Abstract
The expression and localization of four heat shock proteins (Hsp70, Hsp86, Hsp90, and Hsp27) were shown in the heart tissue of pigs transported for 6 h. Immunostaining detected the consistent presence of all Hsps in the pig myocardial cells under both transported and normal housing conditions. Immunohistochemical analysis revealed predominance of Hsp70 (significantly highest levels) and Hsp27 in the cytoplasm of myocardial cells. Hsp90 and Hsp86 were expressed both in the cytoplasm and in the nucleus, preferentially in the cytoplasm, of the myocardial cells. In view of their abundant and uniform distributions in the myocardial cells, the expression and distribution patterns of all detected Hsps within the myocardial cells, mostly limited to the cytoplasm, could be related to their chaperone function for cells with important special activities in this study. The identification of all four Hsps in the blood vessel endothelial cells possibly implies that endothelial cells react to ischemia and hypoxia by expressing Hsps. Immunoblot findings suggest that the level of all Hsps decreased in response to stress due to a 6 h journey. The decrease in Hsp levels in the myocardial cells may indicate that the transport stress may have overcharged the repair mechanisms of the cells. Whether this distinct depletion of Hsps contributes to an increased susceptibility to acute heart failure and the sudden death syndrome in transported pigs should be elucidated in future experiments.
Keywords: Heat shock protein, Heart, Stress, Transportation, Pig
Introduction
Poor environmental conditions during road transport such as truck motion, noise, vibration, centrifugal forces, rapidly changing light conditions, changes in temperature, poor ventilation, and possible lack or shortage of water and food can be very stressful to animals and may potentially lead to tissue damage (Warriss et al. 1994). Porcine stress syndrome (PSS) is a naturally occurring phenomenon and stress appears to be the sole causative factor (Riette et al. 1993). The exact number of deaths due to PSS during road transport is not known. There are estimates that approximately 0.2% of all slaughter pigs die annually during transport in Germany (Schütte 1994), a total number of approximately 160,000 pigs. These figures show that poor road transport does not only affect the welfare of pigs, but it can also lead to considerable economic losses. Stress represents the reaction of a body to stimuli that result in the disturbance of its normal physiological equilibrium or homeostasis. Further, such disturbances are often associated with detrimental effects (Khansari et al. 1990), if the stress impact is too high or if no adequate compensatory mechanisms are in place.
It is generally accepted that HSPs are molecular chaperones known to confer cellular tolerance to I/R injury (Donnelly et al. 1992; Currie et al. 1993; Arrigo 2000; Chiu et al. 2003; Wischmeyer et al. 2003; Gauthaman et al. 2005). Any organism usually reacts by rapid gene transcription and subsequent mRNA translation in order to yield a class of highly conserved proteins known as heat shock proteins (Hsps) when exposed to harmful stimuli (Arrigo 2000). The Hsp species which are usually expressed in animals under stress belong to a large protein family consisting of constitutively expressed and inducible members, which are classified according to their molecular weight (Locke et al. 1996; Liu and Steinacker 2001). Evidence exists suggesting that these Hsps are also expressed in transported pigs (Bao et al. 2002; Yu et al. 2007). However, there seems to be large inter-species variation in the protein induction. For example, Hsp70 expression is known to be activated by several pathological and environmental factors (Kiang and Tsokos 1998). In the small intestine of pigs, 30-min transportation has been shown to cause ischemia (Nabuurs et al. 2001); simultaneously, new depletion of Hsp70 expression in the cells occurred. This may indicate on the one hand that a sufficient induction of Hsp70 could have protected the myocardium from ischemia, and on the other hand that a decreased ability to express Hsp70 during homeostatic disruptions may be seen as a possible mechanism for the increased susceptibility of hearts to ischemic stress (Donnelly et al. 1992; Frier and Locke 2005). Nevertheless, the precise function of the majority of these induced proteins remains unclear, although there is growing appreciation of the protective function of HSPs in the defensive mechanisms against tissue damage and their importance in many regulatory pathways. Furthermore, HSPs have become a focus of physiological research (Natelson et al. 1988; Li et al. 2006; Sepponen and Pösö 2006).
However, the exact contribution of the pathophysiology of ischemic acute heart failure (AHF) and its mechanism remains elusive. In this study, the amount of Hsps from three different HSP families was measured in the heart tissue of transported and control pigs and compared with results from the measurement of conventional markers of stress. Additionally, the distribution of the Hsps in the myocardial cells of the animals was shown.
Materials and methods
Experimental design
A total of 13 pigs of the German Landrace line and weighing 35 ± 1 kg were investigated, eight animals were transported and five served as the control, i.e., were kept under normal housing conditions. Temperatures in the animal house ranged between 18 and 23°C, the outside temperatures were from 14 to 24°C at the transport day (day time between about 8.00 a.m. and 4.00 p.m.). The animals were transported in a commercial trailer pulled by a van up to a period of 6 h (covering a 398-km stretch). The journey included equal periods of transport over local roads; including town traffic, state roads, and federal highways. Prior to transportation, blood samples were collected from all animals in the animal house. The second blood sampling was carried out at the half-way point after approximately 3 h of journey in the trailer. The third sampling was carried out at the end of the transport period in the trailer. After transportation, all animals were anesthetized on the lorry or in the animal house and brought to the operation centre. The samples from the heart were collected and the animals were immediately killed. The specimens for use in morphological studies were fixed in formalin and those for biochemical analysis were frozen in liquid nitrogen. The experiment was undertaken following the guidelines of the Regional Animal Ethics Committee.
