Abstract
Background
Although some observational studies have associated higher calcium intake and especially higher vitamin D intake and 25-hydroxyvitamin D levels with lower breast cancer risk, no randomized trial has evaluated these relationships.
Methods
Postmenopausal women (N = 36 282) who were enrolled in a Women's Health Initiative clinical trial were randomly assigned to 1000 mg of elemental calcium with 400 IU of vitamin D3 daily or placebo for a mean of 7.0 years to determine the effects of supplement use on incidence of hip fracture. Mammograms and breast exams were serially conducted. Invasive breast cancer was a secondary outcome. Baseline serum 25-hydroxyvitamin D levels were assessed in a nested case–control study of 1067 case patients and 1067 control subjects. A Cox proportional hazards model was used to estimate the risk of breast cancer associated with random assignment to calcium with vitamin D3. Associations between 25-hydroxyvitamin D serum levels and total vitamin D intake, body mass index (BMI), recreational physical activity, and breast cancer risks were evaluated using logistic regression models. Statistical tests were two-sided.
Results
Invasive breast cancer incidence was similar in the two groups (528 supplement vs 546 placebo; hazard ratio = 0.96; 95% confidence interval = 0.85 to 1.09). In the nested case–control study, no effect of supplement group assignment on breast cancer risk was seen. Baseline 25-hydroxyvitamin D levels were modestly correlated with total vitamin D intake (diet and supplements) (r = 0.19, P < .001) and were higher among women with lower BMI and higher recreational physical activity (both P < .001). Baseline 25-hydroxyvitamin D levels were not associated with breast cancer risk in analyses that were adjusted for BMI and physical activity (Ptrend = .20).
Conclusions
Calcium and vitamin D supplementation did not reduce invasive breast cancer incidence in postmenopausal women. In addition, 25-hydroxyvitamin D levels were not associated with subsequent breast cancer risk. These findings do not support a relationship between total vitamin D intake and 25-hydroxyvitamin D levels with breast cancer risk.
CONTEXT AND CAVEATS
Prior knowledge
Some observational studies have reported associations of higher calcium intake and especially higher vitamin D intake and 25-hydroxyvitamin D levels with lower breast cancer risk, but these relationships have not been analyzed in randomized trials.
Study design
Randomized double-blind placebo-controlled clinical trial of calcium and vitamin D supplementation vs placebo among postmenopausal women and a nested case–control study of associations between baseline serum 25-hydroxyvitamin D levels, breast cancer risk factors, and risk of breast cancer.
Contribution
Incidence of invasive breast cancer was similar in the two randomized groups after a mean of 7 years. Baseline serum 25-hydroxyvitamin D levels were correlated with supplement use and were higher among women who did more recreational physical activity and had a lower body mass index (BMI), but they were not associated with breast cancer risk after adjustment for BMI and physical activity.
Implications
Vitamin D and calcium supplementation has no detectable effect on the risk of postmenopausal invasive breast cancer.
Limitations
Additional use of calcium and vitamin D supplements was allowed during the study. The duration of calcium and vitamin D supplementation was short compared with how long it takes to develop breast cancer.
From the Editors
Breast cancer is the most common cancer among women in the United States and is the focus of risk reduction efforts (1). Some preclinical (2,3) and observational (4) studies have reported associations between higher calcium intake and higher vitamin D intake (5–7) and reduced breast cancer risk in postmenopausal women, but the results have not been consistent (8–11). No randomized clinical trial has addressed whether calcium and/or vitamin D intake reduce risk of breast cancer.
Against this background, the Women's Health Initiative (WHI) designed a trial to test the hypothesis that calcium plus vitamin D supplementation would reduce risk of hip fracture (as primary endpoint) and of colorectal and breast cancer (as designated secondary endpoints) among postmenopausal women. The hip fracture and colorectal cancer results have been previously reported (12,13). Here, we report the breast cancer findings.
Subjects and Methods
Study Design
Women who were enrolled in the WHI randomized clinical trials evaluating hormone therapy (HT) and dietary modification (DM) (14–16) were invited to join the WHI calcium and vitamin D trial at their first or second annual follow-up visit. Details of eligibility criteria and recruitment have been previously described (12,17). Postmenopausal women aged 50–79 years with life expectancy of more than 3 years, no prior breast cancer, and no other cancer within 10 years were eligible. Women with history of hypercalcemia, kidney stones, and corticosteroid or calcitriol use were excluded. Personal use of calcium and vitamin D during the study was allowed: initially up to 600 IU daily of vitamin D, which was subsequently increased to 1000 IU daily during the course of the study.
Using a permuted block algorithm, eligible women were randomly assigned in a double-blind fashion to active supplement or an identical-appearing placebo (both provided by GlaxoSmith Kline) stratified by clinical center and age. Active tablets contained 500 mg of elemental calcium (as calcium carbonate) combined with 200 IU of vitamin D3. Women were instructed to take two tablets per day in divided doses, with meals to maximize absorption, for a total of 1000 mg of elemental calcium and 400 IU vitamin D3 daily.
The protocol was approved by institutional review boards at each clinical center, and all participants provided written informed consent. Statistical analyses and data management were conducted at the WHI Clinical Coordinating Center. The trial was registered at clinicaltrials.gov (Identifier: NCT 00000611).
All women had both clinical breast examination and mammography and were free of breast cancer at entry in the WHI DM or HT trials 1 or 2 years previously. Mammograms were required annually for women on the HT trials and every 2 years for those on the dietary trial. Use of estrogen or estrogen plus progestin was determined by randomization for HT participants. HT was permitted for DM trial participants. Study pills were discontinued for any of the following: kidney stones, hypercalcemia, kidney dialysis, calcitriol use, or daily personal use of vitamin D supplements greater than 600 IU and later 1000 IU outside the study protocol.
Study Monitoring and Termination
Clinical outcomes were reviewed semiannually by an independent data and safety monitoring board. Final clinical visits occurred as planned between October 1, 2004, and March 31, 2005.
Follow-up Procedures and Ascertainment of Outcomes
One phone contact after 4 weeks assessed symptoms and encouraged adherence. Subsequent semiannual contacts assessed clinical outcomes as well as safety and adherence. Annual clinical visits included weighing returned pill bottles as an adherence measure. Breast cancers were confirmed by both local and central medical record and pathology report review by trained adjudicators who were blinded to randomized allocation, with such records available in 98.2% of cases. Tumor characteristics were coded using the Surveillance, Epidemiology, and End Results (SEER) program guidelines (18).
