Abstract
Integumentary wound healing in early fetal life is regenerative and proceeds without scar formation. Expressomic analysis of this phenomenon by differential display has previously determined that the eta subunit of the cytosolic chaperonin containing T-complex polypeptide (CCT) is downregulated in the healing fetal wound milieu. We now report that no other CCT subunit shares this distinct pattern of gene regulation as determined by limiting dilution reverse transcriptase polymerase chain reaction (RT-PCR); all seven of the remaining CCT subunits demonstrate no change in messenger RNA (mRNA) expression in healing fetal wounds compared to unwounded control tissue. The alpha subunit, however, did evidence reduced message levels in healing adult wound tissue. We herein report on the cloning and sequence of the complementary DNA (cDNA) for rabbit CCT-alpha and confirm its wound specific decrease in adult tissues through quantitative real-time RT-PCR assay. We also confirm that quantitative evaluation of CCT-alpha and CCT-zeta mRNA expression shows no change in healing fetal wounds.
Keywords: CCT, Chaperonin, Real-time PCR, Scarless wound healing
Introduction
Adult (postnatal) mammals heal their injuries with scar. While this allows for the rapid sealing of an injured area, the resulting cicatrix can itself inflict significant morbidity on the organism. In contrast, fetal mammals can heal their injuries without scar into the third trimester. This scarless wound healing is an intrinsic property of healing fetal tissues, not simply a conferred benefit of the protected uterine environment (Lorenz et al. 1992; Armstrong and Ferguson 1995).
We have previously investigated the molecular basis for scarless fetal wound healing in a rabbit model system using several expressomic techniques to identify differentially expressed candidate genes that may be critical to this unique physiology (Darden et al. 2000; Kathju et al. 2006). Differential display identified the eta subunit of the chaperonin-containing T-complex polypeptide (CCT) as specifically downregulated during fetal wound healing. This pattern of expression was confirmed using limiting dilution reverse transcriptase polymerase chain reaction (RT-PCR; Darden et al. 2000).
The CCT molecule is the major cytosolic chaperonin in eukaryotes and has been estimated to interact with up to 15% of all cellular proteins. The structure of the CCT holoenzyme is unique among chaperonins; it consists of two rings each comprised of eight discrete subunits: alpha, beta, gamma, delta, epsilon, eta, theta, and zeta (zeta2, a variant of zeta, is highly expressed only in testis; Rommelaere et al. 1993; Kubota et al. 1994, 1995). The molecular weight of the complete assemblage is approximately 900kD, but there is evidence that subunits may also localize and function separately as monomers or oligomers (Roobol and Carden 1999). CCT has especially been implicated in the folding of cytoskeletal proteins such as tubulin and actin (Sternlicht et al. 1993; Grantham et al. 2006; Neirynck et al. 2006). Deletion of any CCT subunit gene in yeast can be lethal, highlighting the importance of this molecule (Kubota 2002).
Although each CCT subunit arises from a separate gene, the subunits do share some amino acid homology (∼30%), which indicates that all derive from a common ancestor gene (Archibald et al. 2000; Fares and Wolfe 2003). CCT is ubiquitously expressed in all eukaryotic tissues thus far examined, and Kubota et al. (1999) describe that, in mouse tissues, all CCT subunits appear to be coordinately regulated. Cyrne et al. (1996) report that the CCT-eta and CCT-gamma messenger RNAs (mRNA)s are co-regulated during ciliary biogenesis and sexual reproduction in Tetrahymena. However, in other systems, there appears to be variance in the relative expression of subunits. Yokota et al. (2001) report that the CCT-alpha, CCT-delta, and CCT-zeta subunits preferentially decline when the cell cycle is arrested at M-phase. Himmelspach et al. (1997), working in a plant system, find a light dependent reduction of CCT-epsilon but not CCT-alpha. Few reports examine all eight major CCT subunits.
Our observation that CCT-eta is decreased in a healing fetal wound milieu leads to an obvious question: Is this a subunit-specific behavior, or do all CCT subunits demonstrate a similar reduction? We undertake in this report to address that question in our rabbit experimental system, which necessitated the development and validation of assays applicable to the rabbit.
