Abstract
Chaperonin 10 (cpn 10) is a small heat-shock protein that is usually intracellular. Early pregnancy factor (EPF), a biologically active protein that was first described in the serum of pregnant mammals, is homologous to cpn 10. EPF/cpn 10 has been reported to have effects on immunomodulation and cell survival and to inhibit activation of toll-like receptors by lipopolysaccharide. We found that recombinant EPF/cpn 10 was able to suppress experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis, which is a disease causing inflammation and demyelination of the brain and spinal cord. This beneficial effect could be due to anti-inflammatory and/or cell survival properties of EPF/cpn 10. We aimed to assess the effects of cpn 10 on cells of the oligodendrocyte lineage because oligodendrocytes are the brain cells that produce myelin and that are depleted in multiple sclerosis. Two forms of recombinant EPF/cpn 10 were prepared in the pGEX expression system and in the baculovirus expression system. Purified O4+ pro-oligodendrocytes were prepared from the brains of day-old Wistar rats and isolated by cell sorting with flow cytometry. Single cells were dispensed into micro-well plates and tested for survival in the presence of a range of concentrations of the two forms of cpn 10. We also studied the effects of bFGF, PDGF, IGF-1 and insulin as controls. With cpn 10 present, there was enhanced survival of O4+ cells.
Keywords: Oligodendrocyte, Multiple sclerosis, cpn 10, Growth factors, Early pregnancy factor
Introduction
Chaperonin 10 (cpn10) is a heat-shock protein (hsp) that functions within mitochondria as a molecular chaperone (Ellis and van-der Vies 1991; Hartmann et al. 1992; Hohfeld and Hartl 1994) and may have a role in protecting cells from stress. Early pregnancy factor (EPF) is a molecule that is present, from soon after conception, in the sera of all pregnant mammals that have been tested (Morton et al. 1992; Cavanagh 1996; Cavanagh 1997); EPF has immunomodulatory properties and growth factor properties (Morton et al. 1982; Morton 1998). EPF was found to be homologous to cpn 10 (Cavanagh and Morton 1994).
Multiple sclerosis (MS) is a disease of the brain and spinal cord that is characterised by areas of inflammation and loss of myelin from around axons. Myelin is produced by oligodendrocytes, which are neuroglial cells. Mature oligodendrocytes can arise from oligodendrocyte precursors in the brain. Surviving oligodendrocytes are necessary for replacement of myelin, and loss of oligodendrocytes leads to lack of remyelination in MS (Raine 1997; Blakemore and Keirstead 1999). Therefore, improving the survival of oligodendrocytes is an important therapeutic goal in MS.
Multiple sclerosis is less severe in pregnancy (Confavreux et al. 1998). The best available animal model of multiple sclerosis is experimental autoimmune encephalomyelitis (EAE), which is an autoimmune disease induced by the inoculation of susceptible animals with central nervous system antigen and adjuvant. EAE is also less severe when induced in pregnant animals (Harness and McCombe 2001). The improvement of MS and EAE in pregnancy is thought to be due to circulating hormones produced in pregnancy. Because EPF/cpn 10 is found in the serum of pregnant animals, we performed studies and found that recombinant cpn 10 partly suppresses the clinical and pathological signs of experimental autoimmune encephalomyelitis (Zhang et al. 2000; Zhang et al. 2003; Harness et al. 2003) and suggested that this was due to immunomodulation (Harness et al. 2003). More recently, the immunomodulatory effects of cpn 10 have been explored further, and highly purified recombinant cpn 10 has been shown to reduce the effects of lipopolysaccharide (LPS) binding to toll-like receptors (Johnson et al. 2005), which represents another mechanism by which cpn 10 could suppress inflammation in EAE.
Because of our previous studies suggesting that EPF/cpn 10 is a growth factor, and because of the possibility that enhanced oligodendrocyte survival might also contribute to improved outcome after EAE, we performed the present study to determine whether recombinant cpn 10 was a survival factor oligodendrocytes, using O4+ cells of the oligodendrocyte lineage, purified from neonatal brain.