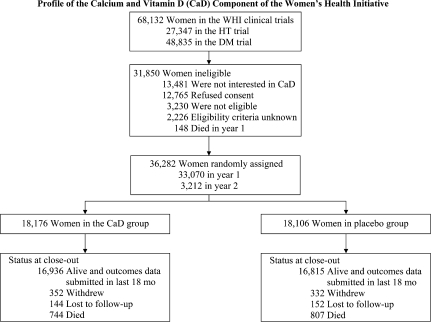
Between 1995 and 2000, of 68 132 postmenopausal women enrolled in the HT or DM trials, 31 850 women were ineligible for or declined participation in the calcium and vitamin D trial. Of the 36 282 women randomly assigned, 25 210 (69%) were in the DM trial, 16 089 (44%) were in one of the HT trials, and 5017 (14%) were in both the DM and the HT trials. At the end of the study, 16 936 women in the calcium and vitamin D group and 16 815 in the placebo group were under active follow-up (Figure 1).
Figure 1.
CONSORT diagram of the Women's Health Initiative randomized trial of calcium and vitamin D. Permission for reproduction was granted from The Publishing Division of the Massachusetts Medical Society (12).
Total calcium intake included dietary intake (assessed with a modified block food frequency questionnaire) and elemental calcium from supplements. Total vitamin D intake included dietary vitamin D (intake largely from fortified dairy products and fatty fish) and vitamin D supplement use.
Retention and Adherence
Adherence (defined as use of 80% or more of study medication) ranged from 60% to 63% during the first 3 years of follow-up, with an additional 13%–21% of participants taking at least half of their study pills. At the end of the trial, 76% were still taking study medication and 59% were taking 80% or more study pills. Over the course of the study, 1551 participants (4.3%) died and 980 (2.7%) either withdrew from the trial or were lost to follow-up.
Vitamin D Level Analyses
Blood samples were collected at baseline after an overnight fast. The DiaSorin Liason chemiluminescent immunoassay (DiaSorin, Stillwater, MN) was used to determine 25-hydroxyvitamin D levels. Samples were assayed in batches with blinded controls with a coefficient of variation of 11.8%. The 25-hydroxyvitamin D levels were used in a nested case–control study that examined whether prerandomization levels either were associated with subsequent breast cancer risk or influenced the effect of calcium and vitamin D supplementation on breast cancer-risk. Case patients were patients with invasive breast cancer (n = 1067), and control subjects (n = 1067) were breast cancer-free and were matched to corresponding case patients on age, latitude of the clinical center, race/ethnicity, and date of blood collection.
Statistical Analysis
Primary results were assessed with time-to-event methods and were based on the intention-to-treat principle. Breast cancer incidence was compared in the two randomization groups using hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) estimated from Cox proportional hazards models (19) that were stratified according to age group at randomization (50–54, 55–59, 60–69, or 70–79 years), prevalent disease (yes or no), and treatment assignment in the HT (conjugated equine estrogen [CEE] therapy, CEE placebo, CEE plus medroxyprogesterone acetate [MPA] therapy, CEE plus MPA placebo, or not randomly assigned) and DM (intervention, comparison, not randomly assigned) trial. Secondary analyses of risk of breast cancer by tumor characteristics used the same Cox proportional hazards models with reported P values from Wald statistics. The proportionality assumption of the Cox models was tested by adding an interaction term for treatment assignment by survival time. No violations of the assumption were found.
Kaplan–Meier estimates describe event rates over time. Sensitivity analyses examining the effect of nonadherence were conducted by repeating Kaplan–Meier analyses after censoring events that occurred 6 months after nonadherence (defined as consuming less than 80% of study pills). Comparisons of baseline characteristics and some breast cancer tumor characteristics were based on chi-square, Fisher's exact test, or t test. Women with missing values were excluded from analyses for that factor.
Differential effects across subgroups were evaluated using the same Cox proportional hazards models, which were extended to include the variable of interest and interaction with group assignment. When possible, continuous variables were used to test for the interaction; otherwise, the subgroup categories were used. Subgroup cut points are based on established groupings or percentiles. Twenty-two subgroups were examined, on which basis one statistically significant interaction test (P < .05) would be expected based on chance alone.
The interaction between serum 25-hydroxyvitamin D at baseline and random assignment to calcium plus vitamin D supplementation or placebo was assessed by unconditional logistic regression. Women with missing data for the adjustment variables were excluded from the models. To avoid bias, only the control group from the nested case–control study was analyzed using linear regression to examine the cross-sectional relationship between self-reported intake of vitamin D and serum 25-hydroxyvitamin D levels.
Results
Participant Characteristics and Nonprotocol Supplement Use
Demographic characteristics, health behaviors, and medical history were balanced between randomization groups (18 176 women in the supplement group and 18 106 in the placebo group). Breast cancer risk factors were also balanced. Use of tamoxifen and raloxifene was low at entry and remained low throughout the study (Table 1).
Table 1.
Descriptive characteristics of participants at baseline by randomization assignment*
| Characteristic | CaD (N = 18 176) |
Placebo (N = 18 106) |
||
| No. | % | No. | % | |
| Age at screening, y | ||||
| 50–59 | 6728 | 37.0 | 6694 | 37.0 |
| 60–69 | 8275 | 45.5 | 8245 | 45.5 |
