Abstract
Heat shock proteins (HSPs) are the best-known endogenous factors that protect against cell injury under various pathological conditions and that can be induced by various physical, chemical, and biological stressors. New research seeks to discover a compound that is clinically safe and can induce the accumulation of HSPs in patients. This paper reports that the oral administration of three doses of bicyclol, a novel antihepatitis drug, induced hepatic HSP27 and HSP70 expression in a time- and dose-dependent manner, and that bicyclol treatment stimulated heat shock factor 1 (HSF1) activation in mice. The inducing effects of bicyclol on HSP27, HSP70 and HSF1 were all blocked by quercetin, an inhibitor of HSP biosynthesis. The cytoprotective effect of HSP27/70 induced by bicyclol against hepatotoxicity of acetaminophen (AP) was assessed in mice. The prior administration of bicyclol markedly suppressed AP-induced liver injury as indicated by the reduction in the elevation of serum alanine aminotransferase and aspartate aminotransferase, in liver necrosis, in the release of cytochrome c and apoptosis-inducing factor from mitochondria, as well as in hepatic deoxyribonucleic acid fragmentation in mice. However, all the above actions of bicyclol against AP-induced mouse liver injuries were significantly attenuated by quercetin. This is the first report to show that bicyclol induces hepatic HSP27/70 expression via activation of HSF1 and that the cytoprotective action of bicyclol against liver injury is mediated by its induction of HSP27/70. These results provide new evidence for elucidating the mechanism of the hepatoprotective action of bicyclol in animals and patients.
Keywords: Acetaminophen, Bicyclol, Heat shock factor-1, Heat shock protein, Liver injury, Quercetin
Introduction
Heat shock proteins (HSPs) belong to a highly diverse and conserved family of proteins containing both constitutive and inducible members. HSPs are the major regulatory proteins in cells and have vital functions, such as maintenance of the cell cycle (Helmbrecht et al. 2000). Both in vivo and in vitro studies have shown that various stressors transiently induce the production of HSPs to protect against harmful insults (Kultz 2005). The presence of sublethal stressful stimuli induces production of HSPs, which can protect cells from injury upon exposure to subsequent lethal insults. This suggests that the increase in expression of HSPs constitutes an important cellular defense mechanism. The cytoprotective effects of HSPs are most often attributed to their ability to bind nonnative proteins and chaperone their refolding or elimination (Bukau and Horwich 1998). In the cells undergoing chemical or physical protein denaturation or adduction (i.e., proteotoxicity), HSPs are believed to enhance the solubility of denatured proteins by preventing the aggregation of exposed hydrophobic areas (Beck and De Maio 1994).
Some chemicals, such as geranylgeranylacetone (Hirakawa et al. 1996), bimoclomol (Vigh et al. 1997), herbimycin-A (Morris et al. 1996), rebamipide (Hahm et al. 1997), and carbenoxolone (Nagayama et al. 2001), were reported to have a cytoprotective effect through the stimulation of HSPs inductions, suggesting that it may be of potential therapeutic benefit to discover compounds that safely and selectively induce HSPs. Bicyclol, formulated as 4,4′-dimethoxy-5,6,5′,6′-bis(methylene-dioxy)-2-hydroxy-methyl-2′-methoxycarbonyl biphenyl, is a novel antihepatitis drug and has been approved to treat chronic viral hepatitis in China since 2004. The drug has significantly reduced elevated serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels and has also inhibited hepatitis virus B replication to a certain degree in patients suffering from chronic viral hepatitis B (Yao et al. 2002). No noticeable side effects have been observed. Pharmacologically, bicyclol has a protective action against experimental liver injury. For example, bicyclol reduced liver necrosis induced by CCl4 (Liu et al. 2005) and by acetaminophen (AP; Li et al. 2001), as well as by nuclear deoxyribonucleic acid (DNA) damage and apoptosis induced by concanavalin A in mice (Zhao and Liu 2001). Bicyclol also inhibited hepatitis virus replication in duck viral hepatitis and in a HepG2.2.15 cell line (Liu 2001; Yao et al. 2002). Since HSP is an endogenous defense mechanism in protecting against cell injury under various pathological conditions, we hypothesized that the hepatoprotective effect of bicyclol might result from the induction of hepatic HSPs. To test the hypothesis, we first investigated the effect of bicyclol on hepatic HSPs expression in mice and then studied the protective action of HSP27/70 induced by bicyclol against the hepatotoxicity of AP in mice. Our results indicated that bicyclol significantly induced liver HSP27/70 expression via activation of heat shock factor 1 (HSF1) and that the hepatoprotective action of bicyclol is mediated by its induction of HSP27/70 expression.
Materials and methods
Animals
Male Kunming mice weighing 20–22 g were supplied by the Animal Center of the Chinese Academy of Medical Sciences. Mice were maintained in a 12-h light/dark cycle at 24°C in a relative humidity of 60% and received food and water ad libitum. All experimental procedures were performed in accordance with the guidelines of the Beijing Municipal Ethic Committee for the Care and Use of Laboratory Animals.
