Abstract
Familial Alzheimer’s disease (FAD)-linked presenilin (PS) mutations show gain-of-toxic-function characteristics. These FAD PS mutations are scattered throughout the PS molecule, reminiscent of the distribution of cystic fibrosis transmembrane conductance regulator and p53 mutations. Because of the scattered distribution of PS mutations, it is difficult to infer mechanistic insights about how these mutations cause the disease similarly. Recent careful reexamination of γ-secretase activity indicates that some PS mutations decrease the proteolytic activity of γ-secretase, suggesting a loss-of-function nature of PS mutations. To extend this observation to all known PS mutations, a large number of PS mutations were evaluated using bioinformatic tools. The analyses reveal that as many as one third of PS1 residues are highly conserved, that about 75% of FAD mutations are located to the highly conserved residues, and that most PS mutations likely damage the activity of PS. These results are consistent with the idea that the majority of PS mutations lower the activity of PS/γ-secretase.
Keywords: Alzheimer’s disease, Presenilin mutations, Protein misfolding, Sequence analyses
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disease affecting millions of people usually at a later stage of life. Affected individuals suffer from difficulties in memory, judgment, abstraction, and language (see Walsh and Selkoe 2004 for review). These behavioral manifestations are closely linked to lesions in the medial temporal lobe and cortical networks which play a crucial role in long-term, declarative memory (see Buckner 2004 for review). These regions of the brain are invariably studded with characteristic aggregates such as extracellular neuritic plaques and intracellular neurofibrilary tangles in AD patients (Walsh and Selkoe 2004). A major constituent of the amyloid plaque is small Aβ peptide fragments derived from the β-amyloid precursor protein (APP). APP is cleaved along the secretory pathway by several proteases, two of which, β- and γ-secretases, generate the Aβ peptide. Another pathological hallmark of AD is the neurofibrillary tangles that are found in cell bodies and apical dendrites. The major proteinaceous component is abnormally phosphorylated tau proteins (Walsh and Selkoe 2004). A currently accepted model is that soluble forms of extracellular amyloid peptides signal cells to form intraneuronal tangles, which doom neurons to a destructive fate (Haass and Selkoe 2007).
A small fraction of AD is inherited in an autosomal dominant fashion (Price and Sisodia 1998) and is genetically heterogeneous. Most familial AD (FAD) cases are due to mutations in APP and presenilin 1 and 2 (PS1 and PS2). PS is a catalytic subunit of γ-secretase that processes APP (Selkoe and Wolfe 2007). γ-Secretase generates amyloid peptides such as Aβ40 and Aβ42 in concert with β-secretase from APP. FAD-linked APP mutations act either by increasing overall production of Aβ40 and Aβ42 or by enhancing propensity of amyloid fragments to aggregate, or by specifically overproducing Aβ42 (Walsh and Selkoe 2004). Most FAD-linked PS1 mutations tested so far increase the ratio of Aβ42/Aβ40, consistent with the idea that increases of the relative or absolute level of toxic Aβ42 may trigger AD (Wolfe 2007), while some FAD-linked PS1 mutations fail to increase neurotoxic Aβ42 or the Aβ42/Aβ40 ratio (Shioi et al. 2007).
Because many APP or PS mutants change the level of Aβ production, one can expect that any gene or reagent that regulates Aβ metabolism may also affect AD pathology. Many cell-based assays monitoring Aβ metabolism have contributed to the identification of genes or chemicals that modulate Aβ synthesis (Kukar et al. 2005; Zhou et al. 2005; Chen et al. 2006; Weggen et al. 2007).
At the molecular level, it is challenging to figure out how such mutations affect Aβ generation. Most APP mutations are found near cleavage sites of α-, β-, and γ-secretases implying that these mutations compromise the cleavage action by each secretase. On the other hand, more than 100 FAD-linked PS1 mutations are spread throughout the molecule, defeating simple explanations. It is interesting to note that this scattered mutation pattern has been observed in other proteins such as cystic fibrosis transmembrane conductance regulator (CFTR) and p53 (Gelman and Kopito 2002). Remarkably, more than 1,500 mutations in the CFTR gene (1,480 amino acids) have been linked to cystic fibrosis (http://www.genet.sickkids.on.ca/cftr). The mutations in CFTR and p53 cause a varying degree of loss of function biochemically and genetically. On the other hand, PS mutations are considered a gain of toxic function genetically. For example, the ablation of one copy of PS1 is asymptomatic in mice (although not confirmed in human), and missense mutations dominantly predispose carriers to AD (De Strooper 2007). It has been recently shown that a few selected PS mutations cause a loss of function biochemically (for example, reduced proteolytic activity; Bentahir et al. 2006) and that the reduction in proteolytic activity of PS may be mechanistically linked to an increased ratio of Aβ42/Aβ40 (Wolfe 2007). Thus, it will be important to examine whether the majority of all known PS mutations reduces proteolytic activity of γ-secretase.
To test whether the characteristics of FAD PS mutations provide an insight with regard to structure and function of PS/γ-secretase, 14 PS sequences from distantly related species were analyzed using bioinformatic approaches. The results suggest that most FAD PS mutations lead to a loss of structure and function of PS/γ-secretase biochemically.
Conservation of PS and pattern of FAD-linked PS mutations
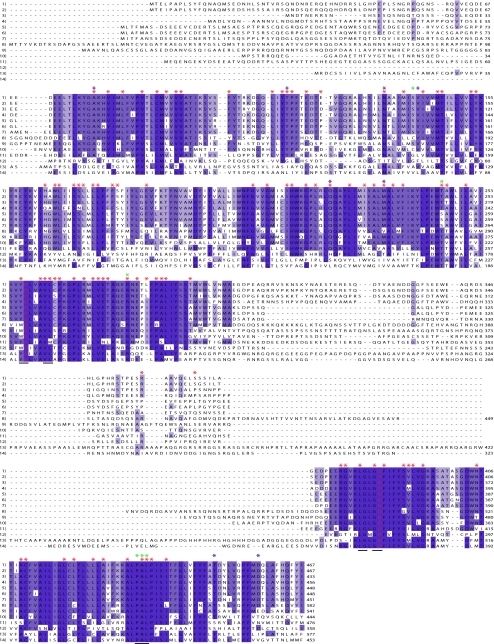
Because not all the functionally and structurally critical residues of PS are known, it was necessary to find a way to recognize such residues. On the assumption that critical residues would be conserved across different species, 14 PS sequences from various species including Chlamydomonas and Arabidopsis were compared (Fig. 1). This analysis resulted in 43 conserved residues in all 14 species. In support of the assumption, Aβ production was significantly altered when the PS1 Y288 residue (one of the 43 conserved residues) was artificially mutated (Laudon et al. 2004), although this residue has not been linked to AD so far.
Fig. 1.
