggers LRP5/6 hyperphosphorylation and Axin recruitment to the receptor complex at the cell surface, sequestering it away from the now inactivated destruction complex. Several recent reports also suggest a role of G-proteins in the disassembly of the destruction complex (Quaiser et al. 2006). Together, these events allow for cytoplasmic β-catenin stabilization and its subsequent translocation to the nucleus, where it regulates transcriptional activity via complex formation with the TCF/LEF family of DNA binding proteins (Figure 1A) (Logan and Nusse 2004, Miller et al. 1999, Willert and Jones 2006).
Early studies in Drosophila indicated a critical role of wg in cardiac development (Wu et al. 1995) and further identified Dsh and the β-catenin homolog Armadillo as mediators of cardiogenic activities of wg (Park et al. 1996). However, microinjection of mRNA encoding the canonical Wnt inhibitors Dkk-1 and Crescent induced cardiac-specific gene expression and beating in explants from noncardiogenic ventral mesoderm in Xenopus (Schneider and Mercola 2001). Consistently, injection of canonical Wnt3a or Wnt8 into the cardiogenic dorsal marginal zone explants inhibited cardiac induction as evidenced by downregulation of Nkx2.5 expression (Schneider and Mercola 2001). Similar results were obtained in chick embryonic explants where Dkk-1 or Crescent induced cardiogenesis in noncardiogenic posterior mesoderm, and ectopic expression of Wnt3a blocked cardiac gene expression in the precardiac anterior mesoderm (Marvin et al. 2001). In a subsequent study (Manisastry et al. 2006), Manisastry et al. exposed chick embryos to Wnt3a, the canonical Wnt activator lithium chloride (LiCl, an inhibitor of GSK3β), or a GSK3β inhibitor SB415286 at different stages of cardiac development. The exposure to LiCl, Wnt3a-conditioned medium, or SB415286 at early Hamburger Hamilton (HH) stage 3 resulted in no identifiable cardiac tissue (defined as a lack of MF20 expression) in a majority of embryos. This inhibition of cardiogenesis decreased markedly when embryos were exposed to canonical Wnt activators at mid to late HH stage 3 and at HH stage 4. Together, the above results (Schneider and Mercola 2001, Marvin et al. 2001, Manisastry et al. 2006) led to the widely accepted notion that canonical Wnt inhibition is required for heart development in vivo.
On the contrary, a cardiogenic role of canonical Wnts is supported by data from studies of cardiac differentiation in embryonic stem cell (ESC) lines in vitro. Specifically, functional activation of the canonical pathway has been shown to be required for DMSO-induced cardiac differentiation in a mouse embryonal carcinoma cell line (P19CL6) (Nakamura et al. 2003), and a role of PI3-K-Akt pathway in the maintenance of canonical activity has also been reported (Naito et al. 2005). Although direct inhibition of β-catenin can promote cardiac differentiation in murine ESCs (Singh et al. 2007), a growing body of evidence supports the notion that canonical Wnt/β-catenin signaling acts biphasically during cardiogenesis (Yamashita et al. 2005, Naito et al. 2006, Ueno et al. 2007). In the study by Ueno et al., canonical Wnt/β-catenin activation at the pregastrula stages (up to 5 h postfertilization (5 hpf) increased Nkx2.5 expression, yet activation during gastrulation (6–9 hpf) inhibited the same. In this regard, it should be emphasized that the specific time windows for the stimulatory as well as the inhibitory effects of canonical Wnt on cardiac induction both belong to the ‘early’ stages of cardiogenesis, often temporally spanning only a few hours depending on the species. Further complexity to this time-specific role of canonical Wnt signaling has been added by recent reports, which indicate that although Wnt/β-catenin signaling specifically promotes the expansion of secondary heart field derivatives (Ai et al. 2007, Klaus et al. 2007), it inhibits heart tube formation (Klaus et al. 2007). Despite these effects in embryonic tissue/cells, attempts to induce cardiomyogenic differentiation in adult progenitor cells with Wnt3a have not been successful thus far (Koyanagi et al. 2005, Flaherty et al. 2008).