Materials and methods
Preparation of recombinant EPF/cpn 10
Recombinant human cpn 10 was produced using prokaryotic (Escherichia coli) and eukaryotic (Baculovirus) protein expression systems. With the E. coli expression system, a modified protein, with alanine–serine–methionine added at the N-terminus, was obtained (Zhang et al. 2000). With the Baculovirus expression system, an unmodified protein sequence with an acetylated N-terminal residue (as in the native molecule) was produced (Somodevilla-Torres et al. 2003). In brief, for expression in E. coli, the complete open reading frame (ORF) of human EPF/cpn 10 (Summers et al. 1996) was cloned into the plasmid pGEX-2T (Pharmacia Biotechnology, Uppsala, Sweden), which directs the synthesis of foreign polypeptides as fusion proteins at the C-terminus of glutathione-S-transferase (GST). An intervening thrombin cleavage site facilitates recovery of the recombinant protein. Cells were transformed and grown as described previously (Zhang et al. 2000). Isolation of the fusion protein and purification of by thrombin cleavage was performed using Glutathione-Sepharose 4B (Pharmacia) and thrombin (T6884, Sigma Chemical Co., St Louis, MO, USA) at the rate of 1 ml gel and 25 units per litre original culture, respectively. pGEX-rEPF/cpn 10, recovered in the supernatant and from two subsequent washes of the gel, was then completely purified by reversed-phase high-performance liquid chromatography (RP-HPLC). After addition of trifluoroacetic acid (TFA; Sequenal grade, Pierce Chemical Company, Rockford, IL, USA; final concentration 0.1% [v/v]), the sample was applied to a Resource™ RPC column (3 ml; Pharmacia Biotechnology) previously equilibrated with solvent A (0.1% TFA) and the column was eluted with a gradient from 100% solvent A to 15% solvent B (0.1% TFA in acetonitrile) applied over 5 min, followed by a gradient from 15% solvent B to 40% solvent B applied over 25 min; a flow rate of 1 ml/min was maintained throughout. Fractions were monitored by SDS-PAGE on 10–20% pre-cast Tris–Tricine gels (Novex, San Diego, CA, USA).
For expression in eukaryote cells, a recombinant baculovirus incorporating the complete ORF of human EPF/cpn 10 was prepared using the transfer vector pBlu-bac 4.5 (Invitrogen, San Diego, CA, USA) (Somodevilla-Torres et al. 2003). This was used to infect insect cells (Spodoptera frugiperda) growing in spinner culture in SF900II serum-free medium (Gibco), following the manufacturer’s instructions. After 48 hours, cells were harvested and recombinant protein was purified from cell pellets using cation exchange chromatography. Briefly, cells were pelleted (1200×g, 15 min, 4°C), resuspended in 1/100 vol ice-cold 0.02-M MES buffer pH 6.2 (buffer A) containing EDTA, PMSF, leupeptin and pepstatin (all from Sigma Chemical Co.; final concentration 5 mM, 1 mM, 2 nM and 2 nM respectively), lysed by sonication and cellular debris removed (75,600×g, 30 min, 4°C). After adjustment to pH 6.2, the lysate was applied to a High-S cartridge (5 ml; Bio-Rad, Hercules, CA, USA.) previously equilibrated with buffer A. The cartridge was washed with 10-vol buffer A and eluted with a gradient of NaCl (0 – 1 M) in buffer A, applied over 60 min. A flow rate of 1 ml/min was maintained throughout. Fractions were monitored by SDS-PAGE as above.
For both preparations, EPF/cpn 10 -containing fractions were pooled and the protein concentration was determined (Bio-Rad Protein Assay with bovine serum albumin [BSA] as standard). BSA (crystalline grade; Sigma, A4161) was then added (final concentration 0.1% [w/v]) and the solution dialysed against three changes of PBS, followed by three changes of DMEM over 48 hours. EPF concentration was determined by sandwich enzyme-linked immunosorbent assay (ELISA) and EPF activity by the bioassay, the rosette inhibition test (Morton et al. 1976; Cavanagh and Morton 1995). Samples were stored at −30°C and thawed only once immediately before use.