Multiple protein sequence alignment of PS from various species was performed using ClustalW (Thompson et al. 1994, http://www.ebi.ac.uk/Tools/clustalw/). Conserved residues were marked with blue shades. Amino acid sequences are from (1) PS1 Homo sapiens (NP_000012), (2) PS1 Mus musculus (NP_032969), (3) PS1 Xenopus tropicalis (NP_001027027), (4) PS1 Danio rerio (NP_571099), (5) PS2 Homo sapiens (P49810), (6) PS2 Mus musculus (NP_035313), (7) PS2 Xenopus tropicalis (NP_001017181), (8) PS Helix lucorum (AAG28518), (9) PS Drosophila melanogaster (O02194), (10) Sel-12 Caenorhabditis elegans (P52166), (11) PS Ephydatia fluviatilis (BAE19681), (12) Hop-1 Caenorhabditis elegans (O02100), (13) PS Chlamydomonas reinhardtii (EDP06639), (14) Arabidopsis thaliana (NP_172346). FAD-linked PS1 residues are marked with red asterisks, FAD PS2 residues with blue asterisks, and PS1 residues identified by random mutagenesis (Nakaya et al. 2005) with green asterisks. Only missense mutations were considered. Two putative active site resides are shown in red. Putative catalytic core regions are underlined (Weihofen et al. 2002)
Conserved residues were classified according to the degree of conservation (Table 1; when a residue is conserved in 12 PS sequences among 14 sequences, it is represented as 12/14). As expected, previously identified motifs such as the active site residues and their surrounding residues were found among the conserved residues (Fig. 1; Wolfe et al. 1999; Weihofen et al. 2002). It is interesting to note that 21 out of 43 absolutely conserved residues (14 of 14) were linked to FAD (48.8%; Table 1). Considering the possibility that more naturally occurring FAD PS1 mutations would be discovered in the future, it is reasonable to predict that the number of FAD mutations in this category will continue to increase. Sixty-eight among 91 FAD PS1 residues (74.7%) were found in highly conserved residues (degree of conservation from 14 of 14 to 11 of 14). Other FAD PS1 mutations (20 residues out of 91; 22%) were found mostly in moderately conserved residues (degree of conservation from 10 of 14 to 7 of 14). Therefore, most FAD PS1 mutations (88 out of 91; 96.7%) were mapped to highly or moderately conserved residues. Only 3 out of 91 FAD mutations (3%) were found in less conserved residues. These observations indicate that the preferred spots for FAD PS1 mutations are the highly conserved residues.
Table 1.
Highly conserved residues of PS are preferred targets for FAD mutation
| Degree of conservationa | Number of conserved residues | Number of FAD-linked PS1 residues | Mutation rate (%) of PS1 residuesb | Number of FAD-linked PS2 residues | Mutation rate (%) of PS2 residuesc | Number of PS1 residues hit by random mutagenesis | Hit rate (%) of PS1 residuesd |
|---|---|---|---|---|---|---|---|
| 14/14 | 43 | 21 | 48.8 | 4 | 9.3 | ||
| 13/14 | 30 | 12 | 40 | 1 | 3.3 | ||
| 12/14 | 32 | 13 | 40.6 | 1 | 3.2 | ||
| 11/14 | 54 | 22 | 40.7 | 4 | 21.1 | 1 | 1.8 |
| 10/14 | 32 | 5 | 15.6 | ||||
| 9/14 | 34 | 5 | 14.7 | 2 | 5.9 | ||
| 8/14 | 35 | 6 | 17.1 | 1 | 2.9 | ||
| 7/14 | 22 | 4 | 18.2 | ||||
| 6/14 | 19 | 1 | 5.3 | ||||
| 5/14 to 0/14 | 166 | 2 | 1.2 | ||||
| Total | 467 | 91 | 19.5% |
aFor example, when a residue is conserved in 12 PS sequences among 14 sequences (Fig. 1), it is shown as 12 of 14.
bThe mutation rate was calculated based on number of FAD-linked PS1 residues from number of conserved residues under a given degree of conservation.
cThe mutation rate was calculated based on number of FAD-linked PS2 residues from number of conserved residues under a given degree of conservation.
dThe mutation rate was calculated based on number of PS1 residues hit by random mutagenesis from number of conserved residues under a given degree of conservation.
The multiple sequence alignment also showed that many PS1 residues are highly conserved (159 out of 467; 34%; Fig. 1 and Table 1). The percentage of conservation was increased to 60.3% when moderately conserved residues were also counted (123 out of 467; 26.3%). This high conservation is consistent with the fact that PS has multiple domains/functions for proteolytic cleavage, Ca2+ ion channeling, PI3K signaling, and cytoskeletal regulation (Selkoe and Wolfe 2007; Baki et al. 2004; Fraering et al. 2005; Tu et al. 2006; Khandelwal et al. 2007; Zhao et al. 2008 and references therein) and suggests that these domains/functions are important across many different species. Because highly conserved residues span over the PS molecule and a majority of FAD mutations fall onto these residues, it is natural to observe a scattered distribution of FAD PS mutations.
Based on the pattern of FAD PS1 mutations, we asked whether or not FAD PS2 mutations follow this trend. Indeed, despite a small pool of FAD-linked mutations, a similar pattern was observed with 75% (six out of eight residues) of FAD mutations being located to highly conserved residues (Table 1). Strikingly, Nakaya et al. (2005) identified residues affecting proteolytic activity of PS1 by randomly mutagenizing the PS1 gene. When these mutations were mapped (Fig. 1), five out of six residues were actually highly conserved residues according to the current analysis (Table 1). The percentage is comparable to that from naturally occurring mutations of PS1 and PS2. These results show that FAD PS mutations involve highly conserved residues.
The core region (CR) of p53 is highly enriched with missense mutations (Soussi and Wiman 2007). All residues of the CR have been found to be mutated in human cancers. This is because the CR of p53 is highly flexible and cycles between folded and unfolded states, rendering fragility to the protein (Joerger and Fersht 2007) and making the CR of p53 a binding site for Hsp90 (Rüdiger et al. 2002). The mutations on the CR lead to a loss of function ranging from partial to complete loss of activity (Kato et al. 2003). In fact, it has been shown that the probability of a random mutation to cause a genetic disease increases as the degree of conservation goes up (Vitkup et al. 2003). Similarly, many FAD PS mutations found in the highly conserved regions of PS also likely cause a varying degree of loss of activity of PS because conserved residues likely play an important role for proper structure and function of the protein (Ng and Henikoff 2006).