Results/ Discussion
Little sequence data is as yet available for rabbit CCT subunit genes; only CCT-zeta sequence has been reported for rabbit, rendering primer design for RT-PCR of the remaining subunits difficult. We therefore used the available mouse and human CCT gene sequences to identify regions that are exactly (or nearly exactly) conserved between the two, reasoning that these would likely be similarly shared by the rabbit by dint of their proven tendency toward maximal evolutionary conservation across mammalian species. Primer sets to each of the seven chaperonin subunits were then designed and are presented in Table 1.
Table 1.
List of primers used for limiting dilution RT-PCR of CCT subunits
| Genes | Primer | Primer sequence | |
|---|---|---|---|
| 1 | CCT-alpha | F | 5′-AACTGGTGCCAATGTTATTC-3′ |
| R | 5′-GATCAGCTCATCATCACAAA-3′ | ||
| 2 | CCT-beta1 | F | 5′-GCATGATGCTCTTTGTGTTC-3′ |
| R | 5′-CTGTCGCTTCACTTGAAAAC-3′ | ||
| 3 | CCT-beta2 | F | 5′-CCATCGCCATTGGAGACTTG-3′ |
| R | 5′-TCTTGAACCCTTGACATATC-3′ | ||
| 4 | CCT-gamma | F | 5′-CAATAATCGCATTGCTAGAG-3′ |
| R | 5′-CATTGTTCCACACCAGTCAT-3′ | ||
| 5 | CCT-delta | F | 5′-GCTGGGTTCTGCTGAGTTAG-3′ |
| R | 5′-ACCACCTCCTGCAATAAGAG-3′ | ||
| 6 | CCT-epsilon | F | 5′-ATGAAGCCAATCACTTACTT-3′ |
| R | 5′-CCAATCAAGGTTTCTATGAC-3′ | ||
| 7 | CCT-zeta | F | 5′-CAGGCGGATCTCTACATTTC-3′ |
| R | 5′-GTGAGGATGTACGCATCTTC-3′ | ||
| 8 | CCT-theta | F | 5′-CAATCTGATGGATGACATAG-3′ |
| R | 5′-GTTTTGCCATGATGATCTGA-3′ |
We initially tested our derived primer pairs in RT-PCR on 200ng of rabbit fetal control RNA. RT-PCR was performed using the same conditions described in Kathju et al. (2006); after amplification, the resulting PCR products were electrophoresed through agarose gels. Each subunit pair yielded a single predominant amplimer of the expected molecular weight, indicating that we had successfully targeted the correct CCT template molecule in each case. PCR without RT yielded no amplicons, confirming that our RT-PCR products derived from RNA substrate and not incidental DNA. In addition, all of the eight primer pairs (with two pairs of primer sets for CCT-beta) loci spanned intron/exon boundaries as determined for their cognate mouse CCT subunit genes (Table 1; Kubota et al. 1999), an additional safeguard against amplification from contaminating DNA.
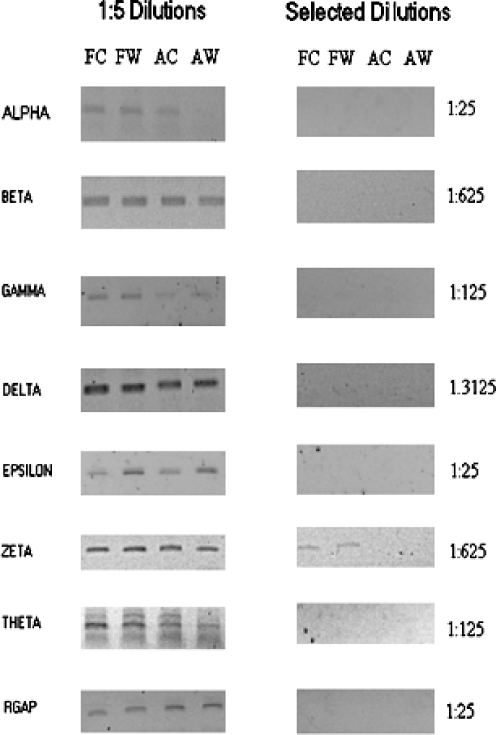
We then tested our primer pairs in limiting dilution RT-PCR across rabbit fetal and adult wound and control tissues. Limiting dilution RT-PCR allows for semi-quantitative assessment of relative message abundance without need for precise intervening sequence information or costly fluorescent-coupled probes. We have previously used this technique to confirm the differential expression of the CCT-eta subunit and a glycophorin-like transcript in healing fetal wounds (Darden et al. 2000). Figure 1 shows the results of limiting dilution RT-PCR assay for each of the remaining seven CCT subunits. The majority of subunits (beta, gamma, delta, epsilon, theta) show no differential expression in any tissue type. CCT-zeta appears to be more abundant in fetal tissues than adult but shows no wound-specific alteration in gene expression in either fetal or adult tissues. In contrast, CCT-alpha is specifically decreased in adult wound tissue. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) control was unaffected across tissue type. These data demonstrate that the reduction of CCT-eta we have previously observed in fetal wounds is a specific behavior not shared by any other CCT subunit.
