Abstract
NF-κB is a transcription factor implicated in pathological responses that develop during diabetes mellitus, including skeletal muscle atrophy. Given that NF-κB activation, protein composition, and content within diabetic skeletal muscle remain generally uncharacterized, a streptozotocin (STZ) model was used to assess NF-κB activation, composition, and content. Sprague-Dawley rats were injected with STZ (55 mg/kg) and after 30 days the soleus (SOL), plantaris (PL), red gastrocnemius (RG), and white gastrocnemius (WG) muscles were assessed by electrophoresis mobility shift assay and western blotting. NF-κB activation was detected in all muscles examined, but was reduced in RG muscles from diabetic animals. Supershifts indicated NF-κB was composed primarily of p50 in diabetic and control animals. The content of both p65 and p52 was elevated in SOL and PL muscles, while p52 was decreased in RG. The coactivating protein, Bcl-3, was increased in WG and RG, but decreased in PL. Both p50 and RelB remained unchanged in all tissues examined. All muscles from diabetic animals demonstrated reduced mass when compared to controls, but only the gastrocnemius demonstrated atrophy as reflected by a reduced muscle-to-body mass ratio. In conclusion, diabetic alterations to the contents and activation of the NF-κB protein were tissue-specific, but did not appear to alter dimer composition of constitutively bound NF-κB. These results indicate that diabetes may alter NF-κB activity and expression in a muscle-specific manner.
Keywords: Diabetes mellitus, NF-κB, Diabetes-induced atrophy, Gastrocnemius
Introduction
Diabetes mellitus (DM) is a metabolic pathology characterized by diminished insulin secretion and/or effect at the cellular level resulting in elevated blood glucose. Diabetes-related hyperglycemia is associated with elevated levels of proinflammatory factors, cytokines, increased advanced glycation end-products, increased free radical production, and is generally coupled with a chronic inflammatory state in diabetic subjects (reviewed by Engstrom et al. 2003). Specifically, hyperglycemia-induced inflammation appears to require the activation of nuclear factor kappa-B (NF-κB), a dimerized transcription factor composed from its five member proteins, including p50, p52, p65, Rel-B, and c-Rel. Under normal conditions, NF-κB remains sequestered in the cytosol by an inhibitor (IκB). When IκB is phosphorylated and subsequently degraded, NF-κB is released, allowing for nuclear translocation and subsequent DNA binding (reviewed by Hoffmann et al. 2006). This can lead to the expression of gene products involved in inflammation, immune responses, or apoptosis. The most typically observed, transcriptionally active dimer (the canonical or classical pathway) appears to be the p50–p65 heterodimer, whereas the noncanonical or alternative pathway is composed of the p52-RelB subunits (reviewed by Gilmore 2006), although other subunit combinations are known to bind DNA (reviewed by Hoffmann et al. 2006). The known complement of genes transcribed by the various NF-κB dimer combinations is continually expanding and may explain mechanisms by which selective pathways are activated in any number of pathologies.
Certain pathological conditions may preferentially signal the activation of atrophic pathways within skeletal muscles (Lecker et al. 2004). Specifically, skeletal muscle atrophy observed in diabetes, disuse, and cancer cachexia has been linked to NF-κB-mediated inflammatory conditions (Wyke and Tisdale 2005). Specifically, pathological conditions leading to atrophy appear to activate the canonical p50–p65 NF-κB pathway (Guttridge et al. 2000). In addition, there is evidence that during disuse atrophy, Bcl-3, normally an inhibitor of NF-κB activation, may serve a contradictory role as a transcriptional activator of the p50 homodimer (Hunter and Kandarian 2004). Hence, although NF-κB appears to be activated in various conditions leading to atrophy, the catabolic consequence common to a number of pathologies appears not to arise from the activation of a single NF-κB dimer, but may derive from specific NF-κB dimer combinations unique to any one disease.
Diabetes, induced by streptozotocin (STZ), has long been known to result in attenuated muscle growth and a preferential atrophy of fast fibers compared to the slow fibers (Armstrong et al. 1975). Hence, specific muscles appear to be more susceptible to diabetes-induced atrophy. Moreover, the mechanisms, composition, and activation of NF-κB in diabetic muscle remain generally uncharacterized. Since the consequence of hyperglycemia-induced inflammatory signaling and hence local fiber atrophy remains unknown, the purpose of the present study was to characterize NF-κB in muscles of varying fiber type by measuring NF-κB activation, determining subunit composition, and quantifying the content of NF-κB protein family members within various skeletal muscles from diabetic animals. It was hypothesized that NF-κB would demonstrate a muscle-specific pattern of activation and expression that would be associated with atrophy in diabetic muscle.
Materials and methods
The present study used a total of ten male Sprague-Dawley rats (286–330 g; Charles River, Quebec). Animals were maintained on a 12-h light–dark cycle at 20 ± 1°C, with an ambient relative humidity of 50%. Food and water were provided ad libitum. All experimental procedures were approved by The Animal Care Committee of the University of Toronto. Animals were randomly assigned to two groups; a control group (C, n = 5), and a diabetic group (D, n = 5). Rats from the D group received a tail vein injection with STZ (55 mg/kg body weight) and were allowed to develop diabetes for 30 days while age-matched animals (3 months old) from the C group served as controls. Thirty days after the STZ treatment, animals (n = 10) from both groups were anesthetized and muscle tissues were harvested following cardiac excision. All tissues were immediately frozen in liquid nitrogen and stored until preparation.