Prediction for activity of PS variants based on bioinformatic approaches
To predict the extent to which PS1 missense mutations affect the activity of γ-secretase, three independent prediction methods were employed (Tables 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, and 12). These methods score whether an amino acid substitution at a certain position is tolerated based on sequence homology and physicochemical properties of amino acids (Sorting Intolerant From Tolerant [SIFT], http://blocks.fhcrc.org/sift/SIFT.html; Protein Analysis Through Evolutionary Relationships [PANTHER] PSEC, http://www.pantherdb.org/; Align-Grantham variation–Grantham deviation [GVGD], http://agvgd.iarc.fr/). These approaches have been used to evaluate how missense mutations abrogate or increase/decrease protein function (Vitkup et al. 2003; Brunham et al. 2005; Mathe et al. 2006). When these analyses were applied to PS1 missense mutations, SIFT, PANTHER, and A-GVGD predicted 94, 90, and 139 mutations (out of 145 mutations) to be deleterious (Tables 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, and 12). As a control, nonpathogenic mutations (R35Q, F175S, and E318G) were also analyzed. The PANTHER prediction turned out to be the most stringent of the three methods. This is consistent with the report that PANTHER yields a high false-negative rate (59%; the percentage of substitutions predicted to be functionally neutral on a set of amino acid substitutions that are known to affect protein function) compared to SIFT (31%; Ng and Henikoff 2006). The accuracy of the Align-GVGD on p53 for deleterious and neutral mutants was 88.1% and 71.2%, respectively (Mathe et al. 2006). Similarly, the Aligh-GVGD prediction for deleterious and neutral mutants of PS1 was 95.9% (139 of 145) and 67% (two of three), respectively. These results suggest that most FAD-linked PS1 mutations reduce the activity of PS1.
Table 2.
Effects of FAD PS1 mutations on PS1/γ-secretase activity
| Degree of conservation (14/14)a | Mutation | Functional significance (SIFT)b | Functional significance (PANTHER)c | Functional significance (GV/GD)d |
|---|---|---|---|---|
| V89 | L | 0.00e | −3.62373e | 0.00/30.92e |
| C92 | S | 0.00 | −3.44456 | 0.00/111.67 |
| T147 | I | 0.00 | −1.87486 | 0.00/89.28 |
| G209 | R | 0.00 | −4.56674 | 0.00/125.13 |
| G209 | E | 0.00 | −4.41973 | 0.00/97.85 |
| G209 | V | 0.00 | −3.91328 | 0.00/109.55 |
| L250 | S | 0.00 | −3.35853 | 0.00/144.08 |
| A260 | V | 0.00 | −3.97167 | 0.00/65.28 |
| V261 | F | 0.00 | −4.59946 | 0.00/48.95 |
| P264 | L | 0.00 | −4.06675 | 0.00/97.78 |
| P267 | S | 0.00 | −2.25417 | 0.00/73.35 |
| P267 | L | 0.00 | −3.84044 | 0.00/97.78 |
| L271 | V | 0.00 | −3.05205 | 0.00/30.92 |
| V272 | A | 0.00 | −3.13143 | 0.00/65.28 |
| R278 | K | 0.00 | −3.05745 | 0.00/26.00 |
| R278 | T | 0.00 | −3.91372 | 0.00/70.97 |
| R278 | S | 0.00 | −3.70071 | 0.00/109.21 |
| L381 | V | 0.00 | −3.99591 | 0.00/30.92 |
| G384 | A | 0.00 | −4.85237 | 0.00/60.00 |
| F386 | S | 0.00 | −5.37049 | 0.00/154.81 |
| G394 | V | 0.00 | −5.85813 | 0.00/109.55 |
| L418 | F | 0.00 | −4.35042 | 0.00/21.82 |
| A431 | E | 0.00 | −4.73687 | 0.00/106.71 |
| A431 | V | 0.00 | −4.08271 | 0.00/65.28 |
| A434 | C | 0.00 | −5.39513 | 0.00/195.00 |
| L435 | F | 0.00 | −4.35042 | 0.00/21.82 |
| P436 | S | 0.00 | −5.25856 | 0.00/73.35 |
| P436 | Q | 0.00 | −6.16212 | 0.00/75.14 |
aThe degree of conservation was according to Table 1 and accurate for SIFT. For PANTHER PSEC and Align-GVGD, it may change due to different set of homologous sequences.
bNormalized probabilities. Positions with normalized probabilities less than 0.05 are predicted to be deleterious; those greater than or equal to 0.05 are predicted to be tolerated. The analysis was performed using PS sequences in Fig. 1 (Ng and Henikoff 2006).
cPANTHER subPSEC values were acquired using its own set of PS1 homologous sequences from its database (http://www.pantherdb.org/; Thomas et al. 2003). A subPSEC score of −3 or less corresponds to a deleterious mutation. This prediction method reveals gain-of-function mutations in some instances (Thomas et al. 2003). The sequences were from PS1_HUMAN (P49768, Homo sapiens), Q6RH31_CANFA (Q6RH31, Canis lupus familiaris), Q6RH32_CANFA (Q6RH32, Canis familiaris), PSN1_BOVIN (Q9XT97, Bos Taurus), PSN1_RAT (P97887, Rattus norvegicus), PSN1_MOUSE (P49769, Mus musculus), Q90X08_CHICK (Q90X08, Gallus gallus), PSN1_XENLA (O12976, Xenopus laevis), PSN1_BRARE (Q9W6T7, Danio rerio), PSN_CAEEL (P52166, Caenorhabditis elegans), HOP-1 (O02100, Caenorhabditis elegans), SPE-4 (Q01608, Caenorhabditis elegans), PSNA_ARATH (O64668, Arabidopsis thaliana), and PSNB_ARATH (Q9SIK7, Arabidopsis thaliana).
dGrantham variation (GV) measures the degree of biochemical variation among amino acids found at a given position in the multiple sequence alignment, and Grantham deviation (GD) scores the biochemical distance of the mutant amino acid from the observed amino acid at a particular position (Grantham 1974; Mathe et al. 2006). The criteria for prediction are described in Mathe et al. (2006). The current Align-GVGD analysis used PS sequences in Fig. 1 except sequences from Arabidopsis thaliana (NP_172346) and Chlamydomonas reinhardtii (EDP06639) because adding more distantly related sequences decreased the number of mutants predicted as deleterious (Mathe et al. 2006). However, the current set of PS sequences is still more divergent than the set of p53 sequences used to develop the Align-GVGD method (Mathe et al. 2006).
eValues predicted to damage the activity of PS1 are shown in italics.
Table 3.
Effects of FAD PS1 mutations on PS1/γ-secretase activity
| Degree of conservation (13/14)a | Mutation | Functional significance (SIFT)b | Functional significance (PANTHER)c | Functional significance (GV/GD)d |
|---|---|---|---|---|
| V96 | F | 0.01e | −2.55121 | 0.00/48.95e |
| V97 | L | 0.03 | −2.14694 | 0.00/30.92 |
| N135 | D | 0.11 | −3.19811e | 0.00/23.01 |
| N135 | S | 0.11 | −3.11384 | 0.00/46.24 |
| L174 | M | 0.03 | −2.56868 | 0.00/14.30 |
| L174 | R | 0.00 | −5.26216 | 0.00/101.88 |
| L262 | F | 0.02 | −4.09855 | 0.00/21.82 |
| E273 | A | 0.02 | −3.73922 | 0.00/106.71 |
| P284 | S | 0.03 | −3.16234 | 0.00/73.35 |
| P284 | L | 0.02 | −3.35216 | 0.00/97.78 |
| A285 | V | 0.01 | −3.10351 | 0.00/65.28 |
| S390 | I | 0.00 | −5.42803 | 0.00/141.80 |
| L392 | V | 0.02 | −3.54057 | 0.00/30.92 |
| L392 | P | 0.00 | −5.82317 | 0.00/97.78 |
| A409 | T | 0.01 | −3.66939 | 0.00/58.02 |
| C410 | Y | 0.00 | −5.50834 | 0.00/193.72 |
a, b, c, d, eSee legend of Table 2.