Noncanonical Wnt Pathways
One widely accepted approach for depicting noncanonical signaling is to represent it as two distinct signaling modules, a calcium-dependent protein kinase C (PKC)-mediated Wnt/Ca2+ pathway and a Dsh-dependent c-jun-terminal kinase (JNK)-mediated planar cell polarity (PCP) pathway (Figure 1B) (Miller et al. 1999, Kuhl et al. 2000, Eisenberg and Eisenberg 2006). These second messenger systems responsible for transducing Wnt signals were first identified in Zebrafish embryos, in which Xenopus-derived XWnt5a enhanced intracellular Ca2+ transients likely arising from phosphatidylinositol cycle activity that was β-catenin-independent (Slusarski et al. 1997). Subsequently, it was shown that noncanonical Wnts also modulate PKC localization, and stimulate PKC activity via a heterotrimeric G-protein-linked Ca2+-dependent mechanism (Sheldahl et al. 1999), one that also involves Ca2+/calmodulin-dependent protein kinase II (CaMKII) (Figure 1B) (Sheldahl et al. 2003, Eisenberg and Eisenberg 2006). Analogous to the β-catenin pathway, members of the Wnt5a (noncanonical) family also activate cytoplasmic Dsh likely via cooperative interaction between Wnt/Fz and transmembrane receptor strabismus (stbm), although the precise interaction/mechanism remains unclear (Sheldahl et al. 2003, Veeman et al. 2003). Downstream signaling via CaMKII/calcineurin regulates cytoskeletal processes, dorsal-ventral patterning, specification of embryonic myoblasts, and other developmental events in a JNK-independent manner (Wnt/Ca2+ pathway); while signaling via the Rho family of small GTPases (RhoA, Rac, and Cdc42) results in activation of JNK (PCP pathway) (Figure 1B) (Miller et al. 1999, Tada and Smith 2000, Eisenberg and Eisenberg 2006, Veeman et al. 2003).
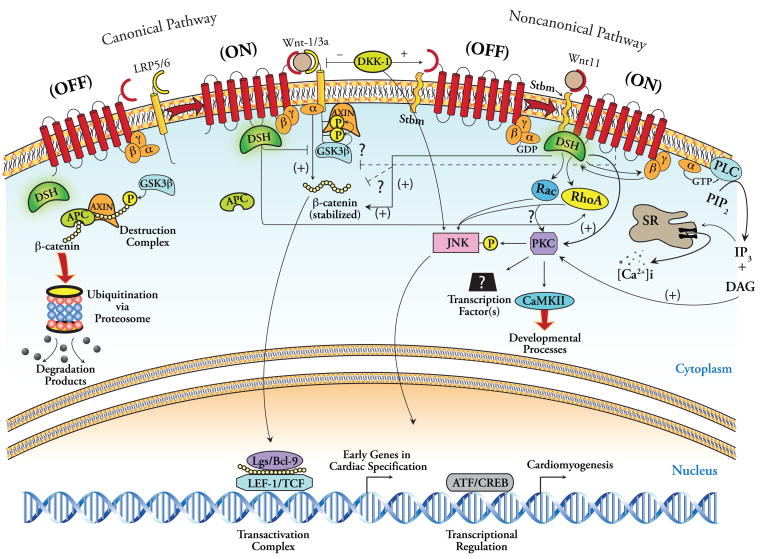
However, emerging evidence indicates that substantial overlap exists not only between the supposedly discrete noncanonical modules, but also between Wnt/β-catenin and noncanonical signaling in general. As depicted in Figure 2, noncanonical signaling involves the cytoplasmic protein Dsh, as does Wnt/β-catenin signaling. However, unlike canonical transduction, noncanonical activation of Dsh results in its recruitment to the cell membrane, where it plays a pivotal role in both Wnt/Ca2+ and Rho/Rac signaling by itself, in cooperation with Stbm (though the mechanism remains poorly understood) and as a component of the G-protein linked Frz receptor pathway (Sheldahl et al. 2003, Veeman et al. 2003). Regarding Fz-mediated pathways, there is evidence that Dsh is responsible for the activation of Ca2+-dependent PKC and CaMKII signaling, either by promoting intracellular Ca2+ influx, likely via its direct interaction with the βγG-protein subunits, and/or by interacting directly with PKC forming a Dsh/PKC protein complex that drives PKC-dependent JNK signaling (Kinoshita et al. 2003, Sheldahl et al. 2003, Veeman et al. 2003). Although the contribution of Rho kinases in JNK activation remains unclear, current evidence suggests that Dsh requires recruitment of the bridging molecule Daam1 at the cell membrane for activation of JNK signaling, either in cooperation or by itself. Whether Rho/Rac requires interaction with PKC downstream to mediate JNK signaling is unclear (Veeman et al. 2003). Thus, Dsh, a tripartite multifunctional effector, appears to be at the center of all Wnt signaling (Figure 2). However, despite the identification of this interdigitating nature of Wnt signaling networks, the precise interaction(s) between canonical and noncanonical modules specifically in the context of cardiogenesis remains to be elucidated in greater detail.
Figure 2.