STZ administration
Animals were placed into a blanket-covered plexiglass constraint. A single dose of streptozotocin (55 mg/kg prepared in 0.1 M NaCl buffer) injected into the tail vein was administered. The development of diabetes was determined by blood glucose accruement over 30 days. Age-matched animals from the C group not receiving the STZ treatment served as controls. Body weight and morning, nonfasting blood glucose concentration obtained from the tail vein of each animal were measured weekly using the One Touch Basic Blood Glucose Monitoring System (Lifescan, Canada) with One Touch test strips (Lifescan, Canada; range = 0–600 mg/dL).
Electrophoretic mobility shift assay
Portions of muscle tissue (50 mg) were homogenized in 15 vol of extraction buffer (25% glycerol, 0.42 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA (pH 8.0), 20 mM HEPES (pH 7.9), 0.5 mM dithiothreitol (DTT), 0.5 mM phenylmethylsufonylfluoride) at 4°C. Homogenates were centrifuged at 14,000 rpm at 4°C for 20 min in an Eppendorf centrifuge. The supernatant was removed and protein concentration was determined by the method described by Lowry et al. (1951). Analyses of NF-κB–oligonucleotide binding in extracts were performed using an electrophoretic mobility shift assay (EMSA) according to the procedure described by Locke and Tanguay (1996). Protein extracts (50 ug) from muscles were incubated with a 32P-labeled, NF-κB oligonucleotide (5’- AGT TGA GGG GAC TTT CCC AGG C-3’; E3291, Promega) in binding buffer (10% glycerol, 50 mM NaCl, 1.0 mM EDTA (pH, 8.0), 20 mM Tris (pH, 8.0), 1.0 mM DTT, 0.3 mg/ml BSA) with approximately 0.1 ng (50,000 cpm) of 32P-labeled oligonucleotide and 2.0 μg poly dI dC (Pharmacia Fine Chemicals, Piscataway, NJ, USA) for at least 30 min at room temperature. Samples were electrophoresed on 4% acrylamide gel at 200V for 2–3 h. Gels were dried using a BioRad Slab dryer (Model 433) for 45 min and exposed to radiographic film (Amersham-ECL, Mississauga, Ontario, Canada) for 1 or 2 days at −70°C. Films were scanned using an Agfa Arcus II scanner. Recombinant NF-κB (p50) [CAS# (56-81-5) Promega, Madison, WI, USA] and oligo was electrophoresed on each gel to determine the positive identification and location of the bound NF-κB–oligonucleotide complex. Correct NF-κB–oligonucleotide interaction was confirmed by the absence of NF-κB-activation following the addition of a 200-fold molar excess of nonlabeled NF-κB oligonucleotide. Supershift assays were performed to determine the composition of the activated NF-kB complex. Briefly, 2 μg of an antibody specific for a single member of the NF-kB family [p50 (NLS; sc-114 X), p52 (K-27; sc-298 X), p65 (C-20; sc-372 X), RelB (C-19; sc-226 X), C-Rel (C; sc-71 X), and Bcl-3 (C-14; sc-185 X); Santa Cruz Biotechnology, CA, USA] was added to the labeled oligo/protein extract for 30 min prior to electrophoresis. The occurrence of an NF-kB member protein within the dimer complex was determined by upward movement of the protein–DNA complex.
Polyacrylamide gel electrophoresis and immunoblotting
Muscle portions of ~50 mg were homogenized in 20× volume of 600 mM NaCl, 15 mM Tris pH 7.5 and protein concentration determined by the method described by Lowry et al. (1951). One dimensional (1-D) sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis (PAGE) was performed according to the method described by Laemmli (1970), except that the separating gel (0.15 × 4.5 × 8 cm) consisted of a 5–15% polyacrylamide gradient. Prestained standards were used as markers (catalogue 161-0324, Bio-Rad, Mississauga, Ontario, Canada). Samples were electrophoresed using a mini protein II (Bio-Rad, Mississauga, Ontario, Canada) at 55 V for ~30 min followed by 90 V for ~1 h or until the dye arrived at the bottom of the separating gel.
Following electrophoretic separation, gels and nitrocellulose membranes were equilibrated in transfer buffer solution (1× SDS-PAGE running buffer with 20% methanol [pH 8.3]) for 20 min. Following equilibration, proteins were transferred to nitrocellulose membranes (0.22 μm pore size, Bio-Rad Laboratories) as described by Towbin et al. (1979) using the Bio-Rad mini-protean II gel transfer system at a voltage of 55 V for 3 h. The transfer buffer was cooled by hourly changes of an ice pack. Following protein transfer, nitrocellulose paper was blocked in Blotto (5% nonfat skim milk powder in Tris buffer saline (TBS, 500 mM NaCl and 20 mM Tris, pH 7.5) for 1 h to overnight. Nitrocellulose membranes were rinsed in three washes of TTBS (TBS plus 0.05% Tween-20) followed by incubation with polyclonal antibodies (see EMSA procedure), diluted 1:500 in TTBS with 2% Blotto (Locke et al 1995). Immunoblots were visualized by reacting an HRP-conjugated, goat anti-rabbit secondary antibody (diluted 1:2,000 and incubated for 1 h) via enhanced chemiluminescence (Amersham Biosciences Corp, Piscataway, NJ, USA). Immunoblots were scanned using an Agfa Arcus II scanner and quantification of bands from the immunoblots was performed using Kodak 1D Image Analysis Software (Kodak Scientific Imaging Systems, New Haven, CT, USA). The content of p50, p52, p65, RelB, and Bcl-3 was determined by analyzing the intensities of bands on Western blots.
Statistical analysis
Independent T tests were performed (SigmaStat, San Jose, USA) using diabetes as an independent variable for each experimental group. Differences between groups were considered statistically significant at a level of P ≤ 0.05.
