Abstract
Noninvasive in vivo imaging is an emerging specialty in experimental radiology aiming at developing hardware and appropriate contrast agents to visualize the molecular basis and pathophysiological processes of many pathological conditions, including atherosclerosis. The list of potentially useful tracers and targets for in vivo molecular imaging in the cascade of early atherosclerotic events has been narrowed down to some very promising endothelial factors, i.e., cell adhesion molecules, macrophages, apoptosis, lipoproteins, heat shock proteins, and others. In this review, we will update on the progress of recent developments in the field of noninvasive molecular imaging in experimental atherosclerosis.
Keywords: Atherosclerosis, In vivo, Molecular imaging, Radiology, Vascular stress
Molecular and cellular mechanisms in the autoimmune hypothesis of atherogenesis
Cardiovascular diseases (CVD) cause approximately 40% of deaths in Western industrialized countries (http://epp.eurostat.cec.eu.int). Of these, atherosclerosis is considered a paradigmatic age-associated disease, and a preponderance of research in this area has focused on the elderly, as atherosclerosis progresses slowly and manifests clinically significant symptoms primarily in this segment of the population. However, more recent evidence suggests that the atherosclerotic process begins at a much earlier age and thus may be accessible to early diagnostic approaches and therapeutic interventions (Akerblom et al. 1991; Berenson et al. 1998; Knoflach et al. 2003a; Millonig et al. 2002).
It is now common knowledge that inflammatory–immunological processes play a major role during atherogenesis (Hansson 2005), but most of the available data on the in situ situation in atherosclerotic lesions are derived from analyses of advanced stages, i.e., atherosclerotic plaques in surgically excised specimens.
During the past 15 years, a new “autoimmune hypothesis” for atherogenesis has been developed in our laboratory supported by solid experimental animal and clinical data (Wick et al. 2004). This hypothesis is based on observations that early stages of atherosclerosis are of an immunological–inflammatory nature, often still without the presence of foam cells, and represented at young age as arterial intimal infiltration with mononuclear cells at known predilection sites, i.e., primarily at areas of arterial branching (Gerrity 1981; Millonig et al. 2001; Schwartz et al. 1985; Xu et al. 1990).
The exact initiating factors of progredient atherosclerotic wall changes have not yet been fully elucidated, but have been narrowed down to the effect of well-known classical risk factors. Notwithstanding the proven importance of these factors, the autoimmune hypothesis of atherogenesis postulates that they first act as endothelial stress factors when individuals still appear clinically healthy. Thus, in vitro and in vivo experiments provided evidence that cigarette smoke (Bernhard et al. 2003), proinflammatory cytokines (Amberger et al. 1997), and mechanical stress in vitro or high blood pressure in vivo (Hochleitner et al. 2000) are among the risk factors that lead to the expression of pro-atherogenic stress proteins, called heat shock proteins (HSPs), as well as various pro-inflammatory mediators (Frostegard et al. 1999). HSPs are ubiquitous and structurally highly conserved molecules (Young and Elliott 1989) that have important physiological functions in intracellular protein assembly, folding, and transport as well as the prevention of protein aggregation and misfolding upon stress (chaperone function; Benjamin and McMillan 1998). HSPs also play important roles in a number of diseases, including cancer and autoimmune diseases (Hightower 1991; Snoeckx et al. 2001; Young and Elliott 1989).
There is increasing evidence that, in atherosclerosis, as an immune-mediated disease, members of the HSP families may be the main antigens triggering the anti-vascular immune response (Xu et al. 1992; Xu and Wick 1996). In particular, microbial HSP60 is a major antigen recognized by the innate and adaptive immune systems, e.g., during bacterial or parasite infections. As HSP60 is phylogenetically highly conserved, the extensive sequence homology between microbial and human HSP60 may result in a cross-reactive immune response against the latter, when it is produced by arterial endothelial cells stressed by classical atherosclerosis risk factors (Wick et al. 2004; Young and Elliott 1989). HSP60 is a mitochondrial protein with a chaperone function that is also transported to the cell surface, creating a target for an immunological attack. The presence of HSP60 on the endothelial cell surface was demonstrated both by immunohistological (Xu et al. 1994) and metabolic (Soltys and Gupta 1997, 2000) labeling techniques. The immune system recognizes HSP60 as a “danger signal” on stressed endothelial cells resulting in their destruction via preexisting innate and adaptive cellular and humoral immune reactions. HSP60 also circulates in the blood (soluble HSP60–sHSP60), probably derived from necrotic endothelial cells (Xu et al. 2000). It should be emphasized that HSP60 is not only produced by endothelial cells themselves, but sHSP60 can also bind to the endothelial cell surface, e.g., via Toll-like receptors (TLRs), specifically TLR-4 in combination with CD14 as well as TLR-2 in association with TLR-6 (Bulut et al. 2002; Habich et al. 2002; Kol et al. 1999). Both anti-HSP60 antibodies and T-cells may detect HSP60 on the endothelial cell surface (Seitz et al. 1996), the latter in a major histocompatibility complex (MHC)-restricted fashion (α/β T-cell receptor positive cells—α/β TCR+ cells) or in a non-MHC restricted fashion (γ/δ TCR+ cells). Stressed, but not unstressed, endothelial cells are lysed by anti-HSP60 antibodies via complement activation or antibody-dependent cellular cytotoxicity (ADCC). This is true both for purified antibodies from patients with atherosclerosis and monoclonal murine antibodies to eukaryotic HSP60 (Mayr et al. 1999; Schett et al. 1995).
As the lifelong infectious load has been shown to correlate significantly with the risk of developing atherosclerosis (Mayr et al. 2000; Ridker et al. 2000), bacterial toxins may be taken as surrogate endothelial stressors (Mayr et al. 1999). Injection of bacterial lipopolysaccharide (LPS) has already been shown to be a potent inducer of the simultaneous expression of HSP60 and intracellular adhesion molecule-1 (ICAM-1) in rats (Seitz et al. 1996). As mentioned, it has been demonstrated that classical atherosclerosis risk factors also lead to the simultaneous expression of adhesion molecules (ICAM-1, endothelial lymphocyte adhesion molecule-1 = ELAM-1, vascular cell adhesion molecule-1 = VCAM-1; Cybulsky and Gimbrone 1991; Iiyama et al. 1999; Libby 2002) and HSP60 (Amberger et al. 1997; Seitz et al. 1996). This is important because interaction of HSP60-specific T cells with the endothelial target is only possible when appropriate adhesion molecules have also been expressed. The invasion of mononuclear effector cells from the arterial lumen (lymphocytes and macrophages) as well as smooth muscle cells (SMC) from the media into the vascular intima represents the beginning of the early inflammatory stage of atherosclerosis (Xu et al. 1990). The Atherosclerosis Risk-Factors in Male Youngsters (ARMY) Study in 17- to 18-year-old clinically healthy men in Austria showed that sonographically demonstrable early atherosclerotic changes correlated significantly to the presence of HSP60-specific T cells and anti-HSP60 antibodies in the peripheral blood (Knoflach et al. 2003a). A similar analysis in 50- to 60-year-old men within the framework of the prospective Bruneck Study showed a correlation of HSP60 antibodies with the presence and extent of sonographically demonstrable atherosclerotic lesions, but no such correlation emerged with respect to HSP60-specific T cells in the peripheral blood (Knoflach et al. 2007).
