Summary
The Tec (tyrosine kinase expressed in hepatocellular carcinoma) family of non-receptor tyrosine kinases consists of five members: Tec, Btk (Bruton’s tyrosine kinase), Itk (inducible T-cell kinase), Rlk/Txk (resting lymphocyte kinase), and Bmx/Etk (bone marrow-expressed kinase). Although their functions are probably best understood in antigen receptor signaling, where they participate in the phosphorylation and regulation of phospholipase C-γ (PLC-γ), it is now appreciated that these kinases contribute to signaling from many receptors and that they participate in multiple downstream pathways, including regulation of the actin cytoskeleton. In T cells, three Tec kinases are expressed, Itk, Rlk/Txk, and Tec. Itk is expressed at highest amounts and plays the major role in regulating signaling from the T-cell receptor (TCR). Recent studies provide evidence that these kinases contribute to multiple aspects of T-cell biology and have unique roles in T-cell development that have revealed new insight into the regulation of conventional and innate T-cell development. We review new findings on the Tec kinases with a focus on their roles in T-cell development and mature T-cell differentiation.
Keywords: T cells, Th1/Th2/Th17 cells, asthma, thymus, protein kinases/phosphatases
Introduction
Approximately 15 years ago, two groups discovered that mutations of a novel non-receptor tyrosine kinase, now termed Bruton’s tyrosine kinase (Btk), were the cause of a severe immunodeficiency X-linked agammaglobulinemia (XLA), which is characterized by impaired B-cell development, low to absent serum immunoglobulins (Igs), and recurrent infections (1, 2). It was recognized at that time that Btk was most homologous to two other previously cloned kinases, Tec (tyrosine kinase expressed in hepatocellular carcinoma) and Itk/Emt (inducible T-cell kinase/expressed mainly in T cells) (3). The Tec family of kinases now consists of five related members: Btk, Tec, Itk, Rlk (resting lymphocyte kinase), also known as Txk, and Bmx/Etk (bone marrow-expressed kinase), most of which are expressed primarily in a variety of hematopoietic cells (4–6). In naive T cells and thymocytes, Itk is the highest expressed family member, while Rlk is expressed at several-fold lower levels. Tec is expressed 20–100 fold lower at the level of messenger RNA in naive T cells, but its expression increases upon activation (7–9). These expression patterns are likely to contribute to their relative importance in T-cell function: Itk deficiency leads to the greatest defects in T-cell development and function (10–12). These defects can be worsened by Rlk deficiency, but to date, no major defects in T cell function have been reported in Tec-deficient mice (13).
Similar to several other families of tyrosine kinases, the Tec kinases possess modular structures that include a COOH-terminal kinase catalytic domain, proceeded by a Src homology 2 (SH2) protein interaction domain that interacts with phosphorylated tyrosine residues, and an SH3 domain that generally interacts with proline-rich sequences (4). However, unlike other tyrosine kinases, the Tec kinases are marked by two distinct amino-terminal domains. Most Tec kinase contain an amino-terminal Pleckstrin homology (PH) domain, which can interact with products of phosphoinositide 3-kinase (PI3K), as well as other phosphoinositides and probably other proteins. These interactions lead to recruitment of the Tec kinases to the plasma membrane; data suggest that such interactions with the PH domain are a pre-requisite for Itk activation (14, 15). Rlk is the sole family member that lacks a PH domain and instead possesses a palmitoylated cysteine-string motif that is required for membrane localization (16). Tec kinases also contain one or two proline-rich regions that are important for intra- and intermolecular interactions that help regulate kinase activity (4, 17). These distinct features help define the Tec kinases, which are now recognized as important modulators of lymphocyte development and function.
Tec kinases and T-cell receptor signaling
Activation of Tec kinases
Like other non-receptor tyrosine kinases, the Tec kinases are regulated by a number of intramolecular interactions between their protein interaction domains as well as intermolecular interactions with phospholipids or other proteins. Over the last several years, biochemical and structural data has revealed complex features of Itk regulation (4). Requirements for activation of Itk include the following: (i) interaction of the pleckstrin homology domain with phospholipids required for membrane association, (ii) disruption of intramolecular protein interactions, (iii) phosphorylation of a tyrosine in the activation loop by Src family kinases, and (iv) interactions with other proteins via its SH2 and kinase domains. Structural studies have recently provided new insight into the requirements for activation of Itk--details of these studies can be found in the article by Andreotti and colleagues in this issue.
Similar to Btk, Itk is activated in response to antigen receptor engagement and is involved in signaling downstream from antigen receptors in T cells. Studies of Itk-deficient mice initially revealed that Itk was required for T-cell receptor (TCR)-induced proliferation and IL-2 production (10–12). Further studies demonstrated that Itk, like Btk in B-cell receptor signaling, is required for TCR-induced phosphorylation of PLC-γ and Ca2+ mobilization (11, 12).
In T cells, Itk participates in TCR-induced PLC-γ activation by its association with the LAT-SLP-76 signalosome (4, 18, 19) (Fig. 1). Upon TCR activation, Lck is activated, leading to the phosphorylation of ITAM motifs on the intracellular tail of the CD3 chains and the recruitment of ZAP-70 (20). Together, these kinases phosphorylate the two key adaptor molecules LAT and SLP-76, which then interact via their association with the adaptor GADS and form a platform for the recruitment of other signaling molecules, including Itk, PLC-γ1, the guanine nucleotide exchange factor Vav, the adaptor Nck, the adaptor Grb2 (which binds the guanine nucleotide factor SOS) and the adaptor ADAP (21). Formation of this signaling platform is critical for TCR signaling: mutations that affect LAT or SLP-76 essentially eliminate TCR signaling and T-cell development at the stage of pre-TCR signaling (22, 23). Itk is recruited to this signaling complex via an interaction between its SH2 domain and a phosphorylated tyrosine on SLP-76 (18, 19). However, other interactions within this complex also occur and there is also evidence that Itk may participate in phosphorylating certain residues of LAT (24). Recent evidence argues that Itk kinase activity requires interaction of its SH2 domain with SLP-76 in the absence of this interaction, Itk kinase activity is markedly decreased (25).
Fig. 1. Itk and TCR signaling complexes.
Itk functions in TCR signaling via its interactions in the LAT-SLP-76 signaling complex, where it participates in activation of PLC-γ and the downstream generation of DAG and IP3, as well as regulation of the actin cytoskeleton via interactions with Vav. Rlk is also likely to participate in this context.
Activation of PLC-γ
Once part of the LAT-SLP-76 signalosome, Itk serves to phosphorylate PLC-γ1, a critical enzyme in antigen receptor signaling that catalyzes the cleavage of PI4,5P2 to inositol 3 phosphate (IP3) and diacylglycerol (DAG), two of the key second messengers in TCR signaling (26). Mutations affecting Itk decrease tyrosine phosphorylation of PLC-γ1, including phosphorylation of Y783, a critical tyrosine for PLC-γ function that allows PLC-γ activation (11, 12, 27). Rlk can also phosphorylate this tyrosine and studies suggest that Rlk can compensate for Itk in Ca2+ regulation when overexpressed (28, 29). Whether Itk affects PLC-γ activation by other means is not known; however, evidence suggests that Btk participates in a positive feedback loop by helping recruit Phosphoinositide-5-kinase to the membrane, thus generating more PI4,5P2, which is the substrate for both PI3K and for PLC-γ. This mechanism thereby both increases PLC-γ activation and provides it with more substrate (30). Itk is also part of a positive feedback loop in which IP4, the product of IP3-3 kinase, enhances the binding of PI(3,4,5)P3 to the Itk PH domain, amplifying Itk’s activation after the initial generation of IP3 by PLC-γ (31).
Once PLC-γ is activated, its product, IP3, binds to receptors on intracellular organelles including the endoplasmic reticulum, leading to Ca2+ release from these intracellular stores (32). In turn, this leads to the activation of the Ca2+ release activated channel (CRAC) via the STIM protein-mediated multimerization of the Orai channel proteins on the plasma membrane, leading to sustained Ca2+ influx (33, 34). This sustained Ca2+ influx in turn leads to activation of Calmodulin and the Ca2+-sensitive phosphatase Calcineurin, leading to downstream activation of transcription factors (35). DAG leads to activation of MAPK pathways via its activation of RasGRP, a guanine nucleotide exchange factor for Ras that contains a DAG-binding domain and which leads to activation of the Ras-Raf-ERK pathway (36), as well as amplification of this pathway via GRB2-SOS-mediated pathways (37). DAG also leads to activation of Protein Kinase Cs (38), including the novel PKC-θ, which is important for activation of JNK and NF-κB. Many of the downstream consequences of TCR stimulation can be mimicked by treatment of cells with PMA (which activate DAG-mediated pathways) and ionomycin, a Ca2+ ionophore (20). The activation of PLC-γ is therefore a critical step of TCR signaling.
Nonetheless, it should be noted that mutation of Itk in primary T cells does not eliminate Ca2+ mobilization and T-cell responses but rather just decreases these responses (Fig. 2). These defects are worsened in T cells from mice deficient both Itk and Rlk, although Ca2+ mobilization is still present, even in T cells from the double knockouts (12). Whether these partial defects are due to redundancy between the Tec kinases and residual Tec expression, or due to other pathways that can contribute to PLC-γ phosphorylation in T cells is not yet clear. While evidence argues that there is some degree of functional redundancy between Tec kinases, it is also likely that there are unique signaling features for each of these kinases and that they participate in signaling cascades downstream of other receptors. Nonetheless, it is of interest that expression of a kinase-inactive mutant of Itk was found to decrease the intensity of TCR-induced Ca2+ mobilization, whereas a kinase-inactive Lck mutant eliminated Ca2+ signaling when expressed at high enough levels (but did not lead to intermediate levels of Ca2+ mobilization) (39). Together these data suggest that, unlike the more proximal tyrosine kinases in the TCR signaling cascade, the Tec kinases appear to play a more modulatory role in regulating PLC-γ activation and may serve more as amplifiers or modulators of T cell responses.
Fig. 2. Naive CD4+ T cells from TCR transgenic Itk−/− mice show defects in TCR-induced Ca2+ influx.

CD4+ cells from Itk−/− and control 5CC7 TCR transgenic mice were stimulated with biotinylated anti-CD3 plus streptavidin and Ca2+ mobilization measured by flow cytometry. Lower panel, expression of CD4 on purified CD4+ cells used in Ca2+ experiments. Percentage of CD4 cells that were CD62Lhi (naive) in these experiments are indicated.
Downstream consequences: activation of transcription
As noted above, activation of PLC-γ is a critical step in TCR signaling and much of the downstream consequences of TCR signaling, including T-cell proliferation and IL-2 production, can be mimicked by treatment with PMA and ionomycin. In part, this is secondary to their effects on the activation of downstream transcription factors. Sustained Ca2+ influx leads to the activation of the phosphatase Calcineurin which dephosphorylates a number of key transcription factors, most notably the nuclear factor of activated T cells (NFATs) (35). Inhibition of calcineurin severely impairs T cell function and is the basis of the function of two key immunosuppressants, cyclosporine A and FK506.
DAG activates several pathways, including those leading to ERK, which increases Elk 1-driven expression of Fos, a component of AP-1 transcription factors (20). AP-1, in turn, heterodimerizes with NFAT to activate NFAT-AP1 complexes important for cytokine regulation. Imbalances in these process can occur when one pathway is differentially activated or inhibited as can happen in the absence of CD28 costimulation, when T cells are stimulated with Ionomycin alone, or when DAG Kinase expression is modified (40, 41). Erk can also participate in positive feedback loops in TCR signaling by preventing recruitment of the SHP1 phosphatase to Lck (42). DAG is also required for activation of Protein kinase C’s (38). In T cells, PKC-θ is important for activating the third arm of T cell transcription, NF-κB, via the Carma-1/Bcl-10/Malt-1 complex (43). PKC-θ also contributes to AP-1 activation via activation of JNK and phosphorylation of Jun, another component of AP-1 complexes.
Consistent with the requirement for Tec kinases in activating PLC-γ and its downstream effectors, T cells from mice mutant in Itk or Itk and Rlk have defective activation of NFAT and AP-1 (44, 45). Moreover, since NFATc1 positively affects its own transcription, defects in NFAT function are further amplified by effects on NFAT expression (46). Effects on NF-κB have been less well documented and may depend on the context of activation.
Together, these defects in TCR signaling in cells lacking Itk or Itk and Rlk lead to alterations in T-cell development and function. In particular, mice deficient in Itk have been found to have impaired Th2 polarization and Th2 cytokine production in response to Th2-inducing agents and infections in vivo (44, 45, 47). Mice deficient in Itk or Itk and Rlk also have altered T cell development that specifically affects the selection and development of conventional T cells, but promotes development of a non-conventional T cell compartment (48). These distinct features will be discussed below.
Itk deficiency and the actin cytoskeleton
One of the other major consequences of TCR activation is the subsequent polarization of T cells towards the site of activation or contact with the antigen-presenting cell (49, 50). This polarization is critical for T-cell function. In the case of CD8+ T cells, polarization is necessary for the directed secretion of lytic granules and properly directed cytolysis. For CD4+ T cells, secretion of certain cytokines has also been shown to be polarized. However, even earlier in T-cell activation, cell polarization is required for the recruitment of the TCR and costimulatory receptors, as well as signaling molecules and complexes to the site of TCR stimulation in order to activate effective TCR signaling. Cell polarization is also required for the formation of the immune synapse (IS), a highly organized structure that occurs at the site of TCR stimulation with certain antigen-presenting cells (particularly B cells), with a central accumulation of the TCR and certain signaling molecules and a more peripheral accumulation of integrin adhesion molecules (51, 52). Although the function of the IS is not clear, data and mathematical modeling suggest it is likely to be important for TCR downregulation, but also for TCR signal amplification under conditions of suboptimal stimulation (53). Polarization also serves to segregate negative signaling molecules and conversely to aggregate integrins, which mediate cell adhesion and contribute to prolonged T cell interactions. Recent data also suggest that polarization serves to differentiate functional outcomes for daughter cells after cell division (54).
