Abstract
In response to extracellular signals during embryonic development, cells undergo directional movements to specific sites and establish proper connections to other cells to form organs and tissues. Cell extension and migration in the direction of extracellular cues is mediated by the actin and microtubule cytoskeletons, and recent results have shed new light on how these pathways are activated by neurotrophins, Wnt or extracellular matrix. These signals lead to modifications of microtubule-associated proteins (MAPs) and point to glycogen synthase kinase (GSK) 3β as a key regulator of microtubule function during directional migration. This review will summarize these results and then focus on the role of microtubule-binding protein Adenomatous Polyposis Coli (APC) in neuronal polarization and directed migration, and on its regulation by GSK3β.
Keywords: directional cell extension, neuronal polarization, microtubules, adenomatous polyposis coli
1. Introduction
During embryonic development, cells reorganize into complex three-dimensional patterns in response to secreted or surface cues such as growth factors (chemotaxis) and guidance cues provided by cell-matrix and cell-cell adhesion proteins (haptotaxis, contact guidance) [1, 2]. Many of these signals and their role in tissue formation are well known [3-5]. Signals inducing cell extension and migration, such as Wnts and neurotrophins have very immediate and local effects on the cytoskeleton, thereby shaping cell morphology and directionality of cell movement [5, 6]. The microtubule cytoskeleton plays an important role in axonal outgrowth during neuronal polarization [7, 8], and is involved in directional extension in many other cell types [9]. Recent advances in the field show how Wnts, neurotrophins and other extracellular cues, such as integrin activation by extracellular matrix, can activate microtubule-associated proteins (MAPs) at the tip of cell extensions to increase microtubule assembly and stability [6, 10, 11]. These results indicate a major role for components of the Wnt signaling pathway, including Dishevelled, Axin, GSK3β and the microtubule-associated protein APC, in regulating local microtubule reorganization at the cortex [6, 12, 13].
2. Role of microtubules and microtubule-associated proteins in neuronal polarization and directional cell extension
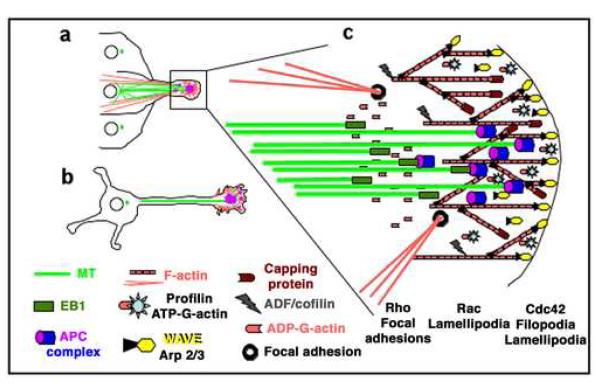
An important problem to understand is how cell extensions required for migration are induced and oriented by extracellular signals. A common feature of this process is cell polarization towards the source of the signal and the asymmetric distribution of signaling complexes and the actin and microtubule cytoskeletons (Fig. 1; [14]). The role of actin in membrane extension and directional cell migration is well established and understood (Fig. 1; [15-17]). Although, it is clear that microtubule orientation and organization at the leading edge are critical for directional cell migration, their role there and how signaling pathways affect microtubule behavior (dynamic instability, cortex interaction and interaction with the actin cytoskeleton) are less well understood (Fig. 1; [9, 14, 18]).
Fig 1. Organization of cytoskeleton and signaling proteins in directional cell migration and extension.
(A) Epithelial or astrocyte migrating into a wound area; (B) neuronal cell extending axon and dendrites; (C) Cytoskeletal organization and signaling proteins at the leading edge: The actin (G- & F-actin) cytoskeleton (red) provides a membrane protrusive force by actin polymerization at the leading edge. The microtubule (MT) cytoskeleton (green) delivers membrane components via the secretory pathway and provides a protrusive force by microtubule polymerization (pioneering) and bundling. The microtubule-organizing center (MTOC, green dots in A and B) orients towards the direction of extension, and microtubules orient along the axis of extension, being laterally guided by cortical actin bundles attached to focal adhesions. Growth factor signals and integrin-matrix adhesion at the leading edge stimulate small GTPases Cdc42, Rac and Rho. Cdc42 and Rac promote actin polymerization by activating the WASP- and WAVE-Arp2/3 actin-nucleation complexes in filopodia and lamellipodia. In epithelial cells, Rho promotes directed migration by inducing actin/myosin contractility in the rear and contraction forces at actin bundle-focal adhesion connections in the front. In neuronal cells, an actin arc separates the central microtubule rich area of the growth cone from peripheral actin rich lamellipodia and filopodia and Rho activity promotes growth cone repulsion. Small GTPases also activate the leading edge-enriched MAP APC. The mechanism of this activation is poorly understood but activated APC promotes directed cell extension probably by interacting with the microtubule plus-end binding protein EB1 and regulating microtubule dynamics at the leading edge. APC can interact with actin either directly via its actin binding domain or indirectly via binding to the CDC42/Rac effector and actin binding protein IQGAP.
The role of microtubules in cell extension is best understood during neuronal polarization [7, 8, 19, 20]. Axonal outgrowth requires microtubule polymer transport into the axon and microtubule assembly at microtubule plus ends in the axonal growth cone [8]. Local actin instability in the growth cone periphery allows microtubule assembly and extension and promotes outgrowth [21]. Microtubule extension into the growth cone periphery precedes growth cone turning at substrate boundaries indicating that it is required for axonal guidance [19].
Several microtubule associated proteins (MAPs) are important for axonal outgrowth [22] including Tau and MAP1B which stabilize microtubules in the axon [23, 24], and Collapsin response mediator protein-2 (CRMP-2) which promotes microtubule assembly and is required for axon formation [25, 26]. Signals that regulate axonal outgrowth and remodeling decrease the level of phosphorylation of Tau, MAP1B and CRMP-2 [23, 24, 27], and inhibition of glycogen synthase kinase 3β (GSK3β) is as a central process in locally regulating these MAPs [6, 23, 24, 27]. Phosphorylation by GSK3β inhibits Tau and CRMP-2 binding to microtubules [27, 28]. Inhibition of GSK3β in the axonal growth cone enables CRMP-2 to bind microtubules and to promote axonal outgrowth [27].