Table 4.
Effects of FAD PS1 mutations on PS1/γ-secretase activity
| Degree of conservation (12/14)a | Mutation | Functional significance (SIFT)b | Functional significance (PANTHER)c | Functional significance (GV/GD)d |
|---|---|---|---|---|
| V82 | L | 0.04e | −2.10435 | 0.00/30.92e |
| Y115 | H | 0.12 | −4.10728e | 0.00/83.33 |
| Y115 | D | 0.37 | −5.28542 | 0.00/159.94 |
| Y115 | C | 0.04 | −5.33748 | 0.00/193.72 |
| M146 | V | 0.28 | −1.63467 | 0.00/20.52 |
| M146 | I | 0.09 | −2.23949 | 0.00/10.12 |
| M146 | L | 0.09 | −2.04936 | 0.00/14.30 |
| L153 | V | 0.05 | −2.32119 | 0.00/30.92 |
| S169 | L | 0.01 | −2.24316 | 0.00/144.08 |
| S169 | P | 0.02 | −3.71787 | 0.00/73.35 |
| G206 | S | 0.22 | −2.33284 | 0.00/55.27 |
| G206 | D | 0.03 | −3.95033 | 0.00/93.77 |
| G206 | A | 0.09 | −2.67628 | 0.00/60.00 |
| G206 | V | 0.02 | −2.10266 | 0.00/109.55 |
| H214 | Y | 0.20 | −2.9983 | 0.00/83.33 |
| G217 | D | 0.04 | −2.90745 | 0.00/93.77 |
| L226 | R | 0.01 | −3.57015 | 0.00/101.88 |
| L226 | F | 0.13 | −3.95858 | 0.00/21.82 |
| L286 | V | 0.03 | −2.53727 | 0.00/30.92 |
| R377 | M | 0.02 | −4.49467 | 0.00/91.64 |
| G378 | V | 0.02 | −2.28787 | 0.00/109.55 |
| G378 | E | 0.02 | −4.13214 | 0.00/97.85 |
| I439 | V | 0.06 | −1.86884 | 0.00/28.68 |
a, b, c, d, eSee legend of Table 2.
Table 5.
Effects of FAD PS1 mutations on PS1/γ-secretase activity
| Degree of conservation (11/14)a | Mutation | Functional significance (SIFT)b | Functional significance (PANTHER)c | Functional significance (GV/GD)d |
|---|---|---|---|---|
| A79 | V | 0.02e | −4.02789e | 0.00/65.28e |
| L85 | P | 0.00 | −4.42532 | 0.00/97.78 |
| L113 | P | 0.02 | −4.94282 | 0.00/97.78 |
| L113 | V | 0.36 | −2.37417 | 0.00/30.92 |
| L113 | Q | 0.01 | −4.64537 | 0.00/112.44 |
| T116 | N | 0.19 | −3.90259 | 0.00/64.77 |
| T116 | I | 0.82 | −4.37086 | 0.00/89.28 |
| P117 | S | 0.14 | −2.65492 | 0.00/73.35 |
| P117 | L | 1.00 | −1.78495 | 0.00/97.78 |
| P117 | R | 0.07 | −3.11267 | 0.00/102.71 |
| E120 | K | 0.06 | −2.11044 | 0.00/56.87 |
| E120 | D | 0.19 | −2.5923 | 0.00/44.60 |
| Y154 | C | 0.00 | −5.3361 | 0.00/193.72 |
| Y154 | N | 0.00 | −4.65004 | 0.00/142.23 |
| W165 | G | 0.00 | −3.80029 | 0.00/183.79 |
| W165 | C | 0.00 | −4.1291 | 0.00/214.36 |
| L166 | H | 0.00 | −4.86668 | 0.00/98.69 |
| L166 | P | 0.01 | −2.81463 | 0.00/97.78 |
| L166 | R | 0.00 | −4.43624 | 0.00/101.88 |
| S170 | F | 0.02 | −4.10627 | 46.24/135.88 |
| L173 | W | 0.00 | −4.97603 | 0.00/60.98 |
| F177 | L | 0.10 | −1.80095 | 0.00/21.82 |
| F177 | S | 0.02 | −3.13367 | 0.00/154.81 |
| I213 | L | 0.36 | −2.09768 | 0.00/4.86 |
| I213 | F | 0.01 | −3.58038 | 0.00/21.28 |
| I213 | T | 0.01 | −3.32339 | 0.00/89.28 |
| L219 | F | 0.17 | −3.17101 | 0.00/21.82 |
| L219 | P | 0.16 | −3.96853 | 0.00/97.78 |
| Q222 | R | 0.29 | −2.66321 | 0.00/42.81 |
| Q222 | H | 0.28 | −2.51753 | 0.00/24.08 |
| A231 | T | 0.02e | −2.73211 | 0.00/58.02 |
| A231 | V | 0.02 | −2.43329 | 0.00/65.28 |
| T245 | P | 0.01 | −4.08141 | 57.75/24.03 |
| R269 | G | 0.01 | −3.65651 | 0.00/125.13 |
| R269 | H | 0.01 | −3.72602 | 0.00/28.82 |
| T274 | R | 0.08 | −3.9915 | 0.00/70.97 |
| E280 | A | 0.06 | −3.19891 | 0.00/106.71 |
| E280 | G | 0.02 | −3.52354 | 0.00/97.85 |
| L424 | H | 0.00 | −5.84841 | 0.00/98.69 |
| L424 | R | 0.01 | −5.33629 | 0.00/101.88 |
| A426 | P | 0.03 | −4.17643 | 0.00/26.87 |
a, b, c, d, eSee legend of Table 2.
Table 6.
Effects of FAD PS1 mutations on PS1/γ-secretase activity
| Degree of conservation (10/14)a | Mutation | Functional significance (SIFT)b | Functional significance (PANTHER)c | Functional significance (GV/GD)d |
|---|---|---|---|---|
| L235 | V | 0.44 | −2.35073 | 0.00/30.92e |
| L235 | P | 0.07 | −4.05115e | 0.00/97.78 |
| F237 | L | 0.53 | −3.04552 | 0.00/21.82 |
| F237 | I | 0.47 | −2.5801 | 0.00/21.28 |
| Y256 | S | 0.01e | −2.17096 | 0.00/143.11 |
| V391 | F | 0.02 | −3.88813 | 20.52/28.53 |
| L420 | R | 0.01 | −4.15521 | 0.00/101.88 |
a, b, c, d, eSee legend of Table 2.