In the past, the major focus of atherosclerosis research was on lipid metabolism alterations (Glass and Witztum 2001; Ross 1993, 1999; Smilde et al. 2001; Steinberg et al. 1989), but recently, inflammatory–immunologic processes during atherogenesis have also received major attention (Hansson 2005; Libby 2002; Wick et al. 2004). Most of this work, however, dealt with the conditions of well-developed lesions with clinically apparent symptoms, such as myocardial infarction, stroke, or peripheral arterial occlusion. In addition, most experimental studies relied on the use of transgenic or knockout mouse models that mimic special hereditary situations in humans, such as functional hypercholesterolemia due to a deficiency of low-density lipoprotein (LDL)-receptor expression (Zhang et al. 1992). In contrast, our interest is focused on the very earliest stages of the disease comprised of mononuclear cell infiltration of the intima in normocholesterolemic humans or experimental animals even in the absence of foam cells (Millonig et al. 2001; Xu et al. 1990).
From these and other data, we concluded that HSP60 expression by endothelial cells is an initiating event that renders them a target for attack by preexisting, in principle protecting, innate and adaptive immunity. Arterial endothelial cells have a lower threshold for HSP60-inducing risk factors as a consequence of lifelong arterial blood pressure compared to venous endothelial cells. Interestingly, endothelial HSP60 expression and mononuclear intima-infiltration are also initial hallmarks of restenosis in coronary and carotid bypass conduits prone to restenosis (Knoflach et al. 2003b).
Molecular imaging of endothelial stress
As the atherosclerotic process begins at an early age, the prolonged course of disease provides a “window of opportunity” for in vivo diagnosis of disease stages before clinical manifestations, as well as the opportunity for early, specifically targeted, therapeutic interventions.
Novel technological developments in the last decade have advanced beyond “classical” radiological techniques to allow imaging of cardiovascular anatomy and physiology on a macroscopic scale, making it possible to image atherogenesis in vivo on the cellular and sometimes even molecular level (Davies et al. 2005a, 2006; Dobrucki and Sinusas 2005; Jaffer et al. 2006a; Jaffer and Weissleder 2004; Lindner 2004a, b; Strauss et al. 2004; Tsimikas 2002; Tsimikas and Shaw 2002; Wickline and Lanza 2003). Noninvasive in vivo imaging is a fast emerging specialty in experimental radiology aiming at developing imaging modalities and appropriate imaging agents to visualize the molecular basis and pathophysiological processes of many pathological conditions, including cardiovascular diseases.
The main difference between the routine application of clinical imaging techniques, such as computed tomography (CT) or magnetic resonance imaging (MRI) and molecular imaging, is that the former primarily focus on assessing anatomical and structural components of atherosclerosis, while the latter aims to visualize the molecular, functional, and pathophysiological process of cardiovascular diseases before the development of clinically overt symptoms. Several serological markers of atherosclerosis, including pro-inflammatory cytokines and vascular stress proteins, are known predictors for, and/or diagnostic biomarkers of, cardiovascular disease and are accepted for both routine and experimental use to monitor patients at risk or after manifestation of cardiovascular symptoms (Danesh et al. 1997; Ridker et al. 2000).
In a prospective context, the development of noninvasive imaging based on the visualization of biologically active markers could be equally important for diagnostic and therapeutic viewpoints, viz: (a) noninvasive diagnostic in vivo imaging of atherosclerosis in patients at risk before the appearance of clinically apparent peripheral arterial-occlusive disease; (b) noninvasive diagnostic in vivo imaging of atherosclerosis in at risk patients in whom classical radiological investigations were inconclusive; (c) using the immunological antigenic function of endothelial cells to monitor possible future medicinal or molecular biological methods for targeted anti-atherosclerotic therapies; (d) providing reproducible well-tolerated noninvasive imaging for initial and/or follow-up biomarkers in longitudinal studies or clinical trials of novel anti-atherosclerotic treatments.
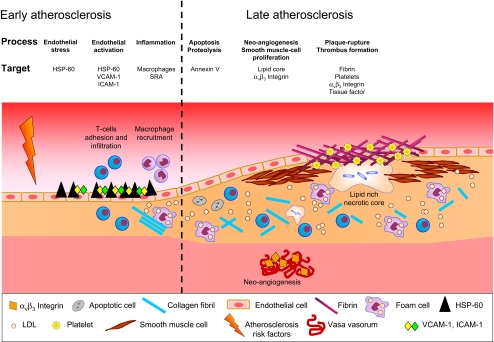
Numerous serological factors are known to be involved in the pathophysiology of atherosclerosis, but not all proved useful targets for molecular imaging techniques. The list of potentially useful factors in the cascade of events occurring in atherosclerosis has been narrowed down to some very promising endothelial targets, shown in the context of the immunological hypothesis of atherogenesis in Fig. 1.
Fig. 1.
Schematic illustration of atherogenesis in the autoimmune hypothesis of atherosclerosis. Promising imaging targets throughout the process can be found for early and later stages in atherogenesis. Importantly, as results from our group showed, effector T cells are the first mononuclear cells to appear in the intima and can then be found through both early and late periods in atherosclerosis. The figure is adapted and modified from Choudhury et al. (2004)
Imaging hardware for in vivo visualization of atherosclerosis
The choice of the imaging platform for an experimental imaging study depends on several factors, including sensitivity, spatial and temporal resolution, depth of tissue penetration, radiation, costs, examination time, and availability at a given research facility. As assessing an atherosclerotic lesion for vascular protein expression represents a much smaller target than, for example, a solid static tumor, vascular in vivo imaging requires highly sensitive and high-resolution strategies that must overcome cardiac and respiratory motion and blood flow. For this purpose, a number of routine clinical radiology hardware platforms can be used, either as stand-alone or fusion technologies.
Ultrasound (US) is widely available, safe, radiation free, and relatively inexpensive, but has insufficient tissue penetration for exact imaging of deep vessels and an overall low spatial resolution and sensitivity.
Single photon emission computed tomography (SPECT) and positron emission tomography (PET) have high sensitivities, but limited spatial resolution, and their data alone do not yield precise anatomic information. Therefore, PET or SPECT examinations need to be combined with, for example, X-ray CT followed by data hybridization of co-registered PET/SPECT and CT image data. Another disadvantage of PET and SPECT is the necessity of radioactive tracers.
CT has a high spatial resolution, but its poor sensitivity to contrast agents has limited its use for molecular imaging to fusion techniques in combination with SPECT or PET. Recently, however, the usefulness of CT for molecular imaging was enhanced by the development of new nanoparticulate contrast agents (Hyafil et al. 2007; Rabin et al. 2006; Winter et al. 2005).
In contrast, MRI has a slightly lower sensitivity than SPECT and PET, but it is considered safe (if individuals do not carry ferromagnetic implants, e.g., a cardiac pacemaker), requires comparable examination times, and provides images with high spatial resolution (Mulder et al. 2005, 2006). A downside of MRI in the context of molecular imaging of atherosclerosis is that direct visualization often needs large amounts of nanoparticles (14 to 56 mg Fe/kg) (Ruehm et al. 2001).
Optical imaging is another frequently used method for molecular imaging, and a variety of different approaches have been described (Ntziachristos et al. 2002; Weissleder and Mahmood 2001). As depth penetration (1–2 mm) is a major limiting factor in in vivo optical imaging, this technology is mainly applied to surface structures only.
Examples of contrast agents for in vivo molecular imaging of atherosclerosis
Molecular imaging of atherosclerosis relies on the use of contrast agents that target specific cells or molecules that are relevant to its pathogenesis (Choudhury et al. 2004). Although most of the available probes for molecular imaging are limited to experimental use, such agents are typically a combination of two major components: (a) a radiologically detectable compound, such as a radioisotope, magnetic particle, fluorochrome, or sonic signal enhancer, and (b) a highly target-specific affinity-ligand moiety, such as an antibody for a given adhesion molecule. In addition, a specific-target cell can take up the probe.