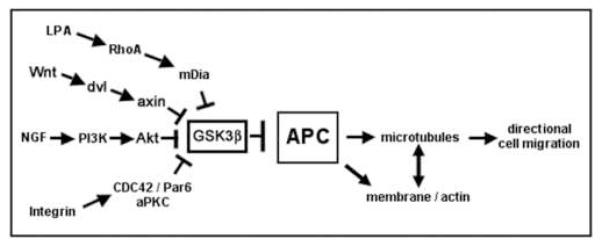
APC is another MAP that is enriched in the axonal growth cone and that is important for axonal outgrowth and is regulated by GSK3β in response to neurotrophins [29]. Phosphorylation of APC by GSK3β inhibits its ability to bundle microtubules in vitro [30] but changes in its phosphorylation state in response to extracellular signals have yet to be investigated and the mechanism of APC inhibition by GSK3 β is not clear (see below). APC is a key regulator of microtubule organization during directed cell extension in other cell types and is targeted by ECM-integrin interaction, Wnts and other extracellular signals (Fig. 2). The following section will describe pathways that affect APC localization and function at the tip of cell extensions.
Fig. 2.
Signaling pathways that induce activation of APC in cell migration.
3. Pathways regulating the microtubule-associated protein APC during cell extension
APC was identified as the product of a tumor suppressor gene mutated in hereditary and somatic colorectal cancers, and some types of brain tumors [31, 32]. There are two mammalian APC genes that encode APC and APC2 (APCL) proteins, and two Drosophila APC genes encoding dAPC and dAPC2 proteins [33-36]. APC plays an important role in regulating β-catenin protein levels and, thereby, β-catenin mediated gene expression in the canonical Wnt pathway [37]. The canonical Wnt pathway is important for cell fate decisions during embryonic development and depletion of both alleles of the mammalian APC gene is embryonic lethal [38]. We will focus here on mammalian APC that has C-terminal microtubule-, EB1- and Dlg- binding sites (see Fig. 4). Results from several laboratories have defined another important function of this APC protein in regulating the microtubule cytoskeleton and in regulating cell extension and migration in response to extracellular signals [29, 39-41].
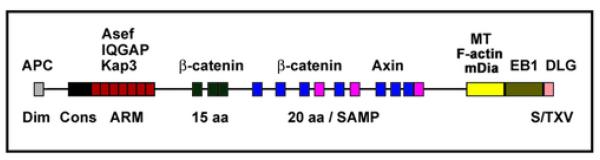
Fig. 4. APC protein-protein interaction domains.
Grey: dimerization domain; Black: conserved APC domain. Red: armadillo repeat domain that binds Asef, IQGAP and Kap3. The N-terminal 1121 amino acids also contain a binding site for the B56 regulatory subunit of PP2A. Dark green and dark purple: 3 repeats of 15 amino acids (15aa) and 7 repeats of 20 amino acids (20 aa), respectively, which bind catenin. Light purple: 3 SAMP domains in between the 20 aa repeats which bind axin. Yellow: microtubule/F-actin/mDia-binding region between amino acids 2219 and 2580. Olive: C-terminal region that binds to the plus-end microtubule binding protein EB1. Pink: C-terminal S/TXV motif binds to PDZ domains of Dlg, PSD-95.
The following sections will describe pathways that modify APC subcellular localization and stimulate APC-dependent directional cell extension (Fig. 2).
3.1. LPA and integrin-extracellular matrix interaction at the cell edge
In fibroblasts, there are at least two pathways, the LPA-Rho-mDia pathway and a non-canonical Wnt pathway, that mediate APC localization to the plus ends of microtubules at the tip of cell extensions and APC-mediated migration after wounding [13, 41]. Both pathways seem to be re-enforced by local integrin-extracellular matrix (ECM) interaction at the wound edge [13, 42]. The non-canonical Wnt pathway will be described later (see 3.3.). Lysophosphatidic acid (LPA) stimulation of Rho GTPase and its effector, the formin mDia leads to capture and stabilization of microtubules in cell extensions by a mechanism that may involve APC interaction with mDia and End-Binding protein 1 (EB1) at microtubule plus ends [41]. Inhibition of GSK3β downstream of Rho-mDia is required for microtubule stabilization but not for reorientation of the microtubule organizing center (MTOC) towards the wound edge [43]. In this aspect the LPA-Rho-mDia pathway seems to be different from the non-canonical Wnt pathway in fibroblasts [13] and another pathway mediating APC-dependent cell extension in astrocytes [40, 44]. In astrocytes, integrin-ECM interaction at the wound edge leads to activation of a Cdc42/Par6/atypical Protein Kinase C (aPKC) complex that inhibits GSK3β and drives localization of APC to microtubule plus ends at the leading edge [40, 44]. In these latter two pathways, both inhibition of GSK3β and the microtubule plus end localization of APC are required for MTOC reorientation [13, 40].
3.2. Neurotrophic factors
The importance of APC in the formation of cell extensions has been best characterized in neuronal cells where APC is essential for neurite extension and axonal outgrowth [29, 45, 46]. In hippocampal neurons, APC localizes to the tip of early neurites and axons and its enrichment in axonal growth cones precedes axonal outgrowth [47]. Polarization of hippocampal neurons requires local activity of a growth factor receptor tyrosine kinase and phosphatidylinositol 3-kinase (PI3K), and formation of a complex of the polarity proteins Par3/Par6 and aPKC at the tip of the axonal outgrowth [48]. APC function is required for localization of Par3 to the axonal growth cone and could mediate transport of Par3 along microtubules by forming a complex with Par3 and kinesin superfamily protein KIF3A [46]. Local inhibition of GSK3β at the axon tip is essential for polarization of hippocampal neurons and Par3 targeting [46]. Although the signal that drives polarization in hippocampal neurons in vivo is not known, polarization of hippocampal neurons can be enhanced in vitro by Brain Derived Neurotrophic Factor (BDNF) or Neurotrophin-3 [27, 49]. Both of these neurothrophic factors inhibit GSK3β and, thereby, reduce phosphorylation of the microtubule-binding site of CRMP-2, another MAP important for axonal outgrowth [27]. It remains to be shown whether and how reduced phosphorylation of APC by GSK3β is involved in APC activation during axonal outgrowth (see 4.1. below).
Following NGF-induced differentiation, APC level increases and APC localizes in clusters at the tip of neurite extensions of PC12 cells [47, 50] and in axonal growth cones of dorsal root ganglion (DRG) neurons [29]. APC function is essential for neurite and axon outgrowth in response to NGF, since APC depletion by RNAi or expression of dominant interfering APC fragments inhibits neurite extension and axonal outgrowth [29, 45]. In DRG neurons, NGF inhibits GSK3β in a pathway that requires activation of PI3K and Integrin-Linked Kinase (ILK). As in hippocampal neurons, local GSK3β inhibition at the neurite tip is essential for APC localization and microtubule stabilization in the axonal growth cone and for axonal outgrowth [29]. However, global GSK3β inhibition with small molecule inhibitors or by RNAi decreases microtubule bundling in the shaft and induces excessive branching and APC accumulation at branch points along the shaft and multiple axon-like extensions [29, 51].
