Abstract
The present study was designed to develop and compare acyclovir containing nano-vesicular liposomes and niosomes based on cholesterol, soya L-α-lecithin and nonionic surfactant, span 20. The effort was made to study in vitro whether acyclovir-loaded nanovesicles could sustain the release of the drug by increasing residence time and thus, acyclovir could reduce its dose-related systemic toxicity. There were good vesicular distributions in both of the niosomes and the liposomes. The obtained vesicles were within 1 μm and about 35% of them were within a size of 100 nm. The percentage of drug loading varied and the niosomal vesicles contained more drug as compared with the liposomes. When the in vitro drug release was compared, it was found that the liposomes released about 90% drug in 150 min whereas the drug release was just 50% from the niosomal vesicles in 200 min. Again, the niosomes showed better stability compared with the liposomes. Thus, niosome could be a better choice for intravenous delivery of acyclovir.
Keywords: acyclovir, liposomes, niosomes, sustained release
Introduction
Liposomes are the unilamellar or multilamellar spherical structures consisting of lipid bilayers arranged in a concentric fashion enclosing an equal number of aqueous compartments (Fang et al 2001; Chetoni et al 2004; Pavelic et al 2005). On the other hand niosomes or nonionic surfactant vesicles are similar to liposomes and they specially contain nonionic surfactant molecules (Fang et al 2001). These are able to encapsulate both hydrophilic and lipophilic molecules. Moreoften, niosomes are preferred as a drug carrier to liposomes because of their advantages related to stability, low cost, and usage of variable purities in their manufacturing with phospholipids (Fang et al 2001).
Acyclovir, an analogue of 2/− deoxyguanosine, has been known for its antiviral activity against varicella zoster virus, Epstein barr virus, cytomegallo virus, herpes simplex virus (HSV), and human herpes virus 6 (Wagstaff et al 1994; Hayden 1996). The oral bioavailability of acyclovir is poor (15%–20%) requiring frequent dosing regimen (Thummel and Shen 2001). Its limited solubility in water makes its intramuscular administration rather difficult (Freeman et al 1986). Many researchers have tried to increase its solubility by modifying chemical structure or by prodrug approach (Bundgared et al 1991; Shao et al 1994; Chikhale et al 1996). But none of the findings are very encouraging. When administered intravenously, most of acyclovir is excreted unchanged through urine since the drug molecules are excreted through kidneys by both glomerular filtration and tubular secretion. The intravenous administration of acyclovir sodium results in thrombophlebitis and has chances of drug crystal formation when excreted through kidneys during the systemic treatment of HSV infections in immuno-competent as well as immuno-compromised patients (Brown et al 2002). The terminal or beta-phase half-life of acyclovir is reported to be 2 to 3 h for adults (Thummel and Shen 2001). Thus, the dose of the drug is high and residence time of the drug in circulation is very less.
So the aim of our research was to develop stable nano-liposomal and nano-niosomal vesicles of acyclovir, which can remain in systemic circulation for a longer time and thus the dose-related toxicities may be reduced (Jain et al 2005). The study was conducted using in vitro methods only.
Methodology
Preparation of liposomes and niosomes
Liposomes and niosomes were prepared by lipid layer hydration method. Briefly, the weighed amounts of soya lecithin, span 20, polyethylene glycol 400 (PEG 400), and cholesterol as per their experimental combinations (Table 1) containing butylated hydroxyanisole (BHA) (2% w/w of lipid) wherever necessary were taken in 250 ml round bottom flask and were dissolved in chloroform by hand-shaking. The mixture was placed in a rotary vacuum evaporator with an aspirator A 3S (Tokyo Rikakikai Co. Ltd., Tokyo, Japan). The rotary vacuum evaporator was fitted with a cold water circulating bath (Spac-N Service, Kolkata, India) and was rotated at 150 rpm. The temperature of the water bath was maintained at 32 °C to evaporate the solvent. After the initial evaporation, the flask was kept in a vacuum desiccator overnight for complete removal of residual chloroform. Acyclovir (1.66 mg/ml) was dissolved in phosphate buffer (pH 7.4) and the desired volume was then taken into a flask containing lipid film. The film was hydrated in a rotary vacuum evaporator maintained at 60 °C and rotated at 100 rpm until the lipid film was dispersed in the aqueous phase. The sizes of the vesicles were reduced by a bath-sonicator (30 ± 2 KHz) (Instrumentation India, Kolkata, India) at 60 °C for 1 h. After sonication, the preparations were kept at room temperature for 1 h for vesicle formation and then they were kept at 4 °C in an inert atmosphere for 24 h. The preparations were then centrifuged at 5000 rpm at 4 °C for 5 min. The supernatant containing the vesicles in each case was taken for further studies as a suspended formulation. The formulations were also lyophilized with a cryo-protectant, sucrose, in different sucrose to lipid wt. ratio (1:1 and 2.5:1). The lyophilization was done using a lyophilizer (Instrumentation India ltd, Kolkata, India). Primary drying was conducted at −35 °C for 8 h under vacuum after the standard prefreezing.