A unifying model of Wnt signaling with regard to cardiomyogenesis. Illustrated at the left is the canonical pathway, similar to that in Figure 1A, with the presence of Dkk-1. Dkk-1 can promote cardiac differentiation via canonical inhibition as well as by activating JNK via unclear mechanisms. The G-proteins have also been linked to the canonical pathway. The illustration to the right provides an alternative portrayal of the noncanonical signaling pathway as a single entity. In this model, activation of the heterotrimeric G-protein-linked Fz receptor by Wnt ligand initiates a multifaceted signaling cascade that includes activation of phospholipase C, which generates inositol triphosphate (IP3) and diacyl glycerol (DAG). IP3 mediates cytoplasmic Ca2+ accumulation via its entry from the extracellular space and its release from the sarcoplasmic reticulum (SR). This Ca2+ influx activates PKC (also activated via DAG) and CaMKII. Activation of Frz receptor also activates Dsh responsible for activation of Ca2+-dependent PKC and CaMKII signaling via direct interaction with the βγG-protein subunits, and/or by forming a Dsh/PKC protein complex that drives PKC-dependent JNK signaling. Rho/Rac may also activate JNK via PKC. Although PKC phosphorylates and activates JNK, cardiogenic signaling is incompletely inhibited with JNK inhibition, suggesting other (unknown) transducing elements downstream from PKC, perhaps ultimately involving (ATF/CREB)-mediated transcriptional regulation.
Noncanonical Wnt11 Signaling and Cardiogenesis
Spatial and temporal regulation of maternal RNA translation is an evolutionarily conserved mechanism in vertebrates that heralds the establishment of intracellular asymmetries required for the formation of a multicellular body pattern. Accordingly, maternally derived axis determinants/factors expressed in the dorsal marginal zone at the Spemann Organizer are likely to be influential in cardiac specification (Ku and Melton 1993). Among all of the Wnt genes, only Wnt11 shows this spatio-temporally regulated expression during gastrulation within or in close proximity to the precardiac mesoderm, which suggests a preeminent role of Wnt11 in cardiac specification (Kispert et al. 1996, Eisenberg et al. 1997, Eisenberg and Eisenberg 1999, Pandur et al. 2002). Consistently, while canonical β-catenin-dependent cardiogenic signaling appears to be restricted to embryonic development and ESCs in vitro, several lines of evidence demonstrate that noncanonical signaling via Wnt11 is sufficient to induce cardiomyocytic commitment in both embryonic (Pandur et al. 2002, Terami et al. 2004, Ueno et al. 2007) and adult stem cell populations (Belema Bedada et al. 2005, Flaherty et al. 2008).
Eisenberg et al. produced the first line of evidence implicating Wnt11 as an important molecular signal during vertebrate cardiogenesis by demonstrating that soluble Wnt11 was sufficient to promote cardiac tissue formation in posterior noncardiac mesoderm of quail embryonic explants (Eisenberg and Eisenberg 1999). Subsequent studies implicated the noncanonical signaling pathway as the transducing apparatus for Wnt11 (Kuhl et al. 2000, Heisenberg et al. 2000). Building on this premise, using elegant loss- and gain-of-function experiments in Xenopus embryos, Pandur and colleagues demonstrated that Wnt11 is required for cardiogenesis and, as such, is sufficient to induce a contractile phenotype (Pandur et al. 2002). This study also showed that, at least in Xenopus, noncanonical Wnt11 signal transduction occurs in a JNK-dependent manner which requires PKC as the upstream mediator (Pandur et al. 2002). Collectively, these results indicate that Wnt11, acting via the noncanonical signaling pathway is indispensable for vertebrate cardiogenesis (Eisenberg and Eisenberg 1999, Pandur et al. 2002).
A few unresolved issues persist regarding the role of Wnt11 as a true ‘inducer’ of cardiogenesis in vivo (Eisenberg and Eisenberg 2007). In this regard, studies in vitro with adult cells offer the advantage of addressing the issue of ‘induction’ more directly by removing the complex molecular milieu inherent in embryonic tissues, and by enabling the use of specific inducing agents alone. Indeed, Wnt11-conditioned medium has been able to enhance cardiomyocytic differentiation in murine bone marrow-derived multipotent adult stem cells (mBM-MASCs) (Belema Bedada et al. 2005) and unfractionated (density gradient-separated) BMMNCs (Flaherty et al. 2008). However, several clarifications are in order to portray the evidence accurately. In mBM-MASCs (Belema Bedada et al. 2005), Wnt11 was unable to induce the expression of α-myosin heavy chain (MHC), the predominant molecular marker of the adult cardiac phenotype; and in Wnt11-treated BMMNCs (Flaherty et al. 2008) sarcomeric organization and contraction could not be documented despite the expression of cardiac-specific MHC, troponin T, and troponin I, markers of advanced cardiac lineage commitment. Moreover, in human peripheral blood-derived circulating progenitor cells (CPCs), Wnt11 alone was unable to induce cardiac differentiation in the absence of co-culture with neonatal rat cardiomyocytes (Koyanagi et al. 2005). Therefore, it is logical to infer that although Wnt11 alone is sufficient to activate a cardiomygenic gene program, synergistic and perhaps sequential signaling initiated by other cardiogenic factors is critical in rendering the mature cardiomyocytic phenotype.