Oligodendrocyte cultures
Primary cultures of neonatal rat brain were prepared from day-old female Wistar rat pups (Cole and de Vellis 1989). Briefly, the rat pups were anaesthetised by placing them on ice, and decapitated using a scalpel. The head was sterilised by wiping with 70% EtOH and immobilised by pinning to a dissection board. The top of the skull was removed and the brain scooped out using forceps. The cerebral cortex was dissected in a Petri dish containing Dulbecco’s modified Eagle’s medium (DMEM) and the meninges removed by rolling the cortex on sterile paper. The cerebral cortex cells were then dissociated by pushing the cortex through a 125-μM mesh (Sigma) using the barrel of a 5-ml syringe. Fetal calf serum (FCS) was added to 10% and the cells cultured in poly-l-lysine (MW 70,000–150,000; Sigma)-coated tissue culture flasks for 10 days at 37°C in an atmosphere of 5% CO2 in air (37°C/5% CO2). The culture media (DMEM/10% FCS) was changed every 3–4 days. At this stage of culture, astrocytes grew as a monolayer attached to the bottom of the tissue culture flasks and oligodendrocytes grew on the top of these cells. Microglial cells were present in suspension and attached directly to the bottom of the flask and on top of the astrocyte monolayer. These cell types were identified by morphology under phase contrast microscopy. Astrocytes appeared as non-granular, phase bright cells, oligodendrocytes as non-granular, phase dark cells and microglia as granular cells.
In our preliminary experiments, oligodendrocytes were isolated as follows. Floating cells (microglia) were discarded and fresh media added to cultures. The culture flasks were then tapped against the bench top five to eight times to dislodge the oligodendrocytes. However, these cells in suspension were approximately 50% oligodendrocytes and 50% microglia. These cells were collected and used in survival experiments with trypan blue exclusion. In subsequent experiments, an improved method of cell purification was employed. This involved the gentle shaking of primary cultures at 200 rpm on a rotor shaker at 37°C for days at a time. With labelling with antibody to microglia, we confirmed that there was microglial contamination of the oligodendrocyte culture.
We therefore used flow cytometry and cell sorting to isolate O4+ cells, and to use single-cell cultures, which are best for determining the effects of growth factors. Oligodendrocytic cells were detached from the astrocyte bed by shaking the cultures at 400 rpm for 2 days at 37°C on an orbital shaker (McCarthy and de Vellis 1980). These cells were then labelled with O4+antibiody by incubating the detached cells with anti-O4 mAb (1/2 dilution of culture supernatant) and then with anti-mouse Ig-rPE conjugate (DAKO, Carpinteria, CA., USA.). O4+cells were then isolated by deposition of single cells in microwells using a Facscalibur flow cytometer and cell sorter (Becton Dickinson).
Antibody to O4
Anti-O4 mAb was produced by hybridoma (generously provided by Dr Trevor Kilpatrick, Walter Eliza Hall Institute of Medical Research, Melbourne, Australia) grown in DMEM/10% heat inactivated FCS. Cultures were grown to exhaustion (5 days) and the culture supernatant harvested. Cellular debris was removed by centrifugation (800×g, 10 min at 4°C) and the culture supernatant frozen and stored at −70°C. O4+cells were prepared from 10–12 day old primary cultures of neonatal rat brain.
Cell survival assays
Initial experiments used trypan blue exclusion as a measure of cell viability in cultures of glial cells. Cells were plated in to 96-well plates and cultured for 48 hours, at which time the cells were stained with trypan blue and the percentage of viable cells was counted. Later, cell viability was measured by identifying cells with metabolic activity assessed using the MTT assay as a means of determining cell viability. Briefly, after 48 or 96 hours of culture, 2 μl of a 5-mg/ml solution of MTT (3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyl tetrazolium bromide; Sigma) in DMEM was added to each well and the plates incubated at 37°C/5%C02 for 4 hours in the absence of light. Viable cells were identified by the presence of dark blue formazan reaction product in their cytoplasm. Total and viable cells were then counted by inverted phase bright field microscopy and the percentage of viable cells determined by counting.