Table 7.
Effects of FAD PS1 mutations on PS1/γ-secretase activity
| Degree of conservation (9/14)a | Mutation | Functional significance (SIFT)b | Functional significance (PANTHER)c | Functional significance (GV/GD)d |
|---|---|---|---|---|
| F105 | I | 0.39 | −2.66139 | 0.00/21.28e |
| F105 | L | 0.68 | −2.39242 | 0.00/21.82 |
| I143 | F | 0.01e | −4.18757e | 0.00/21.28 |
| I143 | N | 0.00 | −5.41713 | 0.00/148.91 |
| I143 | T | 0.01 | −4.00722 | 0.00/89.28 |
| I143 | M | 0.01 | −3.93209 | 0.00/10.12 |
| E184 | D | 0.17 | −2.92255 | 0.00/44.60 |
| M233 | L | 0.25 | −2.4775 | 0.00/14.30 |
| M233 | I | 0.54 | −2.68173 | 0.00/10.12 |
| M233 | V | 1.00 | −2.13273 | 0.00/20.52 |
| M233 | T | 0.40 | −2.39566 | 0.00/81.04 |
| N405 | S | 0.72 | −2.23365 | 0.00/46.24 |
a, b, c, d, eSee legend of Table 2.
Table 8.
Effects of FAD PS1 mutations on PS1/γ-secretase activity
| Degree of conservation (8/14)a | Mutation | Functional significance (SIFT)b | Functional significance (PANTHER)c | Functional significance (GV/GD)d |
|---|---|---|---|---|
| M139 | K | 0.01e | −3.3509e | 0.00/94.49e |
| M139 | T | 0.02 | −2.68458 | 0.00/81.04 |
| M139 | I | 0.20 | −2.23428 | 0.00/10.12 |
| H163 | Y | 0.44 | −3.82384 | 24.08/83.33 |
| H163 | R | 0.57 | −2.45471 | 24.08/28.37 |
| A246 | E | 0.04 | −2.78519 | 58.02/65.50 |
| C263 | R | 0.03 | −1.63645 | 111.67/90.15 |
| C263 | F | 0.02 | −2.90381 | 111.67/124.98 |
| L282 | R | 0.03 | −2.65841 | 4.86/97.59 |
| L282 | V | 0.35 | −1.11025 | 4.86/28.68 |
| R358 | Q | 0.45 | −2.11737 | 111.84/16.48 |
a, b, c, d, eSee legend of Table 2.
Table 9.
Effects of FAD PS1 mutations on PS1/γ-secretase activity
| Degree of conservation (7/14)a | Mutation | Functional significance (SIFT)b | Functional significance (PANTHER)c | Functional significance (GV/GD)d |
|---|---|---|---|---|
| V94 | M | 0.08 | −2.61745 | 28.68/0.00 |
| L171 | P | 0.03e | −4.41596e | 0.00/97.78e |
| I229 | F | 0.08 | −3.62133 | 0.00/21.28 |
| T291 | P | 0.27 | −2.94048 | 58.02/1.62 |
a, b, c, d, eSee legend of Table 2.
Table 10.
Effects of FAD PS1 mutations on PS1/γ-secretase activity
| Degree of conservation (6/14) a | Mutation | Functional significance (SIFT)b | Functional significance (PANTHER)c | Functional significance (GV/GD)d |
|---|---|---|---|---|
| S178 | P | 0.13 | −4.04264e | 57.75/24.03e |
a, b, c, d, eSee legend of Table 2.
Table 11.
Effects of FAD PS1 mutations on PS1/γ-secretase activity
| Degree of conservation (Rest)a | Mutation | Functional significance (SIFT)b | Functional significance (PANTHER)c | Functional significance (GV/GD)d |
|---|---|---|---|---|
| E123 | K | 0.08 | −1.69647 | 117.42/36.71 |
| S365 | Y | 0.15 | −2.12378 | 155.34/25.33 |
a, b, c, dSee legend of Table 2.
Table 12.
Effects of nonpathogenic mutations on PS1/γ-secretase activity
| Mutation | Functional significance (SIFT)a | Functional significance (PANTHER)b | Functional significance (GV/GD)c | |
|---|---|---|---|---|
| R35 | Q | 0.59 | −0.8905 | 147.42/0.00 |
| F175 | S | 0.04d | −2.84132 | 0.00/154.81d |
| E318 | G | 0.55 | −2.6434 | 353.86/0.00 |
aNormalized probabilities. Positions with normalized probabilities less than 0.05 are predicted to be deleterious; those greater than or equal to 0.05 are predicted to be tolerated. The analysis was performed using PS sequences in Fig. 1 (Ng and Henikoff 2006).
bPANTHER subPSEC values were acquired using its own set of PS1 homologous sequences from its database (http://www.pantherdb.org/; Thomas et al. 2003). A subPSEC score of −3 or less corresponds to a deleterious mutation. This prediction method reveals gain-of-function mutations in some instances (Thomas et al. 2003). The sequences were from PS1_HUMAN (P49768, Homo sapiens), Q6RH31_CANFA (Q6RH31, Canis lupus familiaris), Q6RH32_CANFA (Q6RH32, Canis familiaris), PSN1_BOVIN (Q9XT97, Bos Taurus), PSN1_RAT (P97887, Rattus norvegicus), PSN1_MOUSE (P49769, Mus musculus), Q90X08_CHICK (Q90X08, Gallus gallus), PSN1_XENLA (O12976, Xenopus laevis), PSN1_BRARE (Q9W6T7, Danio rerio), PSN_CAEEL (P52166, Caenorhabditis elegans), HOP-1 (O02100, Caenorhabditis elegans), SPE-4 (Q01608, Caenorhabditis elegans), PSNA_ARATH (O64668, Arabidopsis thaliana), and PSNB_ARATH (Q9SIK7, Arabidopsis thaliana).
cGrantham variation (GV) measures the degree of biochemical variation among amino acids found at a given position in the multiple sequence alignment, and Grantham deviation (GD) scores the biochemical distance of the mutant amino acid from the observed amino acid at a particular position (Grantham 1974; Mathe et al. 2006). The criteria for prediction are described in Mathe et al. (2006). The current Align-GVGD analysis used PS sequences in Fig. 1 except sequences from Arabidopsis thaliana (NP_172346) and Chlamydomonas reinhardtii (EDP06639) because adding more distantly related sequences decreased the number of mutants predicted as deleterious (Mathe et al. 2006). However, the current set of PS sequences is still more divergent than the set of p53 sequences used to develop the Align-GVGD method (Mathe et al. 2006).
dValues predicted to damage the activity of PS1 are shown in italics.