3.3. Wnt
The Wnt family of secreted glycoproteins regulates many developmental processes (reviewed in [4]). In the canonical Wnt pathway, Wnt binds Frizzled receptor and Lipoprotein Receptor-Related Protein (LRP) 6 which leads to activation of the cytoplasmic protein dishevelled. Although the function of dishevelled is not fully understood, it seems to co-cluster Wnt receptors with downstream signaling proteins which leads to phosphorylation of LRP6 by Casein kinase 1γ (CK1γ) and GSK3β and binding and recruitment of the APC-binding protein axin to phosphorylated LRP6 at the membrane [52-54]. This process somehow inhibits phosphorylation of β-catenin by GSK3β in the APC/axin/GSK3β destruction complex [4].Under these conditions, stabilized β-catenin can then accumulate in the nucleus and interact with transcription factors of the T-cell factor (TCF) family and activate specific gene expression [4]. In the absence of Wnt signaling, phosphorylation of β-catenin by GSK3β leads to β-catenin ubiquitination and degradation [55]. Understanding the interaction between these components of the canonical Wnt pathway is important for elucidating the regulation of APC and microtubules since many of them are involved in directional cell extension and neuronal polarization.
Non-canonical Wnt signaling does not induce catenin-mediated gene expression and it was long thought that it diverges from canonical Wnt signaling at the level of the dishevelled substrates which in the case of non-canonical signaling include RhoA and Rac and lead to actin cytoskeletal rearrangements and activation of the Jun N-terminal Kinase (JNK) pathway (reviewed in [56]). However, it has been recently shown that non-canonical Wnt5a activates components of the canonical Wnt pathway and promotes hippocampal polarization in a dishevelled- and aPKC- dependent manner [12] and fibroblast migration in a dishevelled- and axin-dependent manner that involves inhibition of GSK3β [13]. There is increasing evidence that Wnts are mediators of axon outgrowth and guidance [6, 57-59] and regulate axonal remodeling through a divergent canonical pathway that includes dishevelled, axin and inhibition of GSK3β [24, 60]. It remains to be shown whether APC regulation of microtubules is part of a Wnt pathway inducing cell extension.
In summary, local inhibition of GSK3β emerges as a common endpoint of several pathways that regulate APC and other MAPs, and microtubule dynamics during directional cell extensions. In astrocytes and neurons, increased phosphorylation of GSK3β on Ser-9 in response to protein kinase B (Akt/PKB) activation by PI3K has been defined as the mechanism of GSK3β inhibition in response to integrin activation at the leading edge or neurotrophic factors [27, 40, 46, 51]. However, recent studies reported that inhibition of GSK3β can occur independently of phosphorylation on Ser-9 or Ser-21 [13, 61]. These studies used fibroblasts and neurons from knock-in mice in which the two isoforms of GSK3 (α and β) had been rendered non-phosphorylatable by Akt/PKB through replacement with Ala [62]. Surprisingly, in GSK3 phospho-mutant fibroblasts, centrosome reorientation, APC relocalization and microtubule polarization occurred normally in response to wounding by inhibition of GSK3β in a Wnt5a-, dishevelled- and axin-dependent mechanism that was independent of Ser-9 or Ser-21 phosphorylation on GSK3β [13]. GSK3β inhibition was also independently regulated during polarization of hippocampal neurons isolated from these mice [61]. Although the pathway leading to mutant GSK3β inhibition in the neurons was not determined, it is intriguing to speculate that Wnt5a could mediate inhibition of GSK3β in these mutant neurons via activation of disheveled [12].
4. APC modification and function during cell extension
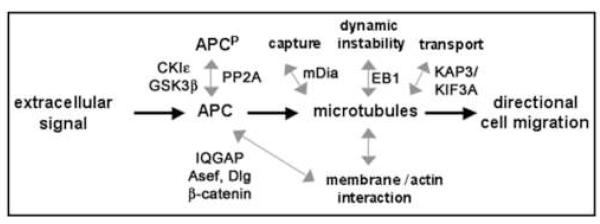
Little is known how APC is modified in response to extracellular signals inducing cell extension or how these signals affect APC interaction with different binding partners and regulate microtubules and cell extension via APC modification (Fig. 3). Does inhibition of GSK3β in response to Wnt or neurotrophins lead to decreased phosphorylation of APC, as has been shown for other MAPs?
Fig. 3. APC as a common target of signals that regulate cell motility.
grey double arrows indicate potential changes in APC phosphorylation and interaction with binding partners that need further investigation.
Several phosphorylation sites for CKIε and GSK3β have been identified in the central 20 amino acid repeats of APC that bind to β-catenin [63-65]. GSK3β phosphorylation of APC needs priming by CKIε and phosphorylation of APC by both kinases increases the affinity of these 20 amino acid repeats for β-catenin [65]. However, it remains unknown how phosphorylation of these APC 20 amino acid repeats affects APC interaction with microtubules and with other binding partners that are involved in microtubule regulation by APC, or whether there are other GSK3β phosphorylation sites in APC. The following paragraphs will summarize what is known about regulation of APC phosphorylation and interactions.
4.1. Regulation of APC-microtubule interactions by phosphorylation
APC is enriched in punctate clusters at the end of microtubule bundles in actively extending membranes in epithelial, neuronal and other cell types [29, 40, 41, 46, 47, 66, 67]. APC binds and stabilizes microtubules, and stimulates microtubule assembly and bundling in vitro [68], but its role in regulating microtubule dynamics in general is poorly understood. In migrating epithelial cells, microtubules decorated with APC at their plus ends spend increased time in growth and decreased time shortening [70]. In polarized epithelial monolayers, APC forms a cortical template of small clusters at the basal membrane along which microtubule networks are organized [71, 72].
Activity of GSK3β is important in regulating APC interactions with microtubules. Studies in vitro show that APC bundling of microtubules is inhibited by protein kinase A (PKA)/GSK3β phosphorylation of APC [30]. However, CKIε, and not PKA, is most likely the priming kinase for GSK3β in the APC complex in vivo [64, 65]. Although phosphorylation by PKA itself does not affect APC binding to microtubules [30], PKA may introduce additional priming sites for GSK3β that reduce APC/microtubule interactions. For example, PKA also phosphorylates the C-terminal EB1-binding site in APC resulting in inhibition of APC interaction with EB1 [74]. There may be additional unidentified GSK3β phosphorylation sites in the C-terminal microtubule and EB1 binding sites of APC and phosphorylation of those sites could inhibit APC interaction with microtubules or EB1 as it is the case for other MAPs [27]. It is also possible that phosphorylation of the central β-catenin binding region inhibits access of the C-terminal domain to microtubules and EB1. As noted above, phosphorylation of the central β-catenin binding region in APC increases the affinity of this APC region for β-catenin, and increased binding of mutant stabilized β-catenin to APC leads to APC hyperphosphorylation and inhibits cell extension [47, 63, 75].
