Abstract
Bacterial endotoxins are known as stress factors for endothelial cells. In 20 normocholesterolemic New Zealand White (NZW) rabbits, endothelial stress was induced by intravenous (i.v.) injection of lipopolysaccharide (LPS), while eight NZW rabbits were sham-treated or served as untreated controls. In vivo molecular imaging was performed using co-registered computer tomography and positron emission tomography 24 h after i.v. injection of 124I-labeled monoclonal anti-HSP60 or 124I-radiolabelled isotype control antibodies. Compared to control animals, in vivo images of rabbit aortae revealed significantly increased endothelial binding of 124I-labeled anti-HSP60 antibodies upon LPS, especially at sites of aortal branching. This was confirmed by immunohistochemistry and autoradiography data. Our results showed, as proof-of-principle, that HSP60-expression in normocholesterolemic rabbits is significantly increased after induction of endothelial stress and that non-invasive in vivo molecular imaging of early aortal HSP60-expression using 124I-labeled anti-HSP60 monoclonal antibodies is possible.
Keywords: Atherosclerosis, Imaging, Isotope labeling, Radiology, Stress proteins
Introduction
Molecular imaging techniques based on magnetic resonance imaging, computer tomography (CT), ultrasound, and positron emission tomography (PET) have been proposed to accurately identify atherosclerotic plaques, plaque formation and vulnerable plaque. In addition to tumors, CVD, especially atherosclerosis, have been shown to be paradigmatic candidates for in vivo imaging (Jaffer et al. 2006a, b). The main reason for this is the fact that i.v.-injected ligands first meet their targets in the vascular tree without having to leave the vascular system. Successful imaging of fully developed atherosclerotic lesions has been achieved in experimental models, notably targeting adhesion molecules such as vascular cell adhesion molecule-1, an adhesion molecule for effector/memory T cells homing to areas of inflammation (Jaffer and Weissleder 2004; Kelly et al. 2005; Nahrendorf et al. 2006, 2008). Most of this work was performed in animals that first underwent invasive endothelial denudation and/or induction of hypercholesterolemia to create vascular lesions (Mettinger et al. 1978; Ord et al. 1992; Tsourkas et al. 2005), as detailed in the accompanying Mini-Review (Wick et al. 2008).
From our own and other groups’ data, it has been shown that heat shock protein (HSP)-60 expression by endothelial cells is an initiating event that renders them a target for attack by pre-existing, in principle protecting, innate and adaptive immunity, even under normocholesterolemic conditions (Frostegard et al. 1999; Wick et al. 1995, 2004a,b). HSP60 has been shown to be expressed as a first response to endothelial stressors even under normocholesterolemic conditions (Seitz et al. 1996) and has not so far been considered an appropriate in vivo imaging target for diagnostic or therapeutic purposes. As indicated, our interest is primarily focussed on the earliest inflammatory stages of atherosclerosis that often occur in the absence of high cholesterol levels, i.e. as presented by mononuclear cell infiltrates in the intima even without concomitant appearance of foam cells. It was thus deemed important to visualise the early endothelial stress response, as previous work in experimental animals and human specimens indicated the potential usefulness of HSP60 as a new imaging target (Hochleitner et al. 2000; Schett et al. 1995; Seitz et al. 1996).
The present project reported herein aimed, as proof-of-principle, at developing radiotracer-based molecular imaging for in vivo visualisation of HSP60 expression in a normocholesterolemic rabbit model.
Materials and methods
Animals
All animal experiments were approved by the Committee for Animal Experimentation of the Austrian Ministry of Science and Research (BMBWK-66.011/0011-BrGT/2005; BMBWK-66.011/0017-BrGT/2006).
All experimental animals used in this study were normocholesterolemic female New Zealand White (NZW) rabbits (Charles River Laboratories, Kisslegg, Germany), fed a standard rabbit chow (N-775) and hey-briquettes until 24 h before the induction of arterial stress or sham-treatment. Drinking water was given automatically ad libitum. All rabbits were 30–35 weeks of age, and their mean bodyweight was 3.1 ± 0.3 kg.
Before the experiments, all animals were kept for at least 4 weeks under routine husbandry in the Central Laboratory Animal Facilities of the Innsbruck Medical University in standard sized cages (Scanbur, Karlslunde, Denmark) in groups of two. After the start of the experiments and until the immunohistochemical and/or imaging analyses, the animals were kept in individual customised plastic cages under routine husbandry with N-775 food, hey-briquettes, and drinking water ad libitum.
Euthanasia of all animals was performed under intramuscular anaesthesia with ketamine at 35 mg/kg and xylazine at 5–10 mg/kg by open heart in vivo perfusion with phosphate-buffered saline (PBS; pH 7.2) followed by perfusion-fixation with 4% PBS-buffered paraformaldehyde (PFA; Merck, Darmstadt, Germany; catalogue no. 2307047). Four-percent PBS-buffered PFA solution was injected into the left heart ventricle, while systemic blood was simultaneously withdrawn from the right ventricle using arterio-venous fistula needles (mod. HTC-15B, 16G; Nipro Corporation, Osaka, Japan).
A total of 28 NZW rabbits were used in this study, performed in two major steps, as detailed below (Fig. 1): Step I was an initial validation trial for in vitro immunohistochemical analyses, and step II was non-invasive in vivo molecular imaging experiments followed by autoradiography and in vitro immunohistochemistry studies. Twelve NZW rabbits were used for part I of this study and 16 NZW rabbits for part II. After sacrifice, aortae were carefully removed intact for microscopic assessment and autoradiography, as described below.
Fig. 1.
Experimental design. Part I, three experimental groups: (a) eight NZW rabbits injected into the ear vein with bacterial LPS (Escherichia coli-endotoxin) at 10 μg/kg bodyweight diluted in 0.2 ml tissue culture medium RPMI 1640; (b) two sham-treated NZW rabbits injected i.v. 0.2 ml RPMI 1640, and c two untreated control animals. Part II, 12 animals injected i.v. with 10 μg/kg LPS diluted in 0.2 ml RPMI 1640, and four untreated control animals
Blood cholesterol level analyses
Two days before the experiments, 2 ml of venous blood was withdrawn by ear vein puncture to determine blood cholesterol levels. Cholesterol (CHOD-PAP, Roche, Mannheim, Germany) was measured with an enzymatic test on a Modular/P analyser (Roche). All rabbits used throughout the study were confirmed normocholesterolemic (below 40 mg/dl cholesterol).