For definitive assays of oligodendrocyte survival, single-cell experiments are preferred (Barres et al. 1992b). O4+oligodendrocytic cells were used in survival assays since cells of this stage of the oligodendrocyte lineage have the ability to divide and may be involved in the remyelination of axons in the CNS following brain injury (Gard and Pfeiffer 1993; Amat et al. 1998; Keirstead and Blakemore 1999). Flow cytometry-purified O4+pro-oligodendrocytes were diluted in DMEM and single cells were dispensed in a volume of 5 μl into wells of microwell plates (10-μl well capacity, 60 wells/plate; Nunc, Rochester, NY, USA.). Wells that contained greater that one cell/well were excluded. pGEX-rEPF, BV-rEPF (as described above), recombinant bovine bFGF, recombinant rat platelet-derived growth factor (PDGF), recombinant human , insulin-like growth factor (IGF-I) (all from R&D Systems, Minneapolis, MN, USA.) and bovine insulin (Sigma) were diluted to twice the desired concentration in a defined medium (2×) consisting of DMEM supplemented with 8 g/L glucose, 20 μg/ml iron-saturated holo-transferrin (Sigma,) and 1 mg/ml bovine serum albumin (crystalline grade; Sigma, A4161). The factors (5 μl) were then added to the wells and the plates incubated at 37°C/5% C02. Control cultures received defined medium (5 μl of 2×) alone. Cell survival was assessed at the commencement of culture and after 20 and 44 hours of culture using MTT reagent as described above (Barres et al. 1992b).
Results
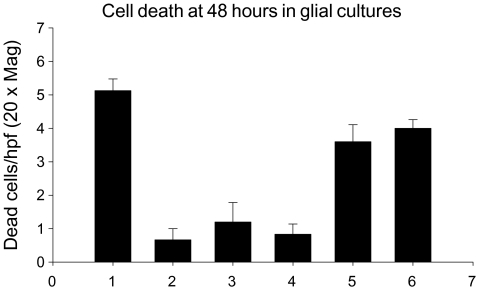
Both recombinant cpn 10 molecules showed similar antibody binding characteristics in an ELISA and, within the semi-quantitative limits of the EPF bioassay (Cavanagh 1996), were equally potent as EPF (data not shown). Initial experiments on mixed glial cultures (50% oligodendrocytes and 50% microglia), as determined by immunohistochemistry, indicated that EPF/cpn 10 was a survival factor for these cells, using trypan blue exclusion to measure cell viability. EPF/cpn 10 was as effective as FCS at inhibiting cell death in these cultures when measured at 48 hours using trypan blue exclusion (Fig. 1). Further studies using trypan blue then explored a wider range of concentrations and found that EPF/cpn 10 promoted the survival of oligodendrocytes in mixed glial culture. In these studies, the effect of EPF/cpn 10 was similar to that of insulin.
Fig. 1.
The effect of EPF and fetal calf serum (FCS) on cell death in mixed glial cultures using trypan blue exclusion. The percentage of dead cells is shown on the y-axis. The bars represent 1) DMEM alone, 2) DMEM + 10% FCS, 3) DMEM + 800 nM EPF, 4) DMEM + 80 nM EPF, 5) DMEM + 8 nM EPF, 6) DMEM + 800 pM EPF
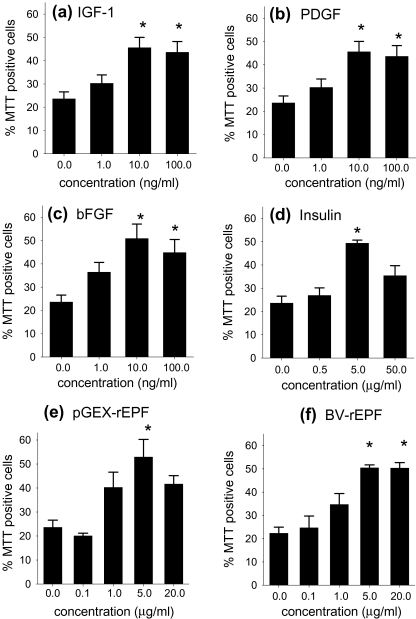
Effects on survival of 04+ oligodendrocytes in single cell cultures were then examined. Cell viability at the beginning of the single cell culturing period was 75.0% ± 4.1. As previously observed (Barres et al. 1992a; Barres et al. 1992b), oligodendrocyte viability declined rapidly in the absence of growth factors, so that after 20 hours of culture the cell viability was 23.6 ± 3.0%. IGF-1 (Fig. 2a), PDGF (Fig. 2b), and bFGF (Fig. 2c) significantly (P < 0.05) promoted the survival of O4+ oligodendrocytes in single cell cultures as previously observed (Barres et al. 1992a). The factors were approximately equipotent with maximal effect observed at 10 ng/ml. Bovine insulin also promoted O4+ oligodendrocyte survival (Fig. 2 d), although effective concentrations (5 μg/ml) of this factor were higher that those required for IGF-1, bFGF and PDGF. pGEX-rEPF/cpn 10 and BV-rEPF/cpn 10 both promoted the survival of O4+ oligodendrocytes when added to single-cell cultures and were equally effective and potent (Fig. 2e,f). Moreover, the effect of both cpn 10 molecules was dose dependent, with a peak effect observed at 5 μg/ml. Cpn 10 was as effective as IGF-1, bFGF, PDGF and insulin in maintaining cell viability (Fig. 2) but less potent than IGF-1, bFGF and PDGF. To maintain viability, the effective concentration of cpn 10 was equivalent to that of insulin (5 µg/ml) but higher than that of IGF-1, bFGF or PDGF (10 ng/ml).