Fig. 1.
Serial-limiting dilution assay of CCT subunits. The wounding protocol for our New Zealand white rabbits and RNA isolation/storage from fetal and adult wound tissues was done as previously described (Darden et al. 2000; Kathju et al. 2006). All animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) and followed guidelines set forth in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. RNA levels were assayed for seven chaperonin subunits using limiting dilution reverse transcription-polymerase chain reaction (RT-PCR) as previously described (Darden et al. 2000). Limiting dilution RT-PCR assays were performed on 200 ng of pooled total RNA from fetal control (FC), fetal wound (FW), adult control (AC), and adult wound (AW) samples; each pool was comprised of equivalent amounts from at least four separate biological samples; assays were performed in triplicate. Undiluted samples were reverse transcribed and subjected to 35 cycles of PCR (using subunit appropriate primers) and compared to serial fivefold dilutions (1:5, 1:25,1:125, 1:625; 1:3125) tested at the same time in the same conditions. Negative controls, that is, PCR reactions without an initial reverse transcriptase step, were also included to ensure that the amplimers seen are derived from expressed mRNA and not contaminating chromosomal DNA. Rabbit GAPDH was used as an invariant internal control. The left panel displays results of 1:5 dilutions for all subunits; the alpha subunit already has lost a band in the AW lane, indicating it is less abundant in adult wound. The right panel shows the result, in each case, of the first dilution at which a band becomes undetectable (indicated at right). Differences in the end dilution points reflect technical variation between subunit assays and are not themselves indicative of differential subunit expression. Rabbit GAPDH (RGAP) is invariant across tissue types
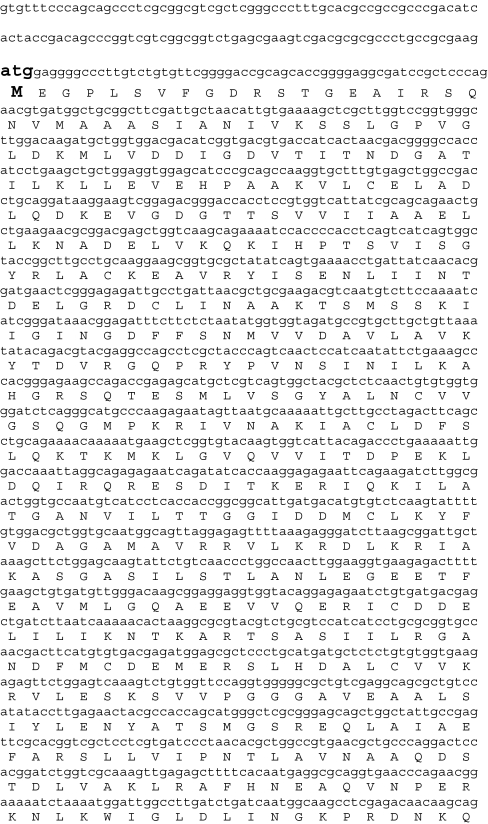
The apparent underexpression of CCT-alpha in healing adult wounds is itself an observation of interest. To verify this pattern with fully quantitative real-time RT-PCR, we first used rapid amplification of cloned ends (RACE) to obtain a full-length rabbit CCT-alpha complementary DNA (cDNA). The sequence of this gene product is presented in Fig. 2. Rabbit CCT-alpha presents a transcript of 1,936bp, which at the nucleotide level, is 85% identical to mouse and 85% identical to cct-alpha human transcript variant 1 (86% identical to cct-alpha human transcript variant 2). Predicted amino acid identity is even higher, with the deduced rabbit protein 96% identical to mouse, and 97% and 96% identical to human transcript variants 1 and 2, respectively. CCT-alpha has a 5′ untranslated region of 120bp and a 3′ UTR of 145bp; a consensus polyadenylation signal is located at nucleotide 1,854bp.