Experimental observations consistent with loss of activities of clinically isolated PS1 variants
The reduction/elimination of the activity of sel-12 (a PS homolog) causes an egg-laying defective phenotype due to defective signaling of the Lin-12/Notch pathway in Caenorhabditis elegans (Sundaram and Greenwald 1993). Normal human PS can substitute for the C. elegans SEL-12 protein in vivo, whereas FAD-linked mutant human PSs have reduced ability to rescue the phenotype caused by sel-12 disruption (Levitan et al. 1996). Proteolytic release of the Notch-1 intracellular domain (NICD) is critical for Notch signaling, and this proteolysis is impaired in PS1 null cells and restored by PS1 expression (Song et al. 1999). However, some FAD-linked PS1 mutations display reduced ability to generate the NICD, consistent with the genetic data obtained from C. elegans (Levitan et al. 1996). FAD-linked PS1 mutations also appear to have a reduced activity toward other PS substrates (Marambaud et al. 2003; Bentahir et al. 2006).
The absence of PS1 causes the accumulation of cytosolic β-catenin, resulting in accelerated entry into the S phase of the cell cycle (Soriano et al. 2001). This hyperproliferative response is rescued by PS1 expression but not by two different FAD-linked PS1 mutants (Soriano et al. 2001). Wild-type PS1 may also activate the PI3K/Akt signaling pathway. In contrast, PS1 FAD mutations inhibit PS1-dependent PI3K/Akt activation, thus promoting glycogen synthase kinase 3 (GSK-3) activity and tau overphosphorylation (Baki et al. 2004).
PS is believed to regulate capacitative calcium entry (CCE) – a mechanism for refilling intracellular Ca2+ through plasma membrane channels. FAD-linked PS variants attenuate CCE (Yoo et al. 2000). PS also appears to be responsible for approximately 80% of passive Ca2+ leak from the endoplasmic reticulum (ER; Tu et al. 2006). Clinical PS mutations are defective in Ca2+ leak from the ER (Tu et al. 2006).
The anterograde fast axonal transport of APP and Trk receptors is impaired in the sciatic nerves of transgenic mice expressing two independent FAD-linked PS1 variants compared to the transport in the comparable nerves of transgenic mice expressing the wild-type human PS1 (Lazarov et al. 2007). The expression of FAD-linked PS1 mutants likely leads to increased GSK-3 activity and the reduction in kinesin-1-based transport of a subset of membrane cargo proteins (Pigino et al. 2003).
However, despite the various defects described in this section, it remains to be discovered how these defective activities of mutant PS contribute to AD.
Defective protein folding as an underlying cause of impaired PS functions
A PS1 mutation seems to cause multiple defects in PS functions. Pleiotropic defects associated with a mutation may be caused by protein misfolding. For example, CFTR ΔF508 displays loss-of-function phenotypes as indicated from defective export from the ER and unstable response to agonists. This is due to the disruption in protein folding (see Gelman and Kopito 2002 for review). The incubation of cells expressing CFTR ΔF508 in a medium containing a high concentration of chemical chaperones such as glycerol and trimethylamine N-oxide (TMAO) increased the steady-state level of mature CFTR at the cell surface and restored a functional Cl− ion channel activity by assisting protein folding (Welch and Brown 1996; Brown et al. 1996; see Brown et al. 1997 for a review). It has been suggested that some FAD PS1 mutations change the conformation of PS1 (Berezovska et al. 2005). It is interesting to note that two FAD PS1 variants, ΔE9 and M146L, displayed a defect in export from the ER (Kim et al. 2007). However, the ER export defect of PS1 ΔE9 was milder than that of CFTR ΔF508 as indicated in the mild disruption of the steady-state distribution of PS1 ΔE9 (Kim et al. 2000). In addition, TMAO partially rescued the ER export defect of PS1 ΔE9 (Kim et al. 2007). This result suggests that indeed some FAD PS mutations alter PS folding, contributing to pleiotropic impairments of PS functions.
Do human genetic data support the loss-of-function theory?
Although our sequence analyses point to a loss-of-function property of PS mutations in a biochemical sense, genetically, FAD PS mutations clearly demonstrate gain-of-toxic-function characteristics as illustrated by De Strooper (2007). Nevertheless, there are a few occasional reports supporting a genetic loss-of-function theory such as PS1 promoter mutations (Theuns et al. 2000, 2003; Lambert et al. 2001) and a PS1 truncation mutation (Tysoe et al. 1998). However, the frequency of these mutations is far less than that of PS missense mutations, and these observations await independent confirmation. Thus, more of such mutations should surface to support the loss-of-function theory of FAD PS mutations from a genetic point of view.
Loss of function and aberrant Aβ production
An important question is how an apparently reduced activity of γ-secretase can result in a gain of toxic function. It has been suggested that the reduced activity of γ-secretase can be linked to an increased ratio of Aβ42/Aβ40 due to the processive nature of this enzyme (Sato et al 2003; Qi-Takahara et al. 2005; Wolfe 2007). For example, γ-secretase consecutively trims Aβ49 or Aβ48 (precursors of Aβ or new cleavage products of APP by γ-secretase) to Aβ46, Aβ43, Aβ40, or to Aβ45, Aβ42, and Aβ39, respectively. If γ-secretase becomes inefficient due to a PS mutation or an APP mutation, γ-secretase fails to process the substrate to smaller fragments and prematurely releases longer forms of amyloid peptide. This will result in an increased ratio of Aβ42/Aβ40. This also explains why deleting one copy of PS1 does not affect the ratio of Aβ42/Aβ40.
Conclusion
In summary, this bioinformatic analysis extends the notion that most FAD-linked PS mutations dampen the activity of γ-secretase. More mutations may be found in highly conserved regions of PS in the future. The scattered distribution of PS mutations appears to reflect the distribution of conserved residues of PS which are structurally and functionally critical to the enzymatic activity of γ-secretase.
Acknowledgments
The author thanks Dr. Simeon Boyadjiev Boyd for critical reading. This work was supported in part by the Korean Research Foundation Postdoctoral Fellowship to S.D. Kim (MOEHRD:KRF-2007-357-E00005).