For this reason, not all serologically useful atherosclerosis biomarkers can function as molecular imaging agents, such as secreted proteins or stationary proteins expressed on endothelial cells only in low densities. In contrast, internalized receptors, abundant extracellular markers, and low-background enzyme-sensing quenched substrates provide high-yield imaging targets. A list of currently favored markers under clinical evaluation as targets for noninvasive imaging in experimental atherosclerosis is given in Table 1.
Table 1.
Currently favored potential markers as targets for noninvasive molecular imaging of atherosclerosis; adapted from Jaffer et al. (2006b)
| Biological Process | Class | Specific molecular target |
|---|---|---|
| Angiogenesis | Increased vascularity | Perfusion markers |
| Endothelium | αvβ3a, E-selectin | |
| Apoptosis | Cell membrane | Phosphatidylserine, Annexin A5, 99mTc-annexin |
| Cell adhesion | Surface receptors | VCAM-1 |
| Lipoproteins | Modified lipoproteins | oxLDL, HDL, others |
| Macrophage activity | Surface receptors | SRA, CD36, dextran receptor, others |
| Metabolism | Hexokinase, GLUT-1 | |
| Proteases | MMP (1, 8, 9, 13), cathepsins-NIRFa (B, S, K) | |
| Peroxidases | MPO | |
| Vascular stress | Stress proteins | HSP-60 |
| Thrombosis | Platelet glycoprotein IIb/IIa receptor | 99mTc-apcitide |
| Fibrin | Fibrin, EP-2104R |
GLUT-1 glucose transporter-1, HSP heat shock protein, MMP matrixmetalloproneinase, MPO myeloperoxydase, oxLDL oxidized low-density lipoprotein, SRA scavenger receptor A, Tc technetium, VCAM-1 vascular cell adhesion molecule-1
aAgents under clinical evaluation
MRI contrast agents
As mentioned, MRI with clinical magnetic field strengths, e.g., at 1.5 T, has a considerably higher sensitivity to contrast agents than CT or US, but the sensitivity is still significantly lower compared to SPETC or PET, which limits its use for in vivo imaging on cellular or molecular levels. Nevertheless, in contrast to SPECT and PET, it can be used without potentially harmful radioactive agents and, in contrast to CT, without X-ray load, which makes a possible future application in humans more likely than other modalities. In addition, recent improvements in the development of appropriate MRI contrast agents and optimized hardware with higher magnetic fields (3 and 4.7 T and higher) have now almost overcome the limited specificity of MRI (Aime et al. 2002a, b).
Other than for SPECT/PET, contrast agents for MRI are not in themselves active signal emitters. They also do not directly modify the MRI signal, as do X-ray contrast agents. Instead, MRI contrast agents lead to an indirect effect on the MRI signal by modifying tissue relaxation properties, which are described by the two time constants T1 and T2. The T1- and T2-relaxation times, together with MRI measurement parameters, determine the contrast in magnetic resonance images. Any MRI contrast agent leads to a reduction of both T1 and T2 and thus to a detectable change in image contrast. The effectiveness of a contrast agent in modifying T1 or T2 is described by the so-called relaxivity of the contrast agent, and the ratio of T2 to T1 relaxivity determines if the contrast agent will produce a positive contrast, i.e., signal increase on T1 weighted images, or a negative contrast, i.e., signal decrease on T2 weighted images. In general, only paramagnetic substances are suitable as contrast agents for MRI. At present, two basic classes of MRI contrast agents are available: (1) contrast agents based on gadolinium [Gd (III)] complexes and (2) contrast agents based on iron oxide. For Gd(III)-based complexes, the ratio of T2 to T1 relaxivity is the order of 1 leading to a positive MRI contrast (Weinmann et al. 1984). In molecular form, iron oxide has considerably less influence on relaxation times than Gd(III) and was originally not considered a contrast agent for MRI. As a nanoparticle, iron oxide becomes superparamagnetic with strongly enhanced relaxivities (Bjornerud et al. 2002; Weissleder et al. 1987). For such superparamagnetic iron oxide preparations, the ratio of T2 to T1 relaxivity is significantly larger than 1, resulting in negative contrast on T2 weighted images.
Targeting molecular imaging applications requires consideration of the potentially low concentrations of the molecular targets of interest (nanomolar to picomolar) that have to be detected within voxels sized in the order of 1 mm3. For basic Gd(III) complexes, it is known that millimolar concentrations of Gd are required at the target site to produce an adequate signal; thus, molecular imaging is difficult to achieve with these contrast agents (Gillies 2002).
With iron oxide nanoparticles, it has been shown that the necessary sensitivity can be obtained (Bulte and Kraitchman 2004), but the detection of negative contrast is frequently considered difficult, and a great deal of effort has been directed into finding methods that provide clear positive contrast mechanisms. For iron oxide nanoparticles, this could be achieved by employing special measurement sequences that give rise to a bright signal at iron accumulation sites (Stuber et al. 2007). For Gd(III)-based contrast agents, the quest for high detection sensitivity has lead to the development of a variety of different Gd-loaded nanoparticles that can deliver up to 50,000 to 90,000 Gd ions per particle (Lanza et al. 2004). A wide variety of Gd-loaded nanoparticles have been described (Mulder et al. 2007) that are based on polymers or polysaccharides (Armitage et al. 1990; Faranesh et al. 2004), proteins (Aime et al. 2002c), dendrimers (Winalski et al. 2002), micelles (Torchilin 2002), liposomes (Mulder et al. 2006; Winter et al. 2003a), and even on viruses (Allen et al. 2005; Manchester and Singh 2006).
As nanoparticulate contrast agents seem mandatory to sufficient sensitivity for targeted molecular MRI, it is important to be aware of the biodistribution of these agents after intravenous administration, when clearance from the blood follows a two-step process based on oponization and phagocytosis by monocytes or macrophages. Due to these processes, colloidal drug carriers are usually cleared from the blood within minutes and accumulate in liver, spleen, and bone marrow. For targeted contrast agents, it is therefore important to slow their clearance from the blood stream, i.e., to extend the circulation time as much as possible by controlling different physico-chemical factors such as size, charge, hydrophilicity/hydrophobicity (Moghimi et al. 2001).
The MRI contrast agents discussed thus far have been based on chelated metal irons or metal particles and had a direct influence on tissue relaxation times. Recently, contrast agents based on magnetization transfer, called chemical exchange saturation transfer (CEST) agents, have been introduced, which in principle, can be totally metal free. By selective excitation of exchangeable protons on these agents, magnetization will be transferred to the free water pool, leading to a drop in signal intensity (Ward et al. 2000). Many small diamagnetic organic compounds, such as sugars or amino acids, have pools of exchangeable protons and may therefore serve as CEST agents. Paramagnetic lanthanide (Eu, Dy, Ho, Er, Tm, Yb) chelates (PARACEST agents) can result in significant detectable saturation transfer effects down to picomolar concentrations (Aime et al. 2005).
PET contrast agents
The most common radiotracer used for molecular imaging of atherosclerosis with PET is the 18fluoro-2-deoxyglucose (18FDG; Davies et al. 2005b; Rudd et al. 2002; Tahara et al. 2007), which competes with glucose for uptake into metabolically active cells, such as macrophages in atheromas. This has been clearly shown in vivo in humans with symptomatic carotid atherosclerosis, where focal 18FDG uptake 3 h after intravenous (i.v.) injection could be correlated with stenosis on co-registered CT images, with heavy macrophage infiltration in histology and with microautoradiography after surgical resection of the respective carotid plaques (Rudd et al. 2002). However, the concept of 18FDG accumulation in inflammatory cells in atherosclerosis was not unequivocally determined (Laurberg et al. 2007), until the recent successful visualization of Nahrendorf et al. (2008) using PET-CT, of macrophages in atherosclerotic lesions in apolipoprotein E-deficient mice using both radioactive 64Cu-trireporter labeled nanoparticles (64Cu-TNP) and 18FDG.