Fig. 2.
Nucleotide and amino acid sequence of rabbit CCT-alpha cDNA. Cloning of the CCT-alpha was accomplished as follows: the primer pairs for CCT-alpha listed in Table 1 were used to generate an RT-PCR amplimer product (as shown in Fig. 1). The nucleotide sequence of this product was then determined. This obtained sequence confirmed homology to mouse and human CCT-alpha sequences on the Basic Local Alignment Search Tool search and was then used to design both forward and reverse primers for 3′ and 5′ RACE, respectively. The primer used for 3′ RACE was 5′-GGACCAAATTAGGCAGAGAGAATC-3′. The primer used for 5′ RACE was 5′-GCCGGTAGCCACTGATGACTGA-3′. 5′ and 3′ RACE reactions were carried out as described in the GeneRacer kit protocol (Invitrogen Corporation, cat. no. L1500-1). RACE amplimers of the expected molecular weight were subcloned by Topo TA ligation into the vector provided; the sequence of these derived amplimers was then determined, and the composite whole CCT-alpha cDNA was assembled therefrom. The full-length nucleotide sequence for the rabbit CCT-alpha cDNA is shown, with the corresponding amino acids in the coding region. The start codon (ATG) and stop codon (TGA) are enlarged and bolded. A consensus polyadenylation signal sequence at 1,854 bp (ATTAAA), appropriately positioned 23 bp upstream of the polyA tail, is similarly enlarged and bolded
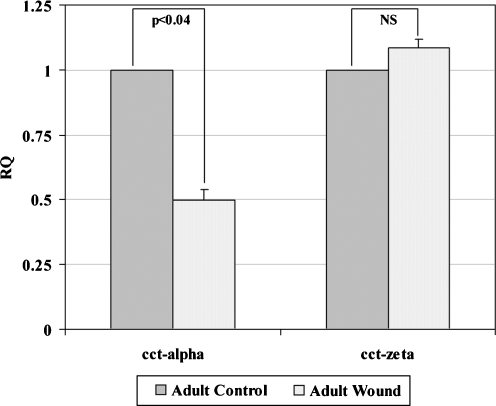
With the full sequence of rabbit CCT-alpha in hand, we designed a real-time RT-PCR assay to further validate the adult wound-specific decrease observed (Table 2). We again verified that the primers used in this assay yielded a single band of the expected molecular weight after gel electrophoresis (data not shown). Quantitative PCR confirmed that CCT-alpha is underexpressed in healing adult wounds versus unwounded adult control tissues (Fig. 3).
Table 2.
Primer and probe sequences for quantitative real time RT-PCR reactions
| Genes | Primer | Primer Sequence | |
|---|---|---|---|
| 1 | CCT-alpha | F | 5′-GCTTCTGGAGCAAGTATTCTGTCA-3′ |
| R | 5′-TGTCCCAACATCACAGCTTCA-3′ | ||
| P | 5′-6FAM-CCCTGGCCAACTTGGAAGGTG-TAMRA-3′ | ||
| 2 | CCT-zeta | F | 5′-ACGTGCTGCTGCATGAAATG-3′ |
| R | 5′-TGAAGTAGTACCATCGCCAGTTATG-3′ | ||
| P | 5′-6FAM-TTCAACACCCAACTGCCTCCTTAATAGCAA-TAMRA-3′ | ||
| 3 | Rabbit GAPDH | F | 5′-CGCCTGGAGAAAGCTGCTAA-3′ |
| R | 5′-CCTCGGTGTAGCCCAGGAT-3′ | ||
| P | 5′-6FAM-AAGCAGGCATCCGAGGGCCC-TAMRA-3′ |
The primers and probes were designed using Primer Express® software v2.0 provided by Applied Biosystems. Primer sequences are named as F and R, representing forward and reverse sequences respectively and P represents probe. Reverse primers (labeled R) are used in the RT reactions.