| 70–79 | 3173 | 17.5 | 3167 | 17.5 |
| Race/ethnicity | ||||
| White | 15 047 | 82.8 | 15 106 | 83.4 |
| Black | 1682 | 9.3 | 1635 | 9.0 |
| Hispanic | 789 | 4.3 | 718 | 4.0 |
| American Indian | 77 | 0.4 | 72 | 0.4 |
| Asian/Pacific Islander | 369 | 2.0 | 353 | 2.0 |
| Unknown | 212 | 1.2 | 222 | 1.2 |
| Education | ||||
| None—some high school | 977 | 5.4 | 925 | 5.1 |
| High school diploma/GED | 3309 | 18.3 | 3364 | 18.7 |
| School after high school | 7216 | 40.0 | 7156 | 39.8 |
| College degree or higher | 6555 | 36.3 | 6543 | 36.4 |
| Gail risk, %/5 y | ||||
| <1.25 | 6355 | 35.0 | 6303 | 34.8 |
| 1.25–1.74 | 5932 | 32.6 | 5938 | 32.8 |
| ≥1.75 | 5889 | 32.4 | 5865 | 32.4 |
| Age at menarche, y | ||||
| ≤11 | 3985 | 22.0 | 3983 | 22.1 |
| 12–13 | 10 011 | 55.3 | 9904 | 54.9 |
| ≥14 | 4109 | 22.7 | 4162 | 23.1 |
| No. of first-degree relatives with breast cancer | ||||
| None | 14 677 | 86.4 | 14 597 | 86.3 |
| 1 | 2112 | 12.4 | 2095 | 12.4 |
| ≥2 | 206 | 1.2 | 230 | 1.4 |
| Prior breast biopsy | ||||
| No | 13 167 | 80.2 | 13 095 | 80.3 |
| Yes, 1 biopsy | 2377 | 14.5 | 2323 | 14.2 |
| Yes, ≥2 biopsies | 867 | 5.3 | 897 | 5.5 |
| Age at the birth of first child, y | ||||
| Never pregnant/no term pregnancy | 1841 | 11.1 | 1909 | 11.5 |
| <20 | 2814 | 17.0 | 2683 | 16.2 |
| 20–29 | 10 676 | 64.3 | 10 807 | 65.1 |
| ≥30 | 1273 | 7.7 | 1204 | 7.3 |
| Oophorectomy | ||||
| No | 12 948 | 73.1 | 12 821 | 72.5 |
| Yes, 1 or part removed | 1474 | 8.3 | 1462 | 8.3 |
| Yes, bilateral oophorectomy | 3304 | 18.6 | 3392 | 19.2 |
| Prior E-only use, y | ||||
| No prior any hormone use | 8788 | 59.4 | 8627 | 58.6 |
| <2 | 1333 | 9.0 | 1341 | 9.1 |
| 2–5 | 1215 | 8.2 | 1217 | 8.3 |
| ≥5 | 3470 | 23.4 | 3538 | 24.0 |
| Prior E+P use, y | ||||
| No prior any hormone use | 8788 | 67.5 | 8627 | 66.9 |
| <2 | 1116 | 8.6 | 1141 | 8.9 |
| 2–5 | 1196 | 9.2 | 1174 | 9.1 |
| ≥5 | 1926 | 14.8 | 1957 | 15.2 |
| Selective estrogen receptor modulators | ||||
| Tamoxifen (current use) | 13 | 0.08 | 10 | 0.06 |
| Raloxifene (current use) | 16 | 0.10 | 17 | 0.11 |
| Body mass index, kg/m2 | ||||
| <25 | 4745 | 26.2 | 4833 | 26.8 |
| 25–<30 | 6472 | 35.8 | 6483 | 36.0 |
| ≥30 | 6867 | 38.0 | 6695 | 37.2 |
| Physical activity, MET-h/week | ||||
| None | 3154 | 19.1 | 3170 | 19.3 |
| >0–3.5 | 2745 | 16.6 | 2669 | 16.2 |
| >3.5–8.0 | 3227 | 19.5 | 3333 | 20.3 |
| >8.0–16.5 | 3613 | 21.8 | 3425 | 20.8 |
| >16.5 | 3807 | 23.0 | 3851 | 23.4 |
| Alcohol use | ||||
| Nondrinker | 1863 | 10.3 | 1891 | 10.5 |
| Past drinker | 3192 | 17.7 | 3209 | 17.8 |
| <1 drink per month | 2529 | 14.0 | 2520 | 14.0 |
| <1 drink per week | 3863 | 21.4 | 3758 | 20.9 |
| 1–<7 drinks per week | 4683 | 26.0 | 4706 | 26.2 |
| ≥7 drinks per week | 1910 | 10.6 | 1900 | 10.6 |
| Smoking | ||||
| Never smoked | 9325 | 51.9 | 9428 | 52.6 |
| Past smoker | 7255 | 40.3 | 7133 | 39.8 |
| Current smoker | 1405 | 7.8 | 1356 | 7.6 |
| NSAID use | ||||
| No | 15 126 | 83.2 | 15 182 | 83.9 |
| Yes | 3050 | 16.8 | 2924 | 16.2 |
| Baseline total vitamin D (supplements + diet), IU | ||||
| <200 | 6827 | 38.3 | 6671 | 37.6 |
| 200–<400 | 3379 | 19.0 | 3423 | 19.3 |
| 400–<600 | 4188 | 23.5 | 4295 | 24.2 |
| ≥600 | 3427 | 19.2 | 3364 | 19.0 |
| Baseline vitamin D supplement use (any) | ||||
| No | 9620 | 52.9 | 9495 | 52.4 |
| Yes | 8556 | 47.1 | 8611 | 47.6 |
| Region of residence at baseline | ||||
| Southern (≤37°N) | 6159 | 33.9 | 6158 | 34.0 |
| Middle (>37–40°N) | 3622 | 19.9 | 3612 | 20.0 |
| Northern (>40°N) | 8395 | 46.2 | 8336 | 46.0 |
| Baseline total calcium (supplements + diet), mg | ||||
| <800 | 6104 | 34.3 | 6003 | 33.8 |
| 800–<1200 | 4715 | 26.5 | 4655 | 26.2 |
| ≥1200 | 7002 | 39.3 | 7095 | 40.0 |
CaD = calcium and vitamin D supplementation; GED = general educational development; E = estrogen; P = progestin; MET = metabolic equivalents; NSAID = nonsteroidal anti-inflammatory drug.
Self-reported baseline total calcium and vitamin D intakes were similar in the randomization groups (Table 1), with nonprotocol vitamin D supplement use of 400 IU/day or greater reported by 37.7% of women in the placebo group and 37.1% of women in the supplement group. During the trial, nonprotocol calcium and vitamin D supplement use was similar in the two randomization groups. At year 6, nonprotocol vitamin D supplement use, mostly in multivitamin preparations, was reported by 52.8% of women in the placebo group and 52.0% of women in the supplement group. During the trial, nonprotocol calcium intake increased by approximately 100 mg daily in both randomization groups.
Breast Cancers
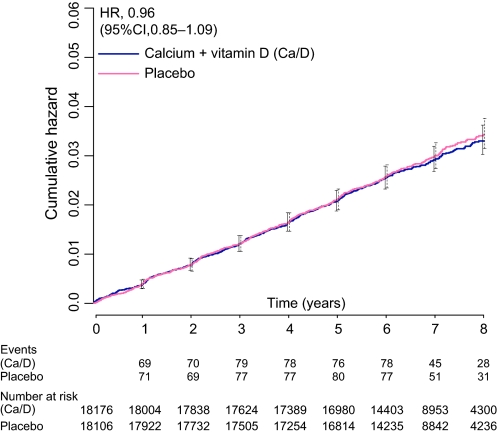
After a mean follow-up of 7 years, a total of 528 invasive breast cancers were diagnosed in the supplement group and 546 in the placebo group (HR = 0.96, 95% CI = 0.85 to 1.09) (Figure 2; Table 2). In addition, 145 in situ breast cancers were seen in the supplement group and 152 in the placebo group (HR = 0.94, 95% CI = 0.75 to 1.18) (Table 2). A sensitivity analysis censoring follow-up of 6 months after nonadherence found 274 invasive breast cancers in the supplement and 315 in the placebo groups (HR = 0.91, 95% CI = 0.77 to 1.07; P = .24). A total of 23 deaths from breast cancer occurred in each randomization group (HR = 0.99, 95% CI = 0.55 to 1.76).
Figure 2.
Kaplan–Meier estimates of the cumulative hazard ratio for invasive breast cancer with supplemental calcium plus vitamin D (Ca/D) as compared with placebo. HR, hazard ratio; CI, confidence interval. Error bars represent 95% CIs of the estimates at each year, using solid and dashed lines for the Ca/D and placebo groups, respectively.
Table 2.