Cytoskeleton polarization, like other effects of TCR signaling, is dependent on the LAT-SLP-76 complex, which in addition to bringing in enzymes and adaptors that contribute to activation of Ca2+ and MAPK pathways, recruits a number of proteins that contribute to cytoskeleton regulation (49, 50). These include the guanine nucleotide exchange factors Vav1, Vav2, and Vav3 (hereafter referred to as Vav), which regulate the small GTPases Rac, Rho, and Cdc42 (55, 56). These critical cytoskeletal regulators lead to the nucleation of distinct types of actin polymerization, as well as reorganization of the microtubule organizing center. Cdc42 activates the Wiskott-Aldrich protein, which binds and activates the Arp2/3 complex to nucleate branched actin filament growth (49). WASP is recruited to the SLP-76 complex via Nck, another adaptor protein that binds tyrosine phosphorylated SLP-76. Rac activates WAVE2, a WASP homologue that is involved in regulation of actin and cell adhesion via integrins (57).
It is now clear that Itk also contributes to TCR-induced cytoskeleton regulation. A role for Itk in actin reorganization was first suggested by experiments from the Tsoukas laboratory (58), which showed that expression of a SH2 mutant of Itk blocked actin polarization in response to TCR stimulation of Jurkat cells. Interestingly, a kinase-dead mutant of Itk did not affect actin in this assay, perhaps accounting for differences with a previous study that concluded that Itk was not involved in actin polarization based on observations that expression of a kinase-dead mutant failed to affect cell polarization (39).
In collaboration with the Burkhardt group, we found that CD4 cells from Itk deficient mice that expressed the AND TCR transgene (which is specific for a peptide from pigeon cytochrome C) showed reduced actin polarization to the site of stimulation with antigen presenting cells (59). This defect was associated with reduced accumulation of activated Cdc42, as determined using a sensor consisting of the WASP GTPase-binding domain (GBD) that binds specifically to activated Cdc42. This defect further correlated with decreased recruitment of Vav1, an upstream activator of Cdc42. We also confirmed this finding in the Jurkat cell line and in primary human T cells in which we knocked-down expression of Itk using siRNA and looked at cell polarization in response to anti-CD3 or anti-TCR coated beads (60). Using the Jurkat system, we were able to probe the functional requirements for Itk in actin polarization and show that rescue of actin polarization in Itk-knockdown cells did not require Itk kinase activity, but did require a functional SH2 domain. Moreover, as the Tsoukas group had previously shown, expression of the SH2 mutant of Itk blocked TCR-induced actin polarization. Again, polarization defects correlated with impaired recruitment of Vav1 to the site of TCR stimulation--expression of an activated membrane-targeted version of Vav1 could rescue cell polarization in siItk-treated cells (60). We further found that SLP-76 did not efficiently co-immunoprecipitate with Vav1 in Itk-knockdown cells. Since Itk binds to SLP-76 via an SH2-mediated interaction, these finding suggested a model where Itk functions in a kinase-independent fashion as an adapter that contributes to the stable association of Vav1 with SLP-76.
Consistent with a role for Itk in regulating Vav1 association with TCR signaling complexes, we and others have found that certain functional defects in mature Itk-deficient T cells, like those in mature T cells deficient in Vav1, can be rescued by mutations affecting the ubiquitin ligase Cbl-b (61, 62). Nonetheless, Vav1 is likely to have additional functions since Vav1-deficient mice have more severe defects in thymic development and T-cell function than Itk-deficient mice (63). Moreover, Ellmeier and colleagues (62) have recently shown that Itk−/−Vav1−/− mice exhibit severe defects in thymic development leading to dramatic decreases in T-cell numbers. Thus, Vav1 is likely to have effects on T-cell biology beyond those involving Itk. Whether Itk also affects actin polarization by other mechanisms awaits further investigation. BTK has been shown to localize with and bind actin directly (64, 65), suggesting another mechanism by which Tec kinases may affect the actin cytoskeleton.
Effects on cell adhesion
One of the other consequences of TCR stimulation and actin reorganization is the activation of integrins, a family of adhesive receptors that help cells interact with other cells and with the extracellular matrix. Adhesion via integrins is activated after TCR stimulation both by long-term upregulation of integrin expression and by an immediate increase in their adhesiveness known as ‘inside-out signaling’ (66). Inside-out signaling is associated with integrin clustering and changes in conformation leading to increased affinity and avidity. These changes occur via actin-dependent processes. Consistent with the role of Itk in regulating Vav recruitment and actin polarization, Itk-deficient cells fail to accumulate integrins at the site of TCR stimulation and do not increase integrin-based adhesion to ICAM (a ligand for β1 integrins) (67). Similarly, defects in binding Fibronectin (a ligand for β2 integrins) were observed in Jurkat cells expressing mutant versions of Itk (68). Once again, whether defective recruitment of Vav is the only mechanism by which Itk affects integrin adhesion is not clear. Ca2+ mobilization, activation of PKCs and other pathways downstream of TCR signaling including those involving ADAP, another component of the SLP-76 complex, all contribute to integrin activation (66). Similarly, how altered cell adhesion may contribute to phenotypes associated with Itk-deficiency is not clear. Itk- and Rlk/Itk-deficient cells exhibit decreased cell adhesion in conjugate assays with a variety of antigen- presenting cells (KLM, unpublished data), suggesting that decreased duration of signaling may also contribute to T cells activation defects in Itk−/− and Rlk−/−Itk−/− mice.
Involvement of Itk in other pathways: chemokine responses
One of the other major signaling pathways that leads to actin reorganization, cell polarization and increased adhesion in lymphocytes, involves responses to chemokines. These small peptides bind to seven-transmembrane G-protein coupled receptors (GPCR) and induce cell migration via pathways involving reorganization of the actin cytoskeleton and modulation of integrin adhesion (69). Activation of GPCR also leads to activation of PI3Ks and, although less well appreciated, can induce tyrosine phosphorylation. Previous data had suggested direct interactions of Btk with Gβγ subunits (70).
The involvement in Tec kinases in actin-based pathways raised the question of whether Itk may participate in other signaling pathways that may require cell polarization in T cells. Indeed, we and A. August and colleagues found that stimulation of cells with SDF-1a led to increased Itk kinase activity and recruitment of Itk to the plasma membrane (71, 72). Similarly, translocations of Btk in response to SDF-1a have also been reported (64, 73). We further showed that Itk-deficient cells show reduced migration to multiple chemokines; these defects were worsened in Rlk−/−Itk−/− T cells (71, 72). Similar findings were seen in Jurkat cells overexpressing mutant versions of Itk. In these cells, we found decreased activation of Cdc42 and Rac in response to SDF-1α (CCL12) (72). Although those biochemical findings have not been confirmed in primary cells, cell transfers into WT mice revealed defects in trafficking of Rlk−/−Itk−/− cells (72). However, it should be noted that these cells also exhibited reduced survival upon transfer, demonstrating the difficulties in evaluating defects in gene-targeted cells where multiple pathways may be affected. Nonetheless, data now suggest that some of the defects in Btk-deficient mice (and perhaps humans) may result from impaired responses to chemokines (74).
Although our findings may be interpreted as there being unique role for the Tec kinases downstream of chemokines, it is now clear that multiple TCR signaling components are required for responses to chemokines. This work includes previous studies showing requirements for ZAP70 and SLP-76, as well as more recent studies demonstrating requirements for TCR ζ chain (75–78). Indeed, it has now been shown that TCR and CD4 stimulation induces recruitment of the chemokine receptor CXCR4 (the receptor for CXCL12/SDF-1α) (79, 80), that chemokines can potentiate IS formation (81), and that CXCL12 can act as a costimulant for TCR-induced proliferation (79). FRET analyses have shown close alignment of zeta chain and CXCR4 and a requirement for zeta chain and ZAP70 in long-term chemokine-induced ERK activation have been reported (77). How these findings fit with previous findings that activation of the TCR can lead to inhibition of responses to certain chemokines is not clear (82), but the requirements for or effects of TCR signaling components could depend on the chemokine and responses examined.
While the cross-talk between and requirements for TCR signaling in chemokine responses is surprising to some, it has actually been known for a while that there must been close proximity between components of the TCR complex and chemokine receptors. In part, this is known because of studies of the human immunodeficiency virus (HIV), which uses CD4 and CXCR4 or CCR5 as coreceptors for entry (83). The fact that these molecules serve as co-receptors for human immunodeficiency virus (HIV) suggests that at least some CD4 molecules must be in close proximity to these chemokine receptors. The implication of Itk in signaling downstream of both TCR and CXCR4 suggested that Itk and other TCR signaling molecules may also affect HIV replication. We review this data below.
Itk’s effects on HIV replication
The HIV is a retrovirus, which has been shown to be the causative agent of acquired immunodeficiency syndrome (AIDS). HIV infects human macrophages and CD4+ T lymphocytes by binding to the CD4 receptor, a component of the TCR complex, and the CXCR4 or CCR5 chemokine receptors, which serve as co-receptors for HIV infection (Fig. 3). These interactions initiate cell signaling events leading to changes in the actin cytoskeleton that allow for the virus to fuse with the cell membrane and release the capsid into the host cell cytoplasm (78, 84). At this point, the capsid protein is degraded and the viral reverse transcriptase enzyme transcribes the single stranded RNA into double stranded DNA, which is then translocated to the nucleus and integrated into the host DNA. Once integrated, the viral mRNA is transcribed and translated into proteins required for efficient viral replication and packaging new virions. Expression of the viral mRNA is dependent on cellular activation and can be influenced by cellular signals. The virus proteins are then assembled in the cytoplasm to package new virions, which are targeted to the cell membrane via the actin cytoskeleton and bud from the surface to form new virus particles. The viral protease enzyme then cleaves the matrix and capsid proteins within the virion to form a fully mature infectious viral particle. It should be noted that HIV can also be transmitted through cell:cell contacts where new virus envelop proteins on the infected cell surface interact with a target cell via a virological synapse that mediates infection.
Fig. 3. Itk affects HIV replication.

The HIV lifecycle is shown with stages affected by Itk highlighted in bold.
Modulators of the actin cytoskeleton play a critical role in HIV infection. Cytoskeleton proteins actin, ezrin/moesin, and cofilin have been shown to be incorporated in the HIV virions (85). Recently it has been shown that inhibition of ezrin, radaxin, moesin, cofilin, and filamin A reduce HIV entry and cell: cell transmission (86–90). Furthermore, PI3K and Rac1 have been implicated as important for HIV infection through CD4 and CXCR4 (91). Given the role of Itk in CXCR4- and TCR-mediated signaling and cytoskeleton regulation, we hypothesized that Itk may be required for productive HIV infection (Fig. 3).
Consistent with this idea, we found that Itk kinase activity is induced upon binding of the HIV envelop protein GP120 to CD4/CXCR4 in Jurkat leukemic T cells (92). To more directly evaluate the effects of Itk on HIV replication, we used siRNA, a chemical inhibitor of Itk and Itk mutants to inhibit Itk expression or function. Using the inhibitor BMS509744 (93) and siRNA directed against Itk (60), we found that loss of Itk expression or function results in reduced HIV replication in primary human CD4+ T lymphocytes and Jurkat T cell line (Fig. 4). To evaluate the mechanism behind the reduction in HIV infection we examined the effects of Itk inhibition on the different stages of infection (92) (Fig. 3). Virus entry was addressed by looking for trypsin resistant p24 protein within hours of exposure to the virus. Cells deficient in Itk activity had reduced levels of tryspsin-resistant p24 suggesting a defect in viral entry. Similar findings were observed using an assay for viral fusion (JAR, unpublished data). Furthermore, siRNA knockdown of Itk reduced f-actin polarization and polymerization in response to the HIV envelop protein GP120. Together, these data suggested that loss of Itk expression or function may affect HIV entry via inhibition of GP120-induced changes in the actin cytoskeleton. Nonetheless, decreases in virus entry did not appear to account for the overall affect on replication, prompting us to look at other processes during HIV infection.
Fig. 4. Inhibition of Itk or Abl reduces HIV infection.

(A) Activated primary human CD4+ T cells were incubated with 10uM STI571, 10uM BMS594744 (Itk inhibitor), or DMSO and then infected with HIV as described previously and stained for intracellular HIV p24Gag (92). Data represents the % cells positive for p24. (B) Cells were incubated with 10uM STI571 or DMSO for 48h and then stained using anti CD4-APC or anti CXCR4-PE (Pharmingen) and analyzed by flow cytometry. (C) Primary human CD4 T cells were activated and rested as described in (92) then treated with either STI571 or DMSO for 1hr prior to activation with GP120 for 0, 1, or 5 min. Cells were stained for intracellular actin using anti-phalloidin FITC antibody and analyzed by flow cytometry.