Results
Blood glucose levels
Blood glucose values for control and STZ-treated animals are shown in Fig. 1. The initial blood glucose value for the control group was 95.7 ± 2 mg/dl in STZ-treated animals and was not significantly different from the initial value measured in the STZ-treated animals (94.2 ± 1 mg/dl). A significant increase (P < 0.05) in blood glucose was measured after 30 days in the STZ-treated animals (470 ± 36 mg/dl), whereas the control animals did not demonstrate a significant change after 30 days (95 ± 2 mg/dl).
Fig. 1.
Blood glucose values after STZ treatment. Glucose values (expressed as a percentage of control) are shown for control and diabetic animals before and after 30 days of STZ treatment. Values are mean ± SEM for glucose (mg/dl). *Significantly different (P < 0.05) from control values
Body mass and muscle mass
Initial body mass values were not significantly different between control and diabetic groups. As expected (from normal growth) a significant increase (P < 0.05) in body mass for both control and diabetic animals was observed after 30 days (Fig. 2a). However, diabetic animals showed a significantly lower body mass (P < 0.05) after 30 days (initial mass, 286 ± 4 g; final mass, 362 ± 11 g) when compared with the control group (initial mass, 302 ± 7 g; final mass, 464 ± 6 g).
Fig. 2.
Diabetes alters body and muscle mass. Panel a 30 days after STZ injection accumulation of body mass is significantly attenuated (P < 0.05). Values are mean ± SEM for total body mass (g). *Significantly different (P < 0.05) from control animals. Panel b Muscle mass accumulation is significantly reduced (P < 0.05) in diabetic animals. Values are percentage of control ± SEM for total muscle mass (g). *Significantly different (P < 0.05) from control animals. Panel c Muscle-to-body mass ratio reflects a muscle specific effect of diabetes. The gastrocnemius demonstrated a significantly attenuated (P < 0.05) muscle-to-body mass ratio. Values are mean muscle-to-body ratio ± SEM (mg/g). *Significantly different (P < 0.05) from control animals
Muscle mass values for the soleus (SOL), plantaris (PL), and gastrocnemius are shown in Fig. 2b. All muscles from diabetic animals demonstrated significantly reduced (P < 0.05) mass when compared with the control group. Both body mass and muscle mass values in diabetic animals were approximately 80% of the values measured in the control animals. To determine if the reduced muscle mass in STZ-treated animals reflected the overall reduction in body mass, muscle mass was expressed relative to body mass (Fig. 2c). The soleus and plantaris muscles did not have a diminished muscle-to-body mass ratio compared with the controls. In contrast, the gastrocnemius muscle-to-body mass ratio was significantly reduced (P < 0.05) in the diabetic animals compared with the control animals, suggesting an atrophic response.
Activation and composition of NF-kB
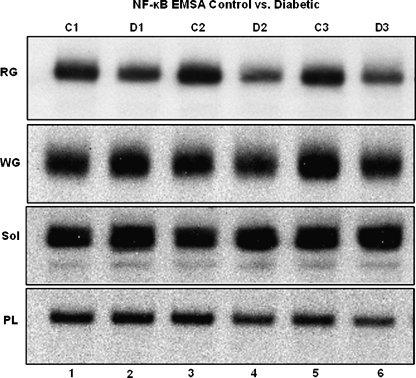
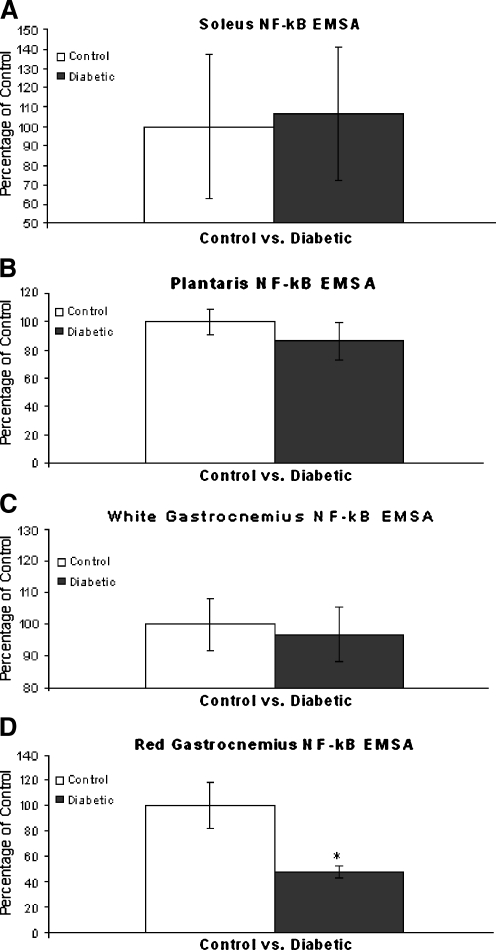
Activation of NF-κB as determined by binding to the NF-κB oligo was measured in muscle extracts from control and diabetic animals by EMSA (Fig. 3). EMSA-derived band quantification indicated NF-κB activation in the SOL, PL, and white gastrocnemius (WG) muscle from diabetic animals was not significantly from between controls (Fig. 4a–c). However, NF-κB activation was significantly (P < 0.05) reduced in the RG of diabetic animals (Fig. 4d), suggesting a unique cellular response in this tissue to the diabetic condition.
Fig. 3.
Transcriptional activation of NF-κB. NF-κB activation in WG, RG, SOL, and PL muscle tissues from control and diabetic animals. Protein extracts are as follows: Lane 1 protein sample from control animal 1, lane 2 protein sample from diabetic animal 1, lane 3 protein sample from control animal 2, lane 4 protein sample from diabetic animal 2, lane 5 protein sample from control animal 3, lane 6 protein sample from diabetic animal 3. NF-κB binding is significantly reduced (P < 0.05) in RG tissues of diabetic animals. *Significantly different (P < 0.05) from control animals
Fig. 4.