Clinical outcomes incidence (annualized %) by randomization assignment group*
| Outcomes | CaD (N = 18 176) |
Placebo (N = 18 106) |
Hazard ratio (95% CI)* | P | ||
| No. | % | No. | % | |||
| Total breast cancer† | 668 | 0.52 | 693 | 0.54 | 0.96 (0.86 to 1.07) | |
| Invasive | 528 | 0.41 | 546 | 0.43 | 0.96 (0.85 to 1.09) | |
| In situ | 145 | 0.11 | 152 | 0.12 | 0.94 (0.75 to 1.18) | |
| Death from breast cancer | 23 | 0.02 | 23 | 0.02 | 0.99 (0.55 to 1.76) | |
| Tumor size, mean (SD), cm | 1.54 | 1.23 | 1.71 | 1.29 | .05 | |
| No tumor found | 1 | <0.01 | 1 | <0.01 | ||
| Microscopic focus or foci, cm | 6 | <0.01 | 14 | 0.01 | 0.43 (0.17 to 1.12) | .08 |
| ≤0.5 | 66 | 0.05 | 55 | 0.04 | 1.17 (0.82 to 1.68) | .38 |
| >0.5–1 | 133 | 0.10 | 107 | 0.08 | 1.24 (0.96 to 1.60) | .10 |
| >1–2 | 179 | 0.14 | 197 | 0.15 | 0.91 (0.74 to 1.11) | .33 |
| >2–5 | 74 | 0.06 | 90 | 0.07 | 0.82 (0.60 to 1.12) | .21 |
| >5 | 7 | 0.01 | 11 | 0.01 | 0.66 (0.25 to 1.70) | .39 |
| Missing | 62 | 0.05 | 71 | 0.06 | ||
| Lymph nodes examined | ||||||
| No | 37 | 0.03 | 55 | 0.04 | 0.67 (0.44 to 1.01) | .06 |
| Yes | 483 | 0.38 | 480 | 0.38 | 1.00 (0.88 to 1.14) | .98 |
| Missing | 8 | 0.01 | 11 | 0.01 | ||
| No. of positive lymph nodes | ||||||
| None | 359 | 0.28 | 362 | 0.28 | 0.99 (0.85 to 1.14) | .87 |
| 1–3 | 90 | 0.07 | 78 | 0.06 | 1.14 (0.84 to 1.55) | .39 |
| ≥4 | 32 | 0.02 | 39 | 0.03 | 0.82 (0.51 to 1.31) | .41 |
| Missing | 47 | 0.04 | 67 | 0.05 | ||
| SEER stage | ||||||
| Localized | 386 | 0.30 | 404 | 0.32 | 0.95 (0.83 to 1.09) | .49 |
| Regional | 127 | 0.10 | 121 | 0.10 | 1.04 (0.81 to 1.34) | .74 |
| Distant | 5 | <0.01 | 9 | 0.01 | 0.53 (0.18 to 1.57) | .24 |
| Missing | 10 | 0.01 | 12 | 0.01 | ||
| Histology | ||||||
| Ductal | 336 | 0.26 | 337 | 0.26 | 0.99 (0.86 to 1.16) | .94 |
| Lobular | 49 | 0.04 | 61 | 0.05 | 0.80 (0.55 to 1.16) | .24 |
| Ductal and lobular | 76 | 0.06 | 78 | 0.06 | 0.96 (0.70 to 1.32) | .81 |
| Tubular | 15 | 0.01 | 26 | 0.02 | 0.59 (0.31 to 1.11) | .10 |
| Other | 49 | 0.04 | 37 | 0.03 | 1.31 (0.85 to 2.00) | .22 |
| Missing | 3 | <0.01 | 7 | 0.01 | ||
| Estrogen receptor assay | ||||||
| Positive | 414 | 0.32 | 403 | 0.32 | 1.02 (0.89 to 1.17) | .75 |
| Negative | 68 | 0.05 | 83 | 0.07 | 0.82 (0.59 to 1.13) | .22 |
| Borderline | 2 | <0.01 | 0 | |||
| Missing | 44 | 0.03 | 60 | 0.05 | ||
| Progesterone receptor assay | ||||||
| Positive | 325 | 0.25 | 329 | 0.26 | 0.98 (0.84 to 1.15) | .84 |
| Negative | 142 | 0.11 | 145 | 0.11 | 0.97 (0.77 to 1.23) | .82 |
| Borderline | 4 | <0.01 | 4 | <0.01 | 0.98 (0.25 to 3.93) | |
| Missing | 57 | 0.04 | 68 | 0.05 | ||
Hazard ratios (HRs) and confidence intervals (CIs) are from unweighted Cox proportional hazards models, stratified by age 50–54, 55–59, 60–69, 70–79 years, prevalent disease, hormone therapy randomization assignment (conjugated equine estrogen [CEE] therapy, CEE + medroxyprogesterone acetate [MPA], CEE + MPA placebo, not randomized), and dietary modification randomization assignment (intervention, comparison, not randomly assigned). P values (two-sided) for the comparison of mean tumor size are from an unadjusted t test. CaD = calcium and vitamin D supplementation; SEER = Surveillance, Epidemiology, and End Results. HRs are not reported for “Missing” categories or when the number of cancers is too few to produce a reliable estimate.
Total breast cancer is the first of either invasive or situ breast cancer.
Breast cancer histology was similar in the two randomization groups. There were 68 estrogen receptor–negative invasive cancers in the supplement group and 83 in the placebo group, but this difference was not statistically significant (HR = 0.82, 95% CI = 0.59 to 1.13). The cancers that were diagnosed in the supplement group were somewhat smaller than those in the placebo group (mean = 1.54 vs 1.71 cm in diameter, respectively, P = .05) but were of similar stage (Table 2). Mammogram findings were comparable in the randomization groups, both at baseline and throughout the study. After 1 year, mammograms with findings suspicious or highly suggestive of malignancy were seen in 269 (1.5%) of the participants in the placebo group and 272 (1.5%) of those in the supplement group.
Subgroup Analyses
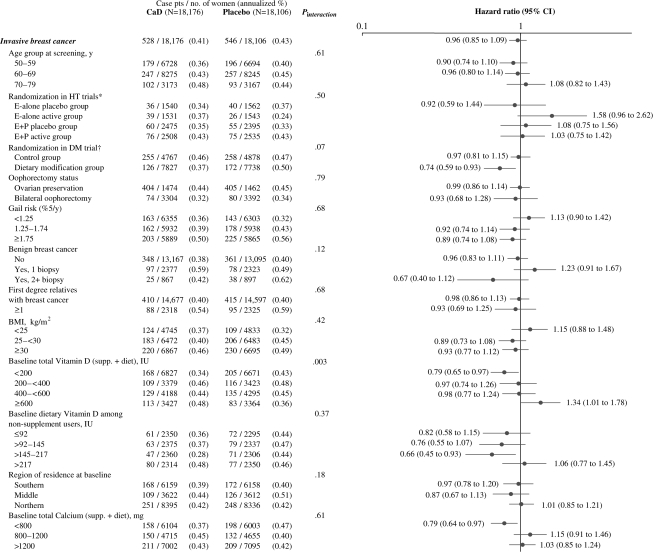
Supplement influence on breast cancer was assessed in 22 subgroups; 12 of the analyses are shown in Figure 3. Among women in the highest quartile of reported total vitamin D intake (diet plus supplement) at baseline, more breast cancers were seen in the supplement group than in the placebo group (HR = 1.34, 95% CI = 1.01 to 1.78); among women in the lowest baseline vitamin D intake quartile, fewer cancers were seen in the supplement group (HR = 0.79, 95% CI = 0.65 to 0.97) (Pinteraction = .003).