Table 1.
Constituents of various formulations containg acyclovir
| Formulation codea | Compositions in ratio (μmole) | % drug load (mean ± SE, n = 3) |
|---|---|---|
| L1 | bSPC:CH = 131.57:28.71 | 8.52 ± 2.11 |
| L2 | bSPC:CH = 131.57:12.82 | 6.49 ± 2.31 |
| L3 | bSPC:CH:PEG 400 = 131.57: 28.71:84.37 | 8.61 ± 1.31 |
| N1 | bSPC:CH:Span20 = 131.57:28.71:44.48 | 28.9 ± 3.53 |
| N2 | Span 20:CH = 75:75 | 20.02 ± 5.21 |
| N3 | Span 20: CH = 100:50 | 14.01 ± 3.34 |
| N4 | Span 20:CH:PEG 400 = 75:75:84 | 9.28 ± 2.31 |
Note: aEach formulation contained 22.2 μmole of acyclovir;
SPC, Soya L-α-Lecithin; CH, cholesterol.
Stability study of liposomes and niosomes containing acyclovir
The stability study was conducted to monitor physical and chemical stabilities of the lyophilized forms as well as the reconstituted lyophilized forms. The stability parameters such as vesicular fusion, variation in pH, size distribution, drug-excipient interaction, and lipid degradation in terms of lipid peroxidation were determined.
The resuspended (reconstituted) lyophilized and lyophilized formulations of liposomes and niosomes were stored at 25 °C (±1 °C), 4 °C (±1 °C), and −20 °C (±1 °C) for three months. Then the above mentioned various parameters were studied. In case of the formulations underwent fusion detected by light microscope and degradation in terms of cracking (visible) of the formulations, no further study was conducted with them.
Morphology
Morphology of the vesicles was performed with the help of scanning electron microscope (SEM) (JSM, JEOL, Tokyo, Japan). Fusions and crystallization were detected by optical microscopy.
pH measurement
pH was measured using a pH meter (Sigma, Toledo, USA) at 25 ± 0.5 °C. Both the reconstituted lyophilized forms and the lyophilized forms stored at various temperatures were studied (but not degraded ones) for pH.
Size distribution and zeta potential
Size distribution and zeta potential measurement was performed with the help of Zetasizer nano ZS (Malvern Instruments Ltd., UK). The image was processed in a Metlab environment (METLAB-7.0). The threshold image was subjected to object analysis using a NIH imaging software (image tools). The classified objects were then subjected to histogram analysis.
Lipid peroxidation (LP) study
LP was measured of all the freshly prepared formulations and of those found stable after storage at different temperatures. The data were given for the stable formulations only along with the corresponding fresh ones. The extent of lipid peroxidation was assessed from the amount of thiobarbituric acid (TBA) reactive substances produced which was spectrophotometrically read at 532 nm as mentioned (Gallova and Szalayova 2004).
Fourier transform, infrared (FTIR) study
The pure drug acyclovir, cholesterol, soya lecithin, and a mixture of drug with cholesterol, and soya lecithin were mixed separately with infrared (IR) grade KBr in the ratio of 100:1 and corresponding pellets were prepared by applying 5.5 metric tons of pressure in a hydraulic press. The pellets were scanned in an inert atmosphere over a wave number range of 4000–400 cm−1 in Magna IR 750 series II (Nicolet, USA) FTIR instrument.
In case of the lyophilized formulations, FTIR study was done using the same method mentioned above. In case of the other formulations, after centrifugation the precipitate was taken and a thin film of it was made in between two discs and placed inside the sample holder.