To bypass the entry defects in Itk deficient cells, we utilized the VSV-G envelope to pseudotype HIV virions and examined subsequent steps in virus replication. Although there were no changes to the process of reverse transcription or integration, inhibition of Itk decreased transcription of HIV mRNA. Conversely, over-expression of Itk increased expression of HIV-luciferase reporter constructs. Using cell lines expressing a kinase dead version of Itk, we were able to show that HIV transcription was dependent on Itk kinase activity. These results were not unexpected, given that Itk has been previously recognized to regulate the activation of NFAT, AP-1, and probably NF-κB, all of which are important activators of HIV transcription. However, surprisingly, when we used an assay to address the assembly/release of the virus we found that co-expression of Itk with the HIV structural Gag proteins in 293T cells enhanced virus-like particle assembly (92). Similar to the requirements for Itk in regulation of TCR-induced actin cytoskeleton rearrangement, this enhancement did not require kinase activity to support enhanced virion production--however enhancement require the PH domain of Itk which suggested Itk needed to be localized to the membrane. An SH2 mutant, which can act as a dominant negative protein for TCR induced actin polarization, was also unable to enhance virion production. These interesting parallels with the requirements for Itk in actin cytoskeleton rearrangement (58, 60), raise the possibility that Itk may influence virion particle production and/or release via effects on the actin cytoskeleton. Interestingly, another protein activated downstream of the TCR, ZAP-70, has been shown to be required in cell: cell transmission of HIV (94).
Finally, we looked to understand if Itk reduce HIV replication in a previously infected population. We found that decreasing Itk expression by treatment with siRNA prevented increased virus production compared to cells treated with a control siRNA. Thus, inhibition of Itk expression is likely to reduce viral production and spread from previously infected cells.
Although Itk-deficient mice have delayed responses to viral infections, they have been able to clear all viral infections examined to date (95, 96). Indeed the major in vivo defects in Itk-deficient mice are impaired responses to Th2-inducing agents and pathogens (44, 45, 47), suggesting that loss of Itk may not impair all immune functions of T cells. These data have prompted multiple pharmaceutical companies to screen for Itk inhibitors for use in treatment of asthma and other diseases associated with hypersensitivity (93, 97–99). The development of such drugs along with the limited cell expression of Itk suggest that Itk has potential as a secondary therapeutic target for HIV infection. However, further testing is required to fully evaluate this issue.
Other kinases and HIV infection
Given our results with Itk, we were curious to understand if other modulators of T-cell activation and actin polarization could affect HIV entry in a manner similar to Itk. One candidate for these studies is the Abl tyrosine kinase. TCR signaling has been shown to activate Abl kinase activity and inhibition of Abl kinases reduced T cell production of IL-2 and proliferation (100). Recent findings suggest that Abl is an important regulator of TCR-induced actin cytoskeleton reorganization via its interactions with a complex containing the actin polymerizing protein WAVE2 (57, 101). Interesting, Abl was also found to be important for chemokine mediated migration.
Abl is currently targeted by the already approved drug STI571 (Gleevec, imatinib meesylate) for use in targeting chronic myelogenous leukemia (CML), which is generally caused by activation of Abl kinase activity by a chromosomal translocation that generates a BCR-Abl fusion protein. STI571works by inhibiting Abl kinase activity, but also inhibits c-kit, and PDGFR (102). Recently, the STI571 inhibitor was used in 2 HIV+ CML patients in concurrence with their HIV drug cocktails to reduce symptoms of CML. These patients showed no adverse drug reactions between the HIV and STI571 (103). STI571 has also been shown to block PDGF receptor induced HIV replication (104).
Given its ability to be used in conjunction with current HIV drugs and its effects on TCR-induced actin polarization and SDF1α mediated chemotaxis, we wanted to look at the ability of STI571 to alter HIV replication. Treatment of activated primary human CD4+ T lymphocytes with STI571 decreased infection with the replication competent NL43 isolate of HIV, as evidenced by reduced virus particle release into the supernatant up to 96 hrs post infection. However, these effects were neither as dramatic nor as long-lasting as those seen with the Itk inhibitor or siRNA to Itk (Fig. 4). Furthermore, expression of CD4 and CXCR4, which are both required for HIV entry, were altered in cells treated with STI571. To evaluate how STI571 affects HIV replication, we examined the effects of STI571 on F-actin accumulation in response to GP120 stimulation. We found that STI571-treatment of primary human CD4+ T cells stimulated with GP120 for 1 or 5 minutes failed to increase f actin accumulation compared to the DMSO control stimulated cells. Similar results for HIV infection, receptor expression, and actin accumulation have been obtained in our laboratory using a second generation Abl inhibitor AMN107 in primary human CD4+ T lymphocytes (JAR, unpublished data).
These results suggest that modulators of TCR and actin cytoskeleton such as Itk and Abl may provide useful targets for HIV therapy. Both inhibition of Abl and Itk reduced f-actin accumulation to the HIV envelope protein GP120. However, our results further suggest that targeting of Abl was less effective in reducing HIV replication compared to targeting Itk. Furthermore, in contrast to results with Itk, Abl inhibition affected CD4 and CXCR4 receptor levels. These data suggest that although both proteins affect actin, they may also inhibit HIV replication independent of actin and may modulate their affects on the actin cytoskeleton in different ways.
Itk and development
Selection of conventional T lymphocytes
The effects of Itk on conventional T-cell development were initially shown in the first publication on Itk-deficient mice, in which Liao and Littman (10) described a defect in positive selection in Itk-deficient mice using the AND class II-specific and the HY Class I-specific TCR transgenes. We subsequently showed that mutation of Rlk worsened these phenotypes, leading to severe defects in both positive and negative selection using the same two TCR transgenes (105). These findings still provide some of the strongest data supporting functional compensation between these kinases. Defects in selection corresponded to decreased expression of CD5, a marker that is upregulated based on the strength of TCR signal received. Berg and colleagues (106) subsequently expanded these findings by showing that the extent of these defects in positive selection depended on the transgene and MHC background examined. Using several TCR transgenes directed against PCC, they observed greatest defects in the 2B4 model but less in the 5CC7 and AND models on the H-2k/k background (106). This conclusion was further supported by examining the extent of the defect in positive selection using the AND transgene on three different genetic backgrounds, H-2b/b, H-2b/k and H-2k/k. However, interpretation of these data needs to be evaluated carefully, since WT AND mice on the H-2k/k background are subject to negative selection, making evaluations based on the numbers and percentages of mature CD4 single positive (SP) cells that develop not straightforward.
The effects of Itk on negative selection are somewhat less clear. We found that Rlk−/−Itk−/− mice showed clear defects using male mice expressing the Von Boehmer HY TCR transgenic model (105). In male Rlk−/−Itk−/− HY+ mice, mature clonotypic (T3.70+) CD8hi cells developed and could be found in the periphery, consistent with negative selection being converted to positive selection. These data are consistent with a model in which TCR signals are dampened in the lack of Tec kinases, leading to a conversion of negative selection to positive selection. Indeed, the thymic profiles of male HY+Rlk−/−Itk−/− mice resembled those from female HY+WT mice, as did their peripheral CD8+ T-cell populations. Although these cells could respond to antigen, they still did so poorly compared to the WT HY+ cells from female mice, consistent with the biochemical defects in TCR signaling observed in these mice. In Itk-deficient HY+ male mice, thymocytes are no longer primarily deleted at the double-negative stage, but instead progressed on to double positive (DP) cells, although few mature CD8+ SP cells developed. Similar findings were found by Berg and colleagues (107). While our findings are similar, interpretations of these results have varied as to whether Itk affects negative selection or rather just delays this process. We interpreted these data as that Itk does affect negative selection, although both views are consistent with the data.
Given the types of biochemical defects observed in Itk- and Rlk/Itk-deficient mice, the effects on positive selection are consistent with findings that positive selection requires prolonged levels of ERK activation (108, 109), which are decreased in Itk-deficient cells due to their impaired activation of PLC-γ. However, defects in adhesion may also contribute to decreased duration of signaling in these cells. Indeed, we find that Itk−/− DP thymocytes show decreased conjugates with antigen presenting cells (KLM, unpublished data). The extent of both these defects are likely to be affected by the strength of signal from the TCR, thus accounting for the differential extent of defects with different TCR trangenes. Negative selection has been more associated with a strong transient burst of ERK activation (108, 109), which is also affected in Itk- and Rlk/Itk-deficient cells, in which both early and sustained ERK activation decreased (105).
Nonetheless, given previous data implicating Ca2+ signaling in pre-TCR signaling (110), it was not clear why early reports failed to see an effect of mutations of these kinases on this process, unlike mutations affecting more proximal TCR signaling molecules. This discrepancy was reconciled by data from Berg and colleagues showing that Itk- and Rlk/Itk-deficient cells failed to compete with WT cells for thymic repopulation in mixed bone chimeras starting at the DN4 stage (111). These data suggest that Itk-deficient mice do have defects in pre-TCR signaling which may account for their reduced thymic cellularity. Why Rlk−/−Itk−/− mice fail to show reduced thymic cellularity is not clear. We initially interpreted the relative increased cellularity compared to Itk-deficient mice as the effect of converting negative to positive selection. Indeed, Rlk−/−Itk−/− have increased percentages and numbers of SP thymocytes, consistent with this hypothesis. However, competitive bone marrow transfers from Lucas et al. (111) revealed that Rlk−/−Itk−/− bone marrow outcompeted WT at earliest stages of DN thymocyte development (this outcompetition is reversed at the DN4 stage). Thus, the increased cellularity could well be due to a second effect of Rlk, most likely on another signaling pathway; responses to cytokines are one such pathway that may affect these early thymocyte stages. Whether Rlk participates in and perhaps negatively regulates other signaling pathways that may contribute to these phenotypes remains unknown. However, recent data suggests that the effects of the Tec kinases, particularly Itk, on later stages of thymocyte development are more complex than originally realized.
Itk and innate T-cell lineages
Although Itk affects positive selection of both MHC class I- an class II-restricted transgenes, one of the surprising features of Itk-deficiency is that although CD4 SP are reduced in Itk−/− mice, the percentages of CD8SP thymocytes are not (10). Indeed, the percentages of CD8 SP cells are increased in Itk−/− mice relative to WT counterparts. This increase is even more pronounced in Rlk−/−Itk−/− thymocytes where there are increased numbers and percentages of SP thymocytes (105). We had originally interpreted these data as potentially resulting from decreased signaling leading to premature termination of TCR signals, and the subsequent generation of CD8 SP cells from class II-restricted cells that had received abbreviated signals that normally lead to CD8 SP cell development (112, 113).
However, recent data from four groups including our own has revealed that these CD8 cells reflect a new feature of the effects of Itk-deficiency on thymocyte development. Examination of CD8 SP thymocytes in Itk−/− and Rlk−/−Itk−/− mice demonstrated that these cells did not resemble conventional CD8 SP cells (114–117). Instead, a large fraction of these cells expressed activation markers CD44 and CD122 (the IL-2Rb chain) and surprisingly, could rapidly express cytokines directly ex-vivo (See Fig. 5). These phenotypes were even more pronounced in the periphery where virtually all of the CD8+ T cells exhibited these phenotypes. Work from Atherly et al and Dubois et al further showed that these cells were dependent on IL-15 (114, 116).
Fig. 5. Itk-deficient mice develop a large population of innate-type CD8+ T cells in the thymus.

A comparison with Vav1-deficient mice is shown. Left: CD4 versus CD8 flow plots of thymi. Right: expression of CD44 on gated CD8 SP cells demonstrating that Itk−/− CD8 SP cells express high levels of the memory marker CD44, whereas Vav−/− CD8 SP cells do not.
All of these features are ones associated with activated or memory CD8+ cells that have previously encountered antigen in the periphery. Consistent with these phenotypes, these cells also expressed high levels of the transcription factor Eomesodermin (114, 118), which is associated with expression of CD122 and other features of CD8+ memory cells in the periphery (119). Indeed, many Itk−/− and Rlk−/−Itk−/−CD8 SP thymocytes are HSAlo, suggesting that they are more mature than control CD8 SP thymocytes. However, fetal thymic organ cultures from Rlk−/−Itk−/− mice demonstrated that CD8 SP cells with these features could develop in the thymus, suggesting that these were not cells that had migrated back in from the periphery (115).
Further evaluation of these CD8 SP cells revealed another surprising finding—these cells could be selected on hematopoietic cells in the thymus (115). Transfer of WT bone marrow into irradiated B2m−/− recipient mice, which lack most MHC class I expression on the thymic stroma, does not permit the development of most CD8 cells. In contrast, transfer of Itk−/− or Rlk−/−Itk−/−/− bone marrow into irradiated B2m-deficient hosts led to the development of large populations of CD8 cells, which again expressed activation markers. Genetic crosses to B2m-deficient mice failed to result in selection of significant numbers of CD8+ cells (in Itk−/−B2m−/− mice), arguing that the generation of these cells required MHC class I expression but that expression could be on hematopoietic cells (115).
Development of most T cells occurs as the result of positive selection on peptide-loaded MHC expressed on cortical thymic epithelial cells (TECs). However, over the last several years it has become apparent that some thymocytes are not selected on the thymic epithelium, but rather are selected on hematopoietic cells in the thymus (reviewed in 4). These cells include invariant NKT cells, a relatively rate, but well studied, T-cell population that responds to the MHC class Ib molecule CD1d in the context of lipid and shares features with innate cells, including expression of activation markers and rapid production of cytokines (120). NKT cells are selected on CD-1d expressed on DP thymocytes. Other innate T-cell lineages include CD8+ innate cell populations that are selected on MHC class Ib, such as H2-M3-restricted cells (4). These subsets of cells respond rapidly to stimulation and are thought to be important for immediate early responses to infection. These MHC class Ib-restricted CD8+ cells can also be selected on hematopoietic cells and data suggest that selection on hematopoietic cells is associated with the cells with innate properties (121). Similarly, mice that express a CIITA (class II transactivator) transgene that drives expression of MHC class II and related molecules on DP cells develop a population of CD4+ cells that are selected on thymocytes and exhibit innate cell properties, including expression of activation/memory markers and rapid expression of cytokines (122, 123). The similarities of the features of the CD8 cells in Itk-deficient mice with those of NKT and other innate T cells, suggested that the CD8 cells in Itk-deficient mice represent an innate T-cell lineage(s) that are selected on hematopoietic cells.