Figure 3.
Estimated effects of supplemental calcium with vitamin D (CaD) on the risk of invasive breast cancer, according to selected baseline characteristics. Modeling for interaction testing used the continuous form of the following variables: age at screening, body mass index (BMI), Gail risk score, and baseline total calcium and vitamin D intake. Data were missing for some variables. HT = hormone therapy; E = estrogen; P = progestin; DM = dietary modification. All models were stratified by age (50–54, 55–59, 60–69, 70–79 years), prevalent disease, and randomization in the dietary modification and HT trials. *Women not randomized in the HT trials are excluded. †Adjusted for age (linear), weight, and baseline percentage of energy from total fat. Women not randomized in the DM trial are excluded. P values are two-sided.
Serum Vitamin D Levels
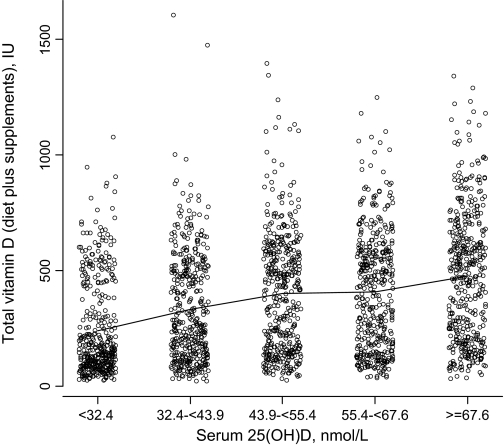
The 25-hydroxyvitamin D levels were obtained at baseline for the nested case–control study from 1067 case patients who developed breast cancer during the trial and 1067 matching control subjects who were breast cancer-free. At baseline, self-reported total vitamin D intake was modestly associated with 25-hydroxyvitamin D levels (Figure 4). There was a somewhat stronger association with 25-hydroxyvitamin D levels for women using only dietary sources (correlation r = 0.21, P < .001) compared with women using vitamin D supplements as well (correlation r = 0.19, P < .001, with a test of the difference in the two regression lines having a P value of .03). Individual vitamin D intakes at baseline are shown in relation to 25-hydroxyvitamin D levels in each 25-hydroxyvitamin D quintile in Figure 4. Few individuals reported total vitamin D intakes greater than 1000 IU, even in the highest quintile. The range of vitamin D intakes substantially overlapped in each 25-hydroxyvitamin D quintile, and an average vitamin D intake difference of only 238 IU/day separated women in the low vs high 25-hydroxyvitamin D quintiles. Although they are potential surrogates for sunlight exposure, 25-hydroxyvitamin D levels were only modestly associated with geographic location (north, middle, or south, defined by °N latitude (P = .03)). They were, however, statistically significantly associated with recreational physical activity and body mass index (BMI) (both P < .001) (Table 3). The 25-hydroxyvitamin D levels were associated with neither breast cancer family history nor prior breast biopsy.
Figure 4.
Self-reported individual vitamin D intake (diet plus supplementation) and serum 25-hydroxyvitamin D levels at baseline. Serum 25-hydroxyvitamin D levels were obtained at baseline entry into the calcium plus vitamin D clinical trial. Results from the 1067 women identified as control subjects from the nested case–control study are shown. Daily intakes of dietary and supplemental vitamin D were determined from self-report. The range of vitamin D intakes substantially overlap in each 25-hydroxyvitamin D quintile, and an average vitamin D intake difference of only 238 IU/day separates women in the low vs high 25-hydroxyvitamin D quintiles. Line segments connect the mean vitamin D intake level in each quintile.
Table 3.
Baseline serum 25-hydroxyvitamin D levels by variables related to selected breast cancer risk factors and sunlight exposure in 1067 women identified as control subjects for the nested case–control analysis*
| Risk factor | No. | 25-hydroxyvitamin D, nmol/L mean (SD) | P | P† |
| Family history of breast cancer | .95 | .76 | ||
| No | 850 | 51.9 (21.1) | ||
| Yes | 160 | 51.8 (10.5) | ||
| No. of breast biopsies | .28 | .20 | ||
| None | 760 | 51.3 (20.8) | ||
| 1 | 138 | 52.8 (22.5) | ||
| ≥2 | 55 | 55.6 (20.9) | ||
| BMI, kg/m2 | <.001 | <.001 | ||
| <25 | 311 | 58.8 (22.2) | ||
| 25–29 | 375 | 52.2 (19.8) | ||
| ≥30 | 379 | 46.1 (19.7) | ||
| Expenditure from physical activity, MET-h/week | <.001 | <.001 | ||
| None | 175 | 45.8 (20.0) | ||
| >0–3.5 | 149 | 48.2 (20.8) | ||
| >3.5–8.0 | 207 | 50.4 (19.2) | ||
| >8.0–16.5 | 210 | 53.4 (21.9) | ||
| >16.5 | 214 | 58.8 (21.2) | ||
| Region of residence at baseline | .26 | .03 | ||
| Southern (≤37°N) | 310 | 52.9 (21.4) | ||
| Middle (>37–40°N) | 241 | 53.2 (22.4) | ||
| Northern (>40°N) | 516 | 50.9 (20.2) |
Blood samples from 1067 women at baseline who remained breast cancer-free identified as control subjects for women who developed breast cancer, matched to corresponding case patients on age, latitude of the clinical center, race/ethnicity, and venipuncture date. Numbers for each risk factor do not sum to the total in the sample because of missing data. BMI = body mass index; MET = metabolic equivalents. P values (two-sided) were calculated from linear regression models.
Adjusted for age and race/ethnicity.
In the nested case–control assessment of 25-hydroxyvitamin D, the mean ± SD baseline 25-hydroxyvitamin D level was 50.0 ± 21.0 nmol/L among the 895 participants who were subsequently diagnosed with invasive breast cancer and 52.0± 21.1 nmol/L among the 898 control subjects (Table 4). In logistic regression analyses adjusted for age, race and/or ethnicity, latitude of the clinical center, breast cancer family history, prior breast biopsies, HT as estrogen alone or combined with progestin, and HT and DM trial participation, higher baseline hydroxyvitamin D levels were associated with lower breast cancer risk (P = .04) (Table 4). However, no association between levels of baseline hydroxyvitamin D and breast cancer risk emerged in analyses that were further adjusted for BMI and physical activity (Ptrend = .20) (Table 4). In addition, there was no interaction between quintile of baseline 25-hydroxyvitamin D levels, randomization group assignment, and breast cancer risk (Pinteraction ≥ .99) (Table 4). Side effects of the calcium and vitamin D supplement were minimal and have been previously reported (12,13).