Drug loading study
Reconstituted lyophilized formulations and the freshly prepared suspensions were undergone centrifugation (Fixed angle ultracentrifuge, 54 k = Avg. RCF = 20, 3300 xg = 54200, Sorvall, USA, run for 8 h) to precipitate liposomes and niosomes. Drug loading estimation was done by simple extraction of drug with 0.1(N) hydrochloric acid from chloroform by breaking the vesicle layers with chloroform. The extract was diluted with 0.1(N) hydrochloric acid and absorbance was measured at 254.5 nm using UV/VIS spectrophotometer (Beckman, USA) against 0.1(N) hydrochloric acid as blank. The same procedure was used for the batch without drug. The absorbance due to drug was the difference between the readings obtained from the respective preparations with drug and without drug. The drug content was then determined from the standard curve.
In vitro release study
In vitro release of acyclovir from niosomes and liposomes was conducted by dialysis in a dialysis sac (Sigma, 12000 MW cut off) with 180 ml of phosphate buffer saline (PBS) buffer at 37 °C following the method published elsewhere (Zhang et al 2005). In a 250 ml conical flask, 180 ml of PBS was taken. 1 ml (250 mg freeze-dried formulation/ml) of a formulation was taken into a dialysis bag. Two ends of the dialysis sac were tightly bound with threads. The sac was hanged inside a conical flask with the help of a glass rod so that the portion of the dialysis sac with the formulation should dip into the buffer solution. The flask was kept on a magnetic stirrer. Stirring was maintained at 300 rpm with the help of a magnetic bead and the temperature of the water was maintained at 37 °C with a thermostatic control. Sampling was done by withdrawing 1 ml from the released medium with the help of micropipette and 1 ml of fresh buffer was added. Samples were analyzed using a spectrophotometer at a wave length of 252 nm. With the help of the standard curve prepared earlier, drug concentration was measured. In case of the freeze-dried formulations, reconstitution was done with PBS buffer just before putting into a dialysis bag for release study.
Results
The prepared liposomes and niosomes were lyophilized with a cryo-protectant, sucrose, in different sucrose to lipid wt. ratio (1:1 and 2.5:1). The formulations containing lipid sucrose ratio 1:1 showed sedimentation, drug crystal formation as well as vesicular fusion (Figure 1). So they were not considered for further study. It was observed that the lyophilized formulations at the sucrose-lipid ratio of 2.5:1 were easily redispersed (when reconstituted) in PBS buffer without any sediment or drug crystals. There was also a good distribution of vesicles. Hence, further study was conducted with those formulations.
Figure 1.
Vesicular fusion and drug crystallization of a formulation with sucrose to lipid ratio 1:1.
After three months of the stability study, it was observed that the formulations both in liquid and lyophilized states became unstable at 25 ± 1 °C and at 4 °C (except niosomes). At 4 °C, the lyophilized-niosomes were found to be stable. All the formulations were stable at −20 °C.
Both the reconstituted lyophilized forms and the lyophilized forms stored at the different temperatures were studied for pH (but not the degraded ones). The formulations kept at 25 °C and 4 °C (except the lyophilized niosomes) were found to be degraded and they were not studied further. There was no major change in pH of the formulations. The pH values (data show mean ± SD, n is the number of experiments) of the lyophilized and the reconstituted lyophilized liposomal formulations containing sucrose-lipid ratio 2.5:1 kept at −20 °C were 6.73 ± 0.8 (n = 5) and 6.58 ± 0.5 (n = 5), respectively. The pH values of the lyophilized and the reconstituted lyophilized niosomal formulations containing sucrose-lipid ratio 2.5:1 kept at −20 °C was 6.77 ± 0.3 (n = 5) and 6.72 ± 0.3 (n = 5), respectively. Those (lyophilized niosomes) kept at 4 °C had the value 6.78 ± 0.7 (n = 5).