Examples of in vivo molecular imaging in atherogenesis
Imaging heat shock proteins
From our own and other data, it has been shown that HSP60 expression by endothelial cells is an initiating event that renders them a target for attack by preexisting, presumably protective, innate and adaptive immunity, even under normocholesterolemic conditions.
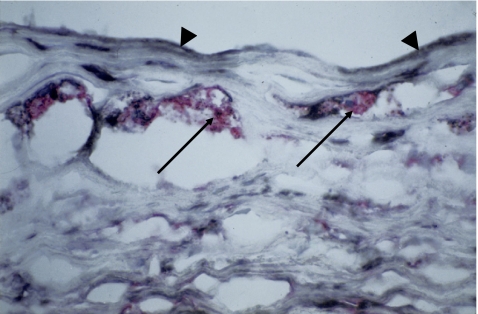
In our radiology studies for in vivo molecular imaging of HSP60 expression, we radiolabeled the murine monoclonal antibody (Mab) II-13, with exquisite specificity for eukaryotic HSP60, and for which in vitro and in vivo reactivity with stressed endothelial cells has been previously demonstrated (Soltys and Gupta 1997; Xu et al. 1994). Endothelial stress was induced in normocholesterolemic New Zealand white rabbits by i.v. injection of bacterial endotoxin (LPS at 10 μg/kg). In vivo molecular imaging was performed using co-registered CT and PET after i.v. injection of 124I-labeled monoclonal anti-HSP60 or 124I-radiolabeled isotype control antibodies. In vitro correlation of in vivo imaging was achieved by en face immunohistochemistry and autoradiography of the aortae. For radiolabeling, 124iodine was selected because of its proven usefulness in the applied imaging systems and its suitable half-life (Davies et al. 2005a, 2006; Rudd et al. 2005). Post-in vivo imaging autoradiography and en face immunohistochemistry convincingly corroborated the data obtained by CT/PET analysis. Importantly, the en face immunohistochemical data also provided additional proof for the concept that HSP60 expression is most intense at arterial branching points, especially at areas subjected to turbulent rather than laminar flow shear stress as shown in several previous studies by immunohistological methods (Fig. 2).
Fig. 2.
Fatty streak in a surgically removed specimen from the aorta of a 50-year-old male patient. Immunohistochemical demonstration of HSP60 (black) by endothelial cells (arrowhead) and cells infiltrating the intima, including foam cells (arrow), and HLA-DR (red) by macrophages transformed into foam cells. Original magnification ×400
Imaging cell adhesion molecules
Endothelial cell stressors lead to the simultaneous expression of HSP60 and adhesion molecules, e.g., ICAM-1, ELAM-1, and VCAM-1 (Amberger et al. 1997), which is a prerequisite for the interaction not only of potentially bacterial/human HSP60 cross-reactive antibodies, as mentioned, but also of T cells with endothelial targets. However, this does not confer immunologic specificity, and adhesion alone does not lead to a cellular immune attack. Cell adhesion molecules are expressed in an early stage of atherosclerosis development and, hence, might serve as diagnostic targets. VCAM-1-internalizing targeted nanoparticles, injected into apoE-knockout mice, have been successfully determined in MRI in experimental atherosclerosis and revealed good correlation with ex vivo histological VCAM-1 staining (Nahrendorf et al. 2006). Using PET, radiolabeled B2702-p, which is a ligand that specifically binds to VCAM-1, also proved to be a promising tracer for noninvasive imaging of adhesion molecules (Broisat et al. 2007). In vivo imaging studies of ICAM-1- or selectin expression in atherosclerosis have not yet been published.
Imaging macrophages
Macrophages can be best imaged with the use of MRI. They phagocyte i.v. injected dextran-coated ultrasmall superparamagnetic ironoxide nanoparticles (USPIOs), which accumulate in atherosclerotic plaques over time, via dextran receptors or scavenger receptors (Kooi et al. 2003; Ruehm et al. 2001). Due to their superparamagnetic properties, USPIOs generate a negative contrast in T2 MRI sequences based on local signal quenching. Amirbekian et al. (2007) targeted macrophages with paramagnetic and fluorescent micelles that specifically bind the macrophage scavenger receptor. Their in vivo images correlated with ex vivo fluorescence microscopy-verified macrophage-rich plaques.
Very recently, Nahrendorf et al. (2008) successfully utilized PET/CT technology for in vivo imaging of macrophages in inflammatory atherosclerosis. Dextranated DTPA-modified magnetofluorescent 20 nm nanoparticles were radiolabeled with 64Cu and its in vivo accumulation in apolipoprotein E-deficient mice correlated with atherosclerotic plaques in specimen autoradiographies and with infiltrating macrophages in ex vivo fluorescence microscopy.
Imaging angiogenesis
Angiogenesis derived from the vasa vasorum is a typical part of the atherosclerotic pathology that occurs predominantly in more advanced stages of the development of an atherosclerotic lesion (Moreno et al. 2006). Contrast agents can detect angiogenic areas by either (a) determination of the permeability of the newly formed vessels with dynamic contrast-enhanced MRI or, more advanced, (b) targeted contrast agents that specifically mark the surface of angiogenically activated endothelial cells (Choudhury et al. 2004). In particular, the vitronectin receptor (αvβ3), which is a member of the integrin superfamily, mediates cell attachment on arginine–glycine–aspartic acid (RGD)-containing adhesive proteins and is a heterodimeric protein demonstrable in human plaques. The αvβ3-integrin has been shown to be a promising and useful target that can be visualized via αvβ3-specific antibodies or the αvβ3-specific RGD peptide (Mulder et al. 2005; Sipkins et al. 1998). Using the first above-mentioned approach, the application of the macromolecular agent gadofluorine, which accumulates in plaques over time, has effectively been detected in lipid-rich experimental plaques using the MRI technique (Sirol et al. 2004).
Based on the second above-mentioned approach for imaging of angiogenesis in atherosclerosis, Lanza et al. (2006) developed nanoparticles with a perfluorocarbon core and a lipid monolayer into which they incorporated Gd-DTPA-bis-oleate, a paramagnetic lipid with high detectability in MRI. Winter et al. (2003b, 2006) used these nanoparticles and specifically targeted them against αvβ3–integrin, which is specifically upregulated on endothelial cells during angiogenesis. Consequently, they were able to specifically detect angiogenesis in cholesterol-fed rabbits with intimal hyperplasia. In another study, they even used this nanoparticle as a vehicle for an anti-angiogenic drug. Other αvβ3-targeted imaging agents have also been designed for PET, SPECT, MRI, and US (Haubner 2006; Haubner et al. 2005; Hua et al. 2005; Meoli et al. 2004; Sipkins et al. 1998).
Imaging apoptosis
Apoptosis, or programmed cell death, is essential for tissue development and homeostasis. Because apoptosis has been found to play a critical role in the etiology of atherosclerosis, in vivo early detection of apoptosis could be of great importance in determining the disease stage (Cederholm and Frostegard 2007). Annexin V targets the phosphatidylserine, which is expressed on the outer layer of the apoptotic cell membrane (Kolodgie et al. 2003; Li et al. 2004; Shiomi et al. 2003). In vivo, apoptosis in atherosclerotic lesions has been detected via radiolabeled and superparamagnetic-iron oxide particle-labeled annexin V (Schellenberger et al. 2002, 2004; Smith et al. 2007).
Imaging lipoproteins
Lipoproteins, particularly LDL and high-density lipoprotein (HDL), play significant roles in the transport of cholesterol and atherosclerotic plaque composition (Ross 1999). While LDL is involved in progressive plaque formation, HDL has the capacity to remove lipids from an atherosclerotic plaque and thus may even serve as a possible therapeutic agent for plaque regression. For molecular imaging purposes, lipoproteins may be labeled with contrast-enhancing agents such as radiotracers, fluorescent dyes, or paramagnetic nanoparticles. Frias et al. (2006, 2004) successfully synthesized a HDL-based paramagnetic contrast agent for MRI and applied it in a mouse model of experimental atherosclerosis wherein in vivo and ex vivo imaging studies provided specific signal enhancement in atherosclerotic lesions in the abdominal aorta.