Table 4.
Odds ratios for invasive breast cancer according to serum 25-hydroxyvitamin D levels and randomization group, as determined in a nested case–control study
| Baseline 25-hydroxyvitamin D, nmol/L |
Main effect OR (95% CI)† | Calcium + vitamin D |
Placebo | Intervention OR (95% CI)‡ | |
| Determinate quintile | Mean (SD) | No. of mpatients/no. of control subjects | |||
| ≥67.6 | 81.9 (13.2) | 1.00 (referent) | 86/109 | 76/86 | 0.89 (0.58 to 1.36) |
| 55.4 ≤ 67.6 | 60.9 (3.5) | 1.15 (0.86 to 1.55) | 95/87 | 86/98 | 1.25 (0.83 to 1.90) |
| 43.9 ≤ 55.4 | 49.2 (3.3) | 1.35 (0.99 to 1.82) | 102/87 | 92/84 | 1.07 (0.70 to 1.62) |
| 32.4 ≤ 43.9 | 38.5 (3.3) | 1.17 (0.86 to 1.60) | 71/84 | 102/87 | 0.69 (0.45 to 1.06) |
| <32.4 | 23.6 (5.9) | 1.22 (0.89 to 1.67) | 94/94 | 91/82 | 0.91 (0.60 to 1.39) |
* To convert values for 25-hydroxyvitamin D to ng/mL, multiply by 0.401. CI = confidence interval. The analyses include 895 case patients and 898 control subjects of the original 1067 sample pairs due to missing data for the covariates.
From an unconditional logistic regression model, adjusted for the matching factors (age, race/ethnicity [white, black, Hispanic, other/unknown]), latitude of clinical center, venipuncture date, and randomization in the hormone therapy (conjugated equine estrogen [CEE] therapy, CEE placebo, estrogen plus progestin therapy, estrogen plus progestin placebo, not randomly assigned) and dietary modification trials (intervention, comparison, not randomly assigned), and body mass index, physical activity (none, >0–3.5, >3.5–8.0, >8.0–16.5, >16.5 MET-h/week), family history of breast cancer (yes or no), history of breast biopsy (none, 1, or 2+), current estrogen plus progestin use (yes or no), and current estrogen-only use (yes or no). The model estimates the main effect of serum 25(OH)D on invasive breast cancer risk. Ptrend = .20. Note: Ptrend = .04 without adjustment for body mass index and physical activity.
From an unconditional logistic regression model, adjusted for the same factors listed above, estimating the effect of vitamin D supplementation (CaD) on invasive breast cancer risk by category of serum 25(OH)D. Pinteraction ≥ .99, computed from an unconditional logistic model including the above listed adjustment factors, the main effects of calcium and CaD randomization arm, and 25(OH)D as a continuous covariate, and their interaction.
Discussion
In this randomized, double-blind, placebo-controlled trial, daily supplementation with 1000 mg of elemental calcium combined with 400 IU of vitamin D3 had no effect on breast cancer incidence. Thus, the main findings do not support a causal relationship between calcium and vitamin D supplement use and reduced breast cancer incidence, despite the association observed in some epidemiological studies.
As reviewed (20–22), although some observational studies (4,5,7) support an inverse association between higher vitamin D intakes and lower breast cancer risk in postmenopausal women, others (8–11,23) do not. A recent meta-analysis of observational studies including premenopausal and postmenopausal women reported that a modest reduction of breast cancer incidence was associated with higher intake of vitamin D (≥400 IU/day) of borderline statistical significance (24). Similarly, an association between both higher calcium intake (7) and higher calcium levels (25) and lower breast cancer risk in postmenopausal women has been observed in some, but not all (10,23), reports. Studies in postmenopausal women of the relationship between circulating 25-hydroxyvitamin D levels and breast cancer risk have also had mixed results. In three studies (26–28), higher circulating 25-hydroxyvitamin D levels were associated with lower breast cancer risk. However, in a similar number of reports, either no association (11,29,30) or only a borderline association (31) was seen between 25-hydroxyvitamin D levels and breast cancer risk. In the current nested case–control study, with 895 case patients and 898 control subjects, no statistically significant association between higher baseline 25-hydroxyvitamin D and subsequent lower breast cancer risk was seen in analyses that adjusted for BMI and physical activity. In the nested case–control analysis that looked at the interaction effects within the trial design, no statistically significant interaction between baseline 25-hydroxyvitamin D levels, random assignment to supplement use, and breast cancer risk emerged, suggesting that even at the highest baseline 25-hydroxyvitamin D level, supplementation with 400 IU/day of D3 together with calcium was not associated with lower breast cancer risk.
Based on the relatively high threshold of about 75 nmol/L of 25-hydroxyvitamin D, which has been associated with low breast cancer risk in some reports (26,27) and is estimated to require about 1700–2000 IU of supplement daily to achieve (27,28), a higher vitamin D dose than used in this trial has been recommended by some as needed to influence the risk of breast cancer (28,32). Although the dose of vitamin D used in this trial remains an issue (33), when baseline vitamin D intakes were examined across 25-hydroxyvitamin D quintiles in the current trial, the distribution of vitamin D intake in each quintile showed substantial overlap. The difference in reported total vitamin D intake between women with the lowest mean and highest quintiles values for 25-hydroxyvitamin D was, surprisingly, only 238 IU/day of vitamin D, yet the mean ± SD 25-hydroxyvitamin D level in the upper quintile was 81.9 ± 13.2 nmol/L. (The IU refers to the intake of vitamin D by participants, the nmol/L refers to the serum 25-hydroxyvitamin D levels associated with the reported intakes.) A technology assessment of 16 prospective trials of the influence of vitamin D supplementation (with or without calcium) on 25-hydroxyvitamin D levels (34) also describes considerable heterogeneity between supplement dose and subsequent magnitude of change in 25-hydroxyvitamin D levels. Such results suggest that factors other than dietary and supplement intake of vitamin D likely influence 25-hydroxyvitamin D levels. In fact, although sunlight exposure is a recognized influence on 25-hydroxyvitamin D levels, a substantial genetic influence on such levels has also been reported (35,36).
The relative contribution of factors influencing 25-hydroxyvitamin D levels remains to be defined, especially for higher concentrations found in individuals who do not report high-dose supplement use. Although this mechanism is speculative, a genetic predisposition to both high 25-hydroxyvitamin D levels and low breast cancer risk could appear as a protective effect of vitamin D on breast cancer. Before future clinical trials of high-dose vitamin D regimens to reduce breast cancer risk are implemented, it will be important to demonstrate that the selected vitamin D dose can definitively increase circulatory 25-hydroxyvitamin D levels to the projected target level. Definitive assessment of factors that influence the relationship between vitamin D supplement use and subsequent changes in circulating 25-hydroxyvitamin D levels are therefore a research priority.