Graphical Illustration of NF-κB Activation. Panel a Representation of NF-κB binding in SOL muscles expressed as percentage of control ± SEM. No significant difference between control and diabetic animals was observed. Panel b Representation of NF-κB binding in PL muscles expressed as percentage of control ± SEM. No significant difference between control and diabetic animals was observed. Panel c Representation of NF-κB binding in WG muscles expressed as percentage of control ± SEM. No significant difference between control and diabetic animals was observed. Panel d Representation of NF-κB binding in RG muscles expressed as percentage of control ± SEM. NF-κB binding was significant reduced (P < 0.05) in RG tissues of diabetic animals. *Significantly different (P < 0.05) from control animals
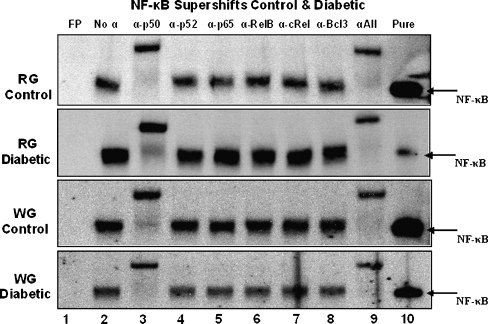
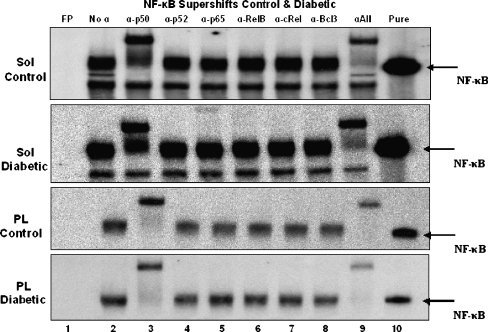
To determine the dimeric composition of the NF-κB transcription factor observed in muscle extracts from control and diabetic animals, EMSA supershifts were performed (Figs. 5 and 6). The addition of antibodies specific for each of the five members of the NF-κB family as well as for Bcl-3 revealed a common pattern for all muscles examined. When incubated with a p50-specific antibody, a slower migration (supershift) of the NF-κB–oligo complex was observed. This was consistent for all control and diabetic muscle extracts. Incubation of muscle samples with other antibodies did not appear to result in any supershifts. When all antibodies were combined the same pattern as p50 only was observed and the disappearance of the NF-κB–oligo complex from its original (antibody-free) location also occurred. Since the entire complex was shifted by the addition of the p50 antibody alone, it suggests that the NF-κB–oligo complex detected in muscles is primarily a p50–p50 homodimer.
Fig. 5.
Supershifts of NF-κB from gastrocnemius muscles from control and diabetic animals. Lane 1 free probe (FP), lane 2 protein sample without antibody, lane 3 protein sample with the addition of 2 μg α-p50, lane 4 protein sample with the addition of 2 μg α-p52, lane 5 protein sample with the addition of 2 μg α-p65, lane 6 protein sample with the addition of 2 μg α-RelB, lane 7 protein sample with the addition of 2 μg α-cRel, lane 8 protein sample with the addition of 2 μg α-Bcl-3, lane 9 protein sample with the addition of 2 μg of each antibody, lane 10 labeled oligo incubated with recombinant p50 (5 ng). Disappearance of the band by a p50-specific antibody indicates a homodimer composed predominately of p50
Fig. 6.
Supershifts of NF-κB from soleus and plantaris muscles from control and diabetic animals. Lane 1 free probe (FP), lane 2 protein sample without antibody, lane 3 protein sample with the addition of 2 μg α-p50, lane 4 protein sample with the addition of 2 μg α-p52, lane 5 protein sample with the addition of 2 μg α-p65, lane 6 protein sample with the addition of 2 μg α-RelB, lane 7 protein sample with the addition of 2 μg α-cRel, lane 8 protein sample with the addition of 2 μg α-Bcl-3, lane 9 protein sample with the addition of 2 μg of each antibody, lane 10 labeled oligo incubated with recombinant p50 (5 ng)
NF-κB protein content
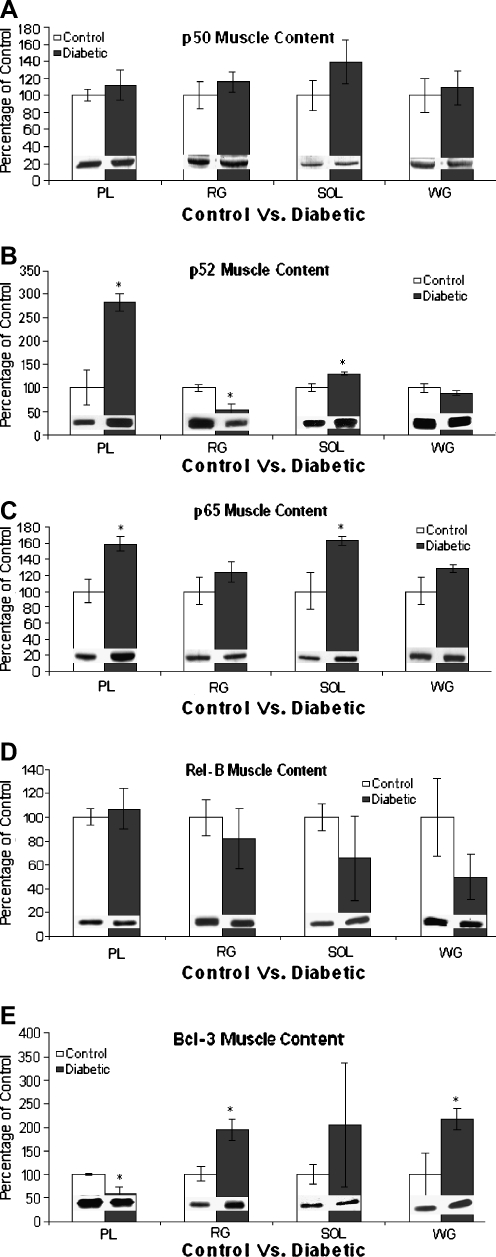
To determine NF-κB protein subunit content in rat hindlimb muscles from both control and diabetic animals (Fig. 7), Western blots were performed for the various NF-κB subunits of the canonical and alternative pathway (p50, p52, p65, Rel-B) and Bcl-3. The main constituent of NF-κB dimerization, p50, demonstrated consistently unchanged protein content in all muscles between control and diabetic animals (Fig. 7a). In contrast, p52 showed a significantly different (P < 0.05) quantity between diabetes and control in the PL, RG, and SOL muscles, whereas the WG remained unchanged (Fig. 7b). However, unlike the PL and SOL muscles, which showed elevated contents of p52 with diabetes, the RG showed reduced p52 content. The PL and SOL muscles also showed significantly elevated (P < 0.05) contents of p65 in diabetic animals, while the WG and the RG remained unchanged (Fig. 7c). Similar to p50, Rel-B content also showed no significant alteration in any muscle in the diabetic condition (Fig. 7d). A muscle-specific protein expression in diabetic tissue was found for Bcl-3 (Fig. 7e), in that the PL muscles from diabetic animals showed a significantly decreased (P < 0.05) Bcl-3 content. In contrast, both the RG and WG muscles showed a significant increase (P < 0.05) in Bcl-3 content. The SOL Bcl-3 content was unchanged. Taken together, there appears to be muscle-specific changes in expression that distinguishes the gastrocnemius muscle from the PL and SOL muscles.