Cycles of phosphorylation and dephosphorylation could also be important in regulating APC functions with microtubules at the cortex. The B56 subunit of protein phosphatase 2A (PP2A) binds to the armadillo domain of APC [63]. PP2A is a likely candidate to regulate phosphorylation of APC complex components by GSK3β and CKIε [63]. Phosphorylation of APC by cyclin dependent kinase p34cdc2 decreases its association with EB1 and this may have a physiological role in regulating APC/EB1 interaction [74, 76].
Despite some insight into the role of several kinases in APC phosphorylation, it remains unclear how changes in APC phosphorylation affect APC binding to, and regulation of microtubules at the leading edge during cell extension.
4.2. Potential roles of other APC binding proteins
Mammalian APC is a 310 kDa protein with multiple protein-protein interaction domains (Fig. 4). In its C-terminal region APC has a basic domain that binds and bundles microtubules in vitro [68]. This domain also binds and bundles F-actin in vitro [77]. Binding to microtubules and F-actin is competitive [77] and this may explain why APC co-localization with F-actin stress fibers in cells is observed only after disruption of microtubules by nocodazole [67, 78]. Interestingly, binding of the basic APC domain to microtubules is inhibited by EB1 interaction with APC [77].
The C-terminus of APC binds the mammalian homolog of Drosophila disc large (Dlg) ([79]; Fig. 4). Dlg co-localizes with APC to cortical clusters at the tip of epithelial cells but also localizes, unlike APC, strongly to established cell-cell contacts [80]. A complex of APC and Dlg seems to regulate directional migration in astrocytes [81]. Both, APC and Dlg co-localize at the leading edge in response to Cdc42/Par6/aPKCζ activation but whereas APC localization depends on GSK3β inactivation, the localization of Dlg is independent of GSK3β inactivation. Mutants of APC or Dlg that disrupt their interaction with each other inhibit directional migration [81].
APC binds EB1, a microtubule plus-end binding protein [82]. Endogenous EB1 and APC overlap in some regions of the cell, but generally, their distributions are different. APC increases EB1’s ability to stimulate microtubule polymerization [74, 83-86]. Microtubule plus-ends show high dynamic instability once they reach the cortex [87, 88]. APC could increase the microtubule rescue frequency at the extension tip or in the growth cone by facilitating EB1 re-localization to shrinking plus-ends. Rho and the formin mDia may also play a role as Rho activation by lysophosphatidic acid (LPA) activates mDia binding to APC and EB1 and their localization to the tip of stabilized microtubules [41].
APC regulation of microtubule stability in cell extensions may involve IQGAP [89, 90]. APC could also indirectly associate with F-actin via binding to IQGAP [89]. IQGAP is a Cdc42/Rac effector that cross-links F-actin, Clip170 and APC when bound to activated Rac [89, 90]. IQGAP and APC form a ternary complex with activated Rac/Cdc42 and depletion of either IQGAP or APC inhibits cell migration [89].
APC also binds Asef, an APC-stimulated Rac-specific guanine nucleotide exchange factor [91, 92]. Asef over-expression reduces cell-cell adhesion and increases cell migration, whereas RNAi depletion of Asef decreases migration; both effects are enhanced by expression of APCΔC [92]. This indicates that Asef can be recruited to, and activated in a complex with APC during cell migration.
The armadillo domain of APC binds Kap3 (kinesin superfamily-associated protein), a component of the kinesin (KIF) 3A/B microtubule plus-end directed motor [93, 94]. Kap3 may facilitate transport of APC and its binding partners to the cortex, since an APC N-terminal fragment (ΔEB1/MT binding sites) moves along microtubules to the cortex and accumulates in cortical clusters, and a Kap3 dominant-negative fragment inhibited APC localization into clusters [67, 85, 94, 95]. It will be important to define how and where in neurons the APC/KAP3 interaction is modified in response to signals mediating neuronal polarization because KAP3 binding may regulate the formation of the KIF3A/Par3/APC complex and APC-mediated transport of Par3 to the axonal growth cone.
5. A role for the APC/axin/GSK3β β-catenin Destruction Complex in Cell Migration?
Recall that in addition to regulating microtubules APC mediates the degradation of catenin in a multi-protein complex containing axin and GSK3β. Are these two functions of APC independent of each other? β-catenin is rarely detected in microtubule-associated APC clusters [66, 96] presumably because it is rapidly targeted to the proteasome for degradation [55]. However, deletion or mutagenesis of the N-terminal CKI/GSK3β phosphorylation sites on β-catenin results in the accumulation of β-catenin in APC clusters [96], and inhibits HGF-induced membrane extensions in MDCK epithelial cells [75] and NGF-induced formation of neurite extensions in PC12 cells [47]. Significantly, expression of stabilized β-catenin mutants increases the level of APC phosphorylation by CKIε and GSK3β [63, 64], and, thereby, may affect microtubule dynamics and stability at the cortex [30]. Other unresolved questions are: does Wnt or neurotrophin signaling locally increase or decrease binding of β-catenin to APC during cell extension or neuronal polarization; can binding of β-catenin to APC in the absence of GSK3β activity have a positive role in cell extension? Understanding the connections between APC functions in β-catenin regulation and in regulation of microtubules will help to elucidate the role of APC in cell extension and neuronal polarization.
6. Conclusions
Although we have a much better understanding of how signals inducing cell extension and migration and neuronal polarization have very immediate and local effects on the microtubule cytoskeleton, there are still many open questions to address. Local inhibition of GSK3β emerges as a common endpoint of several pathways that regulate APC and other MAPs and microtubule dynamics during directional cell extension. A major question to resolve is whether the different pathways of GSK3β inhibition that have been described downstream of neurotrophic factors and integrin activation or downstream of Wnt signaling, respectively, are functionally redundant or cooperate to regulate MAPs and microtubules. Furthermore, there are many gaps in our understanding of how the different components of divergent canonical Wnt signaling interact in their local function as regulators of microtubules during cell extension. It remains unclear how dishevelled inhibits GSK3β, and how axin works as a positive regulator in directional cell extension. It will be also important to learn more about the modification(s) and role(s) of APC as a positive effector of microtubule organization downstream of Wnt signaling and how β-catenin and other binding partners modify this function of APC. Addressing these questions will provide a much clearer picture of how cells reorganize into complex three-dimensional patterns, and could help to develop systems of tissue regeneration in disease or after injury.