Treatment of animals
Bicyclol, a white powder with 99% purity, was kindly provided by Beijing Union Pharmaceutical Manufactory, China. It was dissolved in polyethylene glycol 400 (PEG400) and administered orally to mice. Bicyclol 200 mg/kg was used in the time course study of the inducing effect on HSPs. Bicyclol 100, 200, and 300 mg/kg were also administered to mice in the dose–effect relationship study. The protocol for bicyclol administration was 8:00 a.m. and 6:00 p.m. on the first day and 8:00 a.m. on the second day of the experiment. The mice were killed by decapitation at the indicated time after the last dose of bicyclol. The control mice received a corresponding volume of PEG400 only. Quercetin (Sigma, St. Louis, MO, USA) was suspended in physiologic saline and administered orally at a dosage of 200 mg/kg in association with bicyclol. The dose volume of each compound and vehicle was 10 ml/kg.
AP (Sigma) was dissolved in physiologic saline, and the dose volume was 20 ml/kg. AP 200 mg/kg was intraperitoneally injected 4 h after the last administrations of bicyclol 300 mg/kg and quercetin 200 mg/kg. The control mice received the corresponding volume of physiologic saline only. Ten hours later, the mice were killed for different determinations.
RT-PCR assay
Total ribonucleic acid (RNA) was isolated from liver tissue using Trizol reagent (Invitrogen, Carlsbad, CA, USA) following the protocol recommended by the manufacturer. Reverse transcriptase (RT) polymerase chain reaction (PCR) was performed using a One-Step RT-PCR Kit (Promega, Madison, WI, USA). The reaction system contained avian myeloblastosis virus (AMV)/Tfl reaction buffer 10 μl, deoxy-ribonucleoside triphosphate 0.2 mM, 1 μM of each primer, 1 mM MgSO4, 0.1 U/μl AMV reverse transcriptase and Tfl DNA polymerase, and 2 μg RNA template. The reaction was heated at 45°C for 45 min for reverse transcription and 94°C for 2 min for AMV RT inactivation and RNA/complementary DNA/primer denaturation. Forty cycles were then performed. The denaturation, annealing, and extension steps of HSP27 were 94°C for 30 s, 55°C for 1 min, and 68°C for 2 min. The final extension was at 68°C for 7 min. The same steps were performed for HSP70 with the exception of 59°C for 1 min for annealing. The following primers used in the PCR reactions were synthesized by Shanghai Sangon Biological Engineering Technology and Services (Shanghai, China): HSP27-forward 5′-CCCACCCTCTATCACGGCTAC-3′ and reverse 5′-GGGCTCAACTCTGGCTATCTC-3′, which amplified a 426-bp product, and HSP70-forward 5′-GCGACCTGAACAAGAGCATC-3′ and reverse 5′-GAGCTTGCCCTTGAGACCC-3′, which amplified a 617-bp product. Amplified products were separated on 1% agarose gels in TBE buffer (45 mM Tris borate, 1 mM ethylenediamine tetraacetic acid [EDTA]). RT-PCR bands were photographed with a Kodak Gel Logic 100 Imaging System (Life Technologies, Eastman Kodak, New Haven, CT, USA), and the densities of the bands were determined using Gel-Pro Analyzer 4.0 software.
Western blot analysis
Mouse liver was homogenized in nondenaturing lysis buffer (Applygen Technologies, Beijing, China). Thirty-microgram sample proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis in a 10% polyacrylamide gel and transferred to a polyvinylidene difluoride membrane. The membranes were blocked in 5% skim milk–TBST (20 mM Tris–HCl, pH 7.5, 500 mM NaCl, 0.1% Tween 20) at 4°C overnight. The blots were probed with goat anti-HSP27 (sc-1048, Santa Cruz, CA, USA), mouse anti-inducible and constitutive HSP70 (sc-24, Santa Cruz), rabbit anti-HSP90 (#4875, Cell Signaling, Danvers, MA, USA), rat anti-heat shock factor (HSF1; #RT-407-P0, NeoMarkers, Fremont, CA, USA), mouse anti-cytochrome c (sc-13156, Santa Cruz) and rabbit anti-apoptosis-inducing factor (AIF; sc-5586, Santa Cruz) antibodies in 5% skim milk-TBS-T for 2 h at room temperature and then incubated with horseradish peroxidase-conjugated secondary antibody in skim milk–TBST for 2 h at room temperature. The blot was developed with LAS3000 chemiluminescence system (Fujifilm, Tokyo, Japan), and the densities of the bands were determined using Gel-Pro Analyzer 4.0 software.
Electrophoresis mobility shift assay
The liver nuclear extracts for electrophoresis mobility shift assay (EMSA) were prepared using a nuclear-cytosol extraction kit (Applygen Technologies). Annealed double-stranded heat shock element (HSE) oligonucleotide-321 to oligonucleotide-350 of mouse HSP70 gene (5′-AGA CGC GAA ACT GCT GGA AGA TTC CGT GCC-3′) labeled with biotin was synthesized by IDT (Newark, NJ, USA). An EMSA Kit (Pierce, Rockford, IL, USA) was used to perform the reaction. The binding reaction consists of a 20-μl total volume of 10-μg protein extracts, 20 fmol biotin-labeled DAN, 2.5% glycerol, 5 mM MgCl2, and 50 ng/μl Poly (dI·dC), which was incubated for 20 min at room temperature. DNA–protein complexes were resolved by electrophoresis on 6% polyacrylamide gels at 4°C in 0.5 × TBE buffer (45 mM Tris borate, 1 mM EDTA) and transferred to a nylon membrane. The membrane was then detected with an enhanced LAS3000 chemiluminescence system.