These observations raised the question of whether loss of Itk specifically affects selection or development of MHC class Ib-selected cells? Although Itk−/− CD8+ cell can express low levels of NK1.1 and other NK cell markers, they do not stain with the α gal-cer loaded CD-1d tetramer that recognizes invariant NKT cells, arguing that they are not invariant NKT cells (114, 116) [which are actually reduced in numbers in Itk-deficient mice (124–126)]. To further address this issue, we interbred Itk-deficient mice with KbDb-deficient mice, which lack classical MHC class Ia (127). KbDb-deficient mice develop a very small population of CD8 SP thymocytes and CD8+ cells in the periphery these cells are selected on MHC class Ib and many exhibit characteristics of innate lymphocytes. Studies from Bevan and colleagues have shown that these cells can also be selected on hematopoietic cells (in addition to the thymic stroma); data in the same paper suggested that restricting selection to hematopoietic cells (via bone marrow transfers) led to the development of cells that were enriched in innate phenotypes (121). Examination of Itk−/−Kb−/−Db−/− mice revealed that the percentages and numbers of CD8 SP cells were increased close to 30 fold relative to Kb−/−Db−/− mice that expressed Itk (115). While this is a dramatic increase and does support the idea that Itk increases selection of MHC class Ib-restricted cells, the numbers of CD8 SP cells were still approximately only one third of the numbers of CD8 SP cells in Itk-deficient mice. Thus, it appeared as it the majority of the CD8 SP cells in Itk-deficient mice were still selected on MHC class Ia. Further experiments in which bone marrow from Kb−/−Db−/− or Itk−/−Kb −/−Db−/− mice was transferred into B2m-deficient mice demonstrated that Itk deficiency appeared to specifically increase selection on hematopoietic cells (and thus may increase numbers of class Ib-selected cells because many of them are selected on hematopoietic cells in the thymus) (118). Thus, these data support the concept that selection on class-Ib does not necessarily drive innate phenotypes, but rather it is the selection on hematopoietic cells that induces these characteristics.
Indeed, using reciprocal bone marrow transfers in which B2m−/− or Itk−/−B2m−/− bone marrow was transferred into WT cells, we further demonstrated that the ‘innate-type’ phenotypes of Itk-deficient CD8 cells resulted specifically from selection on hematopoietic cells (118). Transfer of B2m−/− bone marrow into irradiated WT hosts, gives rise to normal numbers of conventional appearing CD8 cells, since these cells are selected on the WT MHC class I-expressing thymic stroma. Transfer of Itk−/−B2m−/− bone marrow also gave rise to normal conventional appearing CD8 SP thymocytes, although these cells were reduced in numbers compared to the numbers of CD8 SP thymocytes found in intact Itk-deficient animals or WT irradiated recipients that had received Itk-deficient bone marrow. Together, these results suggest that Itk specifically increases the selection of CD8 SP thymocytes on hematopoietic cells and that it is the selection on hematopoietic cells that confers innate cell features to these CD8+ cells (Fig. 6). Nonetheless, there still may be secondary requirements for development of innate-cell phenotypes that remain to be explored (see below).
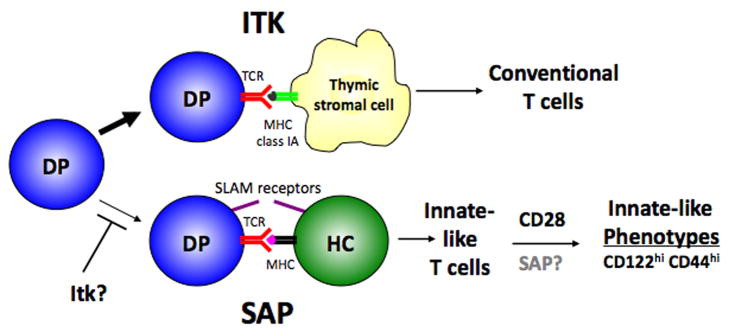
Fig. 6. Itk and SAP reciprocally regulate development of conventional and innate T cells.
Model shows the requirements for Itk and SAP for development of lineages selected on the thymic stroma and hematopoietic cells, respectively. CD28 further contributes to the acquisition of innate-type phenotypes on hematopoietically-selected cells.
Requirements for hematopoietic cell-driven selection in the thymus
So why does selection on hematopoietic cells lead to the development of innate cells properties? One feature of hematopoietic cells is their expression of certain costimulatory molecules. In addition to having distinct properties of selection, development of NKT cells has been shown to require certain signaling molecules that are distinct those required for conventional T-cell development, suggesting NKT cell differentiation relies on other signaling pathway(s). These molecules include the tyrosine kinase Fyn, PKC-θ, Bcl-10, and NF-κB1 (128). Intriguingly, all of these molecules have been implicated in signaling from a distinct series of immunomodulatory receptors expressed on hematopoietic cells, the signaling lymphocyte activation molecule (SLAM) family receptors (129–132). Signaling from SLAM family receptors in T cells requires the association of a small adaptor molecule SLAM-associated protein (SAP), encoded by SH2D1A (129, 131, 132). Strikingly, the absence of SAP leads to a profound defect in NKT cell development both in SAP-deficient mice and in humans with a genetic disorder, X-linked lymphoproliferative disease, caused by mutations affecting SAP (133–135). Although it is not known how the lack of NKT cells may contribute to the diverse phenotypes of XLP, a major feature of which is impaired responses to EBV infection, it is now clear that altered development of NKT cells is also associated with variants of XLP that are caused by mutations in at least one other gene (136).
To examine whether SLAM family receptors are required for the development of the innate CD8+ cell population in Itk-deficient mice, we interbred them with SAP-deficient mice, which lack signaling from SLAM receptors on T cells. Deficiency of SAP reduced the percentages and numbers of CD8+ SP thymocytes, and almost completely eliminated the innate CD8+ SP cell population (118). Furthermore, transfer of Itk−/−SAP−/− bone marrow into irradiated B2m-deficient hosts effectively prevented the development of most SP CD8+ thymocytes. These results suggested to us that SAP (and presumably SLAM family receptors) are required for the selection of these innate T cells on hematopoietic cells. However, we cannot rule out that SAP and SLAM family members are specifically required for the development of hematopoietically selected cells (as opposed to their selection). A recent study from Bendelac and colleagues suggested that SAP was not required for selection of NKT cells, but rather for their development or expansion immediately post-selection, since the authors could identify a very small population of immature cells expressing the invariant TCR associated with NKT cells by tetramer staining in SAP-deficient mice (137).
Costimulation may also play a second role in the development of innate CD8 cells: acquisition of innate cell phenotypes. As we were evaluating the role of costimulatory pathways on the development of innate T cell lineages in Itk−/− mice, we also generated Itk−/−CD28−/− mice. Interestingly, CD28 deficiency also greatly reduced the innate-CD8 cell development associated with Itk-deficiency (118). However, the phenotype of Itk−/−CD28−/− mice was not the same as Itk−/−SAP−/− mice. Large numbers of CD8 SP cells still developed in these mice (as well as increased numbers of CD4+ cells, perhaps due to the effects of CD28 on negative selection); however, these cells more closely resembled conventional CD8 cells. Furthermore, transfer of Itk−/−CD28−/− bone marrow into irradiated B2m-deficient hosts still gave rise to CD8 cells thus hematopoietically derived cells were still being selected and developed. However, these cells failed to develop innate-cell characteristics. Interestingly, we have found that the CD8+ cells that develop in Rlk−/−Itk−/− HY male mice appear to resemble conventional CD8+ T cells, yet bone marrow transfers demonstrated that these cells still can be selected on hematopoietic cells (RH, unpublished data).
These results suggest innate-cell lineages have second levels of requirements for development of innate-cell characteristics. Indeed, a number of mutants have been found to affect the maturation of NKT cells, including CD28. In this regard, it is of interest that the transcription factor PLZF has recently been shown to be required for the acquisition of innate characteristics in iNKT cells (138–140). While a-gal-cer tetramer positive cells develop in PLZF−/− mice, these cells do not have the characteristics of innate lymphocytes and, like conventional CD4+ T cells, are preferentially found in lymphoid organs and not the liver where large populations of iNKT cells normally reside. Whether CD28 is required for optimal expression of PLZF is not known.
Together, these results support a model in which Itk is crucial for the development of conventional T cells, and SAP and SLAM family receptors are required for the development of innate T-cell lineages, while CD28 is required for full acquisition of the innate-T cell phenotype (Fig. 6).
Itk: a negative regulator of hematopoietic cell selection?
One outstanding question from this work is the reason why so many innate-like CD8+ T cells develop in the absence of Itk. Very few innate-like CD8+ T cells develop when control (wildtype) bone marrow is used to reconstitute irradiated mice that do not express MHC class I on the thymic stroma (where MHC class I is only expressed on the hematopoietic cells). These observations suggest that there are pathways, perhaps mediated by Itk, that actively inhibit the selection on hematopoietic cells. As Itk positively regulates TCR signaling and the positive selection of conventional T cells, the results discussed above raise the question of whether the ratio of signals from the TCR and SLAM family members helps determine the balance of conventional versus innate-like T cell development. Supporting this view, when conventional T-cell development is rescued in Itk−/− mice, either by expression of a hypersensitive mutant of ERK or by expression of a strongly selecting TCR transgene, fewer innate cells develop (114, 115).
Nonetheless, it is unclear why Itk-deficiency specifically leads to the development of these innate-like CD8 cells. Although this question has not been examined in depth, the loss of many other TCR signaling molecules does not appear to result in the development of large numbers of innate-like T cells, with the interesting exception of mice expressing a knockin mutation of SLP-76 that affects the putative Itk-binding site (141). One possible explanation is that Itk deficiency only partially blocks TCR signaling, whereas the loss of other TCR signaling molecules might have more severe effects on pre-TCR signaling and/or all positive selection and thus, may block selection of both conventional and innate T-cell lineages. Alternatively, other molecules may be required for both TCR and SLAM family signaling; the guanine nucleotide exchange factor VAV1 has recently been shown to be phosphorylated downstream of Ly108 (142). Indeed, comparison of Itk−/− and Vav−/− mice reveals that very few conventional or innate cells develop in Vav1−/− thymi (62) (Fig. 5). Still, another molecule affecting positive selection, RasGRP, affects positive selection of conventional T cells, without changing numbers of innate-CD8 cells. These observations suggest that Itk may specifically affect pathways required for innate T-cell development.
Itk affects actin cytoskeletal reorganizations and can affect the TCR-induced inside-out signaling that increases integrin adhesion. Accordingly, we find that Itk-deficient T cells and pre-selection DP thymocytes show decreased conjugate formation with antigen presenting cells (KLM, unpublished data). We have recently found that SAP-mediated pathways also affect cell adhesion, but, surprisingly, in a cell-type specific manner. SAP-deficient mice and patients with XLP show defective humoral responses associated with impaired germinal center formation (143–145). Cell transfers and genetic studies in mice indicate that T-cell-intrinsic defects contribute to these phenotypes, yet the basis of the defect was unclear (143, 146, 147). We have recently used intravital multi-photon microscopy combined with in vitro studies to reveal that SAP-deficient T cells show a selective defect in adhesion to activated B cells, whereas these T cells can adhere to and be activated normally by dendritic cells (148). These findings raise the question of whether SAP also affects lymphocyte interactions in the thymus, in particular the DP:DP interactions required for NKT cell development, while leaving DP:thymic epithelial cell (TEC) interactions intact. In contrast, Itk may preferentially affect DP:TEC interactions. Although data suggest that the effects of Itk deficiency on actin reorganization may be mediated by altered localization of Vav, it is likely that this pathway operates primarily downstream from the TCR. As discussed above, Vav1-deficient cells do not show increased innate T-cell development; this could be because Vav1-deficiency more severely affects positive selection of all cells or because of its potential role in signaling from multiple receptors including Ly108, one of the SLAM family receptors implicated in NKT development (137, 142). However, these observations also raise the possibility that Itk actively inhibits signaling from SLAM family members, an intriguing possibility that might account for the phenotypes seen in Itk-deficient mice.
Although the innate phenotype is primarily observed in CD8 cells, innate CD4 cells are also be observed in mice deficient in Itk, although at much lower percentages than the innate-CD8 cells (149, KLM, unpublished data). For NKT cells, selection has been shown to occur on DP thymocytes, which express CD-1d, but do not express MHC class II (120). Although the selecting cell population has not been identified for the innate T cell populations in Itk−/− mice, the low numbers of innate CD4+ cells that develop argue that the innate CD8+ cells are more efficiently selected, perhaps due to larger numbers of cells, such as DP cells that can select them. Thus, the relatively low numbers of innate CD4+ cells compared to the numbers of innate CD8+ cells that develop is likely to result from the relatively few cells in the thymus that express MHC class II. Of note, we have found that SAP deficiency also prevents development of the population of innate-CD4+ T cells found in Itk-deficient mice, again implicating SLAM family signaling in their generation or development (KLM, unpublished data). However, bone marrow transfers in which we transfer WT or Itk-deficient cells into class II-deficient hosts do not give rise to large numbers of CD4+ cells arguing that Itk deficiency may not specifically increase the selection of these cells. These issues may be better addressed in mice that express MHC class II on DP thymocytes as in the CTIIa transgenic mice.