Levels of 25-hydroxyvitamin D at baseline were statistically significantly higher among lean women and/or those with more recreational activity than overweight or obese or less active women. Based on the associations of these breast cancer risk factors with 25-hydroxyvitamin D, 25-hydroxyvitamin D could be a potential mediator of lifestyle influence on breast cancer. Alternatively, lifestyle choices could have led to more sunlight exposure, with higher 25-hydroxyvitamin D levels and lower breast cancer risk as independent processes. The finding that analyses adjusted for BMI and physical activity did not identify an association between baseline 25-hydroxyvitamin D levels and breast cancer risk suggests that the association between 25-hydroxyvitamin D and breast cancer seen in some observational studies could be confounded to some degree by such factors.
Only one of the 22 subgroup analyses we performed demonstrated a statistically significant interaction between randomization group and the selected participant characteristics and suggested a differential calcium plus vitamin D supplement effect on breast cancer incidence according to baseline vitamin D intake. However, in the nested case–control analyses, no statistically significant interactions were observed.
The vitamin D dosage in this trial generally followed recommendations from the Institute of Medicine (37). We cannot assess whether a higher dosage would have changed the outcome of the current study. However, our findings provide some evidence against that hypothesis. Because approximately half of the women were taking an additional 400 IU of nonprotocol vitamin D supplement daily, actual vitamin D supplement intake was greater than 800 IU daily for a substantial number of participants in the supplement group. Nonetheless, no effects on risk of breast cancer overall or in sensitivity analyses that were adjusted for study adherence were observed. Although further study of relationships among calcium plus vitamin D supplement use and breast cancer can be considered, current evidence does not support their use in any dose to reduce breast cancer risk.
Breast cancers in the supplement group were somewhat smaller (P = .05) but were of similar stage to those in the placebo group. Because the frequency of abnormal mammograms and rates of mammography screening were similar in the two randomization groups, differential influence on mammographic breast cancer detection does not explain the cancer findings.
Study strengths include the large, diverse study population, the double-blind placebo-controlled design, comprehensive breast cancer risk assessment, serial mammography monitoring, and central adjudication of breast cancers via pathology report review. Although a limitation is that discontinuation rates were higher than optimal, study supplement adherence was comparable to that in most chronic disease prevention trials, with 76% of participants still taking study pills at the end of the trial. Nonetheless, discontinuation of study pills would decrease the difference between the placebo and treated group in vitamin D intake. Because hip fracture was the primary study endpoint, the intervention included calcium as well as vitamin D. The inclusion of calcium could be considered a limitation, given the more modest information supporting calcium's potential relation to breast cancer.
Given the latency of breast cancer, the 7-year duration of the trial also could be questioned. However, raloxifene and tamoxifen, the two agents with a Federal Drug Administration label indication for breast cancer risk reduction, had efficacy demonstrated in trials of about 5-year duration (38,39). In addition, given difficulties in maintaining long-term drug adherence (40,41), any putative pharmacologic intervention that requires decades-long and continuous exposure would likely have limited public health implications.
Allowing nonprotocol calcium and vitamin D supplement use during the trial represents another limitation. However, nonprotocol supplement use was closely comparable in the two randomization groups and only about 15% of placebo group women could be considered drop-ins (with nonprotocol vitamin D supplement use increased by >400 IU daily during the study course). In addition, the difference in calcium and vitamin D dose between randomization groups was sufficient to statistically significantly increase bone mineral density in the whole population and statistically significantly decrease hip fracture in women older than 65 years (12). Finally, this trial cannot separate calcium and vitamin D influence on breast cancer because these agents were used together in the study.
In summary, calcium and vitamin D supplementation in the dosage provided in this trial did not reduce the incidence of invasive breast cancer in postmenopausal women. In addition, 25-hydroxyvitamin D levels were not associated with subsequent breast cancer risk. These findings do not support a relationship between total vitamin D intake and 25-hydroxyvitamin D levels with breast cancer risk.
Funding
The Women's Health Initiative program was funded by the National Heart, Lung and Blood Institute of the National Institutes of Health, Department of Health and Human Services.
References
- 1.Chlebowski RT, Col N, Winer EP, et al. American Society of Clinical Oncology Technology Assessment of pharmacologic interventions for breast cancer risk reduction including tamoxifen, raloxifene and aromatase inhibitors. J Clin Oncol. 2002;20(15):3328–3343. doi: 10.1200/JCO.2002.06.029. [DOI] [PubMed] [Google Scholar]
- 2.Welsh J. Vitamin D and breast cancer: insight from animal models. Am J Clin Nutr. 2004;80(6 suppl):1721S–1724S. doi: 10.1093/ajcn/80.6.1721S. [DOI] [PubMed] [Google Scholar]
- 3.Ingraham BA, Bragdon B, Noche A. Molecular basis of the potential of vitamin D to prevent cancer. Curr Med Res Opin. 2008;24(1):139–149. doi: 10.1185/030079908x253519. [DOI] [PubMed] [Google Scholar]
- 4.McCullough ML, Rodriquez C, Diver WR, et al. Dairy, calcium, and vitamin D intake and postmenopausal breast cancer risk in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol Biomarkers Prev. 2005;14(12):2898–2904. doi: 10.1158/1055-9965.EPI-05-0611. [DOI] [PubMed] [Google Scholar]
- 5.Braga C, La Vecchia C, Negri E, Franceschi S, Parpinel M. Intake of selected foods and nutrients and breast cancer risk: an age and menopause-specific analysis. Nutr Cancer. 1997;28(3):258–263. doi: 10.1080/01635589709514585. [DOI] [PubMed] [Google Scholar]
- 6.John EM, Schwartz GG, Dreon DM, Koo J. Vitamin D and breast cancer risk: the NHANES I epidemiologic follow-up study, 1971–1975 to 1992: National Health and Nutrition Examination Survey. Cancer Epidemiol Biomarkers Prev. 1999;8(5):399–406. [PubMed] [Google Scholar]
- 7.Kesse-Guyot E, Bertrais S, Duperray B, et al. Dairy products, calcium and the risk of breast cancer: results of the French SU.VI.MAX prospective study. Ann Nutr Metab. 2007;51(2):139–145. doi: 10.1159/000103274. [DOI] [PubMed] [Google Scholar]