Acknowledgements
Work from the Nelson laboratory was supported by a grant from the NIH (GM078270) and Hector Caro was supported by the Cell and Molecular Biology Training Grant, and an NIH grant (GM35527)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Thiery JP. Mechanisms of cell migration in the vertebrate embryo. Cell Differ. 1984;15:1–15. doi: 10.1016/0045-6039(84)90024-1. [DOI] [PubMed] [Google Scholar]
- [2].Hynes RO, Lander AD. Contact and adhesive specificities in the associations, migrations, and targeting of cells and axons. Cell. 1992;68:303–22. doi: 10.1016/0092-8674(92)90472-o. [DOI] [PubMed] [Google Scholar]
- [3].Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, et al. Cell migration: integrating signals from front to back. Science. 2003;302:1704–9. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- [4].Gordon MD, Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem. 2006;281:22429–33. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- [5].Lykissas MG, Batistatou AK, Charalabopoulos KA, Beris AE. The role of neurotrophins in axonal growth, guidance, and regeneration. Curr Neurovasc Res. 2007;4:143–51. doi: 10.2174/156720207780637216. [DOI] [PubMed] [Google Scholar]
- [6].Arevalo JC, Chao MV. Axonal growth: where neurotrophins meet Wnts. Curr Opin Cell Biol. 2005;17:112–5. doi: 10.1016/j.ceb.2005.01.004. [DOI] [PubMed] [Google Scholar]
- [7].Tanaka E, Kirschner MW. The role of microtubule dynamics in growth cone motility and axonal growth. J. Cell Biol. 1995b;128:139–155. doi: 10.1083/jcb.128.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Baas PW. Microtubules and axonal growth. Curr Opin Cell Biol. 1997;9:29–36. doi: 10.1016/s0955-0674(97)80148-2. [DOI] [PubMed] [Google Scholar]
- [9].Watanabe T, Noritake J, Kaibuchi K. Regulation of microtubules in cell migration. Trends Cell Biol. 2005;15:76–83. doi: 10.1016/j.tcb.2004.12.006. [DOI] [PubMed] [Google Scholar]
- [10].Etienne-Manneville S, Hall A. Cell polarity: Par6, aPKC and cytoskeletal crosstalk. Curr Opin Cell Biol. 2003;15:67–72. doi: 10.1016/s0955-0674(02)00005-4. [DOI] [PubMed] [Google Scholar]
- [11].Yoshimura T, Arimura N, Kaibuchi K. Signaling networks in neuronal polarization. J Neurosci. 2006;26:10626–30. doi: 10.1523/JNEUROSCI.3824-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhang X, Zhu J, Yang GY, Wang QJ, Qian L, Chen YM, et al. Dishevelled promotes axon differentiation by regulating atypical protein kinase C. Nat Cell Biol. 2007;9:743–54. doi: 10.1038/ncb1603. [DOI] [PubMed] [Google Scholar]
- [13].Schlessinger K, McManus EJ, Hall A. Cdc42 and noncanonical Wnt signal transduction pathways cooperate to promote cell polarity. J Cell Biol. 2007;178:355–61. doi: 10.1083/jcb.200701083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Etienne-Manneville S. Actin and microtubules in cell motility: which one is in control? Traffic. 2004;5:470–7. doi: 10.1111/j.1600-0854.2004.00196.x. [DOI] [PubMed] [Google Scholar]
- [15].Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–65. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- [16].Disanza A, Steffen A, Hertzog M, Frittoli E, Rottner K, Scita G. Actin polymerization machinery: the finish line of signaling networks, the starting point of cellular movement. Cell Mol Life Sci. 2005;62:955–70. doi: 10.1007/s00018-004-4472-6. [DOI] [PubMed] [Google Scholar]
- [17].Huttenlocher A. Cell polarization mechanisms during directed cell migration. Nat Cell Biol. 2005;7:336–7. doi: 10.1038/ncb0405-336. [DOI] [PubMed] [Google Scholar]
- [18].Rodriguez OC, Schaefer AW, Mandato CA, Forscher P, Bement WM, Waterman-Storer CM. Conserved microtubule-actin interactions in cell movement and morphogenesis. Nat Cell Biol. 2003;5:599–609. doi: 10.1038/ncb0703-599. [DOI] [PubMed] [Google Scholar]
- [19].Tanaka E, Kirschner MW. The role of microtubules in growth cone turning at substrate boundaries. J. Cell Biol. 1995a;128:127–137. doi: 10.1083/jcb.128.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Schaefer AW, Kabir N, Forscher P. Filopodia and actin arcs guide the assembly and transport of two populations of microtubules with unique dynamic parameters in neuronal growth cones. J Cell Biol. 2002;158:139–52. doi: 10.1083/jcb.200203038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bradke F, Dotti CG. The role of local actin instability in axon formation. Science. 1999;283:1931–4. doi: 10.1126/science.283.5409.1931. [DOI] [PubMed] [Google Scholar]
- [22].Takei Y, Teng J, Harada A, Hirokawa N. Defects in Axonal Elongation and Neuronal Migration in Mice with Disrupted tau and map1b Genes. J Cell Biol. 2000;150:989–1000. doi: 10.1083/jcb.150.5.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Stoothoff WH, Johnson GV. Tau phosphorylation: physiological and pathological consequences. Biochim Biophys Acta. 2005;1739:280–97. doi: 10.1016/j.bbadis.2004.06.017. [DOI] [PubMed] [Google Scholar]
- [24].Ciani L, Krylova O, Smalley MJ, Dale TC, Salinas PC. A divergent canonical WNT-signaling pathway regulates microtubule dynamics: dishevelled signals locally to stabilize microtubules. J Cell Biol. 2004;164:243–53. doi: 10.1083/jcb.200309096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Inagaki N, Chihara K, Arimura N, Menager C, Kawano Y, Matsuo N, et al. CRMP-2 induces axons in cultured hippocampal neurons. Nat Neurosci. 2001;4:781–2. doi: 10.1038/90476. [DOI] [PubMed] [Google Scholar]
- [26].Fukata Y, Itoh TJ, Kimura T, Menager C, Nishimura T, Shiromizu T, et al. CRMP-2 binds to tubulin heterodimers to promote microtubule assembly. Nat Cell Biol. 2002;4:583–91. doi: 10.1038/ncb825. [DOI] [PubMed] [Google Scholar]