Antibodies
The polyclonal antibodies for Hsp27 (C-20, sc-1048) and the inducible Hsp70 (K-20, sc-1060) were supplied by Santa Cruz Biotechnology, Inc. Their specificity for porcine Hsps has not been published. The polyclonal antibodies for the inducible Hsp70 (former Hsp72; SPA-812), polyclonal antibodies for Hsp86 (SPS-771; new name: Hsp90α), and monoclonal antibodies for Hsp90 (SPA-835; new name: Hsp90α and β) were supplied by StressGen Biotechnologies Corp. These antibodies are stated by the producer to react with the porcine protein isoform. For both Hsp86 and Hsp90 the previous names were still used in this paper.
Creatine kinase (CK)
The heparinized blood samples of the jugular vein were immediately transported to the laboratory then were centrifuged and separated. The blood plasma was aliquotated and stored at 20°C until the day of photometric enzyme analysis. CK was analyzed using commercial kits (CK-N-acetyl-cysteine Test-kit; Randox Laboratories, UK).
Histopathology
Formalin-fixed, paraffin-embedded heart tissues were serially sectioned into 5-μm sections; each section was routinely stained with hematoxylin and eosin and examined using light microscopy.
Immunohistochemistry
Serial sections of the heart tissues of both transported and control pigs were immunostained by the standard avidin–biotin complex (ABC) immunoperoxidase detection system.
Endogenous peroxidase was blocked using 3%H2O2 for 30 min at room temperature. After washing three times for 5 min in Tris-buffered saline (TBS), the slides were then incubated in preheated 0.25% trypsin at 37°C for 15 min to facilitate the unmasking of antigens. Unspecific binding of the antiserum was blocked by incubating the sections for 20 min at room temperature in a blocking solution containing 20% normal rabbit serum (for Hsp70-sc-1060, Hsp90, and Hsp27) or goat serum (for Hsp70-SPA-812 and Hsp86). The sections were then incubated overnight at 4°C with a 1:25 dilution for the Hsp70-sc-1060 antibody; 1:400 dilution, Hsp70-SPA-812 antibody; 12.5 μg/mL dilution, Hsp86 antibody; 1:100 dilution, Hsp90 antibody; and 1:25 dilution, Hsp27 antibody. After washing three times for 5 min in TBS (500 mM NaCl, 20 mM Tris–Cl, pH 7.6), the slides were incubated for 60 min at room temperature with biotinylated secondary antibodies at 1:300 dilution in TBS (pH 7.1). Subsequently, the sections that were washed three times with TBS were incubated for 60 min in an ABC peroxidase solution, which was prepared according to the manufacturer’s directions and again washed for three times for 5 min in TBS. The sections were then developed with 3–3′ diaminobenzidine tetrachloride (D 5059; Sigma Chemical Co.) in TBS supplemented with hydrogen peroxide. After washing two times for 5 min in TBS and once for 5 min in distilled water, the slides were counterstained for 15 s with Mayer’s hematoxylin. The corresponding negative control sections were prepared by omitting antibody. Actin and desmin were used as positive controls.
Confirmation of Hsps expression by Western blot
Sample preparation The heart samples were pulverized using liquid nitrogen-cooled steel mortar. 1.0 g of each sample was homogenized with POLYTRON® PT 3000 (Kinematik AG) in 10 mL sodium dodecylsulfate (SDS)-containing sample buffer (10% glycerin; 3% SDS; 62 mM upper Tris; 5% mercapthoethanol). The homogenate was centrifuged at 5,000×g for 30 min. The supernatant fractions were denatured at 90°C for 10 min and stored at −30°C until electrophoresis.
SDS-PAGE A one-dimensional SDS polyacrylamide gel electrophoresis (1-D SDS-PAGE) was performed according to the method described by Laemmli (1970); the separating gel consisted of 10% polyacrylamide overlaid with a 4% stacking gel. Appropriate SeeBlue™ prestained standard proteins (Novex; Novel Experimental Technology) were co-electrophoresed. The samples were thawed on ice and heated again for 10 min at 90°C. An aliquot of 5 μL was mixed with 5 μL of the load mix and 20 μL sample buffer; vortexed and heated for 10 min at 90°C. The samples were centrifuged for 1 min to eliminate precipitates and 20 μL of the samples were carefully loaded on the slots. Electrophoresis was carried out at a constant current of 25 mA (50 V).