Although the mechanisms behind these observations await further study, these results suggest that Itk and SAP reciprocally regulate the development of conventional and innate T cell lineages, respectively (Fig. 6). Dissecting the signaling pathways affected by Itk and SAP may therefore help better delineate the mechanisms that regulate the balance between these important T cell lineages.
Itk in peripheral T-cell responses
Effects on peripheral T-cell signaling
The altered development of T cells in Itk-deficient mice raises questions on how this affects the function of peripheral T cells. Indeed, studies by Dubois et al. (116) argued that anti-TCR-induced proliferation of Itk-deficient mature CD8+ cells, while defective compared to naive CD8 cells from WT mice, was very similar to proliferation of the equivalent CD8+CD44hiCD122hi population found in WT mice. The authors therefore suggested that defects observed in TCR-induced responses in Itk−/− T cells result from altered cell populations. This may also be true for responses of CD4+ T cells in the periphery, which also show large percentages of cells with an activated phenotype in Itk-deficient mice. (Although there are only small percentages of CD4 SP cells in the thymus of Itk-deficient mice with an innate-type of phenotype, in the periphery approximately 40–50% of CD4 cells express activation markers.)
To start to address this question, we examined cells from Itk−/− mice expressing a TCR transgene on a RAG-deficient background. We used the 5CC7 MHC class II-restricted TCR transgenic mouse model, which we had interbred to Itk−/− and Rlk−/−Itk−/− mice on a Rag-deficient background. In these mice, we have found that expression of activation markers is normalized, as are TCR levels (79.5% of WT and 82.4% of Itk−/−5CC7 CD4+ cells were CD62Lhi or naive) (Fig. 2). To evaluate the effects of Itk deficiency on signaling, purified CD4+ cells from Itk−/−Rag−/−5CC7+ mice were stimulated with biotinylated anti-CD3 plus streptavidin and examined for Ca2+ influx compared to isolated CD4+ cells from control Rag−/−5CC7+ mice. Evaluation of Ca2+ influx under several conditions clearly demonstrated that the Itk-deficient CD4+ cells still exhibited a defect in Ca2+ mobilization (Fig. 2). These defects, along with defects in proliferation, were even more severe in cells from mice lacking both Itk and Rlk (data not shown). Thus, although Itk−/− and Rlk−/−Itk−/− T cells do contain large numbers of activated cells that have undergone altered development, naive mature CD4+ T cells do still have intrinsic T-cell defects. Although we have not done similar evaluation of cells from a class I-specific transgene on a Rag-background, we have previously examined antigen-induced proliferation of T cells from male HY+TCR transgenic Rlk−/−Itk−/− mice. As discussed above, these mice positively select mature CD8+ cells that express high levels of CD8 (105). We have also found that these cells also do not appear to express activation markers (authors’ unpublished data). However, these cells still proliferate poorly in response to the HY antigen. Thus, these cells still show abnormal responses, although this experiment is not an ideal evaluation, since the cells have been constantly exposed to a low level of antigen and could be anergized. Careful comparison of the function of naive and activated Itk-deficient CD8 cells from either TCR transgenic mice or derived from bone marrow transfers (for example, using cells derived from bone marrow transfers into B2m mice or from B2m−/−Itk−/− into WT mice) will provide further insight into these questions as well as into the function of the innate cells in these mice.
Itk and T-helper cell responses
Upon activation, naive CD4+ T cells can differentiate into a number of distinct effector cell populations that are characterized by the production of distinct classes of cytokines. These effector cell populations include Th1 cells that produce IFN-γ and TNF and are important for promoting cellular responses such as to intracellular pathogens. Th2 cells produce IL-4, IL-5, IL-10, and IL-13 and are important for barrier and allergic responses, as well as responses to extracellular parasites (150). More recently recognized effector classes include Th17 cells that make IL-17A, IL-17F, IL21, and IL-22 and are important for driving inflammatory responses to bacteria and fungi, as well as autoimmunity; induced regulatory T cells (iTregs) which are important for negative regulation of immune responses; and TFH cells, which are effective promoters of B-cell humoral responses (151, 152).
One of the striking features of Itk deficiency has been the observation by several groups of defective Th2 responses in vivo (44, 45, 47). These defects have been observed in response to Leishmania, Nippostrongyloides, Shistosoma eggs, and induced airway hyperreactivity models. In some of these models, impaired TH2 responses have been associated with a switch to a Th1 response (44, 45). While impaired TH2 cytokine production has been observed in vitro in sorted naive CD4+ T cells or in cells from TCR transgenic mice, the results have varied slightly as have the interpretations as to whether there is a primary early defect in Th2 cytokine production versus a secondary defect in the large induction of Th2 cytokines from polarized effector cells (8, 44, 153). Nonetheless, it is clear that all groups recognize defective in vivo responses and there is also a consensus that there are defects in vitro.
In light of recent findings on Itk’s effects on T-cell development, this work raises several questions. The first is whether these defects result from altered development. Fowell et al. (44) originally argued that the defect in IL-4 production was secondary to impaired Ca2+ mobilization since ionomycin could rescue it in their hands. Our findings of impaired Ca2+ in naive CD4+ Itk-deficient T cells would argue that these Ca2+ defects are not secondary to altered development. Similarly, the rescue of Th2 defects by re-expression of Itk by Fowell and colleagues (44) also supports the idea that the defect in Th2 response is not solely the result of altered development. However, it should be noted that transgenic mice that express a CIITA transgene on DP thymocytes and give rise to large innate-type CD4+ population selected on hematopoietic cells, also show defective Th2 responses in allergic asthma models (154). Such observations raise the question of whether innate lymphocytes are defective at allowing full Th2 responses. Unfortunately, it is not clear from the data whether these innate CD4+ T cells may show defective recall responses of all types, which is likely given what is known about H2-M3 responses to Listeria (155)--this remains an important question to address. Further evaluation of Th2 responses in vivo using cells transferred from Itk-TCR transgenic mice may help address some of these issues.
Rlk and Th2 responses
The second major question these Th2 studies brought up is what is the role of Rlk in Th2 responses. Rlk has been described as a TH1 promoting cytokine and there are some very intriguing studies that support this idea. Unlike the other Tec kinases, Rlk lacks a PH domain and instead has a palmitoylated cysteine string motif (16). We also have found evidence for two forms of Rlk that can be generated by alternative translation initiation. The shorter form lacks the cysteine string and when expressed alone, is found in the nucleus. This nuclear localization appears to require a bipartite nuclear localization signal found on both the short and long forms of the protein Furthermore, upon TCR activation, a large proportion of Rlk is rapidly translocated to the nucleus (16, 156). While nuclear translocation has also been reported for Itk and Btk, only a small fraction of these proteins are found there (15% of Itk) (24, 157, 158). Work from Kashiwakura et al. (156) has demonstrated that overexpression of Rlk leads to a large increase in the expression of IFN-γ. Further studies from the same group, under the direction of N. Suzuki (159), have shown that Rlk can bind to a sequence in the IFN-γ promoter to drive increased expression of this Th1 cytokine. Together these studies suggest that Rlk and Itk may play distinct roles in Th cell differentiation.
However, other studies make this conclusion less clear. Rlk mRNA was originally found to be highly expressed in Th1 clones, but not Th2 clones (160). Subsequent work that has more quantitatively examined level of Itk and Rlk mRNA has demonstrated that Rlk mRNA levels are 3–10-fold lower than that of Itk in resting cells. These studies also confirmed that Rlk expression in markedly downregulated upon the differentiation of Th2 cells so that its message can be approximately 100 to greater than 1000-fold lower than Itk message in these cells (7, 8, 161).
The very low levels of expression of Rlk in Th2 cells raises the question of whether Itk is truly a TH2-determining kinase or whether the requirement for Itk in Th2 cells is secondary to the very low levels of other Tec kinases expressed, so that Itk is essentially the only Tec kinase expressed in differentiated Th2 cells. To try to address this question, both our group and that of A. August used published transgenic mice from Sommers and Love, which overexpress Rlk/Txk (CD2TxkTg mice) (28). Both groups crossed these transgenic mice onto the Itk-deficient background to determine how the Itk−CD2TxkTg mice fared in response to Th2 inducing agents in vivo (161). Although these mice express about 10-fold higher levels of Txk/Rlk mRNA than the endogenous gene in resting T cells, in Th2 cells, the Txk/Rlk mRNA levels are similar to those of Itk (approximately twofold lower) (161). Although this experimental system is not nearly as elegant as generating mice in which Rlk is targeted to the Itk locus, where Rlk would be expressed and regulated similarly to Itk, the CD2TxkTg transgenic mice already existed and thus, seemed like a good system to start to address these questions.
Sommers and Love had previously crossed the CD2TxkTg mice onto the Itk-deficient background and had shown that this transgene improved positive selection of both the HY and AND TCR transgenes (two TCR transgenes that have been extensively studied in mice deficient in the Tec kinases, see above) (28). Furthermore, they showed that tyrosine phosphorylation of PLC-γ and TCR-induced Ca2+ mobilization was improved in SP thymocytes from these mice (although Ca2+ was not compared to WT controls in this study). Thus, Rlk/Txk appeared to partially compensate for deficiency of Itk. These results complement our previous studies showing that Rlk−/−Itk−/− mice exhibited more severe defects in positive selection and in Ca2+ mobilization than Itk−/− mice (105).
To evaluate the effects of increased expression of Rlk/Txk on Th2 responses in the Itk-deficient background, Sahu and August challenged these mice with an allergic asthma model of inhaled ova. We challenged the mice with the eggs of Shistosoma mansoni, which home to the lungs and induce an eosinophil-filled granuloma that surrounds the eggs and induce a large amount of Th2 cytokine production from CD4+ T cells in the draining lymph node. In both the challenge with Shistosome mansoni and in the allergic asthma model, our groups found that the CD2Txk transgene was able to substantially improve the development of Th2 responses of Itk-deficient mice (161). In particular, we found that expression of the CD2Txk transgene increased the size of egg-induced granulomas found in the mice, as well as Th2 cytokine production from the draining lymph nodes. Th2 cytokine production was also improved systemically from the spleen. Similar results were found by Sahu and August, who also observed increased recruitment of T cells into the lung. Moreover, in both model systems, neither group observed increased the number of T cells producing the Th1 cytokine IFN-γ (nor the amount of IFN-γ produced), as might be expected if Rlk/Txk was a Th1 inducing kinase (161). Together, these results suggest that Rlk/Txk can substantially rescue TH2 responses in Itk-deficient mice, results that would argue that (i) Rlk/Txk can substitute for Itk under certain circumstances and (ii) the defect in Itk-deficient mice was not due to a specific biochemically-distinct function of Itk, but rather results from the patterns of expression of Itk and the lack of expression of other Tec kinases in Th2 cells. However, while evidence argues that there is some degree of functional redundancy between these kinases, it is also likely that there are unique signaling features for each of these kinases and that these kinases participate in signaling cascades downstream of other receptors.
Whether Rlk plays a role as a Th1-inducing kinase is less clear. Neither group challenged these mice with a Th1-inducing agent or pathogen to see if the CD2Txk transgene led to increased pathology (161). Potential effects of Rlk/Txk on IFN-γ expression may not be observed in strongly Th2-inducing situations, where other ThH1-inducing factors, including transcription factors, may not be induced. Nonetheless, such results may still be difficult to interpret. Ideally, challenge with a pathogen such as Leishmania, which can either induce Th1 or Th2 pathology depending on the strain of mice and stain of bacteria, may be a better system.
Effects of the Txk transgene on development
We also looked further at development in the Itk−/−CD2TxkTg animals. Surprisingly, we found that the CD2Txk transgene did not increase thymic cellularity, nor numbers of peripheral T cells in the spleen if anything, T-cell numbers were decreased (161) (Fig. 7). Those observations suggest that the Txk transgene did not complement the Itk-defect in Th2 responses merely by increasing T-cell numbers. These results also suggest that the decreased T-cell numbers in Itk-deficient mice are not the result from decreased positive selection, which is partially rescued in these mice (28), and support studies from the Berg laboratory implicating an earlier pre-TCR signaling defect as the cause of decreased thymocyte numbers (111). This stage of development may not be affected by the CD2Txk transgene or even may be adversely affected due to increased signaling.
Fig. 7. CD2TxkTg decreases development of innate lymphocytes in Itk−/− mice, yet increased percentages of ‘memory-phenotype’ lymphocytes are still present in the periphery.
Thymocytes (left) and splenocytes (right) stained for CD4 and CD8. Gated SP populations were then examined for CD44 levels. Although innate T-cell development is rescued in the thymus, there are still increased percentages of activated/memory phenotype T cells in the periphery of Itk−/−CD2TxkTg mice.
So how does the Txk transgene affect development of conventional and non-conventional CD8+ and CD4+ T cells? Our initial evaluation of the Itk−/−CD2TxkTg mice suggested that there were still large percentages numbers of innate cells in the periphery, a somewhat surprising finding, but one that suggested that the rescue of Th2 responses could be uncoupled from the numbers of innate T cells. However, further evaluation of thymic development reveals that a smaller percentage of innate-type CD8 cells develop in the thymus (Fig. 7), suggesting that there is a partial rescue of conventional T-cell development (or more accurately a decrease in the development of innate T cells since there are still low numbers of naive T cells in these mice). The same is true with innate CD4+ T cells. The high percentages of activated cells in the periphery may therefore be secondary to the relative lymphopenia in these mice. Thus, it is still possible that alterations in T cell populations contribute to some of the rescue observed in these mice, despite the large percentages of memory-phenotype lymphocytes still present. Whether such memory-phenotype cells function differently than the intrathymically-derived innate-type lymphocytes is not known. How these various T-cell populations respond in primary and secondary infections therefore awaits further evaluation.