Size distribution of the formulations was done by zeta sizer (nanosizer) before and after storage. The zeta potentials were given in Table 2. L1 and L2 gave similar values. N1, N2, and N3 also gave similar zeta potentials. Again, L3 and N4 had similar values. The representatives of each type, L1, N1, and L3 are given in Table 2. Since the zeta potentials of the formulations with and without drug varied, they were mentioned in the table. Zeta potentials of the formulations without drug were strongly negative whereas, those with drug became less negative. In case of N1, N2, and N3, the formulations even showed positive values. No change in zeta potential was detected before and after the storage of the formulations at the different temperatures. The polydispersity index before and after storage for three months was measured. The values were 0.824 ± 0.17 (n = 3) (reconstituted lyophilized) and 0.532 ± 0.19 (n = 3) (lyophilized), and 0.368 ± 0.026 (n = 3) (reconstituted lyophilized) and 0.335 ± 0.063 (n = 3) (lyophilized) of the liposomal formulations before and after storage at −20 °C respectively. In case of the niosomal formulations, the values were 0.757 ± 0.15 (n = 3) (reconstituted lyophilized), 0.645 ± 0.08 (n = 3) (lyophilized) and 0.718 ± 0.022 (n = 3) (reconstituted lyophilized), 0.633 ± 0.05 (n = 3) (lyophilized) before and after the storage at −20 °C, respectively. Niosomes (lyophilized) stored at 4 °C had the values 0.666 ± 0.07 and 0.598 ± 0.02 before and after the storage at 4 °C respectively.
Table 2.
Zeta potential (ZP) data of different formulations
| Formulation compositiond |
ZP(mV) |
|
|---|---|---|
| Without drug | With drug | |
| L1,SPC:CHa | −8.413 | −1.972 |
| N1,SPC:CH:Span 20b | −7.018 | 1.439 |
| L3, SPC:CH:PEG 400c | −17.34 | −11.86 |
Note: Measuring temperature = 25 °C;
SPC:CH = 131.57:28.71 (μmole);
Span 20 = 44.48 μmole;
PEG 400 = 84.37 μmole;
Amount of drug, acyclovir taken for each of the formulations with drug = 22.2 μmole.
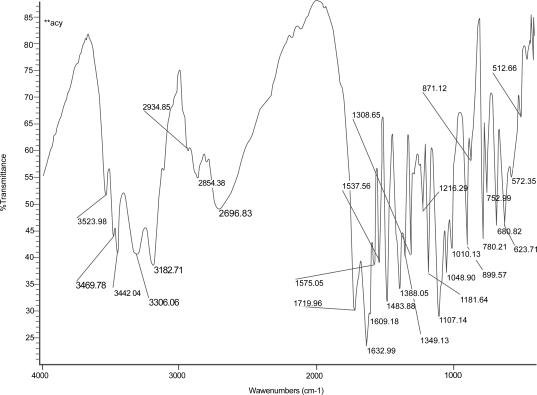
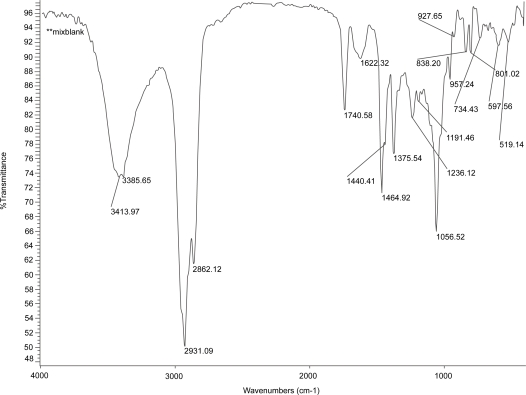