Molecular Mechanisms of Cardiogenesis by Wnt11
An inherent attribute to the study of noncanonical Wnt signaling is its novelty, and as such, alternative paradigms regarding its molecular details are continually unfolding. Figure 2 represents a putative unifying model of Wnt signaling in general, while also attempting to illustrate the bidirectional crosstalk that may occur between canonical and noncanonical transducing elements in cardiogenesis (Pandur et al. 2002, Veeman et al. 2003, Maye et al. 2004, Kuhl et al. 2001, Belema Bedada et al. 2005, Flaherty et al. 2008).
The overall consensus in the literature favors the notion that initial cardiac specification requires balanced expression of both canonical and noncanonical Wnts, but the balance must be tipped in the direction of canonical inhibition for cardiac specification to occur (Schneider and Mercola 2001, Eisenberg and Eisenberg 2006, Ueno et al. 2007) (Eisenberg and Eisenberg 2007) (Figure 2). This is mediated either by specific inhibitors of canonical signaling (e.g., Dkk-1, Crescent, and Wnt11); or by activation of other gene programs that provide negative-feedback inhibition of canonical signaling and/or directly promote cardiomyogenesis (e.g., BMPs, Smads); or perhaps both (Figure 2). Although the ‘Wnt/β-catenin inhibition’ paradigm for cardiogenesis in developmental studies may suggest a merely facilitative role of Dkk-1, Crescent, or Wnt11, subsequent reports have identified a more direct or ‘inductive’ role of noncanonical signaling in this process. Indeed, Dkk-1 and other canonical Wnt inhibitors have been shown to induce a homeodomain transcription factor Hex, which can induce the expression of cardiac markers in a non-cell-autonomous fashion in Xenopus embryos (Foley and Mercola 2005). Although Hex, in turn, is thought to induce expression of a diffusible heart-inducing factor, the identity of this molecule(s) remains unclear.
Several recent studies indicate that activation of PKC and JNK is critical for the induction of cardiac gene program by Wnt11 (Pandur et al. 2002, Belema Bedada et al. 2005, Koyanagi et al. 2005, Flaherty et al. 2008). Following Fz activation, Wnt11 is able to trigger intracellular Ca2+ release, which then activates Ca2+-sensitive kinases PKC, CaMKII, and calcineurin. Downstream from these events, or in parallel, JNK can be activated by Dsh itself, by PKC, or via the Rho kinases. Although inhibition of JNK did not affect Wnt11-induced enhancement of cardiac differentiation in CPCs (Koyanagi et al. 2005), in other adult progenitors (Flaherty et al. 2008) and in Xenopus animal caps (Pandur et al. 2002), inhibition of PKC or JNK could effectively block cardiac commitment. Inhibition of JNK activation by PKC inhibitors also indicates that PKC is upstream of JNK (Figure 2), at least in the context of noncanonical cardiomyogenic signaling (Pandur et al. 2002, Flaherty et al. 2008).
Adding an additional level of complexity, JNK inhibition did not impact cardiomyogenic effects of Wnt11 in CPCs, while in BMMNCs, cardiac gene expression was only partially dependent on JNK activation (Flaherty et al. 2008), thereby suggesting the existence of additional transducing elements downstream of PKC. These additional possibilities include activating transcription factor/cAMP responsive element binding protein (ATF/CREB) family-dependent recruitment of TGFβ2 signaling (Zhou et al. 2007) (Figure 2) and the GATA family of transcription factors. Indeed, a recent report suggested GATA-4/6 as integrators of Wnt signaling in cardiogenesis (Afouda et al. 2008). In this study, β-catenin inhibited GATA-4/6 expression and cardiogenesis in Xenopus animal caps, while GATA-4/6-induced cardiogenesis required Wnt11 expression. However, in adult mBM-MASCs, PKC inhibition slightly increased GATA-4 expression yet abrogated the expression of other cardiac genes (Belema Bedada et al. 2005). These intriguing findings raise the question of whether PKC/JNK signaling in Wnt11-induced cardiomyogenesis is activated in a cell type-specific manner. Finally, partial inhibition of Wnt11/noncanonical induction of troponin I expression by the canonical inhibitor Dkk-1 in BMMNCs (Flaherty et al. 2008) suggests additional intersections between classic canonical and noncanonical pathways in cardiomyocytic commitment of adult progenitors.