Concluding thoughts
The last few years has provided new insight on the Tec kinases expressed in T cells, particularly Itk. Perhaps most surprising are the effects on T-cell development, which reveal new features of the control of thymic selection and development. Although Itk and Rlk/Itk-deficient mice develop nearly normal numbers of T cells, we now realize that many of these T cells exhibit distinct properties and have undergone distinct pathways of selection in the thymus. This work has provided new insight into the pathways controlling thymocyte development and sets the way for further analyses of thymic development. In particular, this work suggests that it may be important to re-evaluate phenotypes of many mouse strains with mutations affecting TCR signaling. Together, these studies may help pave the way for a better understanding of the pathways that regulate the development of non-conventional, innate-type lymphocytes versus those affecting conventional T cells.
How Itk affects T-cell development remains an important question, given that Itk has been actively pursued as a potential therapeutic target. Although this research has primarily been oriented towards drug development for asthma and other Th2-mediated diseases (93), our results with HIV suggest that such drugs could be used for multiple purposes (92). Whether Itk inhibitors ever become useful drugs may depend on how effective they truly are and whether, given the partial phenotypes of Itk-deficiency, they will provide appropriate partial inhibition of T-cell function while balancing effects on T-cell development and other potential unanticipated effects. Ultimately, some of this understanding may have to await identification of humans with Itk deficiencies and evaluation/confirmation of the effects on immune cell function. Given the advances in human genetics, it is likely some of this information will be soon in coming and will provide complementary insight into the role of Tec kinases in T-lymphocyte development and function.
References
- 1.Tsukada S, et al. Deficient expression of a B cell cytoplasmic tyrosine kinase in human X-linked agammaglobulinemia. Cell. 1993;72:279–90. doi: 10.1016/0092-8674(93)90667-f. [DOI] [PubMed] [Google Scholar]
- 2.Vetrie D, et al. The gene involved in X-linked agammaglobulinaemia is a member of the src family of protein-tyrosine kinases. Nature. 1993;361:226–33. doi: 10.1038/361226a0. [DOI] [PubMed] [Google Scholar]
- 3.Desiderio S. Human genetics. Becoming B cells. Nature. 1993;361:202–3. doi: 10.1038/361202a0. [DOI] [PubMed] [Google Scholar]
- 4.Berg LJ, Finkelstein LD, Lucas JA, Schwartzberg PL. Tec family kinases in T lymphocyte development and function. Annu Rev Immunol. 2005;23:549–600. doi: 10.1146/annurev.immunol.22.012703.104743. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt U, Boucheron N, Unger B, Ellmeier W. The role of Tec family kinases in myeloid cells. Int Arch Allergy Immunol. 2004;134:65–78. doi: 10.1159/000078339. [DOI] [PubMed] [Google Scholar]
- 6.Smith CI, Islam TC, Mattsson PT, Mohamed AJ, Nore BF, Vihinen M. The Tec family of cytoplasmic tyrosine kinases: mammalian Btk, Bmx, Itk, Tec, Txk and homologs in other species. Bioessays. 2001;23:436–46. doi: 10.1002/bies.1062. [DOI] [PubMed] [Google Scholar]
- 7.Colgan J, et al. Cyclophilin A regulates TCR signal strength in CD4+ T cells via a proline-directed conformational switch in Itk. Immunity. 2004;21:189–201. doi: 10.1016/j.immuni.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Miller AT, Wilcox HM, Lai Z, Berg LJ. Signaling through Itk promotes T helper 2 differentiation via negative regulation of T-bet. Immunity. 2004;21:67–80. doi: 10.1016/j.immuni.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Tomlinson MG, Kane LP, Su J, Kadlecek TA, Mollenauer MN, Weiss A. Expression and function of Tec, Itk, and Btk in lymphocytes: evidence for a unique role for Tec. Mol Cell Biol. 2004;24:2455–66. doi: 10.1128/MCB.24.6.2455-2466.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liao XC, Littman DR. Altered T cell receptor signaling and disrupted T cell development in mice lacking Itk. Immunity. 1995;3:757–69. doi: 10.1016/1074-7613(95)90065-9. [DOI] [PubMed] [Google Scholar]
- 11.Liu KQ, Bunnell SC, Gurniak CB, Berg LJ. T cell receptor-initiated calcium release is uncoupled from capacitative calcium entry in Itk-deficient T cells. J Exp Med. 1998;187:1721–7. doi: 10.1084/jem.187.10.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schaeffer EM, et al. Requirement for Tec kinases Rlk and Itk in T cell receptor signaling and immunity. Science. 1999;284:638–41. doi: 10.1126/science.284.5414.638. [DOI] [PubMed] [Google Scholar]
- 13.Ellmeier W, et al. Severe B cell deficiency in mice lacking the tec kinase family members Tec and Btk. J Exp Med. 2000;192:1611–24. doi: 10.1084/jem.192.11.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.August A, Sadra A, Dupont B, Hanafusa H. Src-induced activation of inducible T cell kinase (ITK) requires phosphatidylinositol 3-kinase activity and the Pleckstrin homology domain of inducible T cell kinase. Proc Natl Acad Sci U S A. 1997;94:11227–32. doi: 10.1073/pnas.94.21.11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ching KA, Kawakami Y, Kawakami T, Tsoukas CD. Emt/Itk associates with activated TCR complexes: role of the pleckstrin homology domain. J Immunol. 1999;163:6006–13. [PubMed] [Google Scholar]
- 16.Debnath J, et al. rlk/TXK encodes two forms of a novel cysteine string tyrosine kinase activated by Src family kinases. Mol Cell Biol. 1999;19:1498–507. doi: 10.1128/mcb.19.2.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andreotti AH, Bunnell SC, Feng S, Berg LJ, Schreiber SL. Regulatory intramolecular association in a tyrosine kinase of the Tec family. Nature. 1997;385:93–7. doi: 10.1038/385093a0. [DOI] [PubMed] [Google Scholar]
- 18.Bunnell SC, Diehn M, Yaffe MB, Findell PR, Cantley LC, Berg LJ. Biochemical interactions integrating Itk with the T cell receptor-initiated signaling cascade. J Biol Chem. 2000;275:2219–30. doi: 10.1074/jbc.275.3.2219. [DOI] [PubMed] [Google Scholar]
- 19.Su YW, Zhang Y, Schweikert J, Koretzky GA, Reth M, Wienands J. Interaction of SLP adaptors with the SH2 domain of Tec family kinases. Eur J Immunol. 1999;29:3702–11. doi: 10.1002/(SICI)1521-4141(199911)29:11<3702::AID-IMMU3702>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 20.Samelson LE. Signal transduction mediated by the T cell antigen receptor: the role of adapter proteins. Annu Rev Immunol. 2002;20:371–94. doi: 10.1146/annurev.immunol.20.092601.111357. [DOI] [PubMed] [Google Scholar]
- 21.Jordan MS, Singer AL, Koretzky GA. Adaptors as central mediators of signal transduction in immune cells. Nat Immunol. 2003;4:110–6. doi: 10.1038/ni0203-110. [DOI] [PubMed] [Google Scholar]
- 22.Clements JL, et al. Requirement for the leukocyte-specific adapter protein SLP-76 for normal T cell development. Science. 1998;281:416–9. doi: 10.1126/science.281.5375.416. [DOI] [PubMed] [Google Scholar]
- 23.Zhang W, et al. Essential role of LAT in T cell development. Immunity. 1999;10:323–32. doi: 10.1016/s1074-7613(00)80032-1. [DOI] [PubMed] [Google Scholar]
- 24.Perez-Villar J, et al. Phosphorylation of the linker for activation of T-cells by Itk promotes recruitment of Vav. Biochemistry. 2002;41:10732–40. doi: 10.1021/bi025554o. [DOI] [PubMed] [Google Scholar]
- 25.Bogin Y, Ainey C, Beach D, Yablonski D. SLP-76 mediates and maintains activation of the Tec family kinase ITK via the T cell antigen receptor-induced association between SLP-76 and ITK. Proc Natl Acad Sci U S A. 2007;104:6638–43. doi: 10.1073/pnas.0609771104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dennis EA, Rhee SG, Billah MM, Hannun YA. Role of phospholipase in generating lipid second messengers in signal transduction. FASEB J. 1991;5:2068–77. doi: 10.1096/fasebj.5.7.1901288. [DOI] [PubMed] [Google Scholar]
- 27.Wilcox HM, Berg LJ. Itk phosphorylation sites are required for functional activity in primary T cells. J Biol Chem. 2003;278:37112–21. doi: 10.1074/jbc.M304811200. [DOI] [PubMed] [Google Scholar]
- 28.Sommers CL, Rabin RL, Grinberg A, Tsay HC, Farber J, Love PE. A role for the Tec family tyrosine kinase Txk in T cell activation and thymocyte selection. J Exp Med. 1999;190:1427–38. doi: 10.1084/jem.190.10.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Veri MC, et al. Membrane raft-dependent regulation of phospholipase Cgamma-1 activation in T lymphocytes. Mol Cell Biol. 2001;21:6939–50. doi: 10.1128/MCB.21.20.6939-6950.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saito K, et al. BTK regulates PtdIns-4,5-P2 synthesis: importance for calcium signaling and PI3K activity. Immunity. 2003;19:669–78. doi: 10.1016/s1074-7613(03)00297-8. [DOI] [PubMed] [Google Scholar]
- 31.Huang YH, et al. Positive regulation of Itk PH domain function by soluble IP4. Science. 2007;316:886–9. doi: 10.1126/science.1138684. [DOI] [PubMed] [Google Scholar]
- 32.Oh-hora M, Rao A. Calcium signaling in lymphocytes. Curr Opin Immunol. 2008;20:250–8. doi: 10.1016/j.coi.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luik RM, Wang B, Prakriya M, Wu MM, Lewis RS. Oligomerization of STIM1 couples ER calcium depletion to CRAC channel activation. Nature. 2008;454:538–42. doi: 10.1038/nature07065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Penna A, et al. The CRAC channel consists of a tetramer formed by Stim-induced dimerization of Orai dimers. Nature. 2008;456:116–20. doi: 10.1038/nature07338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17:2205–32. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- 36.Ebinu JO, et al. RasGRP links T-cell receptor signaling to Ras. Blood. 2000;95:3199–203. [PubMed] [Google Scholar]
- 37.Roose JP, Mollenauer M, Ho M, Kurosaki T, Weiss A. Unusual interplay of two types of Ras activators, RasGRP and SOS, establishes sensitive and robust Ras activation in lymphocytes. Mol Cell Biol. 2007;27:2732–45. doi: 10.1128/MCB.01882-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Newton AC. Diacylglycerol’s affair with protein kinase C turns 25. Trends Pharmacol Sci. 2004;25:175–7. doi: 10.1016/j.tips.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 39.Donnadieu E, et al. Differential roles of Lck and Itk in T cell response to antigen recognition revealed by calcium imaging and electron microscopy. J Immunol. 2001;166:5540–9. doi: 10.4049/jimmunol.166.9.5540. [DOI] [PubMed] [Google Scholar]
- 40.Borde M, Barrington RA, Heissmeyer V, Carroll MC, Rao A. Transcriptional basis of lymphocyte tolerance. Immunol Rev. 2006;210:105–19. doi: 10.1111/j.0105-2896.2006.00370.x. [DOI] [PubMed] [Google Scholar]
- 41.Zhong XP, Guo R, Zhou H, Liu C, Wan CK. Diacylglycerol kinases in immune cell function and self-tolerance. Immunol Rev. 2008;224:249–64. doi: 10.1111/j.1600-065X.2008.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stefanova I, Hemmer B, Vergelli M, Martin R, Biddison WE, Germain RN. TCR ligand discrimination is enforced by competing ERK positive and SHP-1 negative feedback pathways. Nat Immunol. 2003;4:248–54. doi: 10.1038/ni895. [DOI] [PubMed] [Google Scholar]
- 43.Marsland BJ, Kopf M. T-cell fate and function: PKC-theta and beyond. Trends Immunol. 2008;29:179–85. doi: 10.1016/j.it.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 44.Fowell DJ, et al. Impaired NFATc translocation and failure of Th2 development in Itk-deficient CD4+ T cells. Immunity. 1999;11:399–409. doi: 10.1016/s1074-7613(00)80115-6. [DOI] [PubMed] [Google Scholar]
- 45.Schaeffer EM, et al. Mutation of Tec family kinases alters T helper cell differentiation. Nat Immunol. 2001;2:1183–8. doi: 10.1038/ni734. [DOI] [PubMed] [Google Scholar]
- 46.Nurieva RI, et al. A costimulation-initiated signaling pathway regulates NFATc1 transcription in T lymphocytes. J Immunol. 2007;179:1096–103. doi: 10.4049/jimmunol.179.2.1096. [DOI] [PubMed] [Google Scholar]
- 47.Mueller C, August A. Attenuation of immunological symptoms of allergic asthma in mice lacking the tyrosine kinase ITK. J Immunol. 2003;170:5056–63. doi: 10.4049/jimmunol.170.10.5056. [DOI] [PubMed] [Google Scholar]
- 48.Berg LJ. Signalling through TEC kinases regulates conventional versus innate CD8(+) T-cell development. Nat Rev Immunol. 2007;7:479–85. doi: 10.1038/nri2091. [DOI] [PubMed] [Google Scholar]
- 49.Burkhardt JK, Carrizosa E, Shaffer MH. The actin cytoskeleton in T cell activation. Annu Rev Immunol. 2008;26:233–59. doi: 10.1146/annurev.immunol.26.021607.090347. [DOI] [PubMed] [Google Scholar]