Protein transfer and immunoblotting Following the electrophoretic separation, the proteins were transferred on to nitrocellulose membranes (0.2 μm, PROTRAN® BA 883, Schleicher & Schuell) and stained by Ponceau S (SERVA). The nitrocellulose transfer membranes were washed three times for 10 min in TBS containing 0.1% Tween-20 (Tris-Buffered Saline Tween-20 (TBST), pH 7.6) and blocked with a blocking solution containing 5% non-fat milk powder (NFDM) and 2% albumin fraction V (Roth, Karlsruhe) for 1 h. Blots were incubated with the appropriate primary antibody against Hsp70-sc-1060 at a 1:500 dilution; Hsp70-SPA-812, 1:20,000; and Hsp27, 1:1,000 for 2 h at room temperature; Hsp86 at 0.4 μg/mL and Hsp90 at 1:2,000 dilution were incubated overnight at 4°C in the blocking solution. After washing the blots three times for 10 min in TBST, they were placed in a blocking solution of specific peroxidase-conjugated secondary antibodies for 1 h. The blots were subsequently washed 2 × 10 min in TBST and TBS. Chemiluminescence was detected using the ECL western blot detection kit (Amersham Pharmacia Biotek UK Limited). We calculated the densities of the Hsps in the western blots relative to its corresponding heart tissue β-actin as the difference.
Statistical analyses
Results are provided as mean ± SD. Significant differences among data were determined by analysis of Student’s t test procedures. P < 0.05 was considered statistically significant.
Results
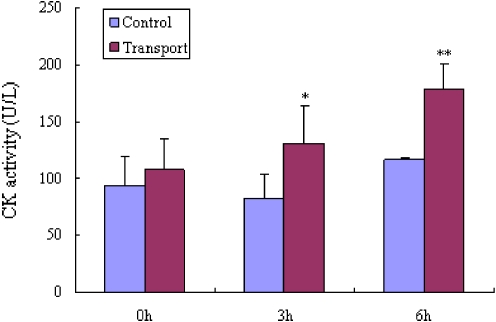
The CK concentration in the blood plasma was used to characterize the muscle fatigue and the stress encountered by the pigs kept under normal housing conditions as compared with those subjected to 6 h transportation. With the length of transport time, the values of CK increased in the blood samples obtained from transported pigs [before transport (P > 0.05), half way (3 h, P < 0.05), and after transport (6 h, P < 0.01)] compared to the control pigs (Fig. 1).
Fig. 1.
CK activity levels (U/L) in blood samples from eight transported (T) and five control (C) pigs. *P < 0.05 vs. C and **P < 0.01 vs. C
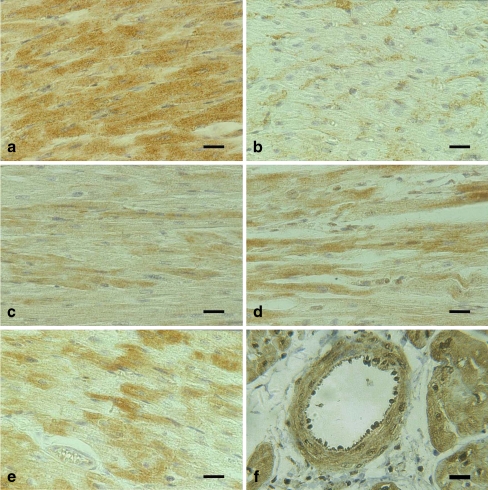
Histologically, slight granular degeneration of the cardiac muscle cells was observed in the heart sections of all transported pigs, whereas no marked lesions were detected in the control pigs. Immunostaining revealed the consistent presence of Hsps in the myocardial cells both in transported (Fig. 2) and control pigs. However, within the stained areas, not all cells were stained positively or to a similar extent. Hsp70 and Hsp27 appeared to be predominant in the cytoplasm. Hsp70 was most abundant in the myocardial cells and was present in rather high levels (Fig. 2a). Approximately half of the myocardial cells showed slight intracytoplasmic labeling and few revealed sparse nuclear labeling by small granular Hsp72 particles (Fig. 2b). In contrast, relatively stronger levels of Hsp27 proteins were detected in the myocardial cells (Fig. 2e) as compared to Hsp70. The intracytoplasmic signals of Hsp90 were most prominent in the myocardial cells; immunostaining of Hsp90 in the nucleus was relatively weak as compared to that in the cytoplasm (Fig. 2c). A similar distribution of Hsp86 was observed in the cytoplasm and nucleus of the myocardial cells (Fig. 2d). We did not specifically identify endothelial cells as a target. However, all for Hsps, Hsp70, Hsp86, Hsp90, and Hsp27, were identified immunohistochemically in the cytoplasm or nucleus of blood vessel endothelial cells as well as in the vascular wall of the heart from transported and control pigs (Fig. 2f). Although Hsp-positive cell types, including endothelial cells, were identified based on histomorphology, immunostaining revealed no marked variation between individual myocardial cells in transported and control pigs. The control sections incubated with secondary antibodies gave consistently negative results.
Fig. 2.