Cell-specific Differences in Cardiomyogenic Effects of Wnt11
Although Wnt/β-catenin-directed cardiomyogenesis has been validated in vitro using ESCs and the P19CL6 cells (Nakamura et al. 2003, Ueno et al. 2007), preservation of ESC pluripotency and self-renewal by Wnt3a has also been reported (Singla et al. 2006). However, these cardiomyogenic properties of canonical Wnt signaling appear to be restricted to pluripotent model systems and could not be applied to adult progenitors (Koyanagi et al. 2005, Flaherty et al. 2008). Conversely, in addition to its ability to initiate a cardiomyogenic program in pluripotent ESCs (Pandur et al. 2002, Terami et al. 2004), Wnt11/noncanonical signaling has also been shown to induce de novo cardiac differentiation in adult BM-derived progenitors (Belema Bedada et al. 2005, Flaherty et al. 2008). Nonetheless, in hematopoietic progenitor cell-enriched avian fetal BM cells and quail mesodermal cell line QCE6, Wnt11 treatment promoted the expansion of red blood cells and monocytes at the expense of macrophages (Brandon et al. 2000). In another study that examined the effects of Wnts on cord blood-derived CD133+ cells, Wnt11 promoted the development of an endothelial-like phenotype (with increased expression of CD31) at the expense of blast cells (Nikolova et al. 2007).
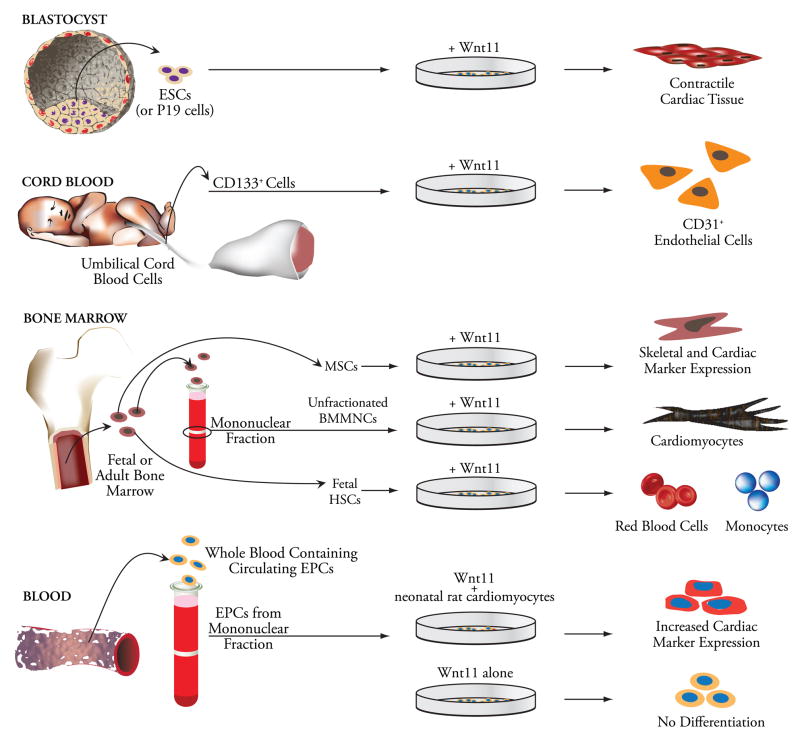
The effects of Wnt11 on cardiac differentiation have been variable in adult cells as well. In mBM-MASCs, although Wnt11 induced expression of cardiac transcription factors, β-MHC, troponin T, and BNP, reproducible induction of α-MHC, a marker of adult cardiomyocyte phenotype, and atrial natriuretic peptide (ANP) was not observed (Belema Bedada et al. 2005). In BMMNCs, ectopically overexpressed Wnt11 was sufficient to induce the expression of GATA-4 and Nkx2.5, troponin T, connexin 43, as well as cardiac-specific MHC and ANP in a pattern characteristic of the cardiac fetal gene program (Flaherty et al. 2008). Moreover, Wnt11 alone is not sufficient to induce cardiomyocytic commitment in all adult progenitor cell populations. This was illustrated in a study that clearly demonstrated the ability of Wnt11 to enhance cardiomyogenic differentiation of adult CPCs only in the presence of co-culture with neonatal cardiomyocytes, but not by itself (Koyanagi et al. 2005). Figure 3 illustrates the differential impact of Wnt11 on cell fate determination in embryonic, cord blood-derived, and BM-, and blood-derived adult progenitor cell populations. These differences perhaps underscore the differential utilization of available signaling pathways by Wnt11 in cell-type-specific manners.
Figure 3.