Induction of stress in experimental animals and controls
For part I, three experimental groups were investigated: (a) Eight NZW rabbits were injected into the ear vein with bacterial LPS (Escherichia coli-endotoxin; serotype 055:B5, L-2880, Sigma, Munich, Germany) at 10 μg/kg bodyweight, diluted in 0.2 ml tissue culture medium RPMI 1640 (Seromed, Berlin, Germany); (b) the second group of two sham-treated NZW rabbits were injected i.v. with 0.2 ml RPMI 1640, and two control animals (c) were untreated.
For part II, 12 animals were injected i.v. 10 μg/kg LPS (Sigma; catalogue no. L2880) diluted in 0.2 ml RPMI 1640 (Invitrogen, Lofer, Germany; catalogue no. BE12-167F), and four animals served as untreated controls.
Experimental design
For the in vitro experiments in part I, the experimental animals were injected at baseline with LPS, as described (Fig. 1). Control animals remained untreated. Prior to and six hours after LPS injection, rectal temperature was measured and the animals behaviour was assessed. Behavioural changes could have been apathy as determined by reduced reaction to light and/or only half-lidded eyes, retreating into the cage corner, refusal of food and water uptake, the development of ruffled fur, etc.. Thereafter, all animals were anaesthetised and sacrificed via in vivo perfusion-fixation with 4% PBS-buffered PFA. After preparation of the aorta, the specimens were stained en face for expression of HSP60 by qualitative immunohistochemical microscopic assessment. For visualisation of the specific immune reaction, a specifically modified immunohistochemical staining procedure was applied to the vessels (see below). The microscopic detection was documented by digital photography using a stereomicroscope.
For the qualitative in vivo molecular imaging experiments in part II, endothelial stress was induced in 12 animals with LPS, as mentioned. Four control animals remained untreated. Again, the biological effect of LPS was determined by rectal body temperature measurement and assessment of behavioural changes at baseline and at 6 h before injection with radiolabelled-monoclonal antibodies (Mabs). Twelve of the 16 animals (10 prior injected with LPS) received radiolabelled-anti-HSP60 Mabs and four of the animals (two LPS-treated and two controls) were injected with radiolabelled-isotype-matched control antibodies.
To determine the systemic chronological distribution of 124I-radioactive-labeled mouse IgG2a anti-HSP60 Mabs and 124I-radioactive-labeled isotype control antibodies, in vivo CT and PET images were also performed in two animals at 2, 12, and 18 h after the injection (=8, 18, and 24 h after the i.v. injection of 10 μg LPS/kg bodyweight).
Twenty-four hours after the injection of the radiolabelled antibodies, all animals were anaesthetised and subjected to CT followed by non-invasive molecular imaging for HSP60 expression using PET, as described below. CT images were co-registered with PET images to improve exact localisation and delineation of aortal tracer activity. Thereafter, the animals were sacrificed via in vivo PFA perfusion fixation.
Autoradiography
After preparation of the aorta, the en face specimens were exposed for 24 h on a storage phosphor screen (high resolution, 12.5 × 25.2 cm; Perkin Elmer, Downers Grove, IL) and analysed using a storage phosphor system (Cyclon Plus, Perkin Elmer). Subsequently, the vessels were stained for expression of HSP60 using specific antibodies (see below).
Radiolabelled antibodies
The in vitro cytotoxic and in vivo atherogenic potential of Mab II-13 has been documented previously (Foteinos et al. 2005; Schett et al. 1995; Soltys and Gupta 2000). The Mabs recognise eukaryotic HSP60 and have been shown to be cytolytic (Schett et al. 1995). Antibody preparations were tested for the absence of endotoxins using limulus–amoebocyte–lysate (LAL) tests (Charles River Laboratories, Wilmington, MA) Endochrome-K Kinetic Chromogenic LAL, catalogue no. 1197) and purified by column chromatography. The specificity of the antibody has been proven by immunohistochemistry on frozen sections of standard control tissues (human kidney, human carcinoma of the colon and advanced atherosclerotic lesions from previous rabbit experiments), Western blots and appropriate absorption studies with recombinant human HSP60. As mouse IgG2a isotype controls, we used commercially available Mabs specific for Aspergillum niger glucose oxidase (Dako, Glostrup, Denmark; catalogue no. X0943).
Mabs were radiolabelled for in vivo imaging of HSP60 and tested en face by autoradiography on in vitro rabbit aorta specimens after in vivo exposure to LPS, as mentioned above. Assessment of their unchanged reactivity and lack of cross-reactivity of control Mabs was performed in Western blots and absorptions with immobilised recombinant human HSP60 (data not shown).
Antibody labeling
Labeling of the anti-HSP60 Mabs and the isotype control antibodies with 124I-iodine was performed using the Iodogen method (Fraker and Speck 1978). In brief, 250 μl of the corresponding antibody (1 mg/ml) and 200 μl PBS (pH 7.4) were added into an Eppendorf cap coated with 150 μg Iodogen (Pierce, Rockford, IL, USA) followed by 75 MBq [124I]NaI in 0.02 N sodium hydroxide (QSA Global, Braunschweig, Germany). After incubation for 25 min at room temperature, the solution was transferred into another Eppendorf cap, and 1 μl was used for quality control [thin-layer chromatography (TLC) with 65% acetone/20% butanol/10% ammonia/5% distilled water as solvent]. If the amount of free iodine was above 8%, a subsequent size exclusion chromatography using a HiTrap desalting column (GE Healthcare Bio-Science, Uppsala, Sweden) was carried out. The column was pre-washed with 10 ml 0.9% sodium chloride solution. Subsequently, the reaction solution was loaded onto the column. The product was eluted with 10 ml 0.9% sodium chloride. Fractions (1 ml each) were measured in a Capintec CRC-15R dose calibrator (Capintec, Ramsey, NJ, USA), and the fractions with the highest activity were pooled (approximately 2–3). Radiochemical purity was controlled by TLC and was between 92% and 98%.