- 50.Gomez TS, Billadeau DD. T cell activation and the cytoskeleton: you can’t have one without the other. Adv Immunol. 2008;97:1–64. doi: 10.1016/S0065-2776(08)00001-1. [DOI] [PubMed] [Google Scholar]
- 51.Cemerski S, Shaw A. Immune synapses in T-cell activation. Curr Opin Immunol. 2006;18:298–304. doi: 10.1016/j.coi.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 52.Dustin ML. T-cell activation through immunological synapses and kinapses. Immunol Rev. 2008;221:77–89. doi: 10.1111/j.1600-065X.2008.00589.x. [DOI] [PubMed] [Google Scholar]
- 53.Cemerski S, et al. The balance between T cell receptor signaling and degradation at the center of the immunological synapse is determined by antigen quality. Immunity. 2008;29:414–22. doi: 10.1016/j.immuni.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chang JT, Reiner SL. Asymmetric Division and Stem Cell Renewal without a Permanent Niche: Lessons from Lymphocytes. Cold Spring Harb Symp Quant Biol. 2008 doi: 10.1101/sqb.2008.73.008. [DOI] [PubMed] [Google Scholar]
- 55.Bustelo XR. Vav proteins, adaptors and cell signaling. Oncogene. 2001;20:6372–81. doi: 10.1038/sj.onc.1204780. [DOI] [PubMed] [Google Scholar]
- 56.Tybulewicz VL. Vav-family proteins in T-cell signalling. Curr Opin Immunol. 2005;17:267–74. doi: 10.1016/j.coi.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 57.Nolz JC, et al. The WAVE2 complex regulates T cell receptor signaling to integrins via Abl- and CrkL-C3G-mediated activation of Rap1. J Cell Biol. 2008;182:1231–44. doi: 10.1083/jcb.200801121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grasis JA, Browne CD, Tsoukas CD. Inducible T cell tyrosine kinase regulates actin-dependent cytoskeletal events induced by the T cell antigen receptor. J Immunol. 2003;170:3971–6. doi: 10.4049/jimmunol.170.8.3971. [DOI] [PubMed] [Google Scholar]
- 59.Labno CM, et al. Itk functions to control actin polymerization at the immune synapse through localized activation of Cdc42 and WASP. Curr Biol. 2003;13:1619–24. doi: 10.1016/j.cub.2003.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dombroski D, et al. Kinase-independent functions for Itk in TCR-induced regulation of Vav and the actin cytoskeleton. J Immunol. 2005;174:1385–92. doi: 10.4049/jimmunol.174.3.1385. [DOI] [PubMed] [Google Scholar]
- 61.Gomez-Rodriguez J, Readinger JA, Viorritto IC, Mueller KL, Houghtling RA, Schwartzberg PL. Tec kinases, actin, and cell adhesion. Immunol Rev. 2007;218:45–64. doi: 10.1111/j.1600-065X.2007.00534.x. [DOI] [PubMed] [Google Scholar]
- 62.Raberger J, Boucheron N, Sakaguchi S, Penninger JM, Ellmeier W. Impaired T-cell development in the absence of Vav1 and Itk. Eur J Immunol. 2008 doi: 10.1002/eji.200838388. [DOI] [PubMed] [Google Scholar]
- 63.Costello PS, et al. The Rho-family GTP exchange factor Vav is a critical transducer of T cell receptor signals to the calcium, ERK, and NF-kappaB pathways. Proc Natl Acad Sci U S A. 1999;96:3035–40. doi: 10.1073/pnas.96.6.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nore BF, et al. Redistribution of Bruton’s tyrosine kinase by activation of phosphatidylinositol 3-kinase and Rho-family GTPases. Eur J Immunol. 2000;30:145–54. doi: 10.1002/1521-4141(200001)30:1<145::AID-IMMU145>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 65.Yao L, et al. Pleckstrin homology domains interact with filamentous actin. J Biol Chem. 1999;274:19752–61. doi: 10.1074/jbc.274.28.19752. [DOI] [PubMed] [Google Scholar]
- 66.Burbach BJ, Medeiros RB, Mueller KL, Shimizu Y. T-cell receptor signaling to integrins. Immunol Rev. 2007;218:65–81. doi: 10.1111/j.1600-065X.2007.00527.x. [DOI] [PubMed] [Google Scholar]
- 67.Finkelstein L, Shimizu Y, Schwartzberg P. Tec kinases regulate TCR-mediated recruitment of signaling molecules and integrin-dependent cell adhesion. J Immunol. 2005;175:5923–30. doi: 10.4049/jimmunol.175.9.5923. [DOI] [PubMed] [Google Scholar]
- 68.Woods ML, Kivens WJ, Adelsman MA, Qiu Y, August A, Shimizu Y. A novel function for the Tec family tyrosine kinase Itk in activation of beta 1 integrins by the T-cell receptor. Embo J. 2001;20:1232–44. doi: 10.1093/emboj/20.6.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wong MM, Fish EN. Chemokines: attractive mediators of the immune response. Semin Immunol. 2003;15:5–14. doi: 10.1016/s1044-5323(02)00123-9. [DOI] [PubMed] [Google Scholar]
- 70.Tsukada S, Simon MI, Witte ON, Katz A. Binding of beta gamma subunits of heterotrimeric G proteins to the PH domain of Bruton tyrosine kinase. Proc Natl Acad Sci U S A. 1994;91:11256–60. doi: 10.1073/pnas.91.23.11256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fischer AM, Mercer JC, Iyer A, Ragin MJ, August A. Regulation of CXC chemokine receptor 4-mediated migration by the Tec family tyrosine kinase ITK. J Biol Chem. 2004;279:29816–20. doi: 10.1074/jbc.M312848200. [DOI] [PubMed] [Google Scholar]
- 72.Takesono A, Horai R, Mandai M, Dombroski D, Schwartzberg PL. Requirement for Tec kinases in chemokine-induced migration and activation of Cdc42 and Rac. Curr Biol. 2004;14:917–22. doi: 10.1016/j.cub.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 73.Gilbert C, et al. Chemotactic factor-induced recruitment and activation of Tec family kinases in human neutrophils. II. Effects of LFM-A13, a specific Btk inhibitor. J Immunol. 2003;170:5235–43. doi: 10.4049/jimmunol.170.10.5235. [DOI] [PubMed] [Google Scholar]
- 74.de Gorter DJ, et al. Bruton’s tyrosine kinase and phospholipase Cgamma2 mediate chemokine-controlled B cell migration and homing. Immunity. 2007;26:93–104. doi: 10.1016/j.immuni.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 75.Inngjerdingen M, Torgersen KM, Maghazachi AA. Lck is required for stromal cell-derived factor 1 alpha (CXCL12)-induced lymphoid cell chemotaxis. Blood. 2002;99:4318–25. doi: 10.1182/blood.v99.12.4318. [DOI] [PubMed] [Google Scholar]
- 76.Kremer KN, Humphreys TD, Kumar A, Qian NX, Hedin KE. Distinct role of ZAP-70 and Src homology 2 domain-containing leukocyte protein of 76 kDa in the prolonged activation of extracellular signal-regulated protein kinase by the stromal cell-derived factor-1 alpha/CXCL12 chemokine. J Immunol. 2003;171:360–7. doi: 10.4049/jimmunol.171.1.360. [DOI] [PubMed] [Google Scholar]
- 77.Kumar A, et al. CXCR4 physically associates with the T cell receptor to signal in T cells. Immunity. 2006;25:213–24. doi: 10.1016/j.immuni.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 78.Ottoson NC, Pribila JT, Chan ASH, Shimizu Y. T cell migration regulated by CXCR4 chemokine receptor signaling to ZAP-70 tyrosine kinase. J Immunol. 2001;167:1857–61. doi: 10.4049/jimmunol.167.4.1857. [DOI] [PubMed] [Google Scholar]
- 79.Molon B, et al. T cell costimulation by chemokine receptors. Nat Immunol. 2005;6:465–71. doi: 10.1038/ni1191. [DOI] [PubMed] [Google Scholar]
- 80.Nguyen DH, Giri B, Collins G, Taub DD. Dynamic reorganization of chemokine receptors, cholesterol, lipid rafts, and adhesion molecules to sites of CD4 engagement. Exp Cell Res. 2005;304:559–69. doi: 10.1016/j.yexcr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 81.Friedman RS, Jacobelli J, Krummel MF. Surface-bound chemokines capture and prime T cells for synapse formation. Nat Immunol. 2006;7:1101–8. doi: 10.1038/ni1384. [DOI] [PubMed] [Google Scholar]
- 82.Bromley SK, Peterson DA, Gunn MD, Dustin ML. Cutting edge: hierarchy of chemokine receptor and TCR signals regulating T cell migration and proliferation. J Immunol. 2000;165:15–9. doi: 10.4049/jimmunol.165.1.15. [DOI] [PubMed] [Google Scholar]
- 83.Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–7. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 84.Gomez C, Hope TJ. The ins and outs of HIV replication. Cell Microbiol. 2005;7:621–6. doi: 10.1111/j.1462-5822.2005.00516.x. [DOI] [PubMed] [Google Scholar]
- 85.Ott DE, et al. Cytoskeletal proteins inside human immunodeficiency virus type 1 virions. J Virol. 1996;70:7734–43. doi: 10.1128/jvi.70.11.7734-7743.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Haedicke J, de Los Santos K, Goff SP, Naghavi MH. The Ezrin-radixin-moesin family member ezrin regulates stable microtubule formation and retroviral infection. J Virol. 2008;82:4665–70. doi: 10.1128/JVI.02403-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jimenez-Baranda S, et al. Filamin-A regulates actin-dependent clustering of HIV receptors. Nat Cell Biol. 2007;9:838–46. doi: 10.1038/ncb1610. [DOI] [PubMed] [Google Scholar]
- 88.Kubo Y, et al. Ezrin, Radixin, and Moesin (ERM) proteins function as pleiotropic regulators of human immunodeficiency virus type 1 infection. Virology. 2008;375:130–40. doi: 10.1016/j.virol.2008.01.047. [DOI] [PubMed] [Google Scholar]
- 89.Naghavi MH, et al. Moesin regulates stable microtubule formation and limits retroviral infection in cultured cells. EMBO J. 2007;26:41–52. doi: 10.1038/sj.emboj.7601475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yoder A, et al. HIV envelope-CXCR4 signaling activates cofilin to overcome cortical actin restriction in resting CD4 T cells. Cell. 2008;134:782–92. doi: 10.1016/j.cell.2008.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pontow SE, Heyden NV, Wei S, Ratner L. Actin cytoskeletal reorganizations and coreceptor-mediated activation of rac during human immunodeficiency virus-induced cell fusion. J Virol. 2004;78:7138–47. doi: 10.1128/JVI.78.13.7138-7147.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Readinger JA, et al. Selective targeting of ITK blocks multiple steps of HIV replication. Proc Natl Acad Sci U S A. 2008;105:6684–9. doi: 10.1073/pnas.0709659105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lin TA, et al. Selective Itk inhibitors block T-cell activation and murine lung inflammation. Biochemistry. 2004;43:11056–62. doi: 10.1021/bi049428r. [DOI] [PubMed] [Google Scholar]
- 94.Sol-Foulon N, et al. ZAP-70 kinase regulates HIV cell-to-cell spread and virological synapse formation. EMBO J. 2007;26:516–26. doi: 10.1038/sj.emboj.7601509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Atherly LO, Brehm MA, Welsh RM, Berg LJ. Tec kinases Itk and Rlk are required for CD8+ T cell responses to virus infection independent of their role in CD4+ T cell help. J Immunol. 2006;176:1571–81. doi: 10.4049/jimmunol.176.3.1571. [DOI] [PubMed] [Google Scholar]
- 96.Bachmann MF, Littman DR, Liao XC. Antiviral immune responses in Itk-deficient mice. J Virol. 1997;71:7253–7. doi: 10.1128/jvi.71.10.7253-7257.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Douhan J, 3rd, et al. A FLIPR-based assay to assess potency and selectivity of inhibitors of the TEC family kinases Btk and Itk. Assay Drug Dev Technol. 2007;5:751–8. doi: 10.1089/adt.2007.9982. [DOI] [PubMed] [Google Scholar]
- 98.Kashem MA, et al. Three mechanistically distinct kinase assays compared: Measurement of intrinsic ATPase activity identified the most comprehensive set of ITK inhibitors. J Biomol Screen. 2007;12:70–83. doi: 10.1177/1087057106296047. [DOI] [PubMed] [Google Scholar]
- 99.Snow RJ, et al. Hit-to-lead studies on benzimidazole inhibitors of ITK: discovery of a novel class of kinase inhibitors. Bioorg Med Chem Lett. 2007;17:3660–5. doi: 10.1016/j.bmcl.2007.04.045. [DOI] [PubMed] [Google Scholar]
- 100.Zipfel PA, et al. Role for the Abi/wave protein complex in T cell receptor-mediated proliferation and cytoskeletal remodeling. Curr Biol. 2006;16:35–46. doi: 10.1016/j.cub.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 101.Huang Y, Comiskey EO, Dupree RS, Li S, Koleske AJ, Burkhardt JK. The c-Abl tyrosine kinase regulates actin remodeling at the immune synapse. Blood. 2008;112:111–9. doi: 10.1182/blood-2007-10-118232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Roskoski R., Jr STI-571: an anticancer protein-tyrosine kinase inhibitor. Biochem Biophys Res Commun. 2003;309:709–17. doi: 10.1016/j.bbrc.2003.08.055. [DOI] [PubMed] [Google Scholar]
- 103.Tsimberidou AM, et al. Chronic myeloid leukemia in a patient with acquired immune deficiency syndrome: complete cytogenetic response with imatinib mesylate: report of a case and review of the literature. Leuk Res. 2004;28:657–60. doi: 10.1016/j.leukres.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 104.Potula R, et al. Association of platelet-derived growth factor-B chain with simian human immunodeficiency virus encephalitis. Am J Pathol. 2004;165:815–24. doi: 10.1016/S0002-9440(10)63344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schaeffer EM, Broussard C, Debnath J, Anderson S, McVicar DW, Schwartzberg PL. Tec family kinases modulate thresholds for thymocyte development and selection. J Exp Med. 2000;192:987–1000. doi: 10.1084/jem.192.7.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lucas JA, Atherly LO, Berg LJ. The absence of Itk inhibits positive selection without changing lineage commitment. J Immunol. 2002;168:6142–51. doi: 10.4049/jimmunol.168.12.6142. [DOI] [PubMed] [Google Scholar]