Differential impact of Wnt11 signaling on cell fate in vitro. While blastocyst-derived pluripotent embryonic stem cells give rise to cardiac tissue with a contractile phenotype, Wnt11-treated cord blood-derived CD133+ progenitors undergo endothelial lineage commitment. Wnt11-treated bone marrow-derived cells show a remarkable degree of divergence with regard to their lineage commitment and the phenotypic characteristics they assume. In fetal hematopoietic progenitors (HSCs), Wnt11 promotes red blood cell commitment and favors monocyte precursor formation. BM-derived multipotent adult stem cells (mBM-MASCs) treated with Wnt11 differentiate along a cardiomyogenic pathway, but fail to adopt an adult phenotype, while Wnt11 is sufficient to produce a cardiomyocytic phenotype in BMMNCs. Finally, in CPCs that adopt a cardiac phenotype when co-cultured with neonatal rat cardiomyocytes, Wnt11 enhances expression of cardiac markers, yet, by itself, is unable to affect the native phenotype.
Future Directions
Over the past three decades, it has become increasingly apparent that Wnt signaling governs a wide and diverse array of biologic processes that range from embryonic patterning to tumor invasion. The translational importance of Wnt11/noncanonical signaling in cardiomyogenesis draws from the acute need to direct differentiation of stem/progenitor cells toward a cardiomyocytic fate so that transplanted cells can repair the infarcted myocardium with greater efficiency (Abdel-Latif et al. 2007). However, the reported data indicate that the cardiomyogenic efficacy of Wnt11 in adult cells is numerically suboptimal, to the tune of 30% of cells on average (Flaherty et al. 2008). In this regard, elucidation of the intracellular signaling steps that emanate from Wnt11-receptor interaction will be critical to enhance Wnt11-induction of cardiomyogenesis possibly with si/shRNA, small molecules, or other agonists/inhibitors of signaling components. Furthermore, precise identification of specific biological molecules that participate in myocyte maturation following Wnt11 induction will facilitate the formulation of an effective predifferentiation strategy to ensure generation of new functional myocytes following cellular transplantation. Finally, characterization of stem/progenitor cells that are more likely to undergo cardiomyocytic differentiation in response to Wnt11 will help identify an optimal cellular population ideally suited for infarct reconstitution. These tasks are daunting, but the potential therapeutic benefits toward myocardial repair will likely more than justify the efforts.
Acknowledgments
This publication was supported in part by NIH grants R01 HL-72410, HL-89939, and R21 HL-89737. We gratefully acknowledge Ms. Heather Jones for expert assistance with graphics design.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdel-Latif A, Bolli R, Tleyjeh IM, et al. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167:989–997. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- Afouda BA, Martin J, Liu F, Ciau-Uitz A, Patient R, Hoppler S. GATA transcription factors integrate Wnt signalling during heart development. Development. 2008;135:3185–3190. doi: 10.1242/dev.026443. [DOI] [PubMed] [Google Scholar]
- Ai D, Fu X, Wang J, et al. Canonical Wnt signaling functions in second heart field to promote right ventricular growth. Proc Natl Acad Sci U S A. 2007;104:9319–9324. doi: 10.1073/pnas.0701212104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belema Bedada F, Technau A, Ebelt H, Schulze M, Braun T. Activation of myogenic differentiation pathways in adult bone marrow-derived stem cells. Mol Cell Biol. 2005;25:9509–9519. doi: 10.1128/MCB.25.21.9509-9519.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand T. Heart development: molecular insights into cardiac specification and early morphogenesis. Dev Biol. 2003;258:1–19. doi: 10.1016/s0012-1606(03)00112-x. [DOI] [PubMed] [Google Scholar]
- Brandon C, Eisenberg LM, Eisenberg CA. WNT signaling modulates the diversification of hematopoietic cells. Blood. 2000;96:4132–4141. [PubMed] [Google Scholar]
- Eisenberg CA, Eisenberg LM. WNT11 promotes cardiac tissue formation of early mesoderm. Dev Dyn. 1999;216:45–58. doi: 10.1002/(SICI)1097-0177(199909)216:1<45::AID-DVDY7>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Eisenberg CA, Gourdie RG, Eisenberg LM. Wnt-11 is expressed in early avian mesoderm and required for the differentiation of the quail mesoderm cell line QCE-6. Development. 1997;124:525–536. doi: 10.1242/dev.124.2.525. [DOI] [PubMed] [Google Scholar]