ALT/AST determination
The serum activities of ALT and AST were determined using commercial kits (Beijing BHKT Clinical Reagent).
Liver histopathology
For histopathological examination, livers were fixed in 10% formalin, embedded in paraffin, and sectioned and stained with hematoxylin and eosin. The specimen was observed under an NIKON E600 digital camera-equipped microscope and was analyzed using image Pro-Plus 7200 software.
DNA fragmentation analysis
The hepatic DNA fragmentation in mice was detected using a DNA ladder detection kit (Applygen Technologies) according to recommended procedures. One hundred milligrams of liver tissue was lysed with Tris–EDTA lysis buffer, and DNA was extracted. Two micrograms of DNA were loaded on 1% agarose gel and run at 50 V for 2 h in 0.5× TBE buffer with 0.5 g/ml ethidium bromide. The bands were photographed with a Kodak Gel Logic 100 Imaging System.
Statistical analysis
Data were expressed as means ± SD. Changes in different assays were analyzed by analysis of variance followed by the Tukey–Kramer test as the post-hoc test. P < 0.05 was considered to be statistically significant.
Results
Time course of bicyclol on the inductions of hepatic HSPs in mice
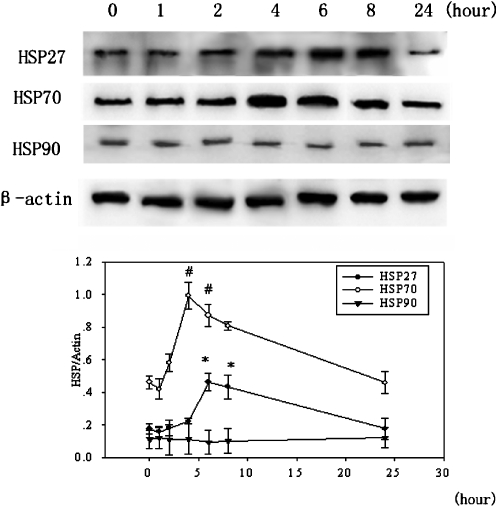
In the study of a time course of the effect of bicyclol on accumulations of hepatic HSP27, HSP70 and HSP 90, three doses of bicyclol 200 mg/kg were administered to mice. As a result, both HSP27 and HSP70 levels increased significantly. The level of HSP27 increased about 2.6-fold within 6 h and remained higher than that of the control at 8 h, then declined to a normal level at 24 h. The expression of HSP70 began to increase within 2 h, reached a maximum level (about 2.1-fold) at 4 h, remained at the higher level for 2 h, and then gradually decreased to the control level at 24 h after the last administration of bicyclol. However, bicyclol did not induce HSP90 accumulation, and only constitute expression was observed at the time point indicated above (Fig. 1).
Fig. 1.
Time course of bicyclol on the induction of hepatic HSPs in mice. Bicyclol 200 mg/kg was administered orally to mice three times in 24 h as described in the “Materials and methods.” The mice were killed at the indicated times after the last administration. Whole liver proteins from different groups of mice were subjected to Western blot analysis. The inductive action of bicyclol on HSP27/70 was in a time-dependent manner, while bicyclol had no effect on HSP90. Each HSP level was quantified by densitometric analysis. A representative of Western blots from each group is shown. Mean ± SD results of three separate experiments are graphed. Asterisk, number sign, P < 0.05 versus 0 h control mice
Dose–effect relationship of bicyclol on hepatic HSP27 and HSP70 expression in mice
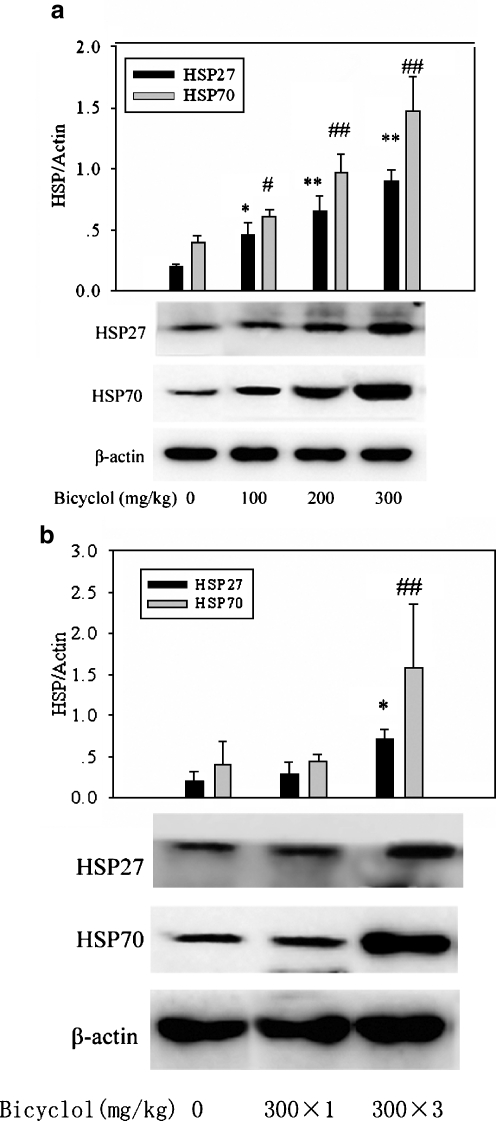
Oral administration of bicyclol 100, 200, and 300 mg/kg all significantly induced the accumulations of HSP27 and HSP70 in mouse liver, and the inducing effect of bicyclol was in a good dose-dependent manner. The effect of bicyclol 300 mg/kg on HSP27 and HSP70 accumulations was more potent than those of 100 and 200 mg/kg (Fig. 2a).