Fig. 3.
Quantification of CCT-alpha and CCT-zeta mRNA levels in healing adult wounds versus adult control assayed by real time RT-PCR. Real time RT-PCR was done to confirm patterns of expression of CCT-alpha and CCT-zeta, the two subunits for which full-length sequence was now available. The primers and Taqman probes were designed using Primer Express software (Applied Biosystems, Foster City, CA, USA) and are listed in Table 2. Forward and reverse primers were purchased from Integrated DNA Technologies (Coralville, IA, USA) and fluorocoupled Taqman probes were purchased from Applied Biosystems. The reverse transcriptase (RT) reaction (using reverse primer) and subsequent real-time PCR assays were performed as previously described (Kathju et al. 2006). Using the comparative critical cycle (Ct) method and using GAPDH as the endogenous control, the expression levels of the target genes were normalized and the relative abundance was calculated. Data were analyzed using the 7,900-HT SDS software version 2.1 provided by Applied Biosystems. Data are shown as mean ± SEM of six independent studies performed in duplicate. Statistical analysis was performed by Student’s t test (*p < 0.05). CCT-alpha displays a significant reduction in the adult wound tissue compared to unwounded control, while CCT-zeta does not
Since a full-length clone of rabbit CCT-zeta cDNA had already been reported, we used this sequence information (Schwartz et al. 2000; GenBank accession no. NM 001082039) to design a quantitative real-time RT-PCR assay for this rabbit CCT subunit as well (Table 2). We again confirmed that the use of our primers in RT-PCR yielded only a single amplimer of the expected size. We then directly examined CCT-zeta, as well as CCT-alpha, in fetal wound and control tissues by quantitative RT-PCR. These assays confirmed that neither CCT-zeta nor CCT-alpha mRNA shows any significant change in abundance in healing fetal wounds compared to unwounded fetal controls (data not shown) nor is CCT-zeta differentially expressed in adult wound versus control tissues (Fig. 3).
We also examined the relative abundance of CCT-alpha and CCT-zeta messages in cultured fetal and adult skin fibroblasts using these same quantitative RT-PCR assays and found no difference in the relative expression of either gene between these cell types (data not shown).
Although the majority of studies on CCT function have focused on the holoenzyme, an increasing body of evidence suggests that the individual subunits of CCT may have an independent function and significance. We have previously reported on the downregulation of CCT-eta in healing fetal wounds, an observation we have subsequently confirmed with quantitative real-time PCR (Satish et al., manuscript in preparation). In this report, we examine the seven remaining CCT subunits in our rabbit model system and find that no other CCT subunit transcript displays any fetal wound-specific reduction. The majority of subunits are unchanged in both fetal and adult wounds. The only other subunit to show a wound-specific change in mRNA expression is CCT-alpha, which is significantly decreased in adult tissues, suggesting a potential role for CCT-alpha in directing scirrhous versus scarless wound healing. This observation raises the intriguing question of whether a similar reduction of CCT-alpha in a fetal milieu would somehow shift the wound healing phenotype from scarless to a more adult, scar-forming pattern.
Additional data from our laboratory using different technology further supports the observation that CCT-theta message expression is unchanged during fetal wound healing. In parallel studies on fetal gene expression, we have constructed a microarray of rabbit cDNAs derived from healing fetal wounds. This microarray has been found to include the CCT-theta subunit, the expression of which was unaltered when fetal wound and fetal control probes were used to interrogate the array (Kathju et al., manuscript in preparation), a result corroborative with our data here.