- Eisenberg LM, Eisenberg CA. Wnt signal transduction and the formation of the myocardium. Dev Biol. 2006;293:305–315. doi: 10.1016/j.ydbio.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Eisenberg LM, Eisenberg CA. Evaluating the role of Wnt signal transduction in promoting the development of the heart. Sci World J. 2007;7:161–176. doi: 10.1100/tsw.2007.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty MP, Abdel-Latif A, Li Q, et al. Noncanonical Wnt11 signaling is sufficient to induce cardiomyogenic differentiation in unfractionated bone marrow mononuclear cells. Circulation. 2008;117:2241–2252. doi: 10.1161/CIRCULATIONAHA.107.741066. [DOI] [PubMed] [Google Scholar]
- Foley AC, Mercola M. Heart induction by Wnt antagonists depends on the homeodomain transcription factor Hex. Genes Dev. 2005;19:387–396. doi: 10.1101/gad.1279405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisenberg CP, Tada M, Rauch GJ, et al. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature. 2000;405:76–81. doi: 10.1038/35011068. [DOI] [PubMed] [Google Scholar]
- Kinoshita N, Iioka H, Miyakoshi A, Ueno N. PKC delta is essential for Dishevelled function in a noncanonical Wnt pathway that regulates Xenopus convergent extension movements. Genes Dev. 2003;17:1663–1676. doi: 10.1101/gad.1101303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kispert A, Vainio S, Shen L, Rowitch DH, McMahon AP. Proteoglycans are required for maintenance of Wnt-11 expression in the ureter tips. Development. 1996;122:3627–3637. doi: 10.1242/dev.122.11.3627. [DOI] [PubMed] [Google Scholar]
- Klaus A, Saga Y, Taketo MM, Tzahor E, Birchmeier W. Distinct roles of Wnt/beta-catenin and Bmp signaling during early cardiogenesis. Proc Natl Acad Sci U S A. 2007;104:18531–18536. doi: 10.1073/pnas.0703113104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyanagi M, Haendeler J, Badorff C, et al. Non-canonical Wnt signaling enhances differentiation of human circulating progenitor cells to cardiomyogenic cells. J Biol Chem. 2005;280:16838–16842. doi: 10.1074/jbc.M500323200. [DOI] [PubMed] [Google Scholar]
- Ku M, Melton DA. Xwnt-11: a maternally expressed Xenopus wnt gene. Development. 1993;119:1161–1173. doi: 10.1242/dev.119.4.1161. [DOI] [PubMed] [Google Scholar]
- Kuhl M, Geis K, Sheldahl LC, Pukrop T, Moon RT, Wedlich D. Antagonistic regulation of convergent extension movements in Xenopus by Wnt/beta-catenin and Wnt/Ca2+ signaling. Mech Dev. 2001;106:61–76. doi: 10.1016/s0925-4773(01)00416-6. [DOI] [PubMed] [Google Scholar]
- Kuhl M, Sheldahl LC, Malbon CC, Moon RT. Ca(2+)/calmodulin-dependent protein kinase II is stimulated by Wnt and Frizzled homologs and promotes ventral cell fates in Xenopus. J Biol Chem. 2000;275:12701–12711. doi: 10.1074/jbc.275.17.12701. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Manisastry SM, Han M, Linask KK. Early temporal-specific responses and differential sensitivity to lithium and Wnt-3A exposure during heart development. Dev Dyn. 2006;235:2160–2174. doi: 10.1002/dvdy.20878. [DOI] [PubMed] [Google Scholar]
- Marvin MJ, Di Rocco G, Gardiner A, Bush SM, Lassar AB. Inhibition of Wnt activity induces heart formation from posterior mesoderm. Genes Dev. 2001;15:316–327. doi: 10.1101/gad.855501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maye P, Zheng J, Li L, Wu D. Multiple mechanisms for Wnt11-mediated repression of the canonical Wnt signaling pathway. J Biol Chem. 2004;279:24659–24665. doi: 10.1074/jbc.M311724200. [DOI] [PubMed] [Google Scholar]
- Miller JR, Hocking AM, Brown JD, Moon RT. Mechanism and function of signal transduction by the Wnt/beta-catenin and Wnt/Ca2+ pathways. Oncogene. 1999;18:7860–7872. doi: 10.1038/sj.onc.1203245. [DOI] [PubMed] [Google Scholar]
- Naito AT, Akazawa H, Takano H, et al. Phosphatidylinositol 3-kinase-Akt pathway plays a critical role in early cardiomyogenesis by regulating canonical Wnt signaling. Circ Res. 2005;97:144–151. doi: 10.1161/01.RES.0000175241.92285.f8. [DOI] [PubMed] [Google Scholar]