For in vivo experiments, 20–40 MBq 124I-labeled antibodies were injected i.v. into the ear vein of the rabbits.
CT and PET protocols
Twenty-four hours after injection of 124I-radiolabelled antibodies, all animals underwent CT (Somatom Sensation, Siemens, Erlangen, Germany) immediately followed by non-invasive molecular imaging of 124I-labeled antibody distribution by a dedicated conventional human PET scanner (Advance, General Electric Medical Systems, Milwaukee, WI, USA). For both CT and PET, the anaesthetised animals were appropriately fixed in supine position on customised boards with detectable reference points attached to avoid body movement during the examinations and allow for an optimal later fusion of CT and PET images datasets (PET–CT fusion). CT images were co-registered with PET images to improve delineation of aortal tracer activity.
In each animal, we performed a native cranio-caudal whole-body multislice CT according to the following protocol: Anterior–posterior scout images were obtained, followed by native CT with a collimation of 40 × 0.6 mm, a gentry speed of 33.6 mm/s, a pitch of 0.7, a tube current of 80 kVp and a wattage of 100 mAs. The reconstructed slice thickness was 1.0 mm.
The PET examinations were done with a 2D protocol in three bed positions. The reconstructed field of view was 24 cm in diameter, and reconstruction parameters comprised interative reconstructions by means of the systems OSEM algorithm (two interations, 32 subsets).
Whole-mount immunohistochemical staining
After PET and CT scan, the anaesthetised animals were killed via in vivo perfusion, as described. After preparation of the aorta, en face specimens were autoradiographed overnight and placed in 4% PBS-buffered PFA for another 2 h. All subsequently performed washing and incubation steps were performed in 50-ml plastic tubes. The en face specimens were washed with PBS (3 × 3 min on an orbital shaker) and incubated with 0.3% H2O2/PBS (20 min) to block endogenous peroxidase reactions. After three PBS washing steps, unspecific binding of antibodies was prevented by further 60-min incubation with a solution of 5% bovine calf serum (BCS; PAA Laboratories, Pasching, Austria; catalogue no. B15-004) in Blocking Reagent (Roche, Mannheim, Germany; catalogue no. 1096176) diluted in PBS-T (0.3% Triton-X-100 (Merck; catalogue no. 1.08603) in PBS). Thereafter, vessels were incubated O/N (minimum of 15 h) either with the mouse IgG2a anti-HSP60 primary antibody or a mouse IgG2a isotype control. Both antibodies were applied at the same protein concentration of 10 μg/ml using 1%BCS/PBS-T as diluent. After further washing steps with PBS (3 × 10 min), vessels were incubated (90 min) with a goat anti-mouse Ig biotin-labeled conjugate (Dako; catalogue no. E0433) diluted 1:300 with PBS-T. Vessels were again washed three times with PBS and incubated for 60 min with horseradish-peroxidase-labeled streptavidin (Dako; catalogue no. P0397) diluted 1:300 with PBS-T. Three final washing steps using PBS were applied, followed by the application of diaminobenzidine-metal enhancer substrate (Sigma; catalogue no. D0426). The vessels were exposed to the substrate under visual control up to 22 min. Colour reaction was stopped by washing the aortae with tap water. Microscopic documentation of the specific antibody and negative control reaction was performed with a stereo-microscope (Leitz) and a digital camera (Nikon).
Testing the specificity of 124I-labeled anti-HSP60 Mabs by Western blot
Recombinant human HSP60 produced in our laboratories [EC-sponsored facility on HSP-reagents; Project BMH4-(198-3935) as described (Ramage et al. 1999)] was blotted in duplicate onto nitrocellulose membranes, 1 μl/spot was applied using a starting concentration of 1 mg/ml and log 10 dilutions thereafter. After blotting, membranes were incubated in 5% fat-free milk/PBS for 1 h. Primary antibody-124I-labeled mouse anti-HSP60 (clone II-13) was diluted 1:1,000 in 5% fat free milk/PBS and incubated with the membrane for 1 h on an orbital shaker. After several washing cycles using 0.1% Tween-20/PBS (Roth, Graz, Austria; catalogue no. 9127.1; 5 × 5 min) and a subsequent wash with PBS (3 × 5 min), the detection was performed on X-ray film for 2 h up to overnight.
Results
Effect of LPS injection
In all 28 experimental animals, baseline rectal measurements revealed a mean body temperature of 38.3 ± 0.4°C, i.e. physiological values before the start of the experiments and cholesterol levels within normal range. Moreover, all experimental animals presented with normal behaviour and appearance of fur at baseline. In animals i.v. injected with LPS, the mean body temperature increased to 40.3 ± 0.3°C 6 h after LPS injection, whereas no increase of body temperature was seen in sham-treated animals or untreated controls. In contrast to the two latter groups, most of the 18 NZW rabbits revealed signs of apathy 6 h after LPS injection, and all LPS-injected animals showed variable degrees of ruffled fur, while no changes were seen in sham-treated animals or untreated controls.
Specificity of radiolabelled antibodies
Using dot blot assays with recombinant human HSP60 as antigenic substrate, the reactivity of anti-HSP60 Mabs II-13 after radiolabelling was found to be unchanged compared to unlabeled antibodies (data not shown). The isotype control antibody did not cross-react with recombinant mammalian HSP60 in either its unlabeled or radiolabelled states.