Histological sections of myocardium tissues of transported pigs. Immunoperoxidase labeling, Mayer’s hematoxylin counterstain. Bar 50 μm. a Immunoreactive Hsp70-sc-1060 is observed to be preferentially present in the cytoplasm of myocardial cells. b Hsp70-SPA-812 positive immunoreactivity was detected in the cytoplasm of the myocardial cells. c Hsp90 (=Hsp90α and b) is observed to be preferentially present in the cytoplasm and to a limited extent in the nuclei of myocardial cells. d Hsp86 (=Hsp90a) is detected preferentially both in the nuclei and in the cytoplasm of the myocardial cells. e Immunoreactive Hsp27 is observed to be preferentially present in the cytoplasm and to a limited extent in the nucleus of heart muscle cells. f Hsp70-sc-1060 identified in the blood wall and the endothelial cells
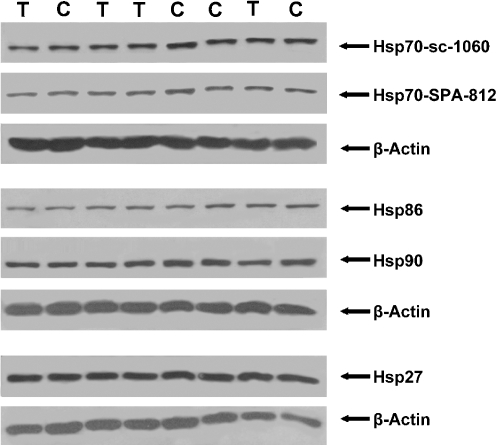
All four Hsps were regularly detected in the heart tissues of both the transported and control pigs (Fig. 3). From these immunoblots, it appears that transportation may not have a distinct effect on the expression of Hsps in pigs. However, semi-quantitative analysis of Hsps expression reveals significant differences after normalized to its corresponding heart tissue β-actin (Table 1). Transport stress significantly induced a drop in the levels of all four Hsps in the heart.
Fig. 3.
Detection of Hsps in the heart of control pigs (C) and 6 h transported pigs (T). Densities of the Hsps in the western blots were normalized to its corresponding heart tissue β-actin as the difference. Here, only a part of the immunoblot that reacted with antibodies specific for Hsp70-sc-1060, Hsp70-SPA-812, Hsp86 (=Hsp90α), Hsp90 (=Hsp90a and βb), and Hsp27 is shown
Table 1.
Densitometric readings of Hsps in the heart tissues of pigs from the control group and after 6 h transport
| Hsp-type | Control (n = 5) | 6-h transport (n = 8) | Changes(%), (P) |
|---|---|---|---|
| Hsp70-sc-1060 | 0.71 ± 0.06 | 0.48 ± 0.01 | −33%, P < 0.01a |
| Hsp70-SPA-812 | 0.78 ± 0.03 | 0.49 ± 0.04 | −37%, P < 0.01a |
| Hsp86 | 0.25 ± 0.08 | 0.15 ± 0.01 | −41%, P < 0.05b |
| Hsp90 | 0.66 ± 0.13 | 0.47 ± 0.02 | −29%, P < 0.05b |
| Hsp27 | 1.43 ± 0.15 | 1.06 ± 0.07 | −26%, P < 0.01a |
The values (means ± SE) given for control and 6 h transport represent the ratio of densitometric scans from immunoblots to the β-actin content of the corresponding heart tissues.
aHighly significant
bSignificant
Discussion
Physiological responses to transport can be used to characterize the effort animals have to put in to cope with the associated stresses. Physical stress such as fatigue increases enzymatic activities in the blood (Klemcke 1994; Warriss et al. 1994) and the CK level in the blood plasma is a particularly good measure of physical stress. High CK levels reflect high physical exertion and, possibly, also suffering of the animals. Our data indicate that CK levels increase with the length of transport. The considerable variation of the results derived from both the control pigs and the transported pigs may have been caused by individual differences of the pigs and, despite careful management, by the blood sampling stress which was, however, the same for both groups. The differences in CK activity levels between control and transported pigs are significant both after 3 h (P < 0.05) and 6 h (P < 0.01) of transport which allows the assessment that the transported pigs were clearly exposed to physical stress.