References
- Baki L, Shioi J, Wen P, Shao Z, Schwarzman A, Gama-Sosa M, Neve R, Robakis NK (2004) PS1 activates PI3K thus inhibiting GSK-3 activity and tau overphosphorylation: effects of FAD mutation. EMBO J 23:2586–2596 [DOI] [PMC free article] [PubMed]
- Bentahir M, Nyabi O, Verhamme J, Tolia A, Horre K, Wiltfang J, Esselmann H, De Strooper B (2006) Presenilin clinical mutations can affect γ-secretase activity by different mechanisms. J Neurochem 96:732–742 [DOI] [PubMed]
- Berezovska O, Lleo A, Herl LD, Frosch MP, Stern EA, Bacskai BJ, Hyman BT (2005) Familial Alzheimer’s disease presenilin 1 mutations cause alterations in the conformation of presenilin and interactions with amyloid precursor protein. J Neurosci 25:3009–3017 [DOI] [PMC free article] [PubMed]
- Brown CR, Hong-Brown LQ, Biwersi J, Verkman AS, Welch WJ (1996) Chemical chaperones correct the mutant phenotype of the ΔF508 cystic fibrosis transmembrane conductance regulator protein. Cell Stress Chaperones 1:117–125 [DOI] [PMC free article] [PubMed]
- Brown CR, Hong-Brown LQ, Welch WJ (1997) Strategies for correcting the delta F508 CFTR protein-folding defect. J Bioenerg Biomembr 29:491–502 [DOI] [PubMed]
- Brunham LR, Singaraja RR, Pape TD, Kejariwal A, Thomas PD, Hayden MR (2005) Accurate prediction of the functional significance of single nucleotide polymorphisms and mutations in the ABCA1 gene. PLOS Genet 1:739–747 [DOI] [PMC free article] [PubMed]
- Buckner RL (2004) Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron 44:195–208 [DOI] [PubMed]
- Chen F, Hasegawa H, Schmitt-Ulms G, Kawarai T, Bohm C, Katayama T, Sanjo N, Glista M, Rogaeva E, Wakutani Y, Pardossi-Piquard R, Ruan X, Tandon A, Checler F, Marambaud P, Hansen K, Westaway D, St. George-Hyslop P, Fraser P (2006) TMP21 is a presenilin complex component that modulates γ-secretase but not ε-secretase activity. Nature 440:1208–1212 [DOI] [PubMed]
- De Strooper B (2007) Loss-of-function presenilin mutations in Alzheimer disease. EMBO Reports 8:141–146 [DOI] [PMC free article] [PubMed]
- Fraering PC, Ye W, LaVoie MJ, Ostaszewski BL, Selkoe DJ, Wolfe MS (2005) γ-Secretase substrate selectivity can be modulated directly via interaction with a nucleotide-binding site. J Biol Chem 280:41987–41996 [DOI] [PMC free article] [PubMed]
- Gelman MS, Kopito RR (2002) Rescuing protein conformation: prospects for pharmacological therapy in cystic fibrosis. J Clin Invest 110:1591–1597 [DOI] [PMC free article] [PubMed]
- Grantham R (1974) Amino acid difference formula to help explain protein evolution. Science 185:862–864 [DOI] [PubMed]
- Haass C, Selkoe DJ (2007) Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid β-peptide. Nat Rev Mol Cell Biol 8:101–112 [DOI] [PubMed]
- Joerger AC, Fersht AR (2007) Structure-function-rescue: the diverse nature of common p53 cancer mutants. Oncogene 26:2226–2242 [DOI] [PubMed]
- Khandelwal A, Chandu D, Roe CM, Kopan R, Quatrano RS (2007) Moonlighting activity of presenilin in plants is independent of γ-secretase and evolutionarily conserved. Proc Natl Acad Sci USA 104:13337–13342 [DOI] [PMC free article] [PubMed]
- Kato S, Han SY, Liu W, Otsuka K, Shibata H, Kanamaru R, Ishioka C (2003) Understanding the function-structure and function-mutation relationships of p53 tumor suppressor protein by high-resolution missense mutation analysis. Proc Natl Acad Sci USA 100:8424–8429 [DOI] [PMC free article] [PubMed]
- Kim S-H, Lah JJ, Thinakaran G, Levey A, Sisodia SS (2000) Subcellular localization of presenilins: association with a unique membrane pool in cultured cells. Neurobiol Dis 7:99–117 [DOI] [PubMed]
- Kim J, Kleizen B, Choy R, Thinakaran G, Sisodia SS, Schekman R (2007) Biogenesis of γ-secretase early in the secretory pathway. J Cell Biol 179:951–963 [DOI] [PMC free article] [PubMed]
- Kukar T, Murphy MP, Eriksen JL, Sagi SA, Weggen S, Smith TE, Ladd T, Khan MA, Kache R, Beard J, Dodson M, Merit S, Ozols VV, Anastasiadis PZ, Das P, Fauq A, Koo EH, Golde TE (2005) Diverse compounds mimic Alzheimer disease-causing mutations by augmenting Aβ42 production. Nat Med 11:545–550 [DOI] [PubMed]
- Lambert J-C, Mann DMA, Harris JM, Chartier-Harlin M-C, Cumming A, Coates J, Lemmon H, StClair D, Iwatsubo T, Lendon C (2001) The -48C/T polymorphism in the presenilin 1 promoter is associated with an increased risk of developing Alzheimer’s disease and an increased Aβ load in brain. J Med Genet 38:353–355 [DOI] [PMC free article] [PubMed]
- Laudon H, Karlström H, Mathews PM, Farmery MR, Gandy SE, Lundkvist J, Lendahl U, Näslund J (2004) Functional domains in presenilin 1. J Biol Chem 279:23925–23932 [DOI] [PubMed]
- Lazarov O, Morfini GA, Pigino G, Gadadhar A, Chen X, Robinson J, Ho H, Brady ST, Sisodia SS (2007) Impairments in fast axonal transport and motor neuron deficits in transgenic mice expressing familial Alzheimer’s disease-linked mutant presenilin 1. J Neurosci 27:7011–7020 [DOI] [PMC free article] [PubMed]
- Levitan D, Doyle TG, Brousseau D, Lee MK, Thinakaran G, Slunt HH, Sisodia SS, Greenwald I (1996) Assessment of normal and mutant human presenilin function in Caenorhabditis elegans. Proc Natl Acad Sci USA 93:14940–14944 [DOI] [PMC free article] [PubMed]
- Marambaud P, Wen PH, Dutt A, Shioi J, Takashima A, Siman R, Robakis NK (2003) A CBP binding transcriptional repressor produced by the PS1/ε-cleavage of N-cadherin is inhibited by PS1 FAD mutations. Cell 114:635–645 [DOI] [PubMed]
- Mathe E, Olivier M, Kato S, Ishioka C, Hainaut P, Tavtigian SV (2006) Computational approaches for predicting the biological effect of p53 missense mutations: a comparison of three sequence analysis based methods. Nucleic Acids Res 34:1317–1325 [DOI] [PMC free article] [PubMed]
- Nakaya Y, Yamane T, Shiraishi H, Wang H-Q, Matsubara E, Sato T, Dolios G, Wang R, De Strooper B, Shoji M, Komano H, Nishimura M (2005) Random mutagenesis of presenilin-1 identifies novel mutants exclusively generating long amyloid β–peptides. J Biol Chem 280:19070–19077 [DOI] [PubMed]