Fig. 2.
Single-cell culture analysis of O4+oligodendrocyte survival after 20 hours in the presence of pGEX-rEPF/cpn 10) and BV-EPF /cpn 10; comparison with other growth factors. All growth factors, (a) IGF-1, (b) PDGF, (c) bFGF, (d) insulin, (e) pGEX-rEPF and (f) BV-rEPF, promoted the survival of single-cell cultures of O4+ oligodendrocytes in a dose dependent manner. Data represent the mean percentage of viable cells after 20 hours of culture + SEM of four to five separate experiments. The significance of difference between means was measured using ANOVA with Tukey’s posttest
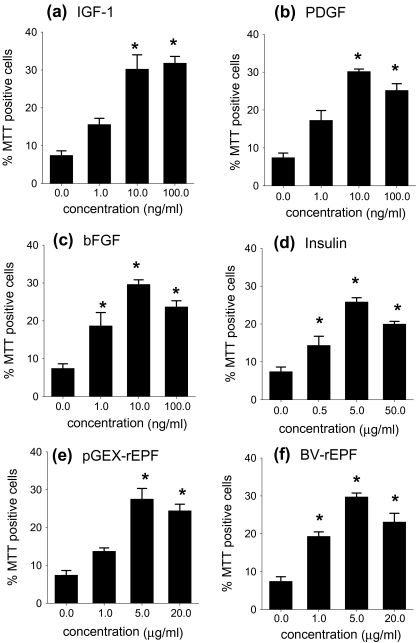
Over a longer period of culture, O4+ oligodendrocyte viability continued to decline in the absence of exogenous growth factors. After 44 hours in culture (Fig. 3), cell viability in the control cultures dropped to approximately 10% of starting cell viability (from 75.0% ± 4.1 to 7.4% ± 1.2). Cell viability also decreased in the presence of the tested growth factors and cpn 10; however, cell death in the presence of these factors was significantly reduced when compared with the control cultures.
Fig. 3.
Single-cell culture analysis of O4+ oligodendrocyte survival after 44 hours in the presence of rEPF; comparison with other growth factors. (a) IGF-1, (b) PDGF, (c) bFGF, (d) insulin, (e) pGEX-rEPF/cpn 10 and BV-rEPF/ cpn 10 all promoted O4+oligodendrocyte survival over prolonged periods (44 hours) of single-cell culture. Data represent the mean percentage of viable cells after 44 hours of culture +SEM of four to five separate experiments. The significance of difference between means was measured using ANOVA with Tukey’s posttest
Discussion
This study was performed to explore the effects of EPF/cpn 10 on survival of cells of the oligodendrocyte lineage, by studying the effects of two forms of recombinant cpn 10 on O4+ pro-oligodendrocytes. To do this we studied the direct effects of cpn 10 on single cells in culture. As positive controls we used a range of growth factors that are recognised to support oligodendrocyte growth. These were bFGF, PDGF, IGF-1 and insulin, which have previously been shown to act on oligodendrocytes and which have specific receptors. While cpn 10 was not as potent as other recognised oligodendrocyte growth factors, it was as effective as the other growth factors in supporting cell survival. The concentration of cpn 10 that was required was of the same order as that of insulin in the same assay. It is likely that the effect of cpn 10 resulted from a direct interaction between cpn 10 and oligodendrocytes, because no other cells were present. One limitation of our study is that we did not use an inactive protein as control. However, as the concentration of cpn 10 required was of the same order as that of insulin, which is a known growth factor, we suggest that we have demonstrated an effect at least equivalent to that of insulin.