- 107.Lucas JA, Miller AT, Atherly LO, Berg LJ. The role of Tec family kinases in T cell development and function. Immunol Rev. 2003;191:119–38. doi: 10.1034/j.1600-065x.2003.00029.x. [DOI] [PubMed] [Google Scholar]
- 108.Mariathasan S, Ho SS, Zakarian A, Ohashi PS. Degree of ERK activation influences both positive and negative thymocyte selection. Eur J Immunol. 2000;30:1060–8. doi: 10.1002/(SICI)1521-4141(200004)30:4<1060::AID-IMMU1060>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 109.McNeil LK, Starr TK, Hogquist KA. A requirement for sustained ERK signaling during thymocyte positive selection in vivo. Proc Natl Acad Sci U S A. 2005;102:13574–9. doi: 10.1073/pnas.0505110102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Aifantis I, Gounari F, Scorrano L, Borowski C, von Boehmer H. Constitutive pre-TCR signaling promotes differentiation through Ca2+ mobilization and activation of NF-κB and NFAT. Nat Immunol. 2001:2. doi: 10.1038/87704. [DOI] [PubMed] [Google Scholar]
- 111.Lucas JA, Felices M, Evans JW, Berg LJ. Subtle defects in pre-TCR signaling in the absence of the Tec kinase Itk. J Immunol. 2007;179:7561–7. doi: 10.4049/jimmunol.179.11.7561. [DOI] [PubMed] [Google Scholar]
- 112.Germain RN. T-cell development and the CD4-CD8 lineage decision. Nat Rev Immunol. 2002;2:309–22. doi: 10.1038/nri798. [DOI] [PubMed] [Google Scholar]
- 113.Singer A, Bosselut R. CD4/CD8 coreceptors in thymocyte development, selection, and lineage commitment: analysis of the CD4/CD8 lineage decision. Adv Immunol. 2004;83:91–131. doi: 10.1016/S0065-2776(04)83003-7. [DOI] [PubMed] [Google Scholar]
- 114.Atherly LO, Lucas JA, Felices M, Yin CC, Reiner SL, Berg LJ. The Tec family tyrosine kinases Itk and Rlk regulate the development of conventional CD8+ T cells. Immunity. 2006;25:79–91. doi: 10.1016/j.immuni.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 115.Broussard C, et al. Altered development of CD8+ T cell lineages in mice deficient for the Tec kinases Itk and Rlk. Immunity. 2006;25:93–104. doi: 10.1016/j.immuni.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 116.Dubois S, Waldmann TA, Muller JR. ITK and IL-15 support two distinct subsets of CD8+ T cells. Proc Natl Acad Sci U S A. 2006;103:12075–80. doi: 10.1073/pnas.0605212103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hu J, Sahu N, Walsh E, August A. Memory phenotype CD8+ T cells with innate function selectively develop in the absence of active Itk. Eur J Immunol. 2007;37:2892–9. doi: 10.1002/eji.200737311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Horai R, et al. Requirements for selection of conventional and innate T lymphocyte lineages. Immunity. 2007;27:775–85. doi: 10.1016/j.immuni.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Intlekofer AM, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–44. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 120.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 121.Urdahl KB, Sun JC, Bevan MJ. Positive selection of MHC class Ib-restricted CD8(+) T cells on hematopoietic cells. Nat Immunol. 2002;3:772–9. doi: 10.1038/ni814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Choi EY, et al. Thymocyte-thymocyte interaction for efficient positive selection and maturation of CD4 T cells. Immunity. 2005;23:387–96. doi: 10.1016/j.immuni.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 123.Li W, Kim MG, Gourley TS, McCarthy BP, Sant’Angelo DB, Chang CH. An alternate pathway for CD4 T cell development: thymocyte-expressed MHC class II selects a distinct T cell population. Immunity. 2005;23:375–86. doi: 10.1016/j.immuni.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 124.Au-Yeung BB, Fowell DJ. A key role for Itk in both IFN gamma and IL-4 production by NKT cells. J Immunol. 2007;179:111–9. doi: 10.4049/jimmunol.179.1.111. [DOI] [PubMed] [Google Scholar]
- 125.Felices M, Berg LJ. The Tec kinases Itk and Rlk regulate NKT cell maturation, cytokine production, and survival. J Immunol. 2008;180:3007–18. doi: 10.4049/jimmunol.180.5.3007. [DOI] [PubMed] [Google Scholar]
- 126.Gadue P, Stein PL. NK T cell precursors exhibit differential cytokine regulation and require Itk for efficient maturation. J Immunol. 2002;169:2397–406. doi: 10.4049/jimmunol.169.5.2397. [DOI] [PubMed] [Google Scholar]
- 127.Perarnau B, et al. Single H2Kb, H2Db and double H2KbDb knockout mice: peripheral CD8+ T cell repertoire and anti-lymphocytic choriomeningitis virus cytolytic responses. Eur J Immunol. 1999;29:1243–52. doi: 10.1002/(SICI)1521-4141(199904)29:04<1243::AID-IMMU1243>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 128.Borowski C, Bendelac A. Signaling for NKT cell development: the SAP-FynT connection. J Exp Med. 2005;201:833–6. doi: 10.1084/jem.20050339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Calpe S, et al. The SLAM and SAP gene families control innate and adaptive immune responses. Adv Immunol. 2008;97:177–250. doi: 10.1016/S0065-2776(08)00004-7. [DOI] [PubMed] [Google Scholar]
- 130.Cannons JL, et al. SAP regulates TH2 differentiation and PKCθ-mediated activation of NF-κB1. Immunity. 2004;21:693–706. doi: 10.1016/j.immuni.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 131.Ma CS, Nichols KE, Tangye SG. Regulation of cellular and humoral immune responses by the SLAM and SAP families of molecules. Annu Rev Immunol. 2007;25:337–79. doi: 10.1146/annurev.immunol.25.022106.141651. [DOI] [PubMed] [Google Scholar]
- 132.Veillette A. Immune regulation by SLAM family receptors and SAP-related adaptors. Nature Rev Immunol. 2006;6:56–66. doi: 10.1038/nri1761. [DOI] [PubMed] [Google Scholar]
- 133.Chung B, Aoukaty A, Dutz J, Terhorst C, Tan R. Signaling lymphocytic activation molecule-associated protein controls NKT cell functions. J Immunol. 2005;174:3153–7. doi: 10.4049/jimmunol.174.6.3153. [DOI] [PubMed] [Google Scholar]
- 134.Nichols KE, et al. Regulation of NKT cell development by SAP, the protein defective in XLP. Nature Med. 2005;11:340–5. doi: 10.1038/nm1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pasquier B, et al. Defective NKT cell development in mice and humans lacking the adapter SAP, the X-linked lymphoproliferative syndrome gene product. J Exp Med. 2005;201:695–701. doi: 10.1084/jem.20042432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rigaud S, et al. XIAP deficiency in humans causes an X-linked lymphoproliferative syndrome. Nature. 2006;444:110–4. doi: 10.1038/nature05257. [DOI] [PubMed] [Google Scholar]
- 137.Griewank K, et al. Homotypic Interactions mediated by Ly108 and SLAM control NKT lineage development. Immunity. 2007 doi: 10.1016/j.immuni.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kovalovsky D, et al. The BTB-zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nat Immunol. 2008;9:1055–64. doi: 10.1038/ni.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Raberger J, et al. The transcriptional regulator PLZF induces the development of CD44 high memory phenotype T cells. Proc Natl Acad Sci U S A. 2008;105:17919–24. doi: 10.1073/pnas.0805733105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Savage AK, et al. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity. 2008;29:1–13. doi: 10.1016/j.immuni.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jordan MS, et al. Complementation in trans of altered thymocyte development in mice expressing mutant forms of the adaptor molecule SLP76. Immunity. 2008;28:359–69. doi: 10.1016/j.immuni.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhong M-C, Veillette A. Control of T lymphocyte signaling by Ly108, a signaling lymphocytic activation molecule receptor implicated in autoimmunity. J Biol Chem. 2008;283:19255–64. doi: 10.1074/jbc.M800209200. [DOI] [PubMed] [Google Scholar]
- 143.Crotty S, Kersh EN, Cannons J, Schwartzberg PL, Ahmed R. SAP is required for generating long-term humoral immunity. Nature. 2003;421:282–7. doi: 10.1038/nature01318. [DOI] [PubMed] [Google Scholar]
- 144.Hron JD, Caplan L, Gerth AJ, Schwartzberg PL, Peng SL. SH2D1A regulates T-dependent humoral autoimmunity. J Exp Med. 2004;200:261–6. doi: 10.1084/jem.20040526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ma CS, et al. Impaired humoral immunity in X-linked lymphoproliferative disease is associated with defective IL-10 production by CD4+ T cells. J Clin Invest. 2005;115:1049–59. doi: 10.1172/JCI23139. Epub 2005 Mar 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cannons JL, et al. SAP regulates T cell-mediated help for humoral immunity by a mechanism distinct from cytokine regulation. J Exp Med. 2006;203:1551–65. doi: 10.1084/jem.20052097. Epub 2006 Jun 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Veillette A, Zhang S, Shi X, Dong Z, Davidson D, Zhong M-C. SAP expression in T cells, not in B cells, is required for humoral immunity. Proc Natl Acad Sci USA. 2008;105:1273–8. doi: 10.1073/pnas.0710698105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP-controlled T-B cell interactions underlies germinal centre formation. Nature. 2008 doi: 10.1038/nature07345. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hu J, August A. Naive and innate memory phenotype CD4+ T cells have different requirements for active Itk for their development. J Immunol. 2008;180:6544–52. doi: 10.4049/jimmunol.180.10.6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Glimcher LH, Murphy KM. Lineage commitment in the immune system: the T helper lymphocyte grows up. Genes and Devel. 2000;14:1693–711. [PubMed] [Google Scholar]
- 151.Vinuesa CG, Tangye SG, Moser B, Mackay CR. Follicular B helper T cells in antibody responses and autoimmunity. Nat Rev Immunol. 2005;5:853–65. doi: 10.1038/nri1714. [DOI] [PubMed] [Google Scholar]
- 152.Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112:1557–69. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Au-Yeung BB, Katzman SD, Fowell DJ. Cutting edge: Itk-dependent signals required for CD4+ T cells to exert, but not gain, Th2 effector function. J Immunol. 2006;176:3895–9. doi: 10.4049/jimmunol.176.7.3895. [DOI] [PubMed] [Google Scholar]
- 154.Li W, et al. Thymic selection pathway regulates the effector function of CD4 T cells. J Exp Med. 2007;204:2145–57. doi: 10.1084/jem.20070321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kerksiek KM, Busch DH, Pilip IM, Allen SE, Pamer EG. H2-M3-restricted T cells in bacterial infection: rapid primary but diminished memory responses. J Exp Med. 1999;190:195–204. doi: 10.1084/jem.190.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kashiwakura J, et al. Txk, a nonreceptor tyrosine kinase of the Tec family, is expressed in T helper type 1 cells and regulates interferon gamma production in human T lymphocytes. J Exp Med. 1999;190:1147–54. doi: 10.1084/jem.190.8.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mohamed AJ, Vargas L, Nore BF, Backesjo CM, Christensson B, Smith CI. Nucleocytoplasmic shuttling of Bruton’s tyrosine kinase. J Biol Chem. 2000;275:40614–9. doi: 10.1074/jbc.M006952200. [DOI] [PubMed] [Google Scholar]
- 158.Webb CF, et al. The transcription factor bright associates with Bruton’s tyrosine kinase, the defective protein in immunodeficiency disease [In Process Citation] J Immunol. 2000;165:6956–65. doi: 10.4049/jimmunol.165.12.6956. [DOI] [PubMed] [Google Scholar]
- 159.Takeba Y, Nagafuchi H, Takeno M, Kashiwakura J, Suzuki N. Txk, a member of nonreceptor tyrosine kinase of Tec family, acts as a Th1 cell-specific transcription factor and regulates IFN-gamma gene transcription. J Immunol. 2002;168:2365–70. doi: 10.4049/jimmunol.168.5.2365. [DOI] [PubMed] [Google Scholar]
- 160.Hu Q, et al. Identification of Rlk, a novel protein tyrosine kinase with predominant expression in the T cell lineage. J Biol Chem. 1995;270:1928–34. doi: 10.1074/jbc.270.4.1928. [DOI] [PubMed] [Google Scholar]
- 161.Sahu N, et al. Selective expression rather than specific function of Txk and Itk regulate Th1 and Th2 responses. J Immunol. 2008;181:6125–31. doi: 10.4049/jimmunol.181.9.6125. [DOI] [PMC free article] [PubMed] [Google Scholar]