Abstract
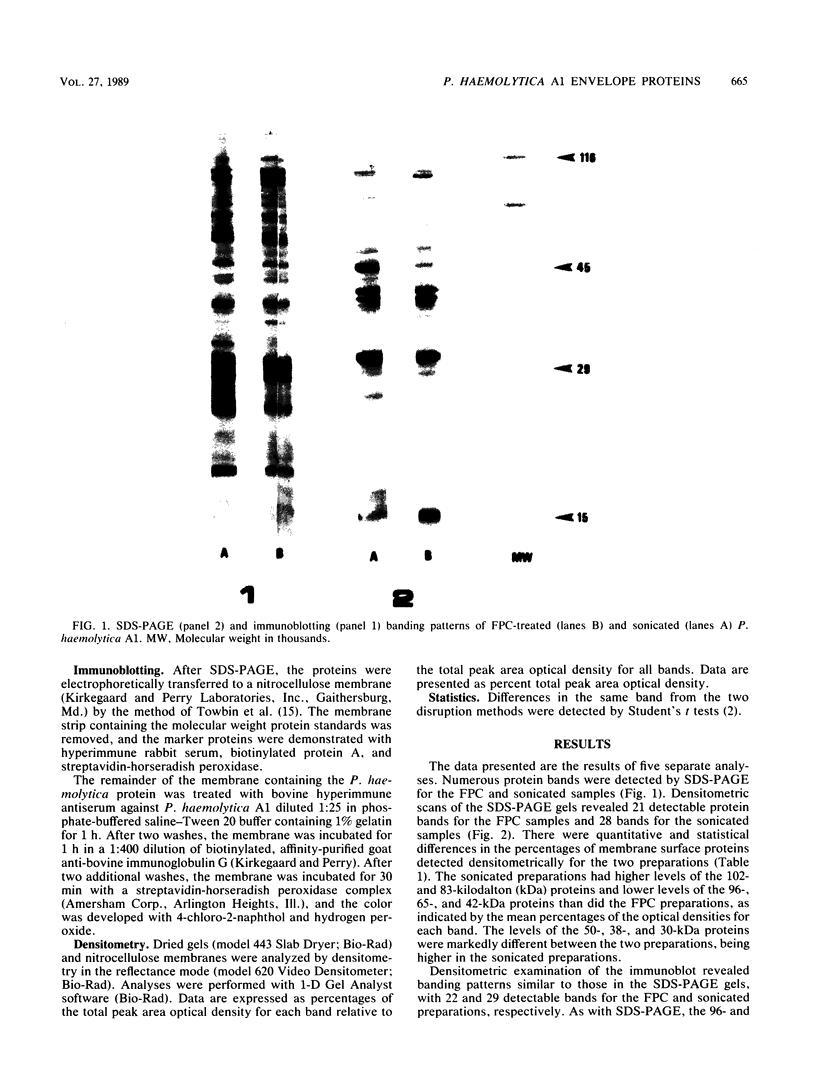
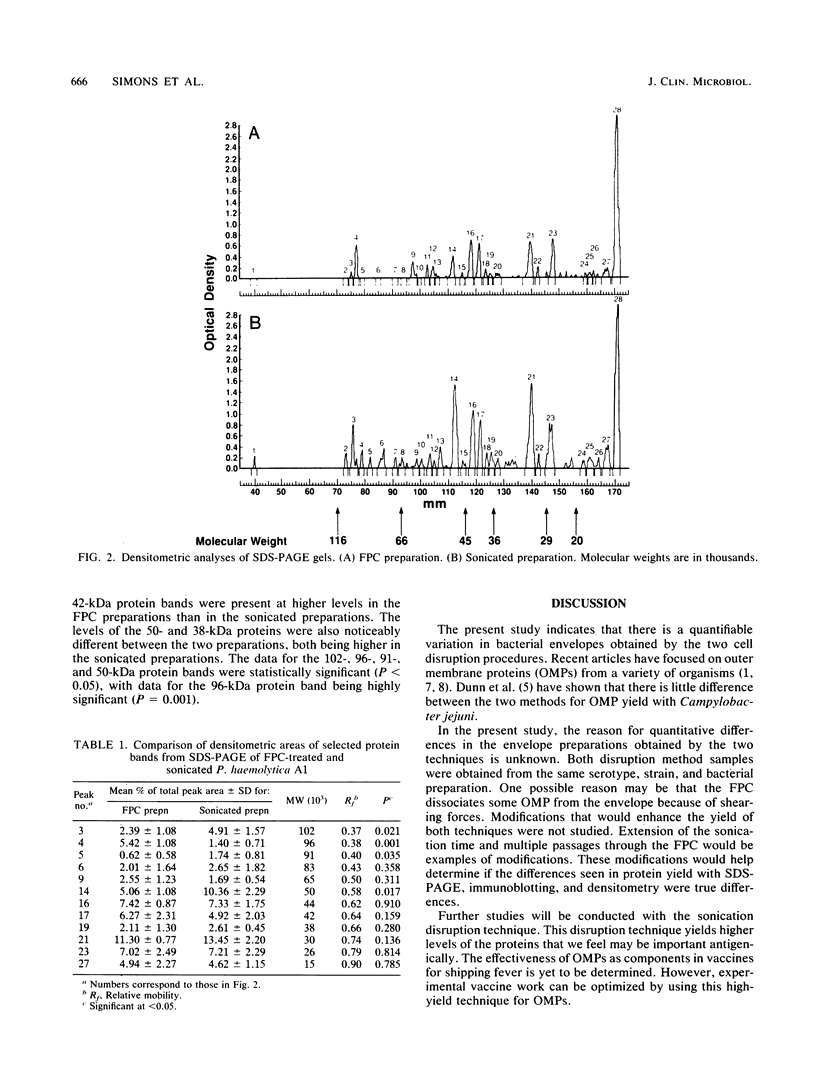
The French pressure cell and sonication methods of bacterial cell disruption were compared for the evaluation of surface proteins from Pasteurella haemolytica A1. Several protein bands were quantitatively different when compared by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, immunoblotting, and densitometry. With densitometry, sonicated preparations had higher concentrations of the 102-, 83-, 50-, 38-, and 30-kilodalton (kDa) proteins; French pressure cell preparations had higher concentrations of the 96-, 65-, and 42-kDa proteins. Qualitative differences between these two disruption methods were evident at the 102-, 96-, 91-, 50-, 38-, and 30-kDa protein bands. However, significant differences (P less than 0.05) were detected between the two methods at only the 102-, 96-, 91-, and 50-kDa bands.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Afzal M., Brodie S. J., Tengerdy R. P., Squire P. G. Isolation and antigenic reactivity of Brucella ovis outer membrane proteins. J Clin Microbiol. 1987 Nov;25(11):2132–2135. doi: 10.1128/jcm.25.11.2132-2135.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser M. J., Hopkins J. A., Berka R. M., Vasil M. L., Wang W. L. Identification and characterization of Campylobacter jejuni outer membrane proteins. Infect Immun. 1983 Oct;42(1):276–284. doi: 10.1128/iai.42.1.276-284.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Confer A. W., Panciera R. J., Fulton R. W., Gentry M. J., Rummage J. A. Effect of vaccination with live or killed Pasteurella haemolytica on resistance to experimental bovine pneumonic pasteurellosis. Am J Vet Res. 1985 Feb;46(2):342–347. [PubMed] [Google Scholar]
- Dunn B. E., Blaser M. J., Snyder E. L. Two-dimensional gel electrophoresis and immunoblotting of Campylobacter outer membrane proteins. Infect Immun. 1987 Jul;55(7):1564–1572. doi: 10.21236/ada265461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour N. J., Angus K. W., Donachie W., Fraser J. Vaccination against experimental pneumonic pasteurellosis. Vet Rec. 1982 May 8;110(19):450–450. doi: 10.1136/vr.110.19.450-a. [DOI] [PubMed] [Google Scholar]