- [27].Yoshimura T, Kawano Y, Arimura N, Kawabata S, Kikuchi A, Kaibuchi K. GSK-3beta regulates phosphorylation of CRMP-2 and neuronal polarity. Cell. 2005;120:137–49. doi: 10.1016/j.cell.2004.11.012. [DOI] [PubMed] [Google Scholar]
- [28].Wagner U, Utton M, Gallo JM, Miller CC. Cellular phosphorylation of tau by GSK-3 beta influences tau binding to microtubules and microtubule organisation. J Cell Sci. 1996;109(Pt 6):1537–43. doi: 10.1242/jcs.109.6.1537. [DOI] [PubMed] [Google Scholar]
- [29].Zhou FQ, Zhou J, Dedhar S, Wu YH, Snider WD. NGF-induced axon growth is mediated by localized inactivation of GSK-3beta and functions of the microtubule plus end binding protein APC. Neuron. 2004;42:897–912. doi: 10.1016/j.neuron.2004.05.011. [DOI] [PubMed] [Google Scholar]
- [30].Zumbrunn J, Kinoshita K, Hyman AA, Näthke IS. Binding of the adenomatous polyposis coli protein to microtubules increases microtubule stability and is regulated by GSK-3β phosphorylation. Current Biology. 2001;11:44–49. doi: 10.1016/s0960-9822(01)00002-1. [DOI] [PubMed] [Google Scholar]
- [31].Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–70. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- [32].Polakis P. The adenomatous polyposis coli (APC) tumor suppressor. Biochim Biophys Acta. 1997;1332:F127–47. doi: 10.1016/s0304-419x(97)00008-5. [DOI] [PubMed] [Google Scholar]
- [33].Nakagawa H, Murata Y, Koyama K, Fujiyama A, Miyoshi Y, Monden M, et al. Identification of a brain-specific APC homologue, APCL, and its interaction with beta-catenin. Cancer Res. 1998;58:5176–81. [PubMed] [Google Scholar]
- [34].van Es JH, Kirkpatrick C, van de Wetering M, Molenaar M, Miles A, Kuipers J, et al. Identification of APC2, a homologue of the adenomatous polyposis coli tumour suppressor. Curr Biol. 1999;9:105–8. doi: 10.1016/s0960-9822(99)80024-4. [DOI] [PubMed] [Google Scholar]
- [35].McCartney BM, Dierick HA, Kirkpatrick C, Moline MM, Baas A, Peifer M, et al. Drosophila APC2 is a cytoskeletally-associated protein that regulates wingless signaling in the embryonic epidermis. J Cell Biol. 1999;146:1303–18. doi: 10.1083/jcb.146.6.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ahmed Y, Nouri A, Wieschaus E. Drosophila Apc1 and Apc2 regulate Wingless transduction throughout development. Development. 2002;129:1751–62. doi: 10.1242/dev.129.7.1751. [DOI] [PubMed] [Google Scholar]
- [37].Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–7. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Moser AR, Shoemaker AR, Connelly CS, Clipson L, Gould KA, Luongo C, et al. Homozygosity for the Min allele of Apc results in disruption of mouse development prior to gastrulation. Dev Dyn. 1995;203:422–33. doi: 10.1002/aja.1002030405. [DOI] [PubMed] [Google Scholar]
- [39].Dikovskaya D, Zumbrunn J, Penman GA, Nathke IS. The adenomatous polyposis coli protein: in the limelight out at the edge. Trends Cell Biol. 2001;11:378–84. doi: 10.1016/s0962-8924(01)02069-4. [DOI] [PubMed] [Google Scholar]
- [40].Etienne-Manneville S, Hall A. Cdc42 regulates GSK-3beta and adenomatous polyposis coli to control cell polarity. Nature. 2003;421:753–6. doi: 10.1038/nature01423. [DOI] [PubMed] [Google Scholar]
- [41].Wen Y, Eng CH, Schmoranzer J, Cabrera-Poch N, Morris EJ, Chen M, et al. EB1 and APC bind to mDia to stabilize microtubules downstream of Rho and promote cell migration. Nat Cell Biol. 2004;6:820–30. doi: 10.1038/ncb1160. [DOI] [PubMed] [Google Scholar]
- [42].Palazzo AF, Eng CH, Schlaepfer DD, Marcantonio EE, Gundersen GG. Localized stabilization of microtubules by integrin- and FAK-facilitated Rho signaling. Science. 2004;303:836–9. doi: 10.1126/science.1091325. [DOI] [PubMed] [Google Scholar]
- [43].Eng CH, Huckaba TM, Gundersen GG. The formin mDia regulates GSK3beta through novel PKCs to promote microtubule stabilization but not MTOC reorientation in migrating fibroblasts. Mol Biol Cell. 2006;17:5004–16. doi: 10.1091/mbc.E05-10-0914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Etienne-Manneville S, Hall A. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell. 2001;106:489–98. doi: 10.1016/s0092-8674(01)00471-8. [DOI] [PubMed] [Google Scholar]
- [45].Dobashi Y, Katayama K, Kawai M, Akiyama T, Kameya T. APC protein is required for initiation of neuronal differentiation in rat pheochromocytoma PC12 cells. Biochem Biophys Res Commun. 2000;279:685–91. doi: 10.1006/bbrc.2000.4015. [DOI] [PubMed] [Google Scholar]
- [46].Shi SH, Cheng T, Jan LY, Jan YN. APC and GSK-3beta are involved in mPar3 targeting to the nascent axon and establishment of neuronal polarity. Curr Biol. 2004;14:2025–32. doi: 10.1016/j.cub.2004.11.009. [DOI] [PubMed] [Google Scholar]
- [47].Votin V, Nelson WJ, Barth AI. Neurite outgrowth involves adenomatous polyposis coli protein and beta-catenin. J Cell Sci. 2005;118:5699–708. doi: 10.1242/jcs.02679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Shi SH, Jan LY, Jan YN. Hippocampal neuronal polarity specified by spatially localized mPar3/mPar6 and PI 3-kinase activity. Cell. 2003;112:63–75. doi: 10.1016/s0092-8674(02)01249-7. [DOI] [PubMed] [Google Scholar]
- [49].Ip NY, Li Y, Yancopoulos GD, Lindsay RM. Cultured hippocampal neurons show responses to BDNF, NT-3, and NT-4, but not NGF. J Neurosci. 1993;13:3394–405. doi: 10.1523/JNEUROSCI.13-08-03394.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Dobashi Y, Bhattacharjee RN, Toyoshima K, Akiyama T. Upregulation of the APC gene product during neuronal differentiation of rat pheochromocytoma PC12 cells. Biochem Biophys Res Commun. 1996;224:479–83. doi: 10.1006/bbrc.1996.1052. [DOI] [PubMed] [Google Scholar]