- Naito AT, Shiojima I, Akazawa H, et al. Developmental stage-specific biphasic roles of Wnt/beta-catenin signaling in cardiomyogenesis and hematopoiesis. Proc Natl Acad Sci U S A. 2006;103:19812–19817. doi: 10.1073/pnas.0605768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Sano M, Songyang Z, Schneider MD. A Wnt- and beta -catenin-dependent pathway for mammalian cardiac myogenesis. Proc Natl Acad Sci U S A. 2003;100:5834–5839. doi: 10.1073/pnas.0935626100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolova T, Wu M, Brumbarov K, et al. WNT-conditioned media differentially affect the proliferation and differentiation of cord blood-derived CD133+ cells in vitro. Differentiation. 2007;75:100–111. doi: 10.1111/j.1432-0436.2006.00119.x. [DOI] [PubMed] [Google Scholar]
- Olson EN, Schneider MD. Sizing up the heart: development redux in disease. Genes Dev. 2003;17:1937–1956. doi: 10.1101/gad.1110103. [DOI] [PubMed] [Google Scholar]
- Pandur P, Lasche M, Eisenberg LM, Kuhl M. Wnt-11 activation of a non-canonical Wnt signalling pathway is required for cardiogenesis. Nature. 2002;418:636–641. doi: 10.1038/nature00921. [DOI] [PubMed] [Google Scholar]
- Park M, Wu X, Golden K, Axelrod JD, Bodmer R. The wingless signaling pathway is directly involved in Drosophila heart development. Dev Biol. 1996;177:104–116. doi: 10.1006/dbio.1996.0149. [DOI] [PubMed] [Google Scholar]
- Quaiser T, Anton R, Kuhl M. Kinases and G proteins join the Wnt receptor complex. Bioessays. 2006;28:339–343. doi: 10.1002/bies.20386. [DOI] [PubMed] [Google Scholar]
- Schneider VA, Mercola M. Wnt antagonism initiates cardiogenesis in Xenopus laevis. Genes Dev. 2001;15:304–315. doi: 10.1101/gad.855601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldahl LC, Park M, Malbon CC, Moon RT. Protein kinase C is differentially stimulated by Wnt and Frizzled homologs in a G-protein-dependent manner. Curr Biol. 1999;9:695–698. doi: 10.1016/s0960-9822(99)80310-8. [DOI] [PubMed] [Google Scholar]
- Sheldahl LC, Slusarski DC, Pandur P, Miller JR, Kuhl M, Moon RT. Dishevelled activates Ca2+ flux, PKC, and CamKII in vertebrate embryos. J Cell Biol. 2003;161:769–777. doi: 10.1083/jcb.200211094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AM, Li FQ, Hamazaki T, Kasahara H, Takemaru K, Terada N. Chibby, an antagonist of the Wnt/beta-catenin pathway, facilitates cardiomyocyte differentiation of murine embryonic stem cells. Circulation. 2007;115:617–626. doi: 10.1161/CIRCULATIONAHA.106.642298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla DK, Schneider DJ, LeWinter MM, Sobel BE. wnt3a but not wnt11 supports self-renewal of embryonic stem cells. Biochem Biophys Res Commun. 2006;345:789–795. doi: 10.1016/j.bbrc.2006.04.125. [DOI] [PubMed] [Google Scholar]
- Slusarski DC, Yang-Snyder J, Busa WB, Moon RT. Modulation of embryonic intracellular Ca2+ signaling by Wnt-5A. Dev Biol. 1997;182:114–120. doi: 10.1006/dbio.1996.8463. [DOI] [PubMed] [Google Scholar]
- Tada M, Smith JC. Xwnt11 is a target of Xenopus brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development. 2000;127:2227–2238. doi: 10.1242/dev.127.10.2227. [DOI] [PubMed] [Google Scholar]
- Terami H, Hidaka K, Katsumata T, Iio A, Morisaki T. Wnt11 facilitates embryonic stem cell differentiation to Nkx2.5-positive cardiomyocytes. Biochem Biophys Res Commun. 2004;325:968–975. doi: 10.1016/j.bbrc.2004.10.103. [DOI] [PubMed] [Google Scholar]
- Ueno S, Weidinger G, Osugi T, et al. Biphasic role for Wnt/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104:9685–9690. doi: 10.1073/pnas.0702859104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell. 2003;5:367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- Willert K, Jones KA. Wnt signaling: is the party in the nucleus? Genes Dev. 2006;20:1394–1404. doi: 10.1101/gad.1424006. [DOI] [PubMed] [Google Scholar]
- Wu X, Golden K, Bodmer R. Heart development in Drosophila requires the segment polarity gene wingless. Dev Biol. 1995;169:619–628. doi: 10.1006/dbio.1995.1174. [DOI] [PubMed] [Google Scholar]
- Yamashita JK, Takano M, Hiraoka-Kanie M, et al. Prospective identification of cardiac progenitors by a novel single cell-based cardiomyocyte induction. FASEB J. 2005;19:1534–1536. doi: 10.1096/fj.04-3540fje. [DOI] [PubMed] [Google Scholar]
- Zaffran S, Frasch M. Early signals in cardiac development. Circ Res. 2002;91:457–469. doi: 10.1161/01.res.0000034152.74523.a8. [DOI] [PubMed] [Google Scholar]
- Zhou W, Lin L, Majumdar A, et al. Modulation of morphogenesis by noncanonical Wnt signaling requires ATF/CREB family-mediated transcriptional activation of TGFbeta2. Nat Genet. 2007;39:1225–1234. doi: 10.1038/ng2112. [DOI] [PMC free article] [PubMed] [Google Scholar]