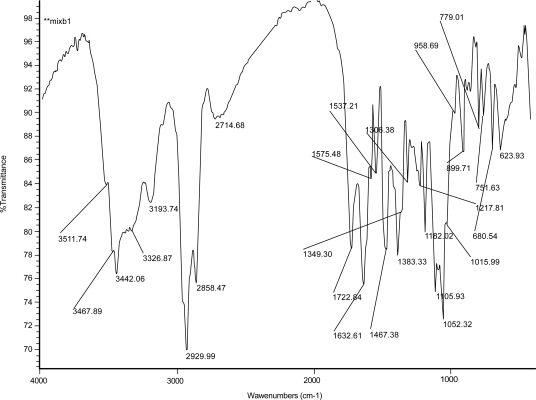
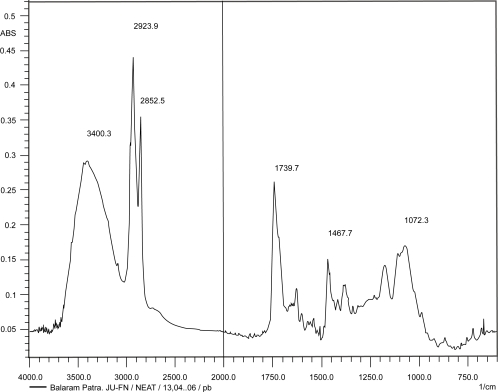
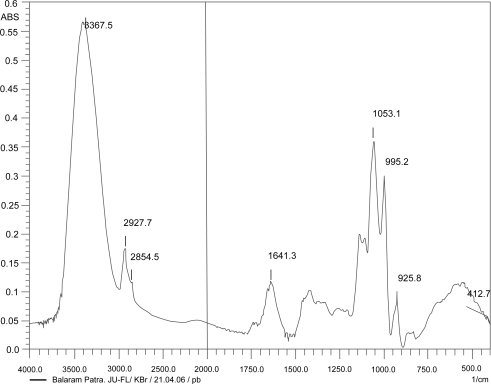
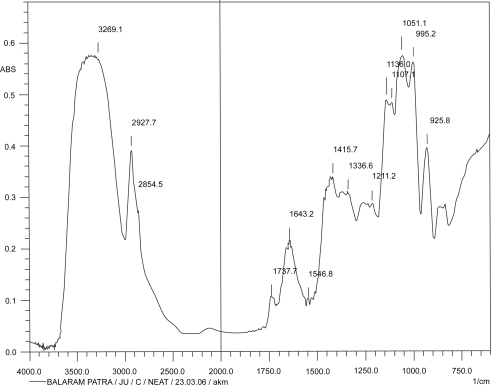
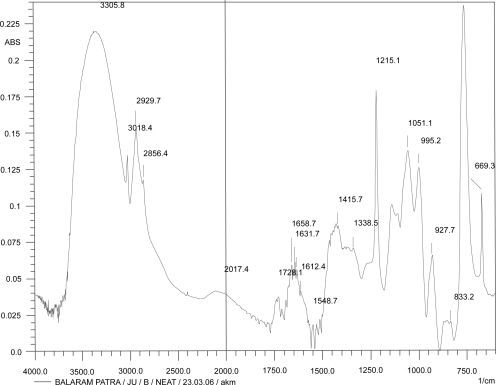
Figures 2, 3, and 4 show the IR spectrum of the drug acyclovir, physical mixture of cholesterol and soya lecithin (wt. ratio = 1:1), and physical mixture of cholesterol, soya lecithin with acyclovir (wt. ratio = 1:1:1) respectively. No predominant drug interaction was detected. Although, there were some mild interactions in the wave number 3600–3200 cm−1 and 1500–800 cm−1. The region 3600–3200 cm−1 is a stretching region of the functional group N-H, C-H of aromatic ring (3100–3000 cm−1), O-H (3200 cm−1) and C-H of alkenes (3100–3000 cm–1) and C-H of alkane (∼3000 cm–1). The region 1500–800 cm−1 is the stretching region of the functional group C-OH of alcohol (1400–1075 cm−1). In the region 1500–800 cm−1, the weak drug interaction was noticed at 1383.33, 985.69, and 899.71 cm−1. The FTIR study of the niosomes after freeze drying (Figure 5), after the storage at 4 °C (the lyophilized niosomes) (Figure 6), lyophilized niosome stored at −20 °C (Figure 7) and the reconstituted lyophilized niosomes stored at −20 °C (Figure 8) for three months were done to observe any chemical interaction between the drug and the excipients. No new different changes in the spectrum were noticed after storing them at different temperatures. This indicates that at those storage temperatures the formulations were stable. No change was noticed in case of liposomes stored at −20 °C (data not shown).
Figure 2.
Infrared spectrum of pure drug, acyclovir.
Figure 3.
Infrared spectrum of physical mixture of excipients.
Figure 4.
Infrared spectrum of physical mixture of excipients and drug, acyclovir.
Figure 5.
Infrared spectrum of fresh freeze-dried niosomes.
Figure 6.
Infrared spectrum of freeze-dried niosomes stored at 4 °C.
Figure 7.
Infrared spectrum of freeze-dried niosomes stored at −20 °C.
Figure 8.
Infrared spectrum of freeze-dried reconstituted niosomes stored at −20 °C.