Fig. 7.
NF-κB subunit content in control and diabetic muscle tissues. Representative Western blots for each protein and muscle are embedded in each bar graph (n = 5). Panel a Representation of p50 content in control and diabetic muscle tissues expressed as percentage of control. Values are percentage of control ± SEM. No significant difference between control and diabetic animals was observed for any tissues examined. Panel b Representation of p52 content in control and diabetic muscle tissues expressed as percentage of control ± SEM. PL and SOL muscles from diabetic animals showed significantly increased (P < 0.05) p52 content. RG contents of p52 from diabetic animals were significantly decreased (P < 0.05). *Significantly different (P < 0.05) from control animals. Panel c Representation of p65 content in control and diabetic muscle tissues expressed as percentage of control ± SEM. PL and SOL muscles from diabetic animals showed significantly increased (P < 0.05) p65 content. No significant difference between control and diabetic animals was observed in either RG or WG tissues. Panel d Representation of Rel-B content in control and diabetic muscle tissues expressed as percentage of control. Values are percentage of control ± SEM. No significant difference between control and diabetic animals was observed for any tissues examined. Panel e Representation of Bcl-3 content in control and diabetic muscle tissues expressed as percentage of control ± SEM. PL muscle from diabetic animals showed significantly decreased (P < 0.05) Bcl-3 content. Both RG and WG contents of Bcl-3 from diabetic animals was significantly increased (P < 0.05). *Significantly different (P < 0.05) from control animals
Discussion
A role for NF-κB and its varying composition has been established in atrophy-associated pathologies, including diabetes. In addition, diabetes appears to obtain a fiber-type specific atrophy leading to a greater loss of fast fibers (Armstrong et al. 1975). Therefore, the present study determined the expression and activation of NF-κB in various skeletal muscles in order to assess any muscle-specific relationship between NF-κB and atrophy under diabetic conditions. There were three main findings in the present study. First, NF-κB activation was reduced in the diabetic RG, but was unchanged in other muscles. Second, NF-κB dimer composition was found to be predominantly p50 homodimers in both control and diabetic muscles. Third, NF-κB subunit contents showed a tissue-specific expression and response to diabetes, revealing a unique response by the gastrocnemius, which was the only muscle to show a diminished muscle-to-body mass ratio.
NF-κB activation is reduced in diabetic muscle
An upregulation of NF-κB appears to be involved in muscle atrophy (Hunter and Kandarian 2004; Cai et al. 2004; Mourkioti et al. 2006). In agreement with this, in the present study only the gastrocnemius muscle demonstrated altered NF-κB activation with diabetes. However, contrary to expectations, the diabetic condition led to unchanged NF-κB activation in WG and a reduced NF-κB activation in RG. NF-κB activation in RG may have been reduced by a generalized reduction in cellular metabolism which may have inhibited NF-κB activation via reduced reactive oxygen species production (Nishikawa et al. 2000). Indeed, treatment with a similar amount of STZ has been shown to reduce oxidative- and glycolytic enzyme activity selectively in the RG (Chen and Ianuzzo 1982). However, in both the SOL and PL, muscle-to-body mass ratio was maintained without a decrease in NF-κB activation; hence RG tissue preservation via reduced NF-κB activation is not supported by the results. Despite the typically reported observation that increased NF-κB activation occurs during stressful conditions, it may not be the case that reduced NF-κB activation found in diabetic RG necessarily indicates less stress. In fact, some pathologies, such as incontinentia pigmenti, are associated with reduced NF-κB activation resulting in excessive apoptosis (Smahi et al. 2000). Nonetheless, the diminished NF-κB activity observed in the RG, combined with previous observations that STZ-induced diabetes preferentially affects the fast glycolytic fibers while preserving slow oxidative fibers (Armstrong et al. 1975), indicates that diminished constitutive NF-κB activation may protect against atrophy in the RG, but not the WG.