The expression of the Hsp in the presence and absence of external stress indicates many potential roles for these proteins in physiologic adaptation (Kilgore et al. 1998). Although the precise function of a majority of these induced proteins is still unclear, many Hsps, as molecular chaperones, provide cells with a mechanism to prevent damage caused by misfolded, damaged, aggregated proteins (Hightower 1991). Several studies have shown that earlier experience with the stressor helps to improve induction (Donnelly et al. 1992; Marber et al. 1993; Yellon et al. 1992; Walker et al. 1993; van Ginneken et al. 2006), transfection (Suzuki et al. 1997), or overexpression of Hsp proteins if the stressor occurs again (Marber et al. 1995; Ooie et al. 2005; Gauthaman et al. 2005; Chiu et al. 2003). It is known that Hsp induction, and particularly Hsp70, can protect the myocardium from ischemia and reduces the infarction size in mice (Wu and Tanguay 2006; Seok et al. 2007). Our immunoblot results show that all five Hsps are detected in the control pigs. After 6 h of transport all Hsp are reduced, possibly because of the increasing physiological demand for keeping body posture and the movement of the trailer. The semi-quantitative analysis (Fig. 3) shows that the levels of Hsp70, Hsp86, Hsp90 and Hsp27 significantly decrease in the heart tissues of 6 h transported pigs when the densities of the Hsps are compared to its corresponding heart tissue β-actin bands. One of the functions of the Hsp70 family protein Hsp73 is, other than thermoprotection, the clathrin uncoating ATPase activity (Chiang et al. 1989; Velez-Granell et al. 1994; Marruchella et al. 2004). Elevated Hsp70 concentrations are closely related to slower rates of adenosine triphosphate depletion (Wang et al. 1996). There is also some evidence that Hsp70 proteins can have regulatory roles as translocases (Deshaies et al. 1988) and molecular chaperones (Yamamoto et al. 1987), which are active in intracellular protein processing and trafficking. Perrault et al. (1996) studied the effects of ischemic preconditioning in cardiac patients and concluded that such interventions may be potentially harmful to the patient. There is obviously a need for the development of additional, less damaging methods of in vivo Hsp70 induction that may be exploited clinically. The major stress inducible Hsp (Hsp70) confers myocardial protection from ischemia (McGuinness et al. 2006). The Hsp kDa family of HSPs displays important functions like stabilizing target proteins in unassembled state (David and Grongnet 2001). However, it cannot be excluded that the abundant Hsp70 reaction can be influenced by cross reactivity with other Hsp members. Hsp27 has been found to be associated with the cytoskeleton and the regulation of actin filament dynamics via different signaling pathways (Arrigo 2000; Lavoie et al. 1995). The shortage of Hsps and associated lesions in the myocardium may cause electromechanical dissociation, resulting in acute heart failure and can lead under poor environmental and management conditions even to death. The apparent molecular mass of Hsp90 is markedly reduced in the hypertrophic cardiomyopathy-affected tissues of pigs that died due to PSS (Lee et al. 1996).
The different expression pattern observed may have functional significance. Stabilizing the intracellular protein structure may be considered a possible mechanism. By immunohistochemical analysis, more than half of the myocardial cells showed intracytoplasmic labeling and few revealed sparse nuclear labeling by Hsp70. Hsp70 was the most abundant Hsp in the myocardial cells of both groups of investigated pigs and was present at significantly higher levels in the cytoplasm of the myocardial cells. Comparatively, rather weak signals of Hsp70 were detected in the myocardial cells using the porcine specific antibody Hsp70-SPA-812. The differences in amount of staining may be due to several possibilities, including recognition of different epitopes on inducible Hsp70 which may be differentially available for binding by the polycolonal antibodies or possibly detection of epitopes on both inducible Hsp70 and constitutive Hsc70 by the Hsp70-sc-1060 antibody. In view of its abundant and uniform distribution in the cytoplasm, it is assumed that the principal function of Hsps would be carried out by Hsp70. It is well known that cells exposed to a large variety of stressful stimuli typically show a translocation of HSPs70 from cytoplasm to nucleus, and particularly to the nucleolus, where such molecules may then exert their cytoprotective functions (Dastoor and Dreyer 2000; Kiang and Tsokos 1998; Li et al. 2006). Our immunohistochemical results also revealed that Hsp90 and Hsp86 were expressed both in the cytoplasm and in the nuclei of myocardial cells. The nuclear immunoreactivity pattern observed may have resulted from cellular redistribution of both Hsp90 and Hsp86, indicating functional significance. There is evidence that the selectively expressed Hsp90 and Hsp86 are involved in transducing a signal or signals for transcription of genes that encode a protein or proteins required for protection against stress (Kilgore et al. 1998). Hsp27 was observed in muscle tissue only, while the other Hsp family proteins (the Hsp70 family and the Hsp90 family) were distributed in all cells of the heart. It is known that Hsp27 is usually expressed in heart muscle tissues under normal conditions where its function can be modified by different cell mechanisms operating either on their expression or their structure depending on cellular localization (Michaud et al. 1997; Anastasiya et al. 2005).
Immunostaining did not reveal any marked variation among individual cells, including endothelial cells obtained from both transported and control pigs. The immunoblot findings suggest that the levels of Hsp70, Hsp86, Hsp90, and Hsp27 investigated in this experiment decreased as the 6-h-long journey progressed. This may indicate that with the length of journey, the protection of the cardiac muscle cells by the Hsps is diminished and may result in hypoxia from blood vessel constriction. It is known that animal’s stress response declines over time in response to repetitive exposure to identical stressors (Klemcke 1994; Natelson et al. 1988). Whether Hsps are playing a role in these adaption processes and whether the decreased level of certain Hsps indicate a damage and/or overuse and whether Hsps actually protect heart tissue from damage and/or overuse should be further investigated in greater detail.