Although we have accumulated substantial data noting the differential expression of select CCT subunits during fetal and adult wound healing, these studies do not address the question of which cell type is responsible for the observed changes in expression. In fetal wounds, where the inflammatory response seen in adult wound healing is nil, the most important candidate cells are fibroblasts and keratinocytes. In adult wounds, the picture is more complicated, with inflammatory cells also contributing to the milieu as a whole. Further studies with in situ probes or rabbit-applicable antibodies for immunohistochemistry will be required to directly address this question.
An even more compelling question is the mechanism by which modulation of CCT expression might affect wound healing. The cell type thought to be the most proximally correlated with scar formation and contracture is the myofibroblast. Myofibroblasts have been found to exhibit an altered actin biology, with a significant increase in cellular actin, especially of the alpha-smooth muscle type (Vande Berg et al. 1989). The known importance of CCT to proper actin folding suggests one pathway by which myofibroblast and, therefore, scar physiology might be regulated. A recent study by Grantham et al. (2002) demonstrates a role for CCT not just in the production of actin monomers but also in their polymerization to F-actin, suggesting that CCT might thereby derivatively regulate myofibroblast biology at multiple points.
Alternatively, it may be that changes in CCT subunits during wound healing, fetal or adult, exert their effects through subunit-specific functions. CCT-eta has recently been described as a biological partner and co-factor for the soluble guanylyl cyclase (Hanafy et al. 2004); changes in -eta expression without similar changes in other subunits might signify a modulation of cyclic guanosine monophosphate-dependent pathways. Although no such specific biological partner pointing to other mechanistic pathways has yet been identified for CCT-alpha, it remains possible that it too has another physiological function apart from its participation in the chaperonin holoenzyme, which is important to wound healing. It may also be that the CCT-alpha subunit specifically provides chaperonin-activity to a substrate protein essential to wound healing or scar formation, similar to the way it appears to act upon huntingtin aggregates (Tam et al. 2006). Additional investigation will be required to clarify these roles.
Acknowledgment
This study was supported by the Allegheny Singer Research Institute, Allegheny General Hospital, and Pittsburgh Tissue Engineering Institute (PTEI). This work was funded from the grants awarded to S.K. (K08 DE014780), J.C.P. (DC 05659), and G.D.E. (DC 04173). We extend our thanks to Ms. Mary O’Toole for her assistance in preparing this manuscript.
References
- Archibald JM, Logsdon JM Jr, Doolittle WF (2000) Origin and evolution of eukaryotic chaperonins: phylogenetic evidence for ancient duplications in CCT genes. Mol Biol Evol 17:1456–1466 [DOI] [PubMed]
- Armstrong JR, Ferguson MWJ (1995) Ontogeny of the skin and transition from scar free to scarring phenotype during wound healing in the pouch young of Monodelphis domestica. Dev Biol 169:242–260 [DOI] [PubMed]
- Cyrne L, Guerreiro P, Cardoso AC, Rodrigues-Pousada C, Soares H (1996) The Tetrahymena chaperonin subunit CCT eta gene is coexpressed with CCT gamma gene during cilia biogenesis and cell sexual reproduction. FEBS Lett 383:277–283 [DOI] [PubMed]
- Darden DL, Hu FZ, Ehrlich MD, Gorry MC, Dressman D, Li HS, Whitcomb DC, Hebda PA, Dohar JE, Ehrlich GD (2000) RNA differential display of scarless wound healing in fetal rabbit indicates downregulation of a CCT chaperonin subunit and upregulation of a glycophorin-like gene transcript. J Pediatr Surg 35:406–409 [DOI] [PubMed]