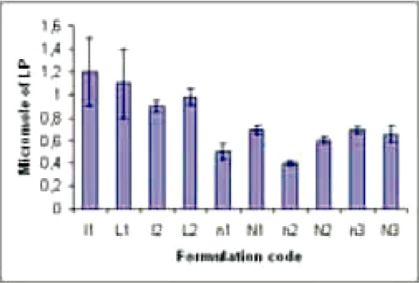
When the reconstituted lyophilized and the lyophilized formulations were compared, it was noticed that all the formulations stored at −20 °C and the lyophilized niosomes stored at 4 °C did not vary LP considerably as compared with the fresh preparations (Figure 9).
Figure 9.
Lipid peroxidation (in micro M) of different formulations. Data show mean± SD (n = 5). l1(reconstituted lyophilized) and l2(lyophilized) are the fresh liposomal and n1(reconstituted lyophilized), n2 (lyophilized), and n3(reconstituted lyophilized niosomes kept at 4 °C for 1 h) are the fresh (not stored except n3) niosomal formulations. Again L1 (reconstituted lyophilized liposomes stored at −20 °C), L2 (lyophilized liposomes stored at −20 °C) and N1 (reconstituted lyophilized niosomes stored at −20 °C), N2 (lyophilized niosomes stored at −20 °C) and N3 (reconstituted lyophilized niosomes stored at 4 °C) are those formulations stored at different temperatures as mentioned.
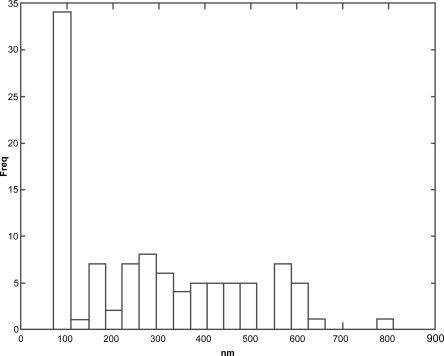
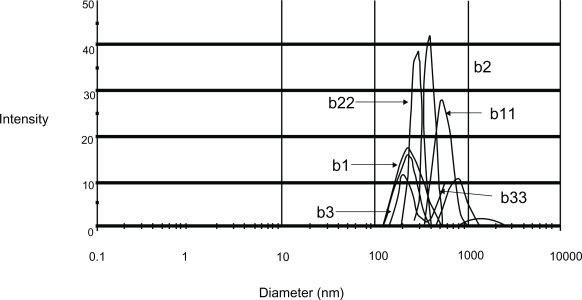
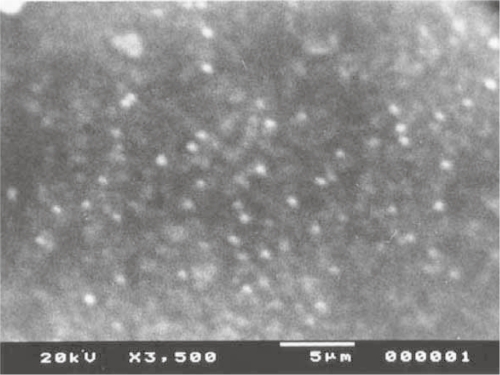
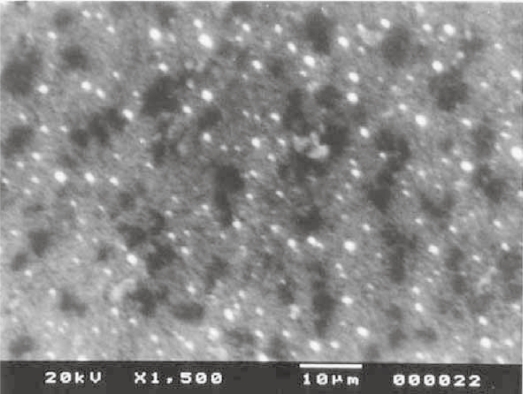
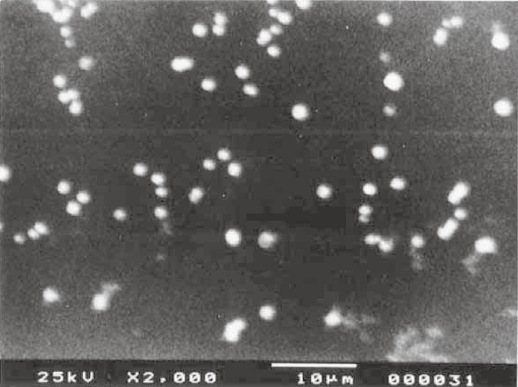
When vesicular size and frequency distribution were studied, it was seen that the maximum frequency distribution (about 35%) was of the vesicles having sizes of 100 nm and below (Figures 10 and 11). Sizes of remaining vesicles were within the size range up to 600 nm with an average frequency percentage of about 5%. The maximum percentage of vesicles was within a size range of 100 nm. Figures 12, 13, and 14 are SEM photographs of N1, L1, and L3 formulations. The vesicle sizes were less than 500 nm in diameter (in N1 and L1), whereas L3 had larger vesicles.