The composition of activated NF-κB
The subunit composition of NF-κB was determined, using EMSA supershifts, to assess muscle-specific differences and if diabetes altered the dimeric composition of NF-κB. For both control and diabetic conditions all muscle NF-κB appeared to be composed of p50, suggesting that the constitutive expression of NF-κB may be the p50–p50 homodimer. It should be noted that the predominant p50 homodimers observed in muscles does not necessarily preclude the existence of other dimers, since the p50 subunit protein itself may be transcribed by a dimer composed of p65 or c-Rel (Cogswell et al. 1993). Likewise, the p52 protein appears to be transcribed by a p52-containing dimer (Liptay et al. 1994), suggesting that the detection of NF-κB proteins in the muscle necessarily indicates that dimers exist within the muscle that are not composed exclusively of p50. Hence, constitutive NF-κB binding by p50 homodimers may only reflect a normal transcriptional repression (Tong et al. 2004) that is rapidly and transiently displaced by other activated dimers (Bosisio et al. 2006). Moreover, the constitutive binding by p50 homodimers in muscles from both normal and diabetic animals suggests that diabetes per se may not be inherently provocative to these muscles. The only muscle that demonstrated reduced muscle-to-body mass ratio (gastrocnemius) also showed a p50 homodimer in control and diabetic conditions in both WG and RG tissue (with a binding decrease in RG only). Hence, the diabetes-induced NF-κB composition and activity observed in the present study may indicate a muscle-specific, treatment-induced change in NF-κB activation, but did not reveal a clear consequence of altered activation and dimer composition relative to atrophy in this condition.
NF-κB subunit quantification
An altered quantity of NF-κB subunits in diabetic muscle supports the concept of a dynamic activation and composition variability. Therefore, altered protein quantities in diabetic tissue may characterize a muscle-specific pattern of NF-κB activity discretely unique to diabetes-related atrophy. Although both p50 and RelB remained unchanged in all tissues, one protein member of the canonical pathway (p65), and one member of the alternate pathway (p52), as well as the p50 homodimer coactivator (Bcl-3), was altered in diabetic muscle. In these cases, changes in protein expression distinguished the gastrocnemius muscle from the SOL and PL muscles. Specifically, p52 was elevated in SOL and PL, but was reduced in RG tissue. Elevated p52 processing, which is normally inhibited (Xiao et al. 2001), has been previously reported in lymphomagenesis (Derudder et al. 2003), a pathology often associated with diabetes (Yui and Rothenberg 2004). In the present study, p52 was reduced in RG but was unchanged in WG which may indicate a mechanism by which the gastrocnemius is protected during diabetes. However, since p52 remained unchanged in the WG, any protection may not be extended to atrophy.
Similarly, quantities of the p65 protein exhibited unchanged levels in WG and RG tissues, but were elevated in SOL and PL muscles. Activated dimers containing p65 have previously been reported to participate in cachexia (Guttridge et al. 2000), oxidative stress (Kefaloyianni et al. 2006), and age-related atrophy (Phillips and Leeuwenburgh 2005). In addition, elevated contents of p65 have been reported in smooth muscle cells cultured in a high glucose environment, which led to increased p65-mediated transcription (Yerneni et al. 1999), a condition observed in vascular pathologies associated with diabetes. In contrast to these catabolic and pathological effects, elevated p65 protein content has also been reported following insulin-like growth factor II-induced myogenesis, a process requiring NF-κB activation (Kaliman et al. 1999), whereas p65 protein expression may remain unchanged during myostatin-induced cachexia (McFarlane et al. 2006). Despite no detection of p65 with EMSA, the possibility remains that p65-associated transcription factors may be transiently activated as evidenced by maintained levels of p50, previously mentioned to be expressed via a p65-associated NF-κB dimer (Cogswell et al. 1993). Therefore, mechanisms of muscle wasting via p65-associated dimer activity may still exist within these muscles.
Bcl-3 was also increased in WG and RG muscles, but diminished in PL, and unchanged in SOL. Normal Bcl-3 expression appears to block cellular arrest and apoptosis (Kashatus et al. 2006), and is upregulated to protect against reduced growth factors (Rebollo et al. 2000). In contrast, reduced expression may diminish both survival against infection and myogenic differentiation (Schwarz et al. 1997; Shiio et al. 1996). Although increased Bcl-3 content and binding with p50 homodimers were previously reported to be required for unloading-induced atrophy (Hunter et al. 2002) its altered expression within the present study was not associated with activated p50-homodimers. Nonetheless, it is possible that Bcl-3 was upregulated within skeletal muscle in response to diabetes, since the gastrocnemius muscle demonstrated both an elevated Bcl-3 protein and a significantly reduced muscle-to-body mass ratio.
Taken together, NF-κB family members demonstrated a tissue-specific expression under diabetic conditions. Although it is not clear what role each protein may play in any diabetes-induced atrophy, there does appear to be a modified pattern of protein expression that distinguishes the gastrocnemius muscle from the SOL and PL muscles.