Fig. 2.
Dose–effect relationship of bicyclol on hepatic HSP27 and HSP70 expression in mice. a Western blot analysis for HSP27/70 induced by bicyclol 100, 200, and 300 mg/kg administered three times. The inductive action of bicyclol 300 mg/kg was more potent than those of 100 and 200 mg/kg. Mean ± SD results of three to four separate experiments are graphed. Asterisk, number sign, P < 0.05; double asterisk, double number sign, P < 0.01 versus control mice. b Western blot analysis for HSP27/70 induced by three doses and one dose of bicyclol. Densitometric analysis showed that three doses of bicyclol 300 mg/kg induced overexpression of HSP27/70 but that one dose did not. Mean ± SD results of four separate experiments are graphed. Asterisk, P < 0.05; double number sign, P < 0.01 versus control mice
As a result of these findings, we further compared the effect of one dose and three doses of bicyclol 300 mg/kg on HSP27 and HSP70 expression. As shown in Fig. 2b, three doses of bicyclol 300 mg/kg significantly upregulated HSP27 and HSP70 expression, while a single dose of bicyclol 300 mg/kg did not significantly induce expression of either HSP27 or HSP70 (Fig. 2b).
Inhibitory effects of quercetin on hepatic HSP27 and HSP70 induced by bicyclol
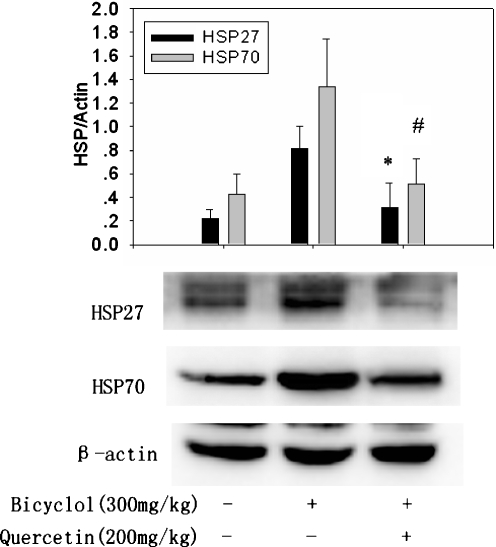
Quercetin, a flavanoid compound, is a known inhibitor of HSP biosynthesis by inhibiting HSF1 activation. In the present study, quercetin was used as a tool to determine whether it could reverse the inducing effect of bicyclol on HSP27 and HSP70 expression. Simultaneous administration of quercetin 200 mg/kg significantly attenuated the expression of HSP27 and HSP70 induced by bicyclol 300 mg/kg, suggesting that bicyclol efficiently induced the synthesis of specific HSPs (Fig. 3).
Fig. 3.
Inhibitory effect of quercetin on the inductions of hepatic HSP27 and HSP70 by bicyclol in mice. Quercetin 200 mg/kg was coadministrated with bicyclol 300 mg/kg to mice. The mice were killed 6 h after the last bicyclol administration. Whole liver proteins were extracted for the Western blot assay. A representative Western blot from each group is shown. Mean ± SD results of three separate experiments are graphed. Asterisk, number sign P < 0.05 versus bicyclol (300 mg/kg)-treated mice
Upregulation of bicyclol on hepatic HSP27 and HSP70 mRNA expression in mice
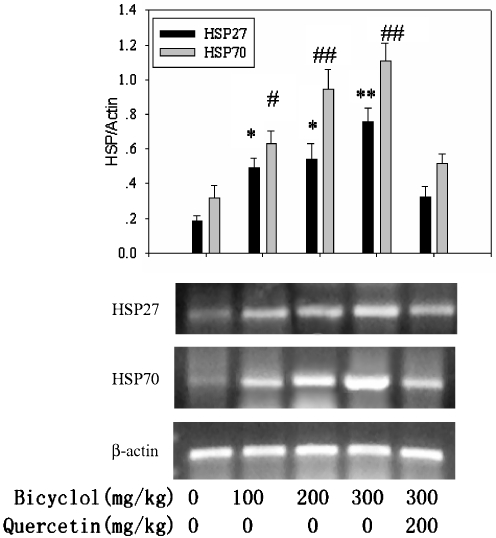
RT-PCR analysis showed that bicyclol upregulated both HSP27 and HSP70 messenger RNA (mRNA) expressions. The expression of HSP27 and HSP70 mRNA induced by bicyclol 300 mg/kg were highly significant. The accumulations of HSP27 and HSP70 were associated with the pronounced expression of mRNA, suggesting that bicyclol induced expression of mouse liver HSP27 and HSP70 by upregulating the transcription of HSP27 and HSP70 genes. This inductive effect of bicyclol on HSP27 and HSP70 mRNA expression was also attenuated by the simultaneous administration of quercetin (Fig. 4).