Systemic distribution of 124I-radiolabelled Mabs
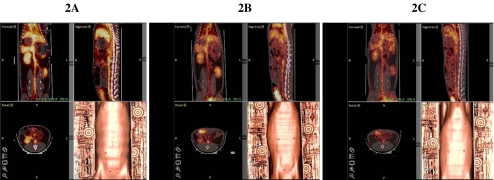
Figure 2A,B and C shows co-registered in vivo CT and PET images of the systemic distribution of i.v.-injected 124I-labeled anti-HSP60 Mab at 2 (Fig. 2A), 12 (Fig. 2B) and 18 (Fig. 2C) h. At 2 h, a strong and homogenous signal of 124I-labeled anti-HSP60 Mabs was detectable in thyroid glands, liver, spleen, kidneys, urinary bladder, heart and aorta, while no radioactivity was detectable in the lungs, intestines or skeletal muscles. The same systemic distribution was seen for 124I-labeled control antibodies (data not shown). Images after 12 and 18 h, respectively, showed continuous elimination of the tracer from the body with neither activity retention nor additional uptake in any of the studied organs.
Fig. 2.
Coronal (left upper panels), sagittal (right upper panels), axial (left lower panels) and 3D surface reconstructions (right lower panels) of co-registered in vivo CT and PET images of the systemic distribution of i.v. injected 124I-labeled anti-HSP60 Mab in a control NZW rabbit at 2 (A), 12 (B), and 18 (C) h
In vivo imaging after i.v. injection of 124I-radiolabelled anti-HSP60 antibodies and isotope controls
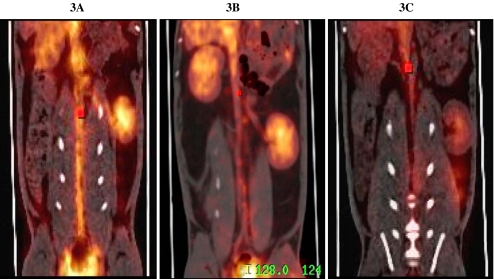
The signals of 124I-radiolabelled anti-HSP60 antibodies visible in the aortae of animals previously injected with LPS showed a scattered pattern that was especially intense at areas of arterial branching (Fig. 3A). In contrast, in animals not injected with LPS, intravascular presence of 124I-labeled anti-HSP60 antibodies was also noted, albeit at low intensity and without the scattered pattern of aortal signals (Fig. 3B). In animals i.v. injected with LPS and 124I-labeled control antibodies, the intensity of signals from 124I-labeled control Mabs in parenchymatous organs and urinary bladder continuously decreased from baseline to 24 h, but no specific binding was observed in the aortae (Fig. 3C). Of note, using the co-registered PET-CT technique, the signals from 124I-labeled anti-HSP60 Mabs were only delineable in the aorta, but not in venous vessels, i.e. the inferior vena cava.
Fig. 3.
Representative in vivo molecular images 24 h after i.v. injection of 124I-radiolabeled anti-HSP60 antibodies and 124I-radiolabeled isotope controls Mabs using co-registered PET-CT technique. A PET-CT of a LPS-treated NZW rabbit after i.v. injection of 124I-radiolabeled anti-HSP60 antibodies. Of note, signals from 124I-labeled anti-HSP60 Mabs were only delineable in the aorta, not in venous vessels, i.e. the inferior vena cava. B LPS-untreated control after i.v. injection of 124I-radiolabeled anti-HSP60 antibodies. C In vivo PET-CT image of a LPS-treated NZW rabbit, 24 h after i.v. injection of 124I-labeled control antibodies
Autoradiography results
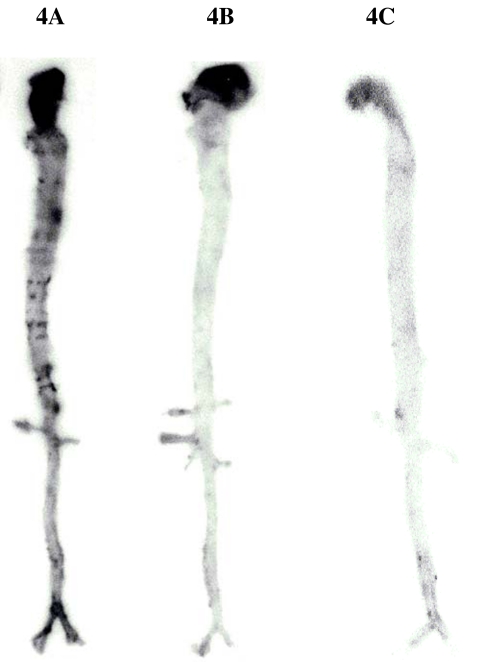
Figure 4A,B and C shows representative autoradiographic documentation of aortae removed from rabbits 24 h after injection of 124I-radiolabelled anti-HSP60 Mabs, i.e. 30 h after injection of LPS (Fig. 4A), an LPS-untreated control 24 h after injection of 124I-radiolabelled anti-HSP60 Mabs (Fig. 4B) and of a LPS-treated rabbit 24 hours after injection of 124I-radiolabelled isotype control Mabs (30 h after injection of LPS; Fig. 4C). Markedly increased signals from 124I-radiolabelled anti-HSP60 Mabs, especially at areas of arterial branching, were only observed in rabbits that had initially been stressed with LPS, whereas no signals from 124I-radiolabelled Mabs were detected in untreated controls or animals that were i.v. injected with 124I-radiolabelled isotype control Mabs.
Fig. 4.