Body mass and muscle mass changes
Body mass is typically reduced in the STZ model of diabetes (Armstrong et al. 1975; Price et al. 1996), therefore, attenuated body mass growth was expected. Likewise, it was expected that hindlimb skeletal muscles would demonstrate reduced mass accumulation. Indeed, impaired body mass growth was paralleled by a proportional, attenuated accruement of muscle mass. The exception was the gastrocnemius muscle, which showed a markedly reduced muscle-to-body mass ratio. Armstrong et al. (1975) showed reduced area in fast glycolytic fibers, but no reduction in the slow oxidative- or fast oxidative fiber area-to-body mass ratio in the gastrocnemius of diabetic rats. This suggests that the significantly reduced gastrocnemius muscle-to-body mass ratio in the present study may be mostly due to a susceptibility of fast glycolytic fibers to STZ treatment (Chaudhury et al. 1994) and may be partly attributable to the upregulation of proteolytic mechanisms (Price et al. 1996; Lee et al. 2004) and/or the loss of anabolic signaling (Chaudhury et al. 1994). However, the atrophic phenomenon observed in the present study was not discernibly fiber-type specific, as the SOL (almost entirely slow fibers) and PL (composed of mostly fast fibers) did not show a diminished muscle-to-body mass ratio. Nonetheless, atrophy was observed in the gastrocnemius. Muscle recruitment patterns indicate that the SOL would be maximally recruited during normal movements while the PL and gastrocnemius would be recruited in parallel with increasing force requirements (Armstrong and Laughlin 1985). However, the gastrocnemius muscle would have a far greater amount of total mass inactive during normal movements compared to both the SOL and PL muscles by virtue of its total mass and quantity of fast fibers. Hence, the possibility exists that the PL and SOL muscles are protected against diabetes-induced atrophy via muscle activity, as a greater percentage of the total mass would be recruited during normal movements whereas the gastrocnemius would remain essentially inactive under the same conditions leading to diabetes-induced, inactivity-mediated atrophy.
Conclusion
DNA binding activity of NF-κB was reduced in diabetic RG muscles only and, in all cases, dimer composition appeared to be composed primarily of p50 homodimers. The finding that p50 homodimers comprised the complement of skeletal muscle NF-κB dimers suggests this to be the constitutive binding dimer, which may act to suppress transcription (Tong et al. 2004), but may not absolutely deny a rapid and transient activation by an assortment of NF-κB transcription factors. While it was proposed that NF-κB would demonstrate muscle-specific activation and would be intimated with skeletal muscle atrophy, the complexity of a transcription factor responsible for the regulation of copious and largely uncharacterized gene expression did not readily provide an unequivocal role for NF-κB activation, composition, and protein expression in STZ-induced atrophy. The paradox that various forms of the NF-κB dimer appear to be a principal player in both pro- and antiapoptosis (Fan et al. 2002), and in hypertrophic and atrophy-related pathways testifies to its complexity and importance in gene regulation. Nonetheless, the unique atrophic effect of diabetes on the gastrocnemius muscle observed in the present study revealed a unique profile of NF-κB regulation and protein expression.
References
- Armstrong RB, Laughlin MH (1985) Metabolic indicators of fibre recruitment in mammalian muscles during locomotion. J Exp Biol 115:201–213 [DOI] [PubMed]
- Armstrong RB, Gollnick PD, Ianuzzo CD (1975) Histochemical properties of skeletal muscle fibers in streptozotocin-diabetic rats. Cell Tissue Res 162:387–394 [DOI] [PubMed]
- Bosisio D, Marazzi I, Agresti A, Shimizu N, Bianchi ME, Natoli G (2006) A hyper-dynamic equilibrium between promoter-bound and nucleoplasmic dimers controls NF-kappaB-dependent gene activity. EMBO J 25:798–810 [DOI] [PMC free article] [PubMed]
- Cai D, Frantz JD, Tawa NE Jr, Melendez PA et al (2004) IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell 119:285–298 [DOI] [PubMed]
- Chaudhury SK, Mandal MB, Deshpande SB, Saxena ID (1994) Effect of streptozotocin-induced diabetes on growth and proteolytic activity of different muscles in rats. Indian J Exp Biol 32:877–880 [PubMed]
- Chen V, Ianuzzo CD (1982) Dosage effect of streptozotocin on rat tissue enzyme activities and glycogen concentration. Can J Physiol Pharmacol 60:1251–1256 [DOI] [PubMed]
- Cogswell PC, Scheinman RI, Baldwin AS Jr (1993) Promoter of the human NF-kappa B p50/p105 gene. Regulation by NF-kappa B subunits and by c-REL. J Immunol 150:2794–2804 [PubMed]
- Derudder E, Laferte A, Ferreira V, Mishal Z, Baud V, Tarantino N, Korner M (2003) Identification and characterization of p100HB, a new mutant form of p100/NF-kappa B2. Biochem Biophys Res Commun 308:744–749 [DOI] [PubMed]
- Engstrom G, Stavenow L, Hedblad B, Lind P, Eriksson KF, Janzon L, Lindgarde F (2003) Inflammation-sensitive plasma proteins, diabetes, and mortality and incidence of myocardial infarction and stroke: a population-based study. Diabetes 52:442–447 [DOI] [PubMed]
- Fan C, Yang J, Engelhardt JF (2002) Temporal pattern of NFkappaB activation influences apoptotic cell fate in a stimuli-dependent fashion. J Cell Sci 115:4843–4853 [DOI] [PubMed]