Cardiovascular diseases are a leading cause of morbidity and death in modern fattening pigs. Several studies in animal models have documented the cytoprotective activity of Hsps against ischemia and reperfusion-associated damage in the heart (Wu and Tanguay 2006; Seok et al. 2007). By immunohistochemical analysis in the endothelial cells and in the wall of the heart blood vessels, Hsp70 and Hsp27 were intracytoplasmically detected. However, Hsp86 and Hsp90 were observed intracytoplasmically or intranuclearly, or both. It may imply that the endothelium reacts to ischemia and hypoxia by expressing Hsps. It is known that human saphenous vein contains significantly higher levels of Hsp27 and pHsp27, and increased levels of phosphorylated Hsp27 might contribute to vasospasm (McLemore et al. 2004, 2005). In ischemia, Hsp90 appears to be associated with hypoxia through several factors including hypoxia factor 1α (Gradin et al. 1996) and involved in artery pressure in the piglet (Viswanathan et al. 1999). The reduction of Hsps levels in the myocardium may cause susceptibility to acute heart failure. This may be one reason for the sudden death of pigs during transportation. Whether these mechanisms associated with transport stress have an effect on the development of diseases in young pigs, which are frequently observed after transportation from breeding farms to fattening units, need further attention. The results clearly show the necessity to further reduce stress during pig transport in order to improve the welfare of the animals and to reduce economic losses.
Acknowledgements
We would like to thank Professor Dirk Pette and Mrs. Elmi Leisner, Faculty of Biology, Konstanz University, Konstanz, Germany and Professor Wolfgang Drommer and Mrs. Danuta Waschke, Institute for Pathology, University of Veterinary Medicine Hanover Foundation, Hanover, Germany for their technical advice.
This work was supported by the Sino-German Agricultural Cooperation Project and by grants (30571400, 30430420) from the National Nature Science Foundation of China.
Contributor Information
Endong Bao, Phone: +86-25-84395316, FAX: +86-25-84398669, Email: b_endong@njau.edu.cn.
J. Hartung, Phone: +49-511-9538832, FAX: +49-511-9538588, Email: itt@tiho-hannover.de
References
- Anastasiya VP, Valeria VM, Ivan SC, Natalia AC, Dmitrii IL, Nikolai BG (2005) Effects of small heat shock proteins on the thermal denaturation and aggregation of F-actin. Bioche Biophy Res Commu 4:1548–1553 [DOI] [PubMed]
- Arrigo AP (2000) sHSP as novel regulators of programmed cell death and tumorigeniticy. Path Bio (Paris) 48:280–288 [PubMed]
- Bao ED, Sultan KR, Nowak B, Hartung J (2002) Localization and expression of heat shock proteins in liver of transport stressed pigs. Sci Agri Sinica 35:1130–1133
- Chiang HL, Terlecky SR, Plant CP, Dice JF (1989) A role for a 70-kilodalton heat shock protein in lysosomal degradation of intracellular proteins. Science 246:382–385 [DOI] [PubMed]
- Chiu JH, Tsou MT, Tung HH, Tai CH, Tsai SK, Chih CL, Lin JG, Wu CW (2003) Preconditioned somatothermal stimulation on median nerve territory increases myocardial heat shock protein 70 and protects rat hearts against ischemia–reperfusion injury. J Thorac Cardiovasc Surg 125:678–685 [DOI] [PubMed]
- Currie RW, Tanguay RM, Kingma JG (1993) Heat-shock response and limitation of tissue necrosis during occlusion/reperfusion in rabbit hearts. Circulation 87:963–971 [DOI] [PubMed]
- Dastoor Z, Dreyer J (2000) Nuclear translocation and aggregate formation of heat shock cognate protein 70 (Hsc70) in oxidative stress and apoptosis. J Cell Sci 113:1845–1854 [DOI] [PubMed]
- David JC, Grongnet JF (2001) Perinatal expression of heat-shock protein 90 in different regions of the brain and in non-neural tissues of the piglet. Biol Neonate 79:131–139 [DOI] [PubMed]
- Deshaies RJ, Koch BD, Werner-Washburne M, Craig EA, Schekman R (1988) A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptides. Nature 332:800–805 [DOI] [PubMed]
- Donnelly TJ, Sievers RE, Vissern FL, Welch WJ, Wolfe CL (1992) Heat shock protein induction in rat hearts: a role for improved myocardial salvage after ischemia and reperfusion. Circulation 85:769–778 [DOI] [PubMed]