Imaging atherosclerotic thrombus
Thrombi can be found in late stage atherogenesis, especially on plaques at the verge of rupture. Because thrombi mainly consist of highly abundant fibrin, specifically anti-fibrin targeted probes can be used. For that purpose, paramagnetic perfluorocarbon nanoparticles that contain Gd and that are conjugated with fibrin-specific antibodies or fibrin-binding Gd-labeled peptides have been used successfully for effective in vivo imaging of thrombi in experimental atherosclerosis after carotid endothelium denudation (Sirol et al. 2005a, b; Yu et al. 2000).
In conclusion, the development of increasingly powerful single platform imaging hardware and rapid progress on the design and successful utilization of highly atherosclerosis-specific targeted contrast agents that are noninvasive and detectable at different stages of atherosclerosis development highlight the crucial role of in vivo molecular imaging of atherosclerosis in future experimental and clinical radiology of cardiovascular disease. Promising targets for detecting the earliest endothelial response to atherosclerosis risk factors (e.g., HSP60 and VCAM-1) as well as intermediate and later stages (morphologically changed vessel walls, e.g., αvβ3–integrin, annexin V, or fibrin) cannot only provide diagnostic indicators of patients at risk for arterial stenosis but, upon their noninvasive-specific radiological detectability, also function as biomarkers to monitor possible future methods for targeted anti-atherosclerotic therapies.
Acknowledgments
This work was supported by the Propter Homines Foundation, Vaduz, FL (to GW); the Medizinische Forschungsförderung Innsbruck MFI (to MCW, Project 9443); the European Union as part of the project Molecular Basis of Vascular Events Leading to Thrombotic Stroke (MOLSTROKE; LSHM-CT-2004–005206), and the Network of Excellence European Vascular Genomics Network (EVGN; LSHM-CT-2003–503254). Editorial assistance from M. Kat Occhipinti-Bender.
Abbreviations
- ADCC
Antibody-dependent cellular cytotoxicity
- ARMY
Atherosclerosis risk factors in male youngsters
- CD
Cluster of differentiation
- CEST
Chemical exchange saturation transfer
- CT
Computed tomography
- CVD
Cardiovascular disease
- ELAM
Endothelial lymphocyte adhesion molecule
- 18FDG
18Fluorodeoxyglucose
- Gd
Gadolinium
- GLUT-1
Glucose transporter-1
- HDL
High-density lipoprotein
- HSP
Heat shock protein
- ICAM
Intracellular adhesion molecule
- LDL
Low-density lipoprotein
- LPS
Lipopolysaccharide
- Mab
Monoclonal antibody
- MHC
Major histocompatibility complex
- MPO
Myeloperoxydase
- MMP
Matrixmetalloproteinase
- MRI
Magnetic resonance imaging
- OxLDL
Oxidized low-density lipoprotein
- PARACEST
Paramagnetic chemical exchange saturation transfer
- PET
Positron emission tomography
- RGD
Arginine-glycine-aspartic acid
- SMC
Smooth muscle cell
- SPECT
Single photon emission tomography
- SPIO
Superparamagnetic iron oxide
- SRA
Scavenger receptor A
- Tc
Technetium
- TCR
T-cell receptor
- TLR
Toll-like receptor
- TNP
Trireporter nanoparticles
- US
Ultrasound
- USPIO
Ultrasmall superparamagnetic iron oxide
- VCAM
Vascular cell adhesion molecule
References
- Aime S, Cabella C, Colombatto S, Geninatti Crich S, Gianolio E, Maggioni F (2002a) Insights into the use of paramagnetic Gd(III) complexes in MR-molecular imaging investigations. J Magn Reson Imaging 16:394–406 [DOI] [PubMed]
- Aime S, Dastru W, Crich SG, Gianolio E, Mainero V (2002b) Innovative magnetic resonance imaging diagnostic agents based on paramagnetic Gd(III) complexes. Biopolymers 66:419–428 [DOI] [PubMed]
- Aime S, Frullano L, Geninatti Crich S (2002c) Compartmentalization of a gadolinium complex in the apoferritin cavity: a route to obtain high relaxivity contrast agents for magnetic resonance imaging. Angew Chem Int Ed Engl 41:1017–1019 [DOI] [PubMed]
- Aime S, Carrera C, Delli Castelli D, Geninatti Crich S, Terreno E (2005) Tunable imaging of cells labeled with MRI-PARACEST agents. Angew Chem Int Ed Engl 44:1813–1815 [DOI] [PubMed]
- Akerblom HK, Uhari M, Pesonen E et al (1991) Cardiovascular risk in young Finns. Ann Med 23:35–39 [DOI] [PubMed]
- Allen M, Bulte JW, Liepold L, Basu G, Zywicke HA, Frank JA, Young M, Douglas T (2005) Paramagnetic viral nanoparticles as potential high-relaxivity magnetic resonance contrast agents. Magn Reson Med 54:807–812 [DOI] [PubMed]
- Amberger A, Maczek C, Jurgens G et al (1997) Co-expression of ICAM-1, VCAM-1, ELAM-1 and Hsp60 in human arterial and venous endothelial cells in response to cytokines and oxidized low-density lipoproteins. Cell Stress Chaperones 2:94–103 [DOI] [PMC free article] [PubMed]
- Amirbekian V, Lipinski MJ, Briley-Saebo KC et al (2007) Detecting and assessing macrophages in vivo to evaluate atherosclerosis noninvasively using molecular MRI. Proc Natl Acad Sci USA 104:961–966 [DOI] [PMC free article] [PubMed]
- Armitage FE, Richardson DE, Li KC (1990) Polymeric contrast agents for magnetic resonance imaging: synthesis and characterization of gadolinium diethylenetriaminepentaacetic acid conjugated to polysaccharides. Bioconjug Chem 1:365–374 [DOI] [PubMed]
- Benjamin IJ, McMillan DR (1998) Stress (heat shock) proteins: molecular chaperones in cardiovascular biology and disease. Circ Res 83:117–132 [DOI] [PubMed]
- Berenson GS, Srinivasan SR, Bao W, Newman WP 3rd, Tracy RE, Wattigney WA (1998) Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med 338:1650–1656 [DOI] [PubMed]
- Bernhard D, Pfister G, Huck CW, Kind M, Salvenmoser W, Bonn GK, Wick G (2003) Disruption of vascular endothelial homeostasis by tobacco smoke: impact on atherosclerosis. Faseb J 17:2302–2304 [DOI] [PubMed]
- Bjornerud A, Johansson LO, Briley-Saebo K, Ahlstrom HK (2002) Assessment of T1 and T2* effects in vivo and ex vivo using iron oxide nanoparticles in steady state–dependence on blood volume and water exchange. Magn Reson Med 47:461–471 [DOI] [PubMed]
- Broisat A, Riou LM, Ardisson V, Boturyn D, Dumy P, Fagret D, Ghezzi C (2007) Molecular imaging of vascular cell adhesion molecule-1 expression in experimental atherosclerotic plaques with radiolabelled B2702-p. Eur J Nucl Med Mol Imaging 34:830–840 [DOI] [PubMed]
- Bulte JW, Kraitchman DL (2004) Iron oxide MR contrast agents for molecular and cellular imaging. NMR Biomed 17:484–499 [DOI] [PubMed]