- Gilmore TD (2006) Introduction to NF-kB: players, pathways, perspectives. Oncogene 25:6680–6684 [DOI] [PubMed]
- Guttridge DC, Mayo MW, Madrid LV, Wang CY, Baldwin AS Jr (2000) NF-kB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science 289:2363–2366 [DOI] [PubMed]
- Hoffmann A, Natoli G, Ghosh G (2006) Transcriptional regulation via the NF-kappaB signaling module. Oncogene 25:6706–6716 [DOI] [PubMed]
- Hunter RB, Kandarian SC (2004) Disruption of either the Nfkb1 or the Bcl3 gene inhibits skeletal muscle atrophy. J Clin Invest 114:1504–1511 [DOI] [PMC free article] [PubMed]
- Hunter RB, Stevenson E, Koncarevic A, Mitchell-Felton H, Essig DA, Kandarian SC (2002) Activation of an alternative NF-kappaB pathway in skeletal muscle during disuse atrophy. FASEB J 16:529–538 [DOI] [PubMed]
- Kaliman P, Canicio J, Testar X, Palacin M, Zorzano A (1999) Insulin-like growth factor-II, phosphatidylinositol 3-kinase, nuclear factor-kappaB and inducible nitric-oxide synthase define a common myogenic signaling pathway. J Biol Chem 274:17437–17444 [DOI] [PubMed]
- Kashatus D, Cogswell P, Baldwin AS (2006) Expression of the Bcl-3 proto-oncogene suppresses p53 activation. Genes Dev 20:225–235 [DOI] [PMC free article] [PubMed]
- Kefaloyianni E, Gaitanaki C, Beis I (2006) ERK1/2 and p38-MAPK signalling pathways, through MSK1, are involved in NF-kappaB transactivation during oxidative stress in skeletal myoblasts. Cell Signal 18:2238–2251 [DOI] [PubMed]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed]
- Lecker SH, Jagoe RT, Gilbert A et al (2004) Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J 18:39–51 [DOI] [PubMed]
- Lee SW, Dai G, Hu Z, Wang X, Du J, Mitch WE (2004) Regulation of muscle protein degradation: coordinated control of apoptotic and ubiquitin-proteasome systems by phosphatidylinositol 3 kinase. J Am Soc Nephrol 15:1537–1545 [DOI] [PubMed]
- Liptay S, Schmid RM, Nabel EG, Nabel GJ (1994) Transcriptional regulation of NF-kappa B2: evidence for kappa B-mediated positive and negative autoregulation. Mol Cell Biol 14:7695–7703 [DOI] [PMC free article] [PubMed]
- Locke M, Tanguay RM (1996) Increased HSF activation in muscles with a high constitutive Hsp70 expression. Cell Stress Chaperones 1:189–196 [DOI] [PMC free article] [PubMed]
- Locke M, Tanguay RM, Klabunde RE, Ianuzzo CD (1995) Enhanced postischemic myocardial recovery following exercise induction of HSP 72. Am J Physiol 269:320–325 [DOI] [PubMed]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275 [PubMed]
- McFarlane C, Plummer E, Thomas M et al (2006) Myostatin induces cachexia by activating the ubiquitin proteolytic system through an NF-kappaB-independent, FoxO1-dependent mechanism. J Cell Physiol 209:501–514 [DOI] [PubMed]
- Mourkioti F, Kratsios P, Luedde T et al (2006) Targeted ablation of IKK2 improves skeletal muscle strength, maintains mass, and promotes regeneration. J Clin Invest 116:2945–2954 [DOI] [PMC free article] [PubMed]
- Nishikawa T, Edelstein D, Du XL et al (2000) Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404:787–790 [DOI] [PubMed]
- Phillips T, Leeuwenburgh C (2005) Muscle fiber specific apoptosis and TNF-alpha signaling in sarcopenia are attenuated by life-long calorie restriction. FASEB J 19:668–670 [DOI] [PubMed]
- Price SR, Bailey JL, Wang X, Jurkovitz C, England BK, Ding X, Phillips LS, Mitch WE (1996) Muscle wasting in insulinopenic rats results from activation of the ATP-dependent, ubiquitin-proteasome proteolytic pathway by a mechanism including gene transcription. J Clin Invest 98:1703–1708 [DOI] [PMC free article] [PubMed]
- Rebollo A, Dumoutier L, Renauld JC, Zaballos A, Ayllon V, Martinez-A C (2000) Bcl-3 expression promotes cell survival following interleukin-4 deprivation and is controlled by AP1 and AP1-like transcription factors. Mol Cell Biol 20:3407–3416 [DOI] [PMC free article] [PubMed]
- Schwarz EM, Krimpenfort P, Berns A, Verma IM (1997) Immunological defects in mice with a targeted disruption in Bcl-3. Genes Dev 11:187–197 [DOI] [PubMed]
- Shiio Y, Sawada J, Handa H, Yamamoto T, Inoue J (1996) Activation of the retinoblastoma gene expression by Bcl-3: implication for muscle cell differentiation. Oncogene 12:1837–1845 [PubMed]
- Smahi A, Courtois G, Vabres P et al (2000) Genomic rearrangement in NEMO impairs NF-kappaB activation and is a cause of incontinentia pigmenti. The International Incontinentia Pigmenti (IP) Consortium. Nature 405:466–472 [DOI] [PubMed]
- Tong X, Yin L, Washington R, Rosenberg DW, Giardina C (2004) The p50-p50 NF-kappaB complex as a stimulus-specific repressor of gene activation. Mol Cell Biochem 265:171–183 [DOI] [PubMed]
- Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Biotechnology 24:145–149 [PubMed]
- Wyke SM, Tisdale MJ (2005) NF-kappaB mediates proteolysis-inducing factor induced protein degradation and expression of the ubiquitin-proteasome system in skeletal muscle. Br J Cancer 92:711–721 [DOI] [PMC free article] [PubMed]
- Xiao G, Harhaj EW, Sun SC (2001) NF-kappaB-inducing kinase regulates the processing of NF-kappaB2 p100. Mol Cell 7:401–409 [DOI] [PubMed]
- Yerneni KK, Bai W, Khan BV, Medford RM, Natarajan R (1999) Hyperglycemia induced activation of nuclear transcription factor kappaB in vascular smooth muscle cells. Diabetes 48:855–864 [DOI] [PubMed]
- Yui MA, Rothenberg EV (2004) Deranged early T cell development in immunodeficient strains of nonobese diabetic mice. J Immunol 173:5381–5391 [DOI] [PubMed]