- Frier B, Locke M (2005) Preservation of heat stress induced myocardial hsp 72 in aged animals following caloric restriction. Exp Gerontol 40:615–617 [DOI] [PubMed]
- Gauthaman K, Banerjee SK, Dinda AK, Ghosh CC, Maulik SK (2005) Terminalia arjuna (Roxb.) protects rabbit heart against ischemic-reperfusion injury: role of antioxidant enzymes and heat shock protein. J Ethnopharmacol 96:403–409 [DOI] [PubMed]
- Gradin M, McGuire J, Wenger RH, Kvietikova L, Whitelaw ML, Toftgard R, Tora L, Gassmann M, Poellinger LA (1996) Function interference between hypoxia and dioxin signal transduction pathways: competition for recruitment of the Arnt transcription factor. Mol Cell Biol 16:5221–5231 [DOI] [PMC free article] [PubMed]
- Hightower LE (1991) Heat shock, stress protein, chaperones, and proteotoxicity. Cell 66:191–197 [DOI] [PubMed]
- Khansari DN, Murgo AJ, Faith RE (1990) Effect of stress on the immune system. Immuno Today 11:170–175 [DOI] [PubMed]
- Kiang JG, Tsokos GC (1998) Heat shock protein 70 kDa: molecular biology, biochemistry, and physiology. Pharmacol Ther 80:183–201 [DOI] [PubMed]
- Kilgore JL, Musch TI, Ross CR (1998) Physical activity, muscle, and the HSP70 response. Can J Appl Physiol 23:245–260 [DOI] [PubMed]
- Klemcke HG (1994) Responses of the porcine pituitary-adrenal axis to chronic intermittent stressor. Domest Anim Endocrinol 11:133–149 [DOI] [PubMed]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T14. Nature 227:680–685 [DOI] [PubMed]
- Lavoie JN, Lambert H, Hickey E, Weber LA, Landry J (1995) Modulation of cellular thermoresistance and actin filament stability accompanies phosphorylation induced in the oligomeric structure of heat shock protein 27. Mol Cell Biol 15:505–510 [DOI] [PMC free article] [PubMed]
- Lee WC, Lin KY, Chiu YT, Lin JH, Cheng HC, Huang HC, Yang PC, Liu SK, Mao SJ (1996) Substantial decrease of heat shock protein 90 in ventricular tissues of two sudden-death pigs with hypertrophic. FASEB J 10:1198–204 [DOI] [PubMed]
- Li YB, Bao ED, Wang ZL, Zhao RQ (2006) Detection of HSP mRNA transcription in transport stressed pigs by fluorescence quantitative RT-PCR. Sci Agri Sinica 39:187–192
- Liu Y, Steinacker JM (2001) Changes in skeletal muscle heat shock proteins: pathological significance. Front Biosci 6:12–25 [DOI] [PubMed]
- Locke M, Tanguay RM, Ianuzzo CD (1996) Constitutive expression of HSP 72 in swine heart. J Mol Cell Cardiol 28:467–474 [DOI] [PubMed]
- Marber MS, Latchman DS, Walker JM, Yellon DM (1993) Cardiac stress protein elevation 24 hours after brief ischemia or heat stress is associated with resistance to myocardial infarction. Circulation 88:1264–1272 [DOI] [PubMed]
- Marber MS, Mestril R, Chi SH, Sayern MR, Yellon DM, Dillmann WH (1995) Overexpression of the rat inducible 70-kD heat stress protein in a transgenic mouse increases the resistance of the heart to ischemia injury. J Clin Invest 95:1446–1456 [DOI] [PMC free article] [PubMed]
- Marruchella G, Di Leonardo M, Di Guardo G, Romanucci M, Marà M, Tiscar PG, Mosca F, Della Salda L (2004) Heat Shock Proteins (HSPs) 27, 72 and 73 in normal and pre-ulcerative mucosa of the gastric pars oesophagea in swine. J Comp Pathol 131:10–17 [DOI] [PubMed]
- McGuinness J, Neilan TG, Sharkasi A, Bouchier-Hayes D, Redmond JM (2006) Myocardial protection using an omega-3 fatty acid infusion: quantification and mechanism of action. J Thorac Cardiovasc Surg 132:72–79 [DOI] [PubMed]
- McLemore EC, Tessier DJ, Komalavilas P, Thresher J, Brophy CM (2004) The yin and yang of vascular smooth muscle tone. J Am Coll Surg 199:106–107 [DOI] [PubMed]
- McLemore EC, Tessier DJ, Thresher J, Komalavilas P, Brophy CM (2005) Role of the small heat shock proteins in regulating vascular smooth muscle tone. J Am Coll Surg 201:30–36 [DOI] [PubMed]
- Michaud S, Marin R, Tanguay RM (1997) Regulation of heat shock gene induction and expression during Drosophila development. Cell and Mol Life Sci 53:104–113 [DOI] [PMC free article] [PubMed]
- Nabuurs MJA, van Essen GJ, Nabuurs P, Niewold TA, van der Meulen J (2001) Thirty minutes transport causes small intestinal acidosis in pigs. Res Vet Sci 70:123–127 [DOI] [PubMed]