- Bulut Y, Faure E, Thomas L et al (2002) Chlamydial heat shock protein 60 activates macrophages and endothelial cells through Toll-like receptor 4 and MD2 in a MyD88-dependent pathway. J Immunol 168:1435–1440 [DOI] [PubMed]
- Cederholm A, Frostegard J (2007) Annexin A5 as a novel player in prevention of atherothrombosis in SLE and in the general population. Ann N Y Acad Sci 1108:96–103 [DOI] [PubMed]
- Choudhury RP, Fuster V, Fayad ZA (2004) Molecular, cellular and functional imaging of atherothrombosis. Nat Rev Drug Discov 3:913–925 [DOI] [PubMed]
- Cybulsky MI, Gimbrone MA Jr (1991) Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science 251:788–791 [DOI] [PubMed]
- Danesh J, Collins R, Peto R (1997) Chronic infections and coronary heart disease: is there a link? Lancet 350:430–436 [DOI] [PubMed]
- Davies JR, Rudd JF, Fryer TD, Weissberg PL (2005a) Targeting the vulnerable plaque: the evolving role of nuclear imaging. J Nucl Cardiol 12:234–246 [DOI] [PubMed]
- Davies JR, Rudd JH, Fryer TD et al (2005b) Identification of culprit lesions after transient ischemic attack by combined 18F fluorodeoxyglucose positron-emission tomography and high-resolution magnetic resonance imaging. Stroke 36:2642–2647 [DOI] [PubMed]
- Davies JR, Rudd JH, Weissberg PL, Narula J (2006) Radionuclide imaging for the detection of inflammation in vulnerable plaques. J Am Coll Cardiol 47:C57–C68 [DOI] [PubMed]
- Dobrucki LW, Sinusas AJ (2005) Cardiovascular molecular imaging. Semin Nucl Med 35:73–81 [DOI] [PubMed]
- Faranesh AZ, Nastley MT, Perez de la Cruz C, Haller MF, Laquerriere P, Leong KW, McVeigh ER (2004) In vitro release of vascular endothelial growth factor from gadolinium-doped biodegradable microspheres. Magn Reson Med 51:1265–1271 [DOI] [PMC free article] [PubMed]
- Frias JC, Williams KJ, Fisher EA, Fayad ZA (2004) Recombinant HDL-like nanoparticles: a specific contrast agent for MRI of atherosclerotic plaques. J Am Chem Soc 126:16316–16317 [DOI] [PubMed]
- Frias JC, Ma Y, Williams KJ, Fayad ZA, Fisher EA (2006) Properties of a versatile nanoparticle platform contrast agent to image and characterize atherosclerotic plaques by magnetic resonance imaging. Nano Lett 6:2220–2224 [DOI] [PubMed]
- Frostegard J, Ulfgren AK, Nyberg P, Hedin U, Swedenborg J, Andersson U, Hansson GK (1999) Cytokine expression in advanced human atherosclerotic plaques: dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis 145:33–43 [DOI] [PubMed]
- Gerrity RG (1981) The role of the monocyte in atherogenesis: II. Migration of foam cells from atherosclerotic lesions. Am J Pathol 103:191–200 [PMC free article] [PubMed]
- Gillies RJ (2002) In vivo molecular imaging. J Cell Biochem Suppl 39:231–238 [DOI] [PubMed]
- Glass CK, Witztum JL (2001) Atherosclerosis. the road ahead. Cell 104:503–516 [DOI] [PubMed]
- Habich C, Baumgart K, Kolb H, Burkart V (2002) The receptor for heat shock protein 60 on macrophages is saturable, specific, and distinct from receptors for other heat shock proteins. J Immunol 168:569–576 [DOI] [PubMed]
- Hansson GK (2005) Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 352:1685–1695 [DOI] [PubMed]
- Haubner R (2006) Alphavbeta3-integrin imaging: a new approach to characterise angiogenesis? Eur J Nucl Med Mol Imaging 33(Suppl 1):54–63 [DOI] [PubMed]
- Haubner R, Weber WA, Beer AJ et al (2005) Noninvasive visualization of the activated alphavbeta3 integrin in cancer patients by positron emission tomography and [18F]Galacto-RGD. PLoS Med 2:e70 [DOI] [PMC free article] [PubMed]
- Hightower LE (1991) Heat shock, stress proteins, chaperones, and proteotoxicity. Cell 66:191–197 [DOI] [PubMed]
- Hochleitner BW, Hochleitner EO, Obrist P, Eberl T, Amberger A, Xu Q, Margreiter R, Wick G (2000) Fluid shear stress induces heat shock protein 60 expression in endothelial cells in vitro and in vivo. Arterioscler Thromb Vasc Biol 20:617–623 [DOI] [PubMed]
- Hua J, Dobrucki LW, Sadeghi MM et al (2005) Noninvasive imaging of angiogenesis with a 99mTc-labeled peptide targeted at alphavbeta3 integrin after murine hindlimb ischemia. Circulation 111:3255–3260 [DOI] [PubMed]
- Hyafil F, Cornily JC, Feig JE et al (2007) Noninvasive detection of macrophages using a nanoparticulate contrast agent for computed tomography. Nat Med 13:636–641 [DOI] [PubMed]
- Iiyama K, Hajra L, Iiyama M, Li H, DiChiara M, Medoff BD, Cybulsky MI (1999) Patterns of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 expression in rabbit and mouse atherosclerotic lesions and at sites predisposed to lesion formation. Circ Res 85:199–207 [DOI] [PubMed]
- Jaffer FA, Weissleder R (2004) Seeing within: molecular imaging of the cardiovascular system. Circ Res 94:433–445 [DOI] [PubMed]
- Jaffer FA, Libby P, Weissleder R (2006a) Molecular and cellular imaging of atherosclerosis: emerging applications. J Am Coll Cardiol 47:1328–1338 [DOI] [PubMed]
- Jaffer FA, Nahrendorf M, Sosnovik D, Kelly KA, Aikawa E, Weissleder R (2006b) Cellular imaging of inflammation in atherosclerosis using magnetofluorescent nanomaterials. Mol Imaging 5:85–92 [PubMed]
- Knoflach M, Kiechl S, Kind M et al (2003a) Cardiovascular risk factors and atherosclerosis in young males: ARMY study (Atherosclerosis Risk-Factors in Male Youngsters). Circulation 108:1064–1069 [DOI] [PubMed]
- Knoflach M, Mayrl B, Mayerl C, Sedivy R, Wick G (2003b) Atherosclerosis as a paradigmatic disease of the elderly: role of the immune system. Immunol Allergy Clin North Am 23:117–132 [DOI] [PubMed]
- Knoflach M, Kiechl S, Mayrl B et al (2007) T-cell reactivity against HSP60 relates to early but not advanced atherosclerosis. Atherosclerosis 195:333–338 [DOI] [PubMed]
- Kol A, Bourcier T, Lichtman AH, Libby P (1999) Chlamydial and human heat shock protein 60s activate human vascular endothelium, smooth muscle cells, and macrophages. J Clin Invest 103:571–577 [DOI] [PMC free article] [PubMed]
- Kolodgie FD, Petrov A, Virmani R et al (2003) Targeting of apoptotic macrophages and experimental atheroma with radiolabeled annexin V: a technique with potential for noninvasive imaging of vulnerable plaque. Circulation 108:3134–3139 [DOI] [PubMed]
- Kooi ME, Cappendijk VC, Cleutjens KB et al (2003) Accumulation of ultrasmall superparamagnetic particles of iron oxide in human atherosclerotic plaques can be detected by in vivo magnetic resonance imaging. Circulation 107:2453–2458 [DOI] [PubMed]
- Lanza GM, Winter PM, Caruthers SD, Morawski AM, Schmieder AH, Crowder KC, Wickline SA (2004) Magnetic resonance molecular imaging with nanoparticles. J Nucl Cardiol 11:733–743 [DOI] [PubMed]