Autoradiographic documentation of aortae removed from: an LPS-stressed rabbit 24 h after injection of 124I-radiolabeled anti-HSP60 Mabs (A), an LPS-untreated control rabbit after injection of 124I-radiolabeled anti-HSP60 Mabs (B), and of a LPS-treated rabbit after the injection of 124I-radiolabeled isotype control Mabs (C)
Immunohistochemical demonstration of HSP60
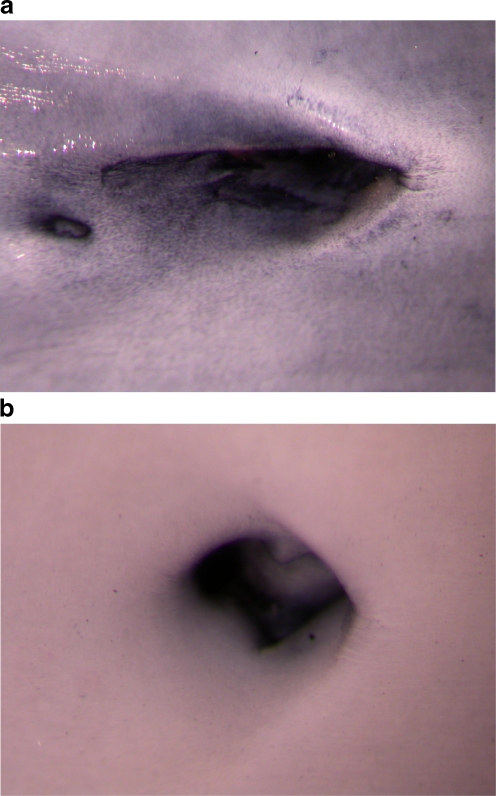
The expression of HSP60 by arterial endothelial cells of animals injected with LPS was also demonstrated immunohistochemically using the en face technique (Fig. 5A,B). The most intensive HSP60 staining was again observed at arterial branching points, i.e. the known predilection sites that are subjected to turbulent rather than laminar shear stress, locations known to be predisposed to the development of atherosclerotic lesions.
Fig. 5.
En face in vitro immunohistochemical demonstration of HSP60 expression of a rabbit aorta branching into an intercostal artery using a mouse IgG2a anti-HSP-60 Mab (clone II-13) 30 h after 10 μg/kg bodyweight LPS injection (a), compared to a negative staining control using unspecific mouse IgG2a (b). Original magnification: 1.6 × 2.0
Discussion
Since cardiovascular diseases in general and atherosclerosis in particular are the number one killers in developed countries, their early diagnosis, prevention and treatment deserve special emphasis. In the past, the major focus of atherosclerosis research was put on changes of the lipid metabolism (Glass and Witztum 2001; Smilde et al. 2001; Steinberg et al. 1989), but more recently, inflammatory-immunologic processes during atherogenesis have received major attention (Hansson 2005; Libby 2002; Wick et al. 2004b). Most of this work, however, dealt with the conditions of well-developed lesions with clinically apparent symptoms, such as myocardial infarction, stroke or peripheral arterial occlusion. In addition, most experimental studies relied on the use of transgenic or knockout mouse models that mimic special hereditary situations in humans, such as functional hypercholesterolemia due to a deficiency of LDL-receptor expression (Zhang et al. 1992). In contrast, our interest is focussed on the very earliest stages of the disease that consists of mononuclear cell infiltration of the intima in normocholesterolemic humans or experimental animals even without foam cells (Millonig et al. 2001; Xu et al. 1990).
As wild-type mice are notoriously resistant to the induction of atherosclerosis by classical atherosclerosis risk factors, we have, in the past, successfully resorted to the rabbit model. In this model, atherosclerosis can be readily induced by subjection to risk factors, including, of course, high blood cholesterol levels.
In previous investigations, we have shown that classical atherosclerosis risk factors first act as endothelial stressors that lead to the simultaneous expression of adhesion molecules and HSP60 (Amberger et al. 1997; Hochleitner et al. 2000; Seitz et al. 1996). We have also shown that normocholesterolemic rabbits develop the initial inflammatory intimal infiltration upon immunisation with HSP60, a phenomenon that can be observed even if no other risk factors, such as high cholesterol blood levels, are present. This initial inflammatory stage is still reversible but can be exaggerated by a high cholesterol diet to a stage that no longer seems reversible, at least during a 32-week observation period (Xu et al. 1996).
In hypercholesterolemic mice, the “baseline” atherosclerosis can be significantly aggravated by immunisation with HSP60 (George et al. 1999), while immunisation with oxidised LDL or malondialdehyde-modified LDL has a protective effect (Caligiuri et al. 2002; George et al. 1998). Interestingly, rats immunised with HSP65 develop adjuvant arthritis resembling human rheumatoid arthritis (van Eden et al. 2005) but no atherosclerosis, while the reverse is true for rabbits (Xu et al. 1992), an observation highlighting the importance of identifying arthritogenic and atherogenic HSP60 epitopes, respectively. As a matter of fact, this goal has already been achieved for arthritogenic and arthritoprotective HSP60 T-cell epitopes (Prakken et al. 1997; van Eden and Waksman 2003), while for atherosclerosis, this is only true for B-cell epitopes (Perschinka et al. 2003, 2007). However, similar to the situation in synovial tissues of animals and humans, accumulation of HSP60-reactive T cells in atherosclerotic plaques compared to peripheral blood was found in humans (Benagiano et al. 2003, 2005) and rabbits (Rossmann et al. 2008; Xu et al. 1993a). In mice, the disease could be transferred by HSP60-specific T cells to syngeneic recipients (George et al. 2001). Furthermore, atherosclerotic lesions can also be produced in mice by the transfer of anti-HSP60 antibodies, both of murine and human origin (Foteinos et al. 2005).
In human prospective longitudinal and cross-sectional studies, a significant correlation of the titer of anti-HSP60 antibodies and the occurrence of sonographically demonstrable atherosclerotic lesions was demonstrated (Xu et al. 1993b). However, this correlation becomes less significant the younger these cohorts were (Knoflach et al. 2003a). In contrast, HSP60 reactive T cells showed a better correlation with increased intima-media thickness the younger the cohort was (Knoflach et al. 2007).
Functional studies of T cells derived from early, clinically inapparent, lesions are so far lacking for obvious reasons.
From these and other data, we concluded that HSP60 expression by endothelial cells is an initiating event that renders them a target for attack by pre-existing, in principle protecting, innate and adaptive immunity (Wick et al. 2004b). Arterial endothelial cells have a lower threshold for HSP60-inducing risk factors due to being subjected to lifelong arterial blood pressure compared to venous endothelial cells. Interestingly, endothelial HSP60 expression and mononuclear intima-infiltration are also initial hallmarks of restenosis in coronary and carotid bypass conduits prone to restenosis (Knoflach et al. 2003b).