- Ng PC, Henikoff S (2006) Predicting the effects of amino acid substitutions on protein function. Annu Rev Genomics Hum Genet 7:61–80 [DOI] [PubMed]
- Pigino G, Morfini G, Pelsman A, Mattson MP, Brady ST, Busciglio J (2003) Alzheimer’s presenilin 1 mutations impair kinesin-based axonal transport. J Neurosci 23:4499–4508 [DOI] [PMC free article] [PubMed]
- Price DL, Sisodia SS (1998) Mutant genes in familial Alzheimer’s disease and transgenic models. Ann Rev Neurosci 21:479–505 [DOI] [PubMed]
- Qi-Takahara Y, Morishima-Kawashima M, Tanimura Y, Dolios G, Hirotani N, Horikoshi Y, Kametani F, Maeda M, Saido TC, Wang R, Ihara Y (2005) Longer forms of β amyloid protein: implications for the mechanism of intramembrane cleavage by γ-secretase. J Neurosci 25:436–445 [DOI] [PMC free article] [PubMed]
- Rüdiger S, Freund SMV, Veprintsev DB, Fersht AR (2002) CRINEPT-TROSY NMR reveals p53 core domain bound in an unfolded form to the chaperone Hsp90. Proc Natl Acad Sci USA 99:11085–11090 [DOI] [PMC free article] [PubMed]
- Sato T, Dohmae N, Qi Y, Kakuda N, Misonou H, Mitsumori R, Maruyama H, Koo EH, Haass C, Takio K, Morishima-Kawashima M, Ishiura S, Ihara Y (2003) Potential link between amyloid β-protein 42 and C-terminal fragment γ 49–99 of β-amyloid precursor protein. J Biol Chem 278:24294–24301 [DOI] [PubMed]
- Selkoe DJ, Wolfe MS (2007) Presenilin: running with scissors in the membrane. Cell 131:215–221 [DOI] [PubMed]
- Shioi J, Georgakopoulos A, Mehta P, Kouchi Z, Litterst CM, Baki L, Robakis NK (2007) FAD mutants unable to increase neurotoxic Aβ 42 suggest that mutation effects on neurodegeneration may be independent of effects on Aβ. J Neurochem 101:674–681 [DOI] [PubMed]
- Song W, Nadeau P, Yuan M, Yang X, Shen J, Yankner BA (1999) Proteolytic release and nuclear translocation of Notch-1 are induced by preseniln-1 and impaired by pathogenic presenilin-1 mutations. Proc Natl Acad Sci USA 96:6959–6963 [DOI] [PMC free article] [PubMed]
- Soriano S, Kang DE, Fu Maofu, Pestell R, Chevallier N, Koo EH (2001) Presenilin 1 negatively regulates β-catenin/T cell factor/lymphoid enhancer factor-1 signaling independently of β-amyloid precursor protein and Notch processing. J Cell Biol 152:785–794 [DOI] [PMC free article] [PubMed]
- Soussi T, Wiman KG (2007) Shaping genetic alterations in human cancer: the p53 mutation paradigm. Cancer Cell 12:303–312 [DOI] [PubMed]
- Sundaram M, Greenwald I (1993) Genetic and phenotypic studies of hypomorphic lin-12 mutants in Casenorhabditis elegans. Genetics 135:755–763 [DOI] [PMC free article] [PubMed]
- Theuns J, Del-Favero J, Dermaut B, van Duijn CM, Backhovens H, Van den Broeck M, Serneels S, Corsmit E, Van Broeckhoven C, Cruts M (2000) Genetic variability in the regulatory region of presenilin 1 associated with risk for Alzheimer’s disease and variable expression. Hum Mol Genet 9:325–331 [DOI] [PubMed]
- Theuns J, Remacle J, Killick R, Corsmit E, Vennekens K, Huylebroeck D, Cruts M, Van Broeckhoven C (2003) Alzheimer-associated C allele of the promoter polymorphism −22C>T causes a critical neuron-specific decrease of presenilin 1. Hum Mol Genet 12:869–877 [DOI] [PubMed]
- Thomas PD, Campbell MJ, Kejariwal A, Mi H, Karlak B, Daverman R, Diemer K, Muruganujan A, Narechania A (2003) PANTHER: a library of protein families and subfamilies indexed by function. Genome Res 13:2129–2141 [DOI] [PMC free article] [PubMed]
- Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680 [DOI] [PMC free article] [PubMed]
- Tu H, Nelson O, Bezprozvanny A, Wang Z, Lee S-F, Hao Y-H, Serneels L, De Strooper B, Yu G, Bezprozvanny I (2006) Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer’s disease-linked mutations. Cell 126:981–993 [DOI] [PMC free article] [PubMed]
- Tysoe C, Whittaker J, Xuereb J, Cairns NJ, Cruts M, Van Broeckhoven C, Wilcock G, Rubinsztein DC (1998) A presenilin-1 truncating mutation is present in two cases with autopsy-confirmed early-onset Alzheimer’s disease. Am J Hum Genet 62:70–76 [DOI] [PMC free article] [PubMed]
- Vitkup D, Sander C, Church GM (2003) The amino-acid mutational spectrum of human genetic disease. Genome Biol 4:R72.1–R72.10 [DOI] [PMC free article] [PubMed]
- Walsh DM, Selkoe DJ (2004) Deciphering the molecular basis of memory failure in Alzheimer’s disease. Neuron 44:181–193 [DOI] [PubMed]
- Weggen S, Rogers M, Eriksen J (2007) NSAIDs: small molecules for prevention of Alzheimer’s disease or precursors for future drug development? Trends Pharmacol Sci 28:536–543 [DOI] [PubMed]
- Weihofen A, Binns K, Lemberg MK, Ashman K, Martoglio B (2002) Identification of signal peptide peptidase, a presenilin-type aspartic protease. Science 296:2215–2218 [DOI] [PubMed]
- Welch WJ, Brown CR (1996) Influence of molecular and chemical chaperones on protein folding. Cell Stress Chaperones 1:109–115 [DOI] [PMC free article] [PubMed]
- Wolfe MS (2007) When loss is gain: reduced presenilin proteolytic function leads to increased Aβ40/Aβ42. EMBO Rep 8:136–140 [DOI] [PMC free article] [PubMed]
- Wolfe MS, Xia W, Ostaszewski BL, Diehl TS, Kimberly WT, Selkoe DJ (1999) Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and γ-secretase activity. Nature 398:513–517 [DOI] [PubMed]
- Yoo AS, Cheng I, Chung S, Grenfell TZ, Lee H, Pack-Chung E, Handler M, Shen J, Xia W, Tesco G, Saunders AJ, Ding K, Frosch MP, Tanzi RE, Kim TW (2000) Presenilin-mediated modulation of capacitative calcium entry. Neuron 27:561–571 [DOI] [PubMed]
- Zhao B, Yu M, Neitzel M, Marugg J, Jagodzinski J, Lee M, Hu K, Schenk D, Yednock T, Basi G (2008) Identification of γ-secretase inhibitor potency determinants on presenilin. J Biol Chem 283:2927–2983 [DOI] [PubMed]
- Zhou S, Zhou H, Walian PJ, Jap BK (2005) CD147 is a regulatory subunit of the γ-secretase complex in Alzheimer’s disease amyloid β-peptide production. Proc Natl Acad Sci USA 102:7499–7504 [DOI] [PMC free article] [PubMed]