Fig. 4.
Effect of bicyclol on the hepatic HSP27 and HSP70 mRNA expression in mice. Mice were administered bicyclol 100, 200, and 300 mg/kg, respectively, for three times in 24 h. Whole liver RNA was extracted for RT-PCR assay (2 μg per lane). The inductive action of bicyclol was in a dose-dependent manner. Mean ± SD results of triplicate experiments are graphed. Asterisk, number sign, P < 0.05; double asterisk, double number sign, P < 0.01 versus control mice, respectively
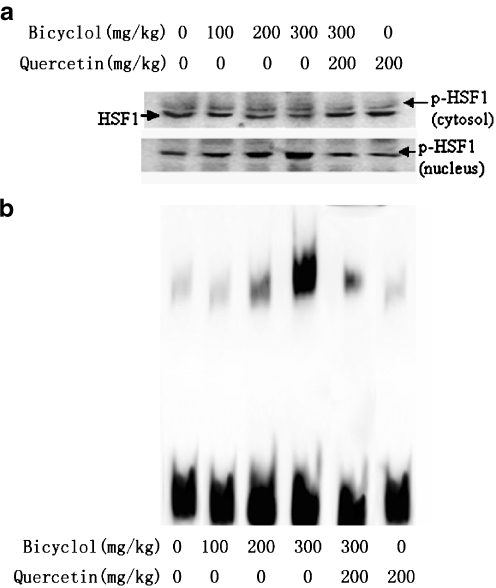
Effect of bicyclol on HSF1 localization and activation
HSF1 mainly presents in the cytosol of resting cells, and its phosphorylation is used as a good indicator of cells under stressful conditions. The phosphorylation of HSF1 was detected as an upward band shift by Western blot analysis. As shown in Fig. 5a, when mice were treated with bicyclol 100, 200, and 300 mg/kg, the cytosol HSF1 level gradually decreased, and concomitantly, the nucleus HSF1 (indicated as p-HSF1) increased. The effect of bicyclol on HSF1 showed a good dose–effect relationship. The specific DNA–protein complexes were detected by gel mobility shift assay using the biotin-labeled oligonucleotide. The treatment of bicyclol produced an HSE-binding activity in 6 h. This activity was confirmed to be an HSF1–HSE complex (Fig. 5b). This effect of bicyclol was also in a dose-dependent manner. Simultaneous administration of quercetin attenuated the effects of bicyclol on nuclear HSF1 and its DNA binding activity.
Fig. 5.
Effect of bicyclol on the activation and nuclear localization of HSF1 in mouse liver. Mice were orally administered bicyclol 100, 200, and 300 mg/kg three times in 24 h. The mice were killed 6 h after the last administration of bicyclol. a Cytosol and nucleus expression of HSF1 and p-HSF1 in mouse liver; a representative of three results is shown. b HSF1-binding activity from whole liver extracts. A representative from three results is shown
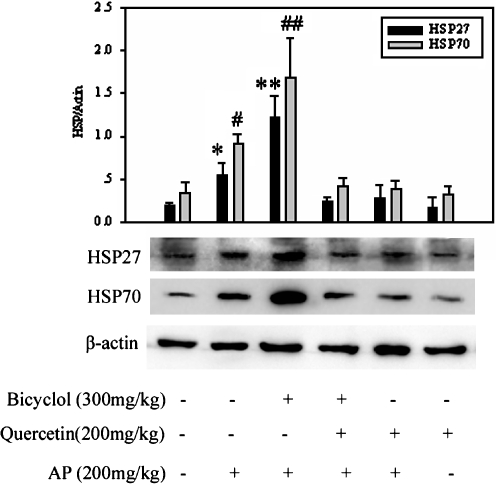
Effect of bicyclol on hepatic HSP27 and HSP70 inductions in AP-treated mice
As shown in Fig. 6, the injection of AP alone induced accumulations of HSP27 and HSP70 in mouse liver, while pretreatment of bicyclol 300 mg/kg before injection of AP caused overexpression of HSP27 and HSP70 at 6 h. The levels of HSP27 and HSP70 induced by bicyclol were double compared with that of AP treated alone. Simultaneous administration of quercetin blocked AP-induced and bicyclol plus AP-induced HSP27 and HSP70 accumulations (Fig. 6).
Fig. 6.