The present paper represents our successful first attempt to provide in vivo proof-of-principle for the concept that an endothelial stressor such as LPS induces HSP60 expression that can be visualised by classical isotope-based imaging techniques. LPS per se is not a known atherosclerosis risk factor but can be taken as one of the possible surrogates for the lifelong infectious load that has been shown to dramatically increase the odds ratio of developing the disease (Danesh et al. 1997; Mayr et al. 1999, 2000, 2003; Ridker et al. 2000; Zhu et al. 2001). In future experiments, we plan to extend the list of in vivo applied endothelial stressors to various classical risk factors, notably cigarette smoke extract (Bernhard et al. 2005).
Finally, post-in vivo imaging autoradiography and en face immunohistochemistry convincingly corroborated the data obtained by CT/PET analysis. Importantly, the en face immunohistochemical data also provided additional proof for the concept that HSP60 expression is most intense at arterial branching points, especially at areas subjected to turbulent rather than laminar flow shear stress.
Besides providing in vivo proof-of-principle for the idea that an endothelial stressor leads to the expression of HSP60, the present results may also form the basis for future practical diagnostic and therapeutic applications. Diagnostic applications are particularly important in view of the alarming observations even in our Alpine region that 28% of clinically healthy 17- to 18-year-old male (Knoflach et al. 2003a) and 20% of 19- to 21-year-old female (unpublished data) volunteers already show a pathologically increased intima-media thickness. As expected, these alterations correlate most significantly with active and passive smoking, followed by peripheral blood T-cell reactivity against human HSP60 and, surprisingly, only in third place with increased blood pressure. We hypothesise that endothelial HSP60 expression precedes the increase in intima-media thickness, which means that a reliable diagnostic tool not necessarily involving radiotracers is highly desirable to identify persons at risk.
For the present studies, we selected the murine monoclonal antibody Mab II-13 with exquisite specificity for eukaryotic HSP60 for which in vitro and in vivo reactivity with stressed endothelial cells has been demonstrated previously (Soltys and Gupta 1997).
For radiolabelling, 124Iodine was selected because of its proven usefulness in the applied imaging systems and its suitable half-life (Davies et al. 2005, 2006; Rudd et al. 2005).
We chose the combination of PET and CT based on the limited anatomic information in PET scans alone that can be complemented by the accurate anatomic capabilities of CT.
Our results may be equally relevant from a future therapeutic viewpoint: It is conceivable that the expression of HSP60, and thus immunological target function of endothelial cells, could be suppressed by molecular biological methods, such as interference RNA (iRNA), or by drugs that have recently been described to either exert a broad spectrum of HSP suppression or to affect the expression of specific HSP family members (Macario and Conway de Macario 2007; Powers and Workman 2007). The transport to, and the local release of, these agents at sites of HSP60 expression in the vascular tree could be achieved by appropriate antibody-coated particles.
Experiments to address these issues, including quantitative evaluations and tracer kinetics, are now under way in our laboratory.
Acknowledgements
This work was supported by the Propter Homines Foundation, Vaduz, FL (to GW), the Medizinische Forschungsförderung Innsbruck MFI (to MCW, Project 9443), the European Union as part of the project Molecular Basis of Vascular Events Leading to Thrombotic Stroke (MOLSTROKE; LSHM-CT-2004-005206), and the Network of Excellence European Vascular Genomics Network (EVGN; LSHM-CT-2003-503254). Editorial assistance from M. Kat Occhipinti-Bender is acknowledged.
References
- Amberger A, Maczek C, Jurgens G et al (1997) Co-expression of ICAM-1, VCAM-1, ELAM-1 and Hsp60 in human arterial and venous endothelial cells in response to cytokines and oxidized low-density lipoproteins. Cell Stress Chaperones 2:94–103 [DOI] [PMC free article] [PubMed]
- Benagiano M, Azzurri A, Ciervo A et al (2003) T helper type 1 lymphocytes drive inflammation in human atherosclerotic lesions. Proc Natl Acad Sci U S A 100:6658–6663 [DOI] [PMC free article] [PubMed]
- Benagiano M, D’Elios MM, Amedei A et al (2005) Human 60-kDa heat shock protein is a target autoantigen of T cells derived from atherosclerotic plaques. J Immunol 174:6509–6517 [DOI] [PubMed]
- Bernhard D, Csordas A, Henderson B, Rossmann A, Kind M, Wick G (2005) Cigarette smoke metal-catalyzed protein oxidation leads to vascular endothelial cell contraction by depolymerization of microtubules. FASEB J 19:1096–1107 [DOI] [PubMed]
- Caligiuri G, Nicoletti A, Poirier B, Hansson GK (2002) Protective immunity against atherosclerosis carried by B cells of hypercholesterolemic mice. J Clin Invest 109:745–753 [DOI] [PMC free article] [PubMed]
- Danesh J, Collins R, Peto R (1997) Chronic infections and coronary heart disease: is there a link? Lancet 350:430–436 [DOI] [PubMed]
- Davies JR, Rudd JF, Fryer TD, Weissberg PL (2005) Targeting the vulnerable plaque: the evolving role of nuclear imaging. J Nucl Cardiol 12:234–246 [DOI] [PubMed]