- Lanza G, Winter P, Cyrus T, Caruthers S, Marsh J, Hughes M, Wickline S (2006) Nanomedicine opportunities in cardiology. Ann N Y Acad Sci 1080:451–465 [DOI] [PubMed]
- Laurberg JM, Olsen AK, Hansen SB, Bottcher M, Morrison M, Ricketts SA, Falk E (2007) Imaging of vulnerable atherosclerotic plaques with FDG-microPET: no FDG accumulation. Atherosclerosis 192:275–282 [DOI] [PubMed]
- Li W, Hellsten A, Jacobsson LS, Blomqvist HM, Olsson AG, Yuan XM (2004) Alpha-tocopherol and astaxanthin decrease macrophage infiltration, apoptosis and vulnerability in atheroma of hyperlipidaemic rabbits. J Mol Cell Cardiol 37:969–978 [DOI] [PubMed]
- Libby P (2002) Inflammation in atherosclerosis. Nature 420:868–874 [DOI] [PubMed]
- Lindner JR (2004a) Microbubbles in medical imaging: current applications and future directions. Nat Rev Drug Discov 3:527–532 [DOI] [PubMed]
- Lindner JR (2004b) Molecular imaging with contrast ultrasound and targeted microbubbles. J Nucl Cardiol 11:215–221 [DOI] [PubMed]
- Manchester M, Singh P (2006) Virus-based nanoparticles (VNPs): platform technologies for diagnostic imaging. Adv Drug Deliv Rev 58:1505–1522 [DOI] [PubMed]
- Mayr M, Metzler B, Kiechl S, Willeit J, Schett G, Xu Q, Wick G (1999) Endothelial cytotoxicity mediated by serum antibodies to heat shock proteins of Escherichia coli and Chlamydia pneumoniae: immune reactions to heat shock proteins as a possible link between infection and atherosclerosis. Circulation 99:1560–1566 [DOI] [PubMed]
- Mayr M, Kiechl S, Willeit J, Wick G, Xu Q (2000) Infections, immunity, and atherosclerosis: associations of antibodies to Chlamydia pneumoniae, Helicobacter pylori, and cytomegalovirus with immune reactions to heat-shock protein 60 and carotid or femoral atherosclerosis. Circulation 102:833–839 [DOI] [PubMed]
- Meoli DF, Sadeghi MM, Krassilnikova S et al (2004) Noninvasive imaging of myocardial angiogenesis following experimental myocardial infarction. J Clin Invest 113:1684–1691 [DOI] [PMC free article] [PubMed]
- Millonig G, Schwentner C, Mueller P, Mayerl C, Wick G (2001) The vascular-associated lymphoid tissue: a new site of local immunity. Curr Opin Lipidol 12:547–553 [DOI] [PubMed]
- Millonig G, Malcom GT, Wick G (2002) Early inflammatory-immunological lesions in juvenile atherosclerosis from the Pathobiological Determinants of Atherosclerosis in Youth (PDAY)-study. Atherosclerosis 160:441–448 [DOI] [PubMed]
- Moghimi SM, Hunter AC, Murray JC (2001) Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol Rev 53:283–318 [PubMed]
- Moreno PR, Purushothaman KR, Sirol M, Levy AP, Fuster V (2006) Neovascularization in human atherosclerosis. Circulation 113:2245–2252 [DOI] [PubMed]
- Mulder WJ, Strijkers GJ, Habets JW et al (2005) MR molecular imaging and fluorescence microscopy for identification of activated tumor endothelium using a bimodal lipidic nanoparticle. Faseb J 19:2008–2010 [DOI] [PubMed]
- Mulder WJ, Strijkers GJ, van Tilborg GA, Griffioen AW, Nicolay K (2006) Lipid-based nanoparticles for contrast-enhanced MRI and molecular imaging. NMR Biomed 19:142–164 [DOI] [PubMed]
- Mulder WJ, Strijkers GJ, Vucic E, Cormode DP, Nicolay K, Fayad ZA (2007) Magnetic resonance molecular imaging contrast agents and their application in atherosclerosis. Top Magn Reson Imaging 18:409–417 [DOI] [PubMed]
- Nahrendorf M, Jaffer FA, Kelly KA, Sosnovik DE, Aikawa E, Libby P, Weissleder R (2006) Noninvasive vascular cell adhesion molecule-1 imaging identifies inflammatory activation of cells in atherosclerosis. Circulation 114:1504–1511 [DOI] [PubMed]
- Nahrendorf M, Zhang H, Hembrador S et al (2008) Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation 117:379–387 [DOI] [PMC free article] [PubMed]
- Ntziachristos V, Tung CH, Bremer C, Weissleder R (2002) Fluorescence molecular tomography resolves protease activity in vivo. Nat Med 8:757–760 [DOI] [PubMed]
- Rabin O, Manuel Perez J, Grimm J, Wojtkiewicz G, Weissleder R (2006) An X-ray computed tomography imaging agent based on long-circulating bismuth sulphide nanoparticles. Nat Mater 5:118–122 [DOI] [PubMed]
- Ridker PM, Hennekens CH, Buring JE, Rifai N (2000) C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 342:836–843 [DOI] [PubMed]
- Ross R (1993) The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 362:801–809 [DOI] [PubMed]
- Ross R (1999) Atherosclerosis—an inflammatory disease. N Engl J Med 340:115–126 [DOI] [PubMed]
- Rudd JH, Warburton EA, Fryer TD et al (2002) Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation 105:2708–2711 [DOI] [PubMed]
- Rudd JH, Davies JR, Weissberg PL (2005) Imaging of atherosclerosis – can we predict plaque rupture? Trends Cardiovasc Med 15:17–24 [DOI] [PubMed]
- Ruehm SG, Corot C, Vogt P, Kolb S, Debatin JF (2001) Magnetic resonance imaging of atherosclerotic plaque with ultrasmall superparamagnetic particles of iron oxide in hyperlipidemic rabbits. Circulation 103:415–422 [DOI] [PubMed]
- Schellenberger EA, Hogemann D, Josephson L, Weissleder R (2002) Annexin V-CLIO: a nanoparticle for detecting apoptosis by MRI. Acad Radiol 9(Suppl 2):S310–S311 [DOI] [PubMed]
- Schellenberger EA, Sosnovik D, Weissleder R, Josephson L (2004) Magneto/optical annexin V, a multimodal protein. Bioconjug Chem 15:1062–1067 [DOI] [PubMed]
- Schett G, Xu Q, Amberger A, Van der Zee R, Recheis H, Willeit J, Wick G (1995) Autoantibodies against heat shock protein 60 mediate endothelial cytotoxicity. J Clin Invest 96:2569–2577 [DOI] [PMC free article] [PubMed]
- Schwartz CJ, Sprague EA, Kelley JL, Valente AJ, Suenram CA (1985) Aortic intimal monocyte recruitment in the normo and hypercholesterolemic baboon (Papio cynocephalus). An ultrastructural study: implications in atherogenesis. Virchows Arch A Pathol Anat Histopathol 405:175–191 [DOI] [PubMed]
- Seitz CS, Kleindienst R, Xu Q, Wick G (1996) Coexpression of heat-shock protein 60 and intercellular-adhesion molecule-1 is related to increased adhesion of monocytes and T cells to aortic endothelium of rats in response to endotoxin. Lab Invest 74:241–252 [PubMed]
- Shiomi M, Ito T, Yamada S, Kawashima S, Fan J (2003) Development of an animal model for spontaneous myocardial infarction (WHHLMI rabbit). Arterioscler Thromb Vasc Biol 23:1239–1244 [DOI] [PubMed]