- [51].Jiang H, Guo W, Liang X, Rao Y. Both the establishment and the maintenance of neuronal polarity require active mechanisms: critical roles of GSK-3beta and its upstream regulators. Cell. 2005;120:123–35. doi: 10.1016/j.cell.2004.12.033. [DOI] [PubMed] [Google Scholar]
- [52].Cliffe A, Hamada F, Bienz M. A role of Dishevelled in relocating Axin to the plasma membrane during wingless signaling. Curr Biol. 2003;13:960–6. doi: 10.1016/s0960-9822(03)00370-1. [DOI] [PubMed] [Google Scholar]
- [53].Zeng X, Tamai K, Doble B, Li S, Huang H, Habas R, et al. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature. 2005;438:873–7. doi: 10.1038/nature04185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Bilic J, Huang YL, Davidson G, Zimmermann T, Cruciat CM, Bienz M, et al. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science. 2007;316:1619–22. doi: 10.1126/science.1137065. [DOI] [PubMed] [Google Scholar]
- [55].Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. beta-catenin is a target for the ubiquitin-proteasome pathway. Embo J. 1997;16:3797–804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Barrow JR. Wnt/PCP signaling: a veritable polar star in establishing patterns of polarity in embryonic tissues. Semin Cell Dev Biol. 2006;17:185–93. doi: 10.1016/j.semcdb.2006.04.002. [DOI] [PubMed] [Google Scholar]
- [57].Lyuksyutova AI, Lu CC, Milanesio N, King LA, Guo N, Wang Y, et al. Anteriorposterior guidance of commissural axons by Wnt-frizzled signaling. Science. 2003;302:1984–8. doi: 10.1126/science.1089610. [DOI] [PubMed] [Google Scholar]
- [58].Lu W, Yamamoto V, Ortega B, Baltimore D. Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell. 2004;119:97–108. doi: 10.1016/j.cell.2004.09.019. [DOI] [PubMed] [Google Scholar]
- [59].Charron F, Tessier-Lavigne M. Novel brain wiring functions for classical morphogens: a role as graded positional cues in axon guidance. Development. 2005;132:2251–62. doi: 10.1242/dev.01830. [DOI] [PubMed] [Google Scholar]
- [60].Krylova O, Messenger MJ, Salinas PC. Dishevelled-1 regulates microtubule stability: a new function mediated by glycogen synthase kinase-3beta. J Cell Biol. 2000;151:83–94. doi: 10.1083/jcb.151.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Gartner A, Huang X, Hall A. Neuronal polarity is regulated by glycogen synthase kinase-3 (GSK-3beta) independently of Akt/PKB serine phosphorylation. J Cell Sci. 2006;119:3927–34. doi: 10.1242/jcs.03159. [DOI] [PubMed] [Google Scholar]
- [62].McManus EJ, Sakamoto K, Armit LJ, Ronaldson L, Shpiro N, Marquez R, et al. Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis. Embo J. 2005;24:1571–83. doi: 10.1038/sj.emboj.7600633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Ikeda S, Kishida M, Matsuura Y, Usui H, Kikuchi A. GSK-3beta-dependent phosphorylation of adenomatous polyposis coli gene product can be modulated by beta-catenin and protein phosphatase 2A complexed with Axin. Oncogene. 2000;19:537–45. doi: 10.1038/sj.onc.1203359. [DOI] [PubMed] [Google Scholar]
- [64].Rubinfeld B, Tice DA, Polakis P. Axin-dependent phosphorylation of the adenomatous polyposis coli protein mediated by casein kinase 1epsilon. J Biol Chem. 2001;276:39037–45. doi: 10.1074/jbc.M105148200. [DOI] [PubMed] [Google Scholar]
- [65].Ha NC, Tonozuka T, Stamos JL, Choi HJ, Weis WI. Mechanism of phosphorylationdependent binding of APC to beta-catenin and its role in beta-catenin degradation. Mol Cell. 2004;15:511–21. doi: 10.1016/j.molcel.2004.08.010. [DOI] [PubMed] [Google Scholar]
- [66].Näthke IS, Adams CL, Polakis P, Sellin JH, Nelson WJ. The adenomatous polyposis coli tumor suppressor protein localizes to plasma membrane sites involved in active cell migration. J Cell Biol. 1996;134:165–79. doi: 10.1083/jcb.134.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Mimori-Kiyosue Y, Shiina N, Tsukita S. Adenomatous Polyposis Coli (APC) Protein Moves along Microtubules and Concentrates at Their Growing Ends in Epithelial Cells. J Cell Biol. 2000;148:505–518. doi: 10.1083/jcb.148.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Munemitsu S, Souza B, Muller O, Albert I, Rubinfeld B, Polakis P. The APC gene product associates with microtubules in vivo and promotes their assembly in vitro. Cancer Res. 1994;54:3676–81. [PubMed] [Google Scholar]
- [69].Smith KJ, Levy DB, Maupin P, Pollard TD, Vogelstein B, Kinzler KW. Wild-type but not mutant APC associates with the microtubule cytoskeleton. Cancer Res. 1994;54:3672–5. [PubMed] [Google Scholar]
- [70].Kita K, Wittmann T, Nathke IS, Waterman-Storer CM. Adenomatous polyposis coli on microtubule plus ends in cell extensions can promote microtubule net growth with or without EB1. Mol Biol Cell. 2006;17:2331–45. doi: 10.1091/mbc.E05-06-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Reilein A, Nelson WJ. APC is a component of an organizing template for cortical microtubule networks. Nat Cell Biol. 2005;7:463–73. doi: 10.1038/ncb1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Reilein A, Yamada S, Nelson WJ. Self-organization of an acentrosomal microtubule network at the basal cortex of polarized epithelial cells. J Cell Biol. 2005;171:845–55. doi: 10.1083/jcb.200505071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Gao ZH, Seeling JM, Hill V, Yochum A, Virshup DM. Casein kinase I phosphorylates and destabilizes the beta-catenin degradation complex. Proc Natl Acad Sci U S A. 2002;99:1182–7. doi: 10.1073/pnas.032468199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Askham JM, Moncur P, Markham AF, Morrison EE. Regulation and function of the interaction between the APC tumour suppressor protein and EB1. Oncogene. 2000;19:1950–8. doi: 10.1038/sj.onc.1203498. [DOI] [PubMed] [Google Scholar]