We have previously found that EPF/cpn 10 is produced by inflammatory cells obtained from the spinal cord of rats with EAE (Harness et al. 2003) and that EPF/cpn 10 leads to enhanced expression of IL-10 and IL-4 mRNA in EAE. We suggested that the beneficial effects of EPF/cpn 10 occur through an immunomodulatory role. The immunomodulatory role of cpn 10 has been further studied and cpn 10 has been found to reduce the toxic effects of lps on toll-like receptors (Johnson et al. 2005). It is known that TLR 4 is needed for lps induced injury of oligodendrocytes (Lehnardt et al. 2002), and it may be that cpn 10 supports the survival of oligodendrocyte precursors in vitro through a mechanism involving toll-like receptors. However, there is also some evidence that there may be a specific receptor for EPF/cpn 10 (Athanasas-Platsis et al. 2004), which provides another possible mode of action.
The suggestion of an extracellular role for heat-shock proteins (hsps) such as cpn 10 is not unprecedented. Hsp responses have been observed in every cell type examined and hsps are among the most conserved proteins known in phylogeny. Heat-shock proteins usually act within the cell or are expressed on the cell surface. However, stress can induce the movement of these proteins to different cellular compartments and, in some cases, outside the cell (Lindquist and Craig 1988; Hightower and Guidon 1989; Ranford et al. 2000). Several heat-shock proteins (hsp110, hsp71, hscp73) have been shown to be released from rat embryo cells, and it was concluded that the rapid release of stress proteins is a homeostatic mechanism for the transfer of some of these proteins from cells capable of mounting a string stress response to neighbouring cells that cannot (Hightower and Guidon 1989). Neuronal tissue may provide a particularly dramatic example of the need for transfer mechanisms for stress proteins (Sprang and Brown 1987). It has been proposed that after heat shock axons may obtain stress protein, needed for repair processes, from adjacent glial cells by cell-to-cell transfer (Tytell 2005). It has been reported that oligodendrocytes in culture upregulate hsps after stress, although hsp 10 was not studied, and it was not documented whether hsps were exported from the cells (Goldbaum and Richter-Landsberg 2001). Extracellular hsps also have immune effects, as shown by the finding that extracellular hsp 70 activates macrophages (Vega et al. 2008) and that hsps act on Toll like receptors (Calderwood et al. 2007). As already mentioned, the immunomodulatory effects of cpn 10 are mediated by its downregulation of signalling through Toll-like receptors (Johnson et al. 2005).
It is possible that EPF/cpn 10 has a direct action to support the survival of oligodendrocytes, and this could be beneficial in multiple sclerosis. Hsps are now recognised as having a role in inflammation (Chen et al. 2007). Cpn 10 has been used successfully in a clinical trial of arthritis (Vanags et al. 2006) and so may become available for therapy in other autoimmune diseases. Our present study showing the role of cpn 10 in cell survival points to an additional possible role for hsp10, and it is possible that cpn 10 may have similar effects on the survival of other cells in culture. We suggest that this is worth further study.
Acknowledgements
The financial support of the National Multiple Sclerosis Society of Australia, the National Health and Medical Research Council of Australia and CSL Ltd is gratefully acknowledged. The recombinant proteins were prepared and purified by Drs A.C. Cavanagh and Ms M Somodevilla-Torres. The assistance of Dr A Mould is gratefully acknowledged.