Effect of bicyclol on the inductions of HSP27 and HSP70 in AP-injected mice. Mice were orally administered bicyclol 300 mg/kg three times in 24 h. They were then subjected to AP 200 mg/kg injection 4 h later and then killed 6 h after the AP injection. Whole liver proteins were extracted for the Western blot assay. A representative Western blot for each group is shown. Mean ± SD results of triplicate experiments are graphed. A representative result is shown. Asterisk, number sign, P < 0.05 versus control mice and AP + bicyclol + quercetin-treated mice; double asterisk, double number sign, P < 0.01 versus control mice and AP + bicyclol + quercetin-treated mice
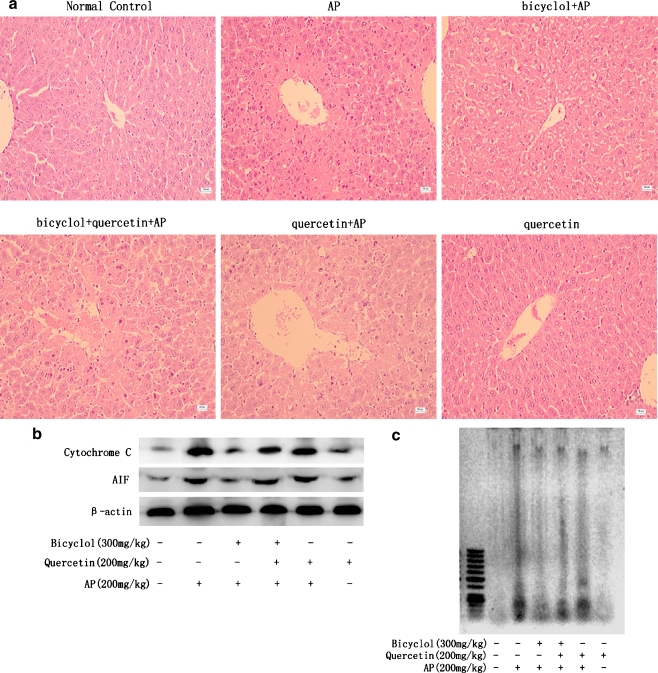
Quercetin attenuated the protective action of bicyclol against AP-induced liver injury in mice
To confirm whether the induction of HSP27 and HSP70 by bicyclol plays a critical role in protecting against AP-induced hepatotoxicity, the HSPs inhibitor quercetin was administered in association with bicyclol in AP-injected mice. Pretreatment of bicyclol 300 mg/kg alone significantly decreased the elevated serum ALT/AST (Table 1) and hepatocyte necrosis (Fig. 7a). Bicyclol also inhibited the leakage of cytochrome c and AIF from mitochondria (Fig. 7b) and inhibited nuclear DNA fragmentation (Fig. 7c) induced by AP in mice. Coadministration of quercetin 200 mg/kg with bicyclol 300 mg/kg before AP injection markedly attenuated the protective effect of bicyclol on serum ALT/AST levels (Table 1), liver necrosis (Fig. 7a), the release of cytochrome c and AIF from damaged mitochondria (Fig. 7b), and hepatic DNA fragmentation (Fig. 7c) in mice. Quercetin itself showed no effect on the above biomarkers both in normal and AP-treated mice.
Table 1.
Protection by bicyclol against AP-induced liver injury was reversed by quercetin as determined by ALT/ AST (U/l)
| ALT | AST | |
|---|---|---|
| Normal control | 35.3 ± 11.0 | 37.0 ± 17.7 |
| AP | 188.2 ± 50.8 | 145.1 ± 23.2 |
| Bicyclol + AP | 73.7 ± 12.4*** | 60.3 ± 17.3### |
| Bicyclol + quercetin + AP | 163.6 ± 42.5 | 135.2 ± 32.5 |
| Quercetin + AP | 152.4 ± 45.2 | 129.3 ± 19.2 |
| Quercetin | 38.0 ± 7.61 | 35.7 ± 10.4 |
Values represent mean ± SD, n = 7 in each group.
***P < 0.001 versus AP group and bicyclol + quercetin + AP group
###P < 0.001 versus AP group and bicyclol + quercetin + AP group
Fig. 7.
Quercetin attenuated the protective action of bicyclol against liver injury induced by AP in mice. Mice were orally administered bicyclol 300 mg/kg three times in 24 h. They were then subjected to AP 200 mg/kg injection 4 h after the last dosing of bicyclol. Serum and liver tissue were obtained 6 h after AP injection for different determinations. a Liver lesions (hematoxylin–eosin stain, scale bar = 50 μm). b Western blot of cytochrome c and AIF leakage from mitochondia performed from mitochondrial extracts. A representative result is shown. c Representative hepatic DNA fragmentation from each animal of one experiment
Discussion
HSPs play important roles in the complex defense mechanism to increase survival under adverse environmental stress as well as in normal cellular homeostasis (Kregel 2002). In the present study, bicyclol was shown to induce hepatic HSP27 and HSP70 accumulations in mice. The synthesis of HSPs can be regulated both at the transcriptional and posttranscriptional levels. In addition, one of the first cellular responses to stress may be an increase in HSP mRNA “half-life” (Knowlton 1995). As to bicyclol, its upregulation of HSP27 and HSP70 synthesis occurred at the transcriptional level as indicated by increases in HSP27 and HSP70 mRNA expression in mouse liver.
The synthesis of HSPs is tightly regulated at the transcriptional level by HSF1. The activation of HSF1 is associated with its oligomerization, its nuclear localization, and its acquisition of binding activity to HSE in the heat shock gene promoter to exert a transcriptional activation (Santoro 2000; Han et al. 2001). The HSE consists of a contiguous array of alternately oriented pentanucleotide 5′-nGAAn-3′ units (Perisic et al. 1989). To explain the mechanism of HSP27 and HSP70 inductions by bicyclol, we examined the effect of bicyclol on the activation HSF1. The gel mobility shift assay showed that bicyclol produced an HSE sequence-specific complex. The nuclear translocation and phosphorylation of HSF1 were accelerated by bicyclol treatment in mice, which resulted in a marked upregulation of the HSE-binding activity. The HSE-binding activity of HSF1 coincided with the expression of HSP27 and HSP70 mRNA in bicyclol-treated mice. These results suggest that bicyclol activates HSF1, which in turn induces HSP27 and HSP70 gene expression. However, further studies are required to explain the molecular mechanism of the activation of HSF1.