- Hansen E. J., Frisch C. F., McDade R. L., Jr, Johnston K. H. Identification of immunogenic outer membrane proteins of Haemophilus influenzae type b in the infant rat model system. Infect Immun. 1981 Jun;32(3):1084–1092. doi: 10.1128/iai.32.3.1084-1092.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsnell J. M., Teale C. M. Characterisation of the outer membrane protein antigens of British field isolates of Moraxella bovis. Vet Microbiol. 1987 Nov;15(3):181–189. doi: 10.1016/0378-1135(87)90072-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lillie L. E. The bovine respiratory disease complex. Can Vet J. 1974 Sep;15(9):233–242. [PMC free article] [PubMed] [Google Scholar]
- Martin S. W. Vaccination: Is it Effective in Preventing Respiratory Disease or Influencing Weight Gains in Feedlot Calves? Can Vet J. 1983 Jan;24(1):10–19. [PMC free article] [PubMed] [Google Scholar]
- Newman P. R., Corstvet R. E., Panciera R. J. Distribution of Pasteurella haemolytica and Pasteurella multocida in the bovine lung following vaccination and challenge exposure as an indicator of lung resistance. Am J Vet Res. 1982 Mar;43(3):417–422. [PubMed] [Google Scholar]
- Purdy C. W., Livingston C. W., Jr, Frank G. H., Cummins J. M., Cole N. A., Loan R. W. A live Pasteurella haemolytica vaccine efficacy trial. J Am Vet Med Assoc. 1986 Mar 15;188(6):589–591. [PubMed] [Google Scholar]
- Squire P. G., Smiley D. W., Croskell R. B. Identification and extraction of Pasteurella haemolytica membrane proteins. Infect Immun. 1984 Sep;45(3):667–673. doi: 10.1128/iai.45.3.667-673.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie B. N., Markham R. J., Shewen P. E. Response of calves to lung challenge exposure with Pasteurella haemolytica after parenteral or pulmonary immunization. Am J Vet Res. 1980 Nov;41(11):1773–1778. [PubMed] [Google Scholar]
- Yates W. D., Stockdale P. H., Babiuk L. A., Smith R. J. Prevention of experimental bovine pneumonic pasteurellosis with an extract of Pasteurella haemolytica. Can J Comp Med. 1983 Jul;47(3):250–256. [PMC free article] [PubMed] [Google Scholar]