- Davies JR, Rudd JH, Weissberg PL, Narula J (2006) Radionuclide imaging for the detection of inflammation in vulnerable plaques. J Am Coll Cardiol 47:C57–C68 [DOI] [PubMed]
- Foteinos G, Afzal AR, Mandal K, Jahangiri M, Xu Q (2005) Anti-heat shock protein 60 autoantibodies induce atherosclerosis in apolipoprotein E-deficient mice via endothelial damage. Circulation 112:1206–1213 [DOI] [PubMed]
- Fraker PJ, Speck JC Jr (1978) Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun 80:849–857 [DOI] [PubMed]
- Frostegard J, Ulfgren AK, Nyberg P, Hedin U, Swedenborg J, Andersson U, Hansson GK (1999) Cytokine expression in advanced human atherosclerotic plaques: dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis 145:33–43 [DOI] [PubMed]
- George J, Afek A, Gilburd B et al (1998) Hyperimmunization of apo-E-deficient mice with homologous malondialdehyde low-density lipoprotein suppresses early atherogenesis. Atherosclerosis 138:147–152 [DOI] [PubMed]
- George J, Shoenfeld Y, Afek A et al (1999) Enhanced fatty streak formation in C57BL/6J mice by immunization with heat shock protein-65. Arterioscler Thromb Vasc Biol 19:505–510 [DOI] [PubMed]
- George J, Afek A, Gilburd B, Shoenfeld Y, Harats D (2001) Cellular and humoral immune responses to heat shock protein 65 are both involved in promoting fatty-streak formation in LDL-receptor deficient mice. J Am Coll Cardiol 38:900–905 [DOI] [PubMed]
- Glass CK, Witztum JL (2001) Atherosclerosis. The road ahead. Cell 104:503–516 [DOI] [PubMed]
- Hansson GK (2005) Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 352:1685–1695 [DOI] [PubMed]
- Hochleitner BW, Hochleitner EO, Obrist P, Eberl T, Amberger A, Xu Q, Margreiter R, Wick G (2000) Fluid shear stress induces heat shock protein 60 expression in endothelial cells in vitro and in vivo. Arterioscler Thromb Vasc Biol 20:617–623 [DOI] [PubMed]
- Jaffer FA, Weissleder R (2004) Seeing within: molecular imaging of the cardiovascular system. Circ Res 94:433–445 [DOI] [PubMed]
- Jaffer FA, Libby P, Weissleder R (2006a) Molecular and cellular imaging of atherosclerosis: emerging applications. J Am Coll Cardiol 47:1328–1338 [DOI] [PubMed]
- Jaffer FA, Nahrendorf M, Sosnovik D, Kelly KA, Aikawa E, Weissleder R (2006b) Cellular imaging of inflammation in atherosclerosis using magnetofluorescent nanomaterials. Mol Imaging 5:85–92 [PubMed]
- Kelly KA, Allport JR, Tsourkas A, Shinde-Patil VR, Josephson L, Weissleder R (2005) Detection of vascular adhesion molecule-1 expression using a novel multimodal nanoparticle. Circ Res 96:327–336 [DOI] [PubMed]
- Knoflach M, Kiechl S, Kind M et al (2003a) Cardiovascular risk factors and atherosclerosis in young males: ARMY study (Atherosclerosis Risk-Factors in Male Youngsters). Circulation 108:1064–1069 [DOI] [PubMed]
- Knoflach M, Mayrl B, Mayerl C, Sedivy R, Wick G (2003b) Atherosclerosis as a paradigmatic disease of the elderly: role of the immune system. Immunol Allergy Clin North Am 23:117–132 [DOI] [PubMed]
- Knoflach M, Kiechl S, Mayrl B et al (2007) T-cell reactivity against HSP60 relates to early but not advanced atherosclerosis. Atherosclerosis 195:333–338 [DOI] [PubMed]
- Libby P (2002) Inflammation in atherosclerosis. Nature 420:868–874 [DOI] [PubMed]
- Macario AJ, Conway de Macario E (2007) Chaperonopathies and chaperonotherapy. FEBS Lett 581:3681–3688 [DOI] [PubMed]
- Mayr M, Xu Q, Wick G (1999) Atherogenic effects of chronic infections: the role of heat shock protein 60 in autoimmunity. Isr Med Assoc J 1:272–277 [PubMed]
- Mayr M, Kiechl S, Willeit J, Wick G, Xu Q (2000) Infections, immunity, and atherosclerosis: associations of antibodies to Chlamydia pneumoniae, Helicobacter pylori, and cytomegalovirus with immune reactions to heat-shock protein 60 and carotid or femoral atherosclerosis. Circulation 102:833–839 [DOI] [PubMed]
- Mayr M, Kiechl S, Mendall MA, Willeit J, Wick G, Xu Q (2003) Increased risk of atherosclerosis is confined to CagA-positive Helicobacter pylori strains: prospective results from the Bruneck study. Stroke 34:610–615 [DOI] [PubMed]
- Mettinger KL, Larsson S, Ericson K, Casseborn S (1978) Detection of atherosclerotic plaques in carotid arteries by the use of 123I-fibrinogen. Lancet 1:242–244 [DOI] [PubMed]
- Millonig G, Schwentner C, Mueller P, Mayerl C, Wick G (2001) The vascular-associated lymphoid tissue: a new site of local immunity. Curr Opin Lipidol 12:547–553 [DOI] [PubMed]
- Nahrendorf M, Jaffer FA, Kelly KA, Sosnovik DE, Aikawa E, Libby P, Weissleder R (2006) Noninvasive vascular cell adhesion molecule-1 imaging identifies inflammatory activation of cells in atherosclerosis. Circulation 114:1504–1511 [DOI] [PubMed]
- Nahrendorf M, Zhang H, Hembrador S et al (2008) Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation 117:379–387 [DOI] [PMC free article] [PubMed]
- Ord JM, Hasapes J, Daugherty A, Thorpe SR, Bergmann SR, Sobel BE (1992) Imaging of thrombi with tissue-type plasminogen activator rendered enzymatically inactive and conjugated to a residualizing label. Circulation 85:288–297 [DOI] [PubMed]
- Perschinka H, Mayr M, Millonig G et al (2003) Cross-reactive B-cell epitopes of microbial and human heat shock protein 60/65 in atherosclerosis. Arterioscler Thromb Vasc Biol 23:1060–1065 [DOI] [PubMed]