Much of the interest in HSPs comes from numerous reports showing that inductions of HSPs correlate with the development of resistance to a variety of cytotoxic insults (Goossens et al. 1995; Baek et al. 2000). The cytoprotective role of HSP27 and HSP70 has been observed in a wide variety of human diseases, including ischemia, metabolic disorders, inflammation, and infection (Sreedhar and Csermely 2004). Recently, HSP27 and HSP70 have also been shown to have a critical involvement in protection against ischemia-induced injury (Kuboki et al. 2007; Matsuo et al. 2005) and against AP-induced hepatotoxicity (Nishida et al. 2006; Sumioka et al. 2004). Since HSPs occur and function endogenously, the upregulation of the function of HSPs via pharmacological mediators provides a potential for designing prophylactic and therapeutic drugs to treat diseases (Latchman 2003), such as inducing HSPs in the treatment of liver injury. An ideal drug with an HSP-inducing capability should activate the stress response by mimicking the effect of the stressor in the complete absence of stress or at a significantly lower threshold of stressful conditions without causing detrimental side effects on the targeted cell or tissue. Bicyclol has been widely used in China since 2004 in the treatment of chronic viral hepatitis. The protective effect of bicyclol against liver injury has been well documented in previous reports from our laboratory (Liu et al. 2005; Li et al. 2001). The present results indicate that the pretreatment of bicyclol significantly induced hepatic HSP27 and HSP70 accumulations and also decreased serum ALT/AST levels, liver necrosis, mitochondrial damage, and hepatic DNA fragmentation due to AP poisoning in mice. It was interesting to ascertain whether the induction of HSP27 and HSP70 by bicyclol is responsible for its cytoprotective action against liver injury. If the HSP27 and HSP70 induced by bicyclol are indeed involved in its hepatoprotection, inhibiting the synthesis of HSP27 and HSP70 should result in the disappearance or weakening of the hepatoprotective activity of bicyclol. Quercetin, an inhibitor of HSPs biosynthesis (Masuda et al. 2003; Jakubowicz-Gil et al. 2002; Hosokawa et al. 1990), was therefore chosen as a tool in this study. As was expected, simultaneous administration of quercetin markedly attenuated all the protective effect of bicyclol on the AP-induced liver injury in mice mentioned above, suggesting that the hepatoprotective action of bicyclol is mediated by its induction of HSP27 and HSP70.
Bicyclol is thus a novel inducer of HSP27 and HSP70 biosynthesis, which provides new evidence for elucidating the mechanism of the cytoprotective effect of bicyclol against liver injury both in animals and in people.
Acknowledgements
This work was supported by grants from the Chinese Ministry of Science and Technology (96-901-01-45) and from the Chinese Medical Board in New York (93-582).
Abbreviations
- HSPs
heat shock proteins
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- AP
acetaminophen
- PEG-400
polyethylene glycol 400
- RT-PCR
reverse transcription-polymerase chain reaction
- dNTP
deoxy-ribonucleoside triphosphate
- TBE
Tris-borate buffer
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electropheresis
- PVDF
polyvinylidene difluoride
- HSF1
heat shock factor-1
- AIF
apoptosis-inducing factor
- EMSA
electrophoretic mobility shift assay
- HSE
heat shock element
- mRNA
messenger ribonucleic acid
References
- Baek SH, Min JN, Park EM, Han MY, Lee YS, Lee YJ, Park YM (2000) Role of small heat shock protein HSP25 in radioresistance and glutathione redox cycle. J Cell Physiol 183:100–107 [DOI] [PubMed]
- Beck SC, De Maio A (1994) Stabilization of protein synthesis in thermotolerant cells during heat shock. Association of heat shock protein-72 with ribosomal subunits of polysomes. J Biol Chem 269:21803–21811 [PubMed]
- Bukau B, Horwich AL (1998) The Hsp70 and Hsp60 chaperone machines. Cell 92:351–366 [DOI] [PubMed]
- Goossens V, Grooten J, De Vos K, Fiers W (1995) Direct evidence for tumor necrosis factor-induced mitochondrial reactive oxygen intermediates and their involvement in cytotoxicity. Proc Natl Acad Sci USA 92:8115–8119 [DOI] [PMC free article] [PubMed]
- Hahm KB, Park IS, Kim YS, Kim JH, Cho SW, Lee SI, Youn JK (1997) Role of rebamipide on induction of heat-shock proteins and protection against reactive oxygen metabolite-mediated cell damage in cultured gastric mucosal cells. Free Radic Biol Med 22:711–716 [DOI] [PubMed]