- Sipkins DA, Cheresh DA, Kazemi MR, Nevin LM, Bednarski MD, Li KC (1998) Detection of tumor angiogenesis in vivo by alphaVbeta3-targeted magnetic resonance imaging. Nat Med 4:623–626 [DOI] [PubMed]
- Sirol M, Itskovich VV, Mani V et al (2004) Lipid-rich atherosclerotic plaques detected by gadofluorine-enhanced in vivo magnetic resonance imaging. Circulation 109:2890–2896 [DOI] [PubMed]
- Sirol M, Aguinaldo JG, Graham PB et al (2005a) Fibrin-targeted contrast agent for improvement of in vivo acute thrombus detection with magnetic resonance imaging. Atherosclerosis 182:79–85 [DOI] [PubMed]
- Sirol M, Fuster V, Badimon JJ, Fallon JT, Moreno PR, Toussaint JF, Fayad ZA (2005b) Chronic thrombus detection with in vivo magnetic resonance imaging and a fibrin-targeted contrast agent. Circulation 112:1594–1600 [DOI] [PubMed]
- Smilde TJ, van Wissen S, Wollersheim H, Kastelein JJ, Stalenhoef AF (2001) Genetic and metabolic factors predicting risk of cardiovascular disease in familial hypercholesterolemia. Neth J Med 59:184–195 [DOI] [PubMed]
- Smith BR, Heverhagen J, Knopp M et al (2007) Localization to atherosclerotic plaque and biodistribution of biochemically derivatized superparamagnetic iron oxide nanoparticles (SPIONs) contrast particles for magnetic resonance imaging (MRI). Biomed Microdevices 9:719–727 [DOI] [PubMed]
- Snoeckx LH, Cornelussen RN, Van Nieuwenhoven FA, Reneman RS, Van Der Vusse GJ (2001) Heat shock proteins and cardiovascular pathophysiology. Physiol Rev 81:1461–1497 [DOI] [PubMed]
- Soltys BJ, Gupta RS (2000) Mitochondrial proteins at unexpected cellular locations: export of proteins from mitochondria from an evolutionary perspective. Int Rev Cytol 194:133–196 [DOI] [PubMed]
- Soltys BJ, Gupta RS (1997) Cell surface localization of the 60 kDa heat shock chaperonin protein (hsp60) in mammalian cells. Cell Biol Int 21:315–320 [DOI] [PubMed]
- Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL (1989) Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med 320:915–924 [DOI] [PubMed]
- Strauss HW, Grewal RK, Pandit-Taskar N (2004) Molecular imaging in nuclear cardiology. Semin Nucl Med 34:47–55 [DOI] [PubMed]
- Stuber M, Gilson WD, Schar M et al (2007) Positive contrast visualization of iron oxide-labeled stem cells using inversion-recovery with ON-resonant water suppression (IRON). Magn Reson Med 58:1072–1077 [DOI] [PubMed]
- Tahara N, Kai H, Yamagishi S et al (2007) Vascular inflammation evaluated by [18F]-fluorodeoxyglucose positron emission tomography is associated with the metabolic syndrome. J Am Coll Cardiol 49:1533–1539 [DOI] [PubMed]
- Torchilin VP (2002) PEG-based micelles as carriers of contrast agents for different imaging modalities. Adv Drug Deliv Rev 54:235–252 [DOI] [PubMed]
- Tsimikas S (2002) Noninvasive imaging of oxidized low-density lipoprotein in atherosclerotic plaques with tagged oxidation-specific antibodies. Am J Cardiol 90:22L–27L [DOI] [PubMed]
- Tsimikas S, Shaw PX (2002) Non-invasive imaging of vulnerable plaques by molecular targeting of oxidized LDL with tagged oxidation-specific antibodies. J Cell Biochem Suppl 39:138–146 [DOI] [PubMed]
- Ward KM, Aletras AH, Balaban RS (2000) A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST). J Magn Reson 143:79–87 [DOI] [PubMed]
- Weinmann HJ, Brasch RC, Press WR, Wesbey GE (1984) Characteristics of gadolinium-DTPA complex: a potential NMR contrast agent. AJR Am J Roentgenol 142:619–624 [DOI] [PubMed]
- Weissleder R, Mahmood U (2001) Molecular imaging. Radiology 219:316–333 [DOI] [PubMed]
- Weissleder R, Stark DD, Compton CC, Wittenberg J, Ferrucci JT (1987) Ferrite-enhanced MR imaging of hepatic lymphoma: an experimental study in rats. AJR Am J Roentgenol 149:1161–1165 [DOI] [PubMed]
- Wick G, Knoflach M, Xu Q (2004) Autoimmune and inflammatory mechanisms in atherosclerosis. Annu Rev Immunol 22:361–403 [DOI] [PubMed]
- Wickline SA, Lanza GM (2003) Nanotechnology for molecular imaging and targeted therapy. Circulation 107:1092–1095 [DOI] [PubMed]
- Winalski CS, Shortkroff S, Mulkern RV, Schneider E, Rosen GM (2002) Magnetic resonance relaxivity of dendrimer-linked nitroxides. Magn Reson Med 48:965–972 [DOI] [PubMed]
- Winter PM, Caruthers SD, Yu X et al (2003a) Improved molecular imaging contrast agent for detection of human thrombus. Magn Reson Med 50:411–416 [DOI] [PubMed]
- Winter PM, Morawski AM, Caruthers SD et al (2003b) Molecular imaging of angiogenesis in early-stage atherosclerosis with alpha(v)beta3-integrin-targeted nanoparticles. Circulation 108:2270–2274 [DOI] [PubMed]
- Winter PM, Shukla HP, Caruthers SD et al (2005) Molecular imaging of human thrombus with computed tomography. Acad Radiol 12(Suppl 1):S9–S13 [DOI] [PubMed]
- Winter PM, Neubauer AM, Caruthers SD et al (2006) Endothelial alpha(v)beta3 integrin-targeted fumagillin nanoparticles inhibit angiogenesis in atherosclerosis. Arterioscler Thromb Vasc Biol 26:2103–2109 [DOI] [PubMed]
- Xu Q, Wick G (1996) The role of heat shock proteins in protection and pathophysiology of the arterial wall. Mol Med Today 2:372–379 [DOI] [PubMed]
- Xu QB, Oberhuber G, Gruschwitz M, Wick G (1990) Immunology of atherosclerosis: cellular composition and major histocompatibility complex class II antigen expression in aortic intima, fatty streaks, and atherosclerotic plaques in young and aged human specimens. Clin Immunol Immunopathol 56:344–359 [DOI] [PubMed]
- Xu Q, Dietrich H, Steiner HJ, Gown AM, Schoel B, Mikuz G, Kaufmann SH, Wick G (1992) Induction of arteriosclerosis in normocholesterolemic rabbits by immunization with heat shock protein 65. Arterioscler Thromb 12:789–799 [DOI] [PubMed]
- Xu Q, Schett G, Seitz CS, Hu Y, Gupta RS, Wick G (1994) Surface staining and cytotoxic activity of heat-shock protein 60 antibody in stressed aortic endothelial cells. Circ Res 75:1078–1085 [DOI] [PubMed]
- Xu Q, Schett G, Perschinka H et al (2000) Serum soluble heat shock protein 60 is elevated in subjects with atherosclerosis in a general population. Circulation 102:14–20 [DOI] [PubMed]
- Young RA, Elliott TJ (1989) Stress proteins, infection, and immune surveillance. Cell 59:5–8 [DOI] [PubMed]
- Yu X, Song SK, Chen J et al (2000) High-resolution MRI characterization of human thrombus using a novel fibrin-targeted paramagnetic nanoparticle contrast agent. Magn Reson Med 44:867–872 [DOI] [PubMed]
- Zhang SH, Reddick RL, Piedrahita JA, Maeda N (1992) Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science 258:468–471 [DOI] [PubMed]