- Perschinka H, Wellenzohn B, Parson W, van der Zee R, Willeit J, Kiechl S, Wick G (2007) Identification of atherosclerosis-associated conformational heat shock protein 60 epitopes by phage display and structural alignment. Atherosclerosis 194:79–87 [DOI] [PubMed]
- Powers MV, Workman P (2007) Inhibitors of the heat shock response: biology and pharmacology. FEBS Lett 581:3758–3769 [DOI] [PubMed]
- Prakken BJ, van der Zee R, Anderton SM, van Kooten PJ, Kuis W, van Eden W (1997) Peptide-induced nasal tolerance for a mycobacterial heat shock protein 60 T cell epitope in rats suppresses both adjuvant arthritis and nonmicrobially induced experimental arthritis. Proc Natl Acad Sci U S A 94:3284–3289 [DOI] [PMC free article] [PubMed]
- Ramage JM, Young JL, Goodall JC, Gaston JS (1999) T cell responses to heat-shock protein 60: differential responses by CD4+T cell subsets according to their expression of CD45 isotypes. J Immunol 162:704–710 [PubMed]
- Ridker PM, Hennekens CH, Buring JE, Rifai N (2000) C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 342:836–843 [DOI] [PubMed]
- Rossmann A, Henderson B, Heidecker B et al (2008) T-cells from advanced atherosclerotic lesions recognize hHSP60 and have a restricted T-cell receptor repertoire. Exp Gerontol 43:229–237 [DOI] [PubMed]
- Rudd JH, Davies JR, Weissberg PL (2005) Imaging of atherosclerosis—can we predict plaque rupture? Trends Cardiovasc Med 15:17–24 [DOI] [PubMed]
- Schett G, Xu Q, Amberger A, Van der Zee R, Recheis H, Willeit J, Wick G (1995) Autoantibodies against heat shock protein 60 mediate endothelial cytotoxicity. J Clin Invest 96:2569–2577 [DOI] [PMC free article] [PubMed]
- Seitz CS, Kleindienst R, Xu Q, Wick G (1996) Coexpression of heat-shock protein 60 and intercellular-adhesion molecule-1 is related to increased adhesion of monocytes and T cells to aortic endothelium of rats in response to endotoxin. Lab Invest 74:241–252 [PubMed]
- Smilde TJ, van Wissen S, Wollersheim H, Kastelein JJ, Stalenhoef AF (2001) Genetic and metabolic factors predicting risk of cardiovascular disease in familial hypercholesterolemia. Neth J Med 59:184–195 [DOI] [PubMed]
- Soltys BJ, Gupta RS (1997) Cell surface localization of the 60 kDa heat shock chaperonin protein (hsp60) in mammalian cells. Cell Biol Int 21:315–320 [DOI] [PubMed]
- Soltys BJ, Gupta RS (2000) Mitochondrial proteins at unexpected cellular locations: export of proteins from mitochondria from an evolutionary perspective. Int Rev Cytol 194:133–196 [DOI] [PubMed]
- Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL (1989) Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med 320:915–924 [DOI] [PubMed]
- Tsourkas A, Shinde-Patil VR, Kelly KA, Patel P, Wolley A, Allport JR, Weissleder R (2005) In vivo imaging of activated endothelium using an anti-VCAM-1 magnetooptical probe. Bioconjug Chem 16:576–581 [DOI] [PubMed]
- van Eden W, Waksman BH (2003) Immune regulation in adjuvant-induced arthritis: possible implications for innovative therapeutic strategies in arthritis. Arthritis Rheum 48:1788–1796 [DOI] [PubMed]
- van Eden W, van der Zee R, Prakken B (2005) Heat-shock proteins induce T-cell regulation of chronic inflammation. Nat Rev Immunol 5:318–330 [DOI] [PubMed]
- Wick G, Schett G, Amberger A, Kleindienst R, Xu Q (1995) Is atherosclerosis an immunologically mediated disease? Immunol Today 16:27–33 [DOI] [PubMed]
- Wick G, Knoflach M, Kind M, Henderson B, Bernhard D (2004a) Heat shock proteins and stress in atherosclerosis. Autoimmun Rev 3(Suppl 1):S30–S31 [PubMed]
- Wick G, Knoflach M, Xu Q (2004b) Autoimmune and inflammatory mechanisms in atherosclerosis. Annu Rev Immunol 22:361–403 [DOI] [PubMed]
- Wick MC, Kremser C, Frischauf S, Wick G (2008) In vivo molecular imaging of vascular stress. Cell Stress Chaperones. DOI 10.1007/s12192-008-0043-3 [DOI] [PMC free article] [PubMed]
- Xu QB, Oberhuber G, Gruschwitz M, Wick G (1990) Immunology of atherosclerosis: cellular composition and major histocompatibility complex class II antigen expression in aortic intima, fatty streaks, and atherosclerotic plaques in young and aged human specimens. Clin Immunol Immunopathol 56:344–359 [DOI] [PubMed]
- Xu Q, Dietrich H, Steiner HJ, Gown AM, Schoel B, Mikuz G, Kaufmann SH, Wick G (1992) Induction of arteriosclerosis in normocholesterolemic rabbits by immunization with heat shock protein 65. Arterioscler Thromb 12:789–799 [DOI] [PubMed]
- Xu Q, Kleindienst R, Waitz W, Dietrich H, Wick G (1993a) Increased expression of heat shock protein 65 coincides with a population of infiltrating T lymphocytes in atherosclerotic lesions of rabbits specifically responding to heat shock protein 65. J Clin Invest 91:2693–2702 [DOI] [PMC free article] [PubMed]
- Xu Q, Willeit J, Marosi M et al (1993b) Association of serum antibodies to heat-shock protein 65 with carotid atherosclerosis. Lancet 341:255–259 [DOI] [PubMed]
- Xu Q, Kleindienst R, Schett G, Waitz W, Jindal S, Gupta RS, Dietrich H, Wick G (1996) Regression of arteriosclerotic lesions induced by immunization with heat shock protein 65-containing material in normocholesterolemic, but not hypercholesterolemic, rabbits. Atherosclerosis 123:145–155 [DOI] [PubMed]
- Zhang SH, Reddick RL, Piedrahita JA, Maeda N (1992) Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science 258:468–471 [DOI] [PubMed]
- Zhu J, Nieto FJ, Horne BD, Anderson JL, Muhlestein JB, Epstein SE (2001) Prospective study of pathogen burden and risk of myocardial infarction or death. Circulation 103:45–51 [DOI] [PubMed]