- Han SI, Oh SY, Woo SH, Kim KH, Kim JH, Kim HD, Kang HS (2001) Implication of a small GTPase Rac1 in the activation of c-Jun N-terminal kinase and heat shock factor in response to heat shock. J Biol Chem 276:1889–1895 [DOI] [PubMed]
- Helmbrecht K, Zeise E, Rensing L (2000) Chaperones in cell cycle regulation and mitogenic signal transduction: a review. Cell Prolif 33:341–365 [DOI] [PMC free article] [PubMed]
- Hirakawa T, Rokutan K, Nikawa T, Kishi K (1996) Geranylgeranylacetone induces heat shock proteins in cultured guinea pig gastric mucosal cells and rat gastric mucosa. Gastroenterology 111:345–357 [DOI] [PubMed]
- Hosokawa N, Hirayoshi K, Nakai A et al (1990) Flavonoids inhibit the expression of heat shock proteins. Cell Struct Funct 15:393–401 [DOI] [PubMed]
- Jakubowicz-Gil J, Rzymowska J, Paduch R, Gawron A (2002) The effect of quercetin on the expression of heat shock proteins and apoptosis induction in monkey kidney cell line GMK. Folia Histochem Cytobiol 40:137–138 [PubMed]
- Knowlton AA (1995) The role of heat shock proteins in the heart. J Mol Cell Cardiol 27:121–131 [DOI] [PubMed]
- Kregel KC (2002) heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J App Physiol 92:2177–2186 [DOI] [PubMed]
- Kuboki S, Schuster R, Blanchard J, Pritts TA, Wong HR, Lentsch AB (2007) Role of heat shock protein 70 in hepatic ischemia–reperfusion injury in mice. Am J Physiol Gastrointest Liver Physiol 292:G1141–1149 [DOI] [PubMed]
- Kultz D (2005) Molecular and evolutionary basis of the cellular stress response. Annu Rev Physiol 67:225–257 [DOI] [PubMed]
- Latchman DS (2003) Protective effect of heat shock proteins: potential for gene therapy. Gene Ther Mol Biol 7:245–254
- Li Y, Dai GW, Li Y, Liu GT (2001) Effect of bicyclol on acetaminophen-induced hepatotoxicity: energetic metabolism and mitochondrial injury in acetaminophen-intoxicated mice. Yao Xue Xue Bao 36:723–726 [PubMed]
- Liu GT (2001) The anti-virus and hepatoprotective effect of bicyclol and its mechanism of action. Chin J New Drugs 10:325–327
- Liu GT, Li Y, Wei HL, Zhang H, Xu JY, Yu LH (2005) Mechanism of protective action of bicyclol against CCl-induced liver injury in mice. Liver Int 25:872–879 [DOI] [PubMed]
- Masuda Y, Sumita S, Fujimura N, Namiki A (2003) Geranylgeranylacetone attenuates septic diaphragm dysfunction by induction of heat shock protein 70. Crit Care Med 31:2585–2591 [DOI] [PubMed]
- Matsuo K, Togo S, Sekido H et al (2005) Pharmacologic preconditioning effects: prostaglandin E1 induces heat-shock proteins immediately after ischemia/reperfusion of the mouse liver. J Gastrointest Surg 9:758–768 [DOI] [PubMed]
- Morris SD, Cumming DVE, Latchman DS, Yellon DM (1996) Specific induction of the 70-kD heat stress proteins by the tyrosine kinase inhibitor herbimycin-A protects rat neonatal cardiomyocytes. A new pharmacological route to stress protein expression? J Clin Investig 97:706–712 [DOI] [PMC free article] [PubMed]
- Nagayama S, Jono H, Suzaki H et al (2001) Carbenoxolone, a new inducer of heat shock protein 70. Life Sci 69:2867–2873 [DOI] [PubMed]
- Nishida T, Matsura T, Nakada J et al (2006) Geranylgeranylacetone protects against acetaminophen-induced hepatotoxicity by inducing heat shock protein 70. Toxicology 219:187–196 [DOI] [PubMed]
- Perisic O, Xiao H, Lis JT (1989) Stable binding of Drosophila heat shock factor to head-to head and tail-to tail repeats of a conserved 5bp recognition unit. Cell 59:767–806 [DOI] [PubMed]
- Santoro MG (2000) Heat shock factors and the control of the stress response. Biochem Pharmacol 59:55–63 [DOI] [PubMed]
- Sreedhar AS, Csermely P (2004) Heat shock proteins in the regulation of apoptosis: new strategies in tumor therapy: a comprehensive review. Pharmaco Ther 101:227–257 [DOI] [PubMed]
- Sumioka I, Matsura T, Kai M, Yamada K (2004) Potential roles of hepatic heat shock protein 25 and 70i in protection of mice against acetaminophen-induced liver injury. Life Sci 74:2551–2561 [DOI] [PubMed]
- Vigh L, Literati PN, Horvath I, Torok Z, Balogh G, Glatz A, Kovacs E, Boros I et al (1997) Bimoclomol: a nontoxic, hydroxylamine derivative with stress protein-inducing activity and cytoprotective effects. Nat Med 3:1150–1154 [DOI] [PubMed]
- Yao GB, Ji YY, Wang QH, Zhou XQ, Xu DZ, Chen XY, Zhang QB (2002) A randomized double-blind controlled trial of bicyclol in treatment of chronic hepatitis B. Chin J New Drugs Clin Rem 21:457–462
- Zhao DM, Liu GT (2001) Protective effect of bicyclol on concanavalin A induced liver nuclei DNA injury in mice. Natl Med J China 81:844–848 [PubMed]