References
- Amat JA, Farooq M, Ishiguro H, Norton WT (1998) Cells of the oligodendrocyte lineage proliferate following cortical stab wounds: an in vitro analysis. Glia 22:64–71 [DOI] [PubMed]
- Athanasas-Platsis S, Somodevilla-Torres MJ, Morton H, Cavanagh AC (2004) Investigation of the immunocompetent cells that bind early pregnancy factor and preliminary studies of the early pregnancy factor target molecule. Immunol Cell Biol 82:361–369 [DOI] [PubMed]
- Barres BA, Hart IK, Coles HS, Burne JF, Voyvodic JT, Richardson WD, Raff MC (1992a) Cell death and control of cell survival in the oligodendrocyte lineage. Cell 70:31–46 [DOI] [PubMed]
- Barres BA, Hart IK, Coles HS, Burne JF, Voyvodic JT, Richardson WD, Raff MC (1992b) Cell death in the oligodendrocyte lineage. J Neurobiol 23:1221–1230 [DOI] [PubMed]
- Blakemore WF, Keirstead HS (1999) The origin of remyelinating cells in the central nervous system. J Neuroimmunol 98:69–76 [DOI] [PubMed]
- Calderwood SK, Mambula SS, Gray PJ Jr. (2007) Extracellular heat shock proteins in cell signaling and immunity. Ann NY Acad Sci 1113:28–39 [DOI] [PubMed]
- Cavanagh AC (1996) Identification of early pregnancy factor as chaperonin 10: implications for understanding its role. Rev Reprod 1:28–32 [DOI] [PubMed]
- Cavanagh AC (1997) An update on the identity of early pregnancy factor and its role in early pregnancy. J Assist Reprod Genet 14:492–495 [DOI] [PMC free article] [PubMed]
- Cavanagh AC, Morton H (1994) The purification of early-pregnancy factor to homogeneity from human platelets and identification as chaperonin 10. Eur J Biochem 222:551–560 [DOI] [PubMed]
- Cavanagh AC, Morton H. Chaperonin 10. International patent application. [PCT/AU94/00740]. (1995) International Application. Ref Type: Patent
- Chen Y, Voegeli TS, Liu PP, Noble EG, Currie RW (2007) Heat shock paradox and a new role of heat shock proteins and their receptors as anti-inflammation targets. Inflammation and Allergy Drug Targets 6:91–100 [DOI] [PubMed]
- Cole R, de Vellis J (1989) Preparation of astrocyte and oligodendrocyte cultures from primary rat glial cultures. In: Shahar A, de Vellis J, vernafakis A, Haber P (eds) A dissection and tissue culture manual of the nervous system. Allan R Liss Inc., New York, pp 121–133
- Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T (1998) Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. N Engl J Med 339:285–291 [DOI] [PubMed]
- Ellis RJ, van-der Vies S (1991) Molecular chaperones. Annu Rev Biochem 60:321–347 [DOI] [PubMed]
- Gard AL, Pfeiffer SE (1993) Glial cell mitogens bFGF and PDGF differentially regulate development of O4+GalC-oligodendrocyte progenitors. Dev Biol 159:618–630 [DOI] [PubMed]
- Goldbaum O, Richter-Landsberg C (2001) Stress proteins in oligodendrocytes: differential effects of heat shock and oxidative stress. J Neurochem 78:1233–1242 [DOI] [PubMed]
- Harness J, Cavanagh A, Morton H, McCombe P (2003) A protective effect of early pregnancy factor on experimental autoimmune encephalomyelitis induced in Lewis rats by inoculation with myelin basic protein. J Neurol Sci 216:33–41 [DOI] [PubMed]
- Harness J, McCombe PA (2001) The effects of pregnancy on myelin basic protein-induced experimental autoimmune encephalomyelitis in Lewis rats: suppression of clinical disease, modulation of cytokine expression in the spinal cord inflammatory infiltrate and suppression of lymphocyte proliferation by pregnancy sera. Am J Reprod Immunol 46:405–412 [DOI] [PubMed]
- Hartmann DJ, Hoogenraad NJ, Condron R, Hoj PB (1992) Identification of a mammalian 10kDa heat shock protein, a mitochondrial chaperonin 10 homologue essential for assisted folding of trimeric ornithine transcarbamoylase in vitro. Proc Natl Acad Sci U S A 89:3394–3398 [DOI] [PMC free article] [PubMed]
- Hightower LE, Guidon PT Jr. (1989) Selective release from cultured mammalian cells of heat-shock (stress) proteins that resemble glia-axon transfer proteins. J Cell Physiol 138:257–266 [DOI] [PubMed]
- Hohfeld J, Hartl FU (1994) Role of the chaperonin cofactor Hsp10 in protein folding and sorting in yeast mitochondria. J Cell Biol 126:305–315 [DOI] [PMC free article] [PubMed]