- Natelson BH, Ottenweller JE, Cook JA, Pitman D, McCarty R, Tapp WN (1988) Effect of stressor intensity on habituation of the adrenocortical stress response. Physiol Behav 43:41–46 [DOI] [PubMed]
- Ooie T, Kajimoto M, Takahashi N, Shinohara T, Taniguchi Y, Kouno H, Wakisaka O, Yoshimatsu H, Saikawa T (2005) Effects of insulin resistance on geranylgeranylacetone-induced expression of heat shock protein 72 and cardioprotection in high-fat diet rats. Life Sci 77:869–881 [DOI] [PubMed]
- Perrault LP, Menasche P, Bel A, de Chaumaray T, Peynet J, Mondry A, Olivero P, Emanoil-Ravier R, Moalic JM (1996) Ischemic preconditioning in cardiac surgery: a word of caution. J Thorac Cardiovasc Surg 112:1378–1386 [DOI] [PubMed]
- Riette LJ, van Laack M, Faustman C, Sebranek JG (1993) Pork quality and the expression of stress protein Hsp70 in swine. J Anim Sci 71:2958–2964 [DOI] [PubMed]
- Schütte A (1994) Transporttauglichkeit von Schweinen. In: Hartung J (ed) Hygiene und Tierschutz beim Tiertransport. Proc. Deutsche Veterinärmedizinische Gesellschaft, Hanover 8–9. März, S: 83–98
- Seok YM, Kim JN, Choi KC, Yoon CH, Boo YC, Park YK, Park KM (2007) Wen-pi-tang-Hab-Wu-ling-san attenuates kidney ischemia/reperfusion injury in mice: a role for antioxidant enzymes and heat-shock proteins. J Ethnopharmacol 112:333–340 [DOI] [PubMed]
- Sepponen K, Pösö AR (2006) The inducible form of heat shock protein 70 in the serum, colon and small intestine of the pig: comparison to conventional stress markers. Vet J 171:519–524 [DOI] [PubMed]
- Suzuki K, Sawa Y, Kaneda Y, Ichikawa H, Shirakura R, Matsuda H (1997) In vivo gene transfection with heat shock protein 70 enhances myocardial tolerance to ischemia–reperfusion injury in the rat. J Clin Invest 99:1645–1650 [DOI] [PMC free article] [PubMed]
- van Ginneken MME, de Graaf-Roelfsema E, Keizer HA, van Dam KG, Wijnberg ID, van der Kolk JH, van Breda E (2006) Effect of exercise on activation of the p38 mitogen-activated protein kinase pathway, c-Jun NH2 terminal kinase, and heat shock protein 27 in equine skeletal muscle. Am J Vet Res 67:837–844 [DOI] [PubMed]
- Velez-Granell CS, Arias AE, Torres-Ruiz JA, Bendayan M (1994) Molecular chaperones in pancreatic tissue: the presence of cpn10, cpn60 and hsp70 in distinct compartments along the secretory pathway of the acinar cells. J Cell Sci 107:539–549 [DOI] [PubMed]
- Viswanathan M, Rivera O, Short BL (1999) Heat-shock protein 90 involved in pulsatile flow induced dilatation of the rat middle cerebral artery. J Vasc Res 36:524–527 [DOI] [PubMed]
- Walker DM, Pasini E, Kucukoglu S, Marber MS, Iliodromitis E, Ferrari R, Yellon DM (1993) Heat stress limits infarct size in isolated perfused rabbit heart. Cardiovasc Res 27:962–967 [DOI] [PubMed]
- Wang D, McMillin JB, Bick R, Buja LM (1996) Response of the neonatal rat cardiomyocyte in culture to energy depletion: effects of cytokines, nitric oxide, and heat shock proteins. Lab Invest 75:809–818 [PubMed]
- Warriss PD, Brown SN, Adams SJM, Corlett IK (1994) Relationship between subjective and objective assessments of stress at slaughter and meat quality in pigs. Meat Sci 38:329–340 [DOI] [PubMed]
- Wischmeyer PE, Jayakar D, Williams U, Singleton KD, Riehm J, Bacha EA, Jeevanandam V, Christians U, Serkova N (2003) Single dose of glutamine enhances myocardial tissue metabolism, glutathione content, and improves myocardial function after ischemia–reperfusion injury. J Parenter Enter Nutr 27:396–403 [DOI] [PubMed]
- Wu T, Tanguay RM (2006) Antibodies against heat shock proteins in environmental stresses and diseases: friend or foe. Cell Stress Chaperon 11:1–12 [DOI] [PMC free article] [PubMed]
- Yamamoto T, McIntyre J, Sell S, Georgopoulos C, Skowyra D, Zylicz M (1987) Enzymology of the pre-priming steps in lambda DNA replication in vitro. J Biol Chem 262:7996–7999 [PubMed]
- Yellon DM, Pasini E, Cargoni A, Marber MS, Latchman DS, Ferrari R (1992) The protective role of heat stress in the ischemic and reperfused rabbit myocardium. J Mol Cell Cardiol 24:342–346 [DOI] [PubMed]
- Yu H, Bao E, Zhao R, Lv Q (2007) Effect of transportation stress on heat shock protein 70 concentration and mRNA expression in heart and kidney tissues and serum enzyme activities and hormone concentrations of pigs. Am J Vet Res 68:1145–1150 [DOI] [PubMed]