- Fares MA, Wolfe KH (2003) Positive selection and subfunctionalization of duplicated CCT chaperonin subunits. Mol Biol Evol 20:1588–1597 [DOI] [PubMed]
- Grantham J, Ruddock LW, Roobol A, Carden MJ (2002) Eukaryotic chaperonin containing T-complex polypeptide 1 interacts with filamentous actin and reduces the initial rate of actin polymerization in vitro. Cell Stress Chaperones 7:235–242 [DOI] [PMC free article] [PubMed]
- Grantham J, Brackley KI, Willison KR (2006) Substantial CCT activity is required for cell cycle progression and cytoskeletal organization in mammalian cells. Exp Cell Res 312:2309–2324 [DOI] [PubMed]
- Hanafy KA, Martin E, Murad F (2004) CCTeta, a novel soluble guanylyl cyclase-interacting protein. J Biol Chem 279:46946–46953 [DOI] [PubMed]
- Himmelspach R, Nick P, Schafer E, Ehmann B (1997) Developmental and light-dependent changes of the cytosolic chaperonin containing TCP-1 (CCT) subunits in maize seedlings, and the localization in coleoptiles. Plant J 12:1299–1310 [DOI] [PubMed]
- Kathju S, Satish L, Rabik C, Rupert T, Oswald D, Johnson S, Hu FZ, Post JC, Ehrlich GD (2006) Identification of differentially expressed genes in scarless wound healing utilizing polymerase chain reaction-suppression subtractive hybridization. Wound Repair Reg 14:413–420 [DOI] [PubMed]
- Kubota H, Hynes G, Carne A, Ashworth A, Willison K (1994) Identification of six TCP-1-related genes encoding divergent subunits of the TCP-1-containing chaperonin. Curr Biol 4:89–99 [DOI] [PubMed]
- Kubota H, Hynes G, Willison K (1995) The eighth CCT gene, Cctq, encoding the theta subunit of the cytosolic chaperonin containing TCP-1. Gene 154:231–236 [DOI] [PubMed]
- Kubota H, Yokota S, Yanagi H, Yura T (1999) Structures and co-regulated expression of the genes encoding mouse cytosolic chaperonin CCT subunits. Eur J Biochem 262:492–500 [DOI] [PubMed]
- Kubota H (2002) Function and regulation of cytosolic molecular chaperone CCT. Vitam Horm 65:313–331 [DOI] [PubMed]
- Lorenz HP, Longaker MT, Perkocha LA, Jennings RW, Harrison MR, Adzick NS (1992) Scarless wound repair: a human fetal skin model. Development 114:253–259 [DOI] [PubMed]
- Neirynck K, Waterschoot D, Vandekerckhove J, Ampe C, Rommelaere H (2006) Actin interacts with CCT via discrete binding sites: a binding transition-release model for CCT-mediated actin folding. J Mol Biol 355:124–138 [DOI] [PubMed]
- Rommelaere H, Van Troys M, Gao Y, Melki R, Cowan NJ, Vandekerckhove J, Ampe C (1993) Eukaryotic cytosolic chaperonin contains t-complex polypeptide 1 and seven related subunits. Proc Natl Acad Sci U S A 90:11975–11979 [DOI] [PMC free article] [PubMed]
- Roobol A, Carden MJ (1999) Subunits of the eukaryotic cytosolic chaperonin CCT do not always behave as components of a uniform hetero-oligomeric particle. Eur J Cell Biol 78:21–32 [DOI] [PubMed]
- Schwartz GJ, Kittelberger AM, Segel GB (2000) Cloning of rabbit Cct6 and the distribution of the Cct complex in mammalian tissues. Exp Nephrol 8:152–160 [DOI] [PubMed]
- Sternlicht H, Farr GW, Sternlicht ML, Driscoll JK, Willison K, Yaffe MB (1993) The T-complex polypeptide 1 complex is a chaperonin for tubulin and actin in vivo. Proc Natl Acad Sci U S A 90:9422–9426 [DOI] [PMC free article] [PubMed]
- Tam S, Geller R, Spiess C, Frydman J (2006) The chaperonin TRiC controls polyglutamine aggregation and toxicity through subunit-specific interactions. Nat Cell Biol 8:1155–1162 [DOI] [PMC free article] [PubMed]
- Vande Berg JS, Rudolph R, Poolman WL, Disharoon DR (1989) Comparative growth dynamics and actin concentration between cultured human myofibroblasts from granulating wounds and dermal fibroblasts from normal skin. Lab Invest 61:532–538 [PubMed]
- Yokota S, Yanagi H, Yura T, Kubota H (2001) Cytosolic chaperonin-containing t-complex polypeptide 1 changes the content of a particular subunit species concomitant with substrate binding and folding activities during the cell cycle. Eur J Biochem 268:4664–4673 [DOI] [PubMed]