- Johnson BJ, Le TT, Dobbin CA, Banovic T, Howard CB, Flores FM, Vanags D, Naylor DJ, Hill GR, Suhrbier A (2005) Heat shock protein 10 inhibits lipopolysaccharide-induced inflammatory mediator production. J Biol Chem 280:4037–4047 [DOI] [PubMed]
- Keirstead HS, Blakemore WF (1999) The role of oligodendrocytes and oligodendrocyte progenitors in CNS remyelination. Adv Exp Med Biol 468:183–197 [DOI] [PubMed]
- Lehnardt S, Lachance C, Patrizi S, Lefebvre S, Follett PL, Jensen FE, Rosenberg PA, Volpe JJ, Vartanian T (2002) The toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J Neurosci 22:2478–2486 [DOI] [PMC free article] [PubMed]
- Lindquist S, Craig EA (1988) The heat-shock proteins. Annu rev Genet 22:631–677 [DOI] [PubMed]
- McCarthy KD, de Vellis J (1980) Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol 85:890–902 [DOI] [PMC free article] [PubMed]
- Morton H (1998) Early pregnancy factor: an extracellular chaperonin 10 homologue. Immunol Cell Biol 76:483–496 [DOI] [PubMed]
- Morton H, Cavanagh AC, Athanasas-Platsis S, Quinn KA, Rolfe BE (1992) Early pregnancy factor has immunosuppressive and growth factor properties. Reprod Fertil Dev 4:411–422 [DOI] [PubMed]
- Morton H, Hegh V, Clunie GJA (1976) Studies of the rosette inhibition test in pregnant mice: evidence of immunosuppression? Proc R Soc Lond B Biol Sci 193:413–419 [DOI] [PubMed]
- Morton H, Rolfe B, Cavanagh A (1982) Early pregnancy factor: biology and clinical significance. In: Grudzinskas B, Teisner B, Seppala M (eds) Pregnancy Proteins. Academic Press, Sydney, pp 391–405
- Raine CS (1997) The Norton lecture: a review of the oligodendrocyte in the multiple sclerosis lesion. J Neuroimmunol 77:135–152 [DOI] [PubMed]
- Ranford JC, Coates AR, Henderson B (2000) Chaperonins are cell-signalling proteins: the unfolding biology of molecular chaperones. Expert Rev Mol Med 2000:1–17 [DOI] [PubMed]
- Somodevilla-Torres MJ, Morton H, Reid S, Cavanagh A (2003) Purification and characterization of functional early pregnancy factor (EPF) expressed in Sf9 insect cells and in Escherichia coli. Protein Exp Purif 32(2):276–287 [DOI] [PubMed]
- Sprang GK, Brown IR (1987) Selective induction of a heat shock gene in fibre tracts and cerebellar neurons of the rabbit brain detected by in situ hybridization. Brain Res 427:89–93 [DOI] [PubMed]
- Summers KM, Murphy RM, Webb GC, Peters GB, Morton H, Cassady AI, Cavanagh AC (1996) The human early pregnancy factor/chaperonin 10 gene family. Biochem Mol Med 58:52–58 [DOI] [PubMed]
- Tytell M (2005) Release of heat shock proteins (Hsps) and the effects of extracellular Hsps on neural cells and tissues. Int J Hypertherm 21:445–455 [DOI] [PubMed]
- Vanags D, Williams B, Johnson B, Hall S, Nash P, Taylor A, Weiss J, Feeney D (2006) Therapeutic efficacy and safety of chaperonin 10 in patients with rheumatoid arthritis: a double-blind randomised trial. Lancet 368:855–863 [DOI] [PubMed]
- Vega VL, Rodriguez-Silva M, Frey T, Gehrmann M, Diaz JC, Steinem C, Multhoff G, Arispe N, De MA (2008) Hsp70 translocates into the plasma membrane after stress and is released into the extracellular environment in a membrane-associated form that activates macrophages. J Immunol 180:4299–4307 [DOI] [PubMed]
- Zhang B, Harness J, Somodevilla-Torres MJ, Hillyard NC, Mould AW, Alewood D, Love SG, Alewood PF, Greer JM, Cavanagh AC, McCombe PA, Morton H (2000) Early pregnancy factor suppresses experimental autoimmune encephalomyelitis induced in Lewis rats with myelin basic protein and in SJL/J mice with myelin proteolipid protein peptide 139–151. J Neurol Sci 182:5–15 [DOI] [PubMed]
- Zhang B, Walsh NM, Nguyen KB, Hillyard NC, Cavanagh AC, McCombe PA, Morton H (2003) Early pregnancy factor treatment suppresses the inflammatory response and adhesion molecule expression in the spinal cord of SJL/J mice with experimental autoimmune encephalomyelitis and the delayed type hypersensitivity reaction to trinitrochlorobenzene in normal BALB/c mice. J Neurol Sci 212:37–46 [DOI] [PubMed]