- [75].Pollack AL, Barth AIM, Altschuler Y, Nelson WJ, Mostov KE. Dynamics of β-catenin interactions with APC protein regulate epithelial tubulogenesis. J Cell Biol. 1997;137:1651–1662. doi: 10.1083/jcb.137.7.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Trzepacz C, Lowy AM, Kordich JJ, Groden J. Phosphorylation of the tumor suppressor adenomatous polyposis coli (APC) by the cyclin-dependent kinase p34. J Biol Chem. 1997;272:21681–4. doi: 10.1074/jbc.272.35.21681. [DOI] [PubMed] [Google Scholar]
- [77].Moseley JB, Bartolini F, Okada K, Wen Y, Gundersen GG, Goode BL. Regulated binding of adenomatous polyposis coli protein to actin. J Biol Chem. 2007;282:12661–8. doi: 10.1074/jbc.M610615200. [DOI] [PubMed] [Google Scholar]
- [78].Mimori-Kiyosue Y, Matsui C, Sasaki H, Tsukita S. Adenomatous polyposis coli (APC) protein regulates epithelial cell migration and morphogenesis via PDZ domainbased interactions with plasma membranes. Genes Cells. 2007;12:219–33. doi: 10.1111/j.1365-2443.2007.01045.x. [DOI] [PubMed] [Google Scholar]
- [79].Matsumine A, Ogai A, Senda T, Okumura N, Satoh K, Baeg GH, et al. Binding of APC to the human homolog of the Drosophila discs large tumor suppressor protein. Science. 1996;272:1020–3. doi: 10.1126/science.272.5264.1020. [see comments] [DOI] [PubMed] [Google Scholar]
- [80].Iizuka-Kogo A, Shimomura A, Senda T. Colocalization of APC and DLG at the tips of cellular protrusions in cultured epithelial cells and its dependency on cytoskeletons. Histochem Cell Biol. 2005;123:67–73. doi: 10.1007/s00418-004-0729-2. [DOI] [PubMed] [Google Scholar]
- [81].Etienne-Manneville S, Manneville JB, Nicholls S, Ferenczi MA, Hall A. Cdc42 and Par6-PKC{zeta} regulate the spatially localized association of Dlg1 and APC to control cell polarization. J Cell Biol. 2005;170:895–901. doi: 10.1083/jcb.200412172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Su LK, Burrell M, Hill DE, Gyuris J, Brent R, Wiltshire R, et al. APC binds to the novel protein EB1. Cancer Res. 1995;55:2972–7. [PubMed] [Google Scholar]
- [83].Morrison EE, Wardleworth BN, Askham JM, Markham AF, Meredith DM. EB1, a protein which interacts with the APC tumour suppressor, is associated with the microtubule cytoskeleton throughout the cell cycle. Oncogene. 1998;17:3471–7. doi: 10.1038/sj.onc.1202247. [DOI] [PubMed] [Google Scholar]
- [84].Mimori-Kiyosue Y, Shiina N, Tsukita S. The dynamic behavior of the APC-binding protein EB1 on the distal ends of microtubules. Curr Biol. 2000;10:865–8. doi: 10.1016/s0960-9822(00)00600-x. [DOI] [PubMed] [Google Scholar]
- [85].Barth AI, Siemers KA, Nelson WJ. Dissecting interactions between EB1, microtubules and APC in cortical clusters at the plasma membrane. J Cell Sci. 2002;115:1583–90. doi: 10.1242/jcs.115.8.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Berrueta L, Tirnauer JS, Schuyler SC, Pellman D, Bierer BE. The APC-associated protein EB1 associates with components of the dynactin complex and cytoplasmic dynein intermediate chain. Curr Biol. 1999;9:425–8. doi: 10.1016/s0960-9822(99)80190-0. [DOI] [PubMed] [Google Scholar]
- [87].Kaverina I, Rottner K, Small JV. Targeting, capture, and stabilization of microtubules at early focal adhesions. J Cell Biol. 1998;142:181–90. doi: 10.1083/jcb.142.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Waterman-Storer CM, Salmon E. Positive feedback interactions between microtubule and actin dynamics during cell motility. Curr Opin Cell Biol. 1999;11:61–7. doi: 10.1016/s0955-0674(99)80008-8. [DOI] [PubMed] [Google Scholar]
- [89].Watanabe T, Wang S, Noritake J, Sato K, Fukata M, Takefuji M, et al. Interaction with IQGAP1 links APC to Rac1, Cdc42, and actin filaments during cell polarization and migration. Dev Cell. 2004;7:871–83. doi: 10.1016/j.devcel.2004.10.017. [DOI] [PubMed] [Google Scholar]
- [90].Fukata M, Watanabe T, Noritake J, Nakagawa M, Yamaga M, Kuroda S, et al. Rac1 and Cdc42 capture microtubules through IQGAP1 and CLIP-170. Cell. 2002;109:873–85. doi: 10.1016/s0092-8674(02)00800-0. [DOI] [PubMed] [Google Scholar]
- [91].Kawasaki Y, Senda T, Ishidate T, Koyama R, Morishita T, Iwayama Y, et al. Asef, a link between the tumor suppressor APC and G-protein signaling. Science. 2000;289:1194–7. doi: 10.1126/science.289.5482.1194. [DOI] [PubMed] [Google Scholar]
- [92].Kawasaki Y, Sato R, Akiyama T. Mutated APC and Asef are involved in the migration of colorectal tumour cells. Nat Cell Biol. 2003;5:211–5. doi: 10.1038/ncb937. [DOI] [PubMed] [Google Scholar]
- [93].Yamazaki H, Nakata T, Okada Y, Hirokawa N. Cloning and characterization of KAP3: a novel kinesin superfamily-associated protein of KIF3A/3B. Proc Natl Acad Sci U S A. 1996;93:8443–8. doi: 10.1073/pnas.93.16.8443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Jimbo T, Kawasaki Y, Koyama R, Sato R, Takada S, Haraguchi K, et al. Identification of a link between the tumour suppressor APC and the kinesin superfamily. Nat Cell Biol. 2002;4:323–7. doi: 10.1038/ncb779. [DOI] [PubMed] [Google Scholar]
- [95].Sharma M, Leung L, Brocardo M, Henderson J, Flegg C, Henderson BR. Membrane localization of adenomatous polyposis coli protein at cellular protrusions: targeting sequences and regulation by beta-catenin. J Biol Chem. 2006;281:17140–9. doi: 10.1074/jbc.M513027200. [DOI] [PubMed] [Google Scholar]
- [96].Barth AI, Pollack AL, Altschuler Y, Mostov KE, Nelson WJ. NH2-terminal deletion of beta-catenin results in stable colocalization of mutant beta-catenin with adenomatous polyposis coli protein and altered MDCK cell adhesion. J Cell Biol. 1997;136:693–706. doi: 10.1083/jcb.136.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]