- 8.Potischman N, Swanson CA, Coates RJ, et al. Intake of food groups and associated micronutrients in relation to risk of early-stage breast cancer. Int J Cancer. 1999;82(3):315–321. doi: 10.1002/(sici)1097-0215(19990730)82:3<315::aid-ijc1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 9.Levi F, Pasche C, Lucchini F, La Vecchia C. Dietary intake of selected micronutrients and breast cancer risk. Int J Cancer. 2001;91(2):260–263. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1041>3.3.co;2-r. [DOI] [PubMed] [Google Scholar]
- 10.Lin J, Mason JE, Lee IM, Cook NR, Buring JE, Zhang SM. Intakes of calcium and vitamin D and breast cancer risk in women. Arch Intern Med. 2007;267(10):1050–1059. doi: 10.1001/archinte.167.10.1050. [DOI] [PubMed] [Google Scholar]
- 11.Robien K, Cutler GJ, Lazovich D. Vitamin D intake and breast cancer risk in postmenopausal women: the Iowa Women's Health Study. Cancer Causes Control. 2007;18(7):775–782. doi: 10.1007/s10552-007-9020-x. [DOI] [PubMed] [Google Scholar]
- 12.Jackson RD, LaCroix AZ, Gass M, et al. Calcium and vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354(7):669–683. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- 13.Wactawski-Wende J, Kotchen JM, Anderson GL, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. 2006;354(7):684–696. doi: 10.1056/NEJMoa055222. [DOI] [PubMed] [Google Scholar]
- 14.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 15.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 16.Prentice RL, Caan B, Chlebowski RT, et al. Low fat dietary pattern and risk of invasive breast cancer. JAMA. 2006;295(6):629–642. doi: 10.1001/jama.295.6.629. [DOI] [PubMed] [Google Scholar]
- 17.Jackson RD, LaCroix AZ, Cauley JA, McGowan J. The Women's Health Initiative calcium-vitamin D trial: overview and baseline characteristics of participants. Ann Epidemiol. 2003;13(suppl):S98–S106. doi: 10.1016/s1047-2797(03)00046-2. [DOI] [PubMed] [Google Scholar]
- 18.National Cancer Institute. About SEER. http://www.seer.cancer.gov/. Accessed August 14, 2007. [Google Scholar]
- 19.Cox DR. Regression models and life-tables. J R Stat Soc [Ser B] 1972;34:187–220. [Google Scholar]
- 20.Garland CF, Garland FC, Gorham ED, et al. The role of vitamin D in cancer prevention. Am J Public Health. 2006;96(2):252–261. doi: 10.2105/AJPH.2004.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cui Y, Rohan TE. Vitamin D, calcium, and breast cancer risk: a review. Cancer Epidemiol Biomarkers Prev. 2006;15(8):1427–1437. doi: 10.1158/1055-9965.EPI-06-0075. [DOI] [PubMed] [Google Scholar]
- 22.Bertone-Johnson ER. Prospective studies of dietary vitamin D and breast cancer: more questions raised than answered. Nutr Rev. 2007;65(10):459–466. doi: 10.1111/j.1753-4887.2007.tb00271.x. [DOI] [PubMed] [Google Scholar]
- 23.Shin MH, Holmes MD, Hankinson SE, et al. Intake of dairy products, calcium, and vitamin D and risk of breast cancer. J Natl Cancer Inst. 2002;94(17):1301–1311. doi: 10.1093/jnci/94.17.1301. [DOI] [PubMed] [Google Scholar]
- 24.Gissel T, Rehnmark L, Mosekilde L, Vestergaard P. Intake of vitamin D and risk of breast cancer—A meta-analysis. J Steroid Biochem Mol Biol. 2008;111(3–5):195–199. doi: 10.1016/j.jsbmb.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Almquist M, Manjer J, Bondeson L, Bondeson AG. Serum calcium and breast cancer risk: results from prospective cohort study of 7,847 women. Cancer Causes Control. 2007;18(6):595–602. doi: 10.1007/s10552-007-9001-0. [DOI] [PubMed] [Google Scholar]
- 26.Abbas S, Linseisen J, Slanger T, et al. Serum 25-hydroxyvitamin D and risk of postmenopausal breast cancer—results of a large case-control study. Carcinogenesis. 2007;29(1):93–99. doi: 10.1093/carcin/bgm240. [DOI] [PubMed] [Google Scholar]
- 27.Lowe LC, Guy M, Mansi JL, et al. Plasma 25-hydroxy vitamin D concentrations, vitamin D receptor genotype and breast cancer risk in UK Caucasian population. Eur J Cancer. 2005;41(8):1164–1169. doi: 10.1016/j.ejca.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 28.Garland CF, Gorham ED, Mohr SB, et al. Vitamin D and prevention of breast cancer: pooled analysis. J Steroid Biochem Mol Biol. 2007;103(3–5):708–711. doi: 10.1016/j.jsbmb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 29.Colston KW, Lowe LC, Mansi JL, Campbell MJ. Vitamin D status and breast cancer risk. Anticancer Res. 2006;26(4A):2573–2580. [PubMed] [Google Scholar]
- 30.Freedman DM, Chang SC, Falk RT, et al. Serum levels of vitamin D metabolites and breast cancer risk in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Biomarkers Prev. 2008;17(4):889–890. doi: 10.1158/1055-9965.EPI-07-2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bertone-Johnson ER, Chen WY, Holick MF, et al. Plasma 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14(8):1991–1997. doi: 10.1158/1055-9965.EPI-04-0722. [DOI] [PubMed] [Google Scholar]
- 32.Vieth R, Bischoff-Ferrari H, Boucher BJ, et al. The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr. 2007;85(3):649–650. doi: 10.1093/ajcn/85.3.649. [DOI] [PubMed] [Google Scholar]
- 33.Holick MF. Calcium plus vitamin D and the risk of colorectal cancer. N Engl J Med. 2006;354(21):2287–2288. doi: 10.1056/NEJMc060753. [DOI] [PubMed] [Google Scholar]
- 34.Cranney A, Horsley T, O’Donnell S, et al. Effectiveness and Safety of Vitamin D in Relation to Bone Health. Evidence Report/Technology Assessment. 2007. 2007:1–343. Ottawa, Canada: University of Ottawa Evidence based Pratice Center. [PMC free article] [PubMed] [Google Scholar]
- 35.Livshits G, Yakovenko C, Seibel M. Substantial genetic effects involved in determination of circulating levels of calciotropic hormones in human pedigrees. Biochem Genet. 2003;41(9–10):269–289. doi: 10.1023/b:bigi.0000006029.01736.64. [DOI] [PubMed] [Google Scholar]
- 36.Ramos-Lopez E, Bruck P, Jansen T, et al. CYP2R1 (vitamin D 25-hydroxylase) gene is associated with susceptibility to type 1 diabetes and vitamin D levels in Germans. Diabetes Metab Res Rev. 2007;23(8):631–636. doi: 10.1002/dmrr.719. [DOI] [PubMed] [Google Scholar]
- 37.Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes. A Risk Assessment Model for Establishing Upper Intake Levels for Nutrients. Washington, DC: National Academy Press; 1998. [PubMed] [Google Scholar]
- 38.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90(18):1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 39.Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast caner and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295(23):2727–2741. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 40.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 41.McDonald HP, Garg AX, Haynes B. Interventions to enhance patient adherence to mediation prescriptions. JAMA. 2002;288(22):2868–2879. doi: 10.1001/jama.288.22.2868. [DOI] [PubMed] [Google Scholar]