Figure 10.
Size distribution data of N1 formulation using image analyzer.
Figure 11.
Size distribution data of the formulation N1. Here “b1, b2, b3” and “b11, b22, b33” were the various size range between 100 nm and 1 μm.
Figure 12.
Photograph of nonionic vesicular dispersions using scanning electron microscopy.
Figure 13.
Photograph of distribution of liposome vesicles using scanning electron microscopy.
Figure 14.
Scanning electron microscopic photograph of liposome dispersion taking from supernatant of the formulation before centrifugation.
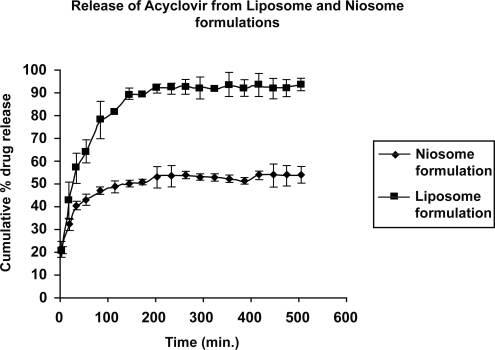
When the in vitro release of acyclovir was studied from the liposomes and the niosomes, it was found that about 90% release of drug was obtained from the liposomal formulation (L1) in 150 min whereas about 50% drug release was achieved in case of the niosomal formulation (N1) in about 200 min (Figure 15). About 95% release was monitored from N1 only after 7 h (data not shown).
Figure 15.
In vitro drug release profile from one niosomal (N1) and one liposomal (L1) formulations. Data shows mean ± SD (n = 3).
Discussion
Colloidal nanovesicular delivery systems of acyclovir were tried here. Various formulations were prepared with various combinations of cholesterol and soya L-α-lecithin in one case (formulations, L1, L2, and L3) and cholesterol and span 20 in the other case (N2, N3, and N4). In case of L3 formulation, PEG 400 was used along with the cholesterol and soya L-α-lecithin. In N1, soya L-α-lecithin, cholesterol and span 20 were used.
Before the development of the formulations, FTIR studies were conducted to determine the drug-excipient interactions. Drug-excipient interaction study is one of the most important parameters, which depicts much information including the stability of formulations, drug release from them and drug availability patterns (Mukherjee et al 2005). The results suggest that the weak bond formations took place between –OH group of Span 20 and/or cholesterol with a nitrogen atom present in the purine ring of the drug. Minor variation of the peak when compared with the cholesterol standard indicates that the interaction was a mild one. When spectrum of the various figures were compared in the wave number region between 3600 cm−1 and 3200 cm−1, it was found that there were some weak interactions between the drug and phospholipids. There may be a formation of hydrogen bonds or some other weak bonds due to van der Waal forces, dipole moment, etc amongst the functional groups N-H, C=O, -OH, -NH2 of the drug and phospholipid moieties. However, these interactions may favor the formation of vesicular shape, stabilizing the structures, and slower release of drug (Zhao and Feng 2005). Among the nonionic surfactant esters, Span 20 has C11 saturated alkyl chain which is believed to assist in the formation of smaller vesicles because of the smaller alkyl chain of Span 20 as compared with the other varieties (Hao et al 2002). This has once again been proved to be true since smaller vesicles were obtained in N1 and N2 (<500 nm). Again along with the same composition of N2 in N4 when PEG 400 was used, the vesicle size became larger (1000–1500 nm), although the drug loading did not improve. PEG in the formulation membranes generally occupies an additional surface hydration thickness. It thus produced the size of vesicles larger with a size distribution between 1000–1500 nm. However, PEG 400 was found to increase the stability of the formulations by preventing aggregation of vesicles. This was further confirmed by SEM study. Although the other physico-chemical data of this formulation were not shown as it had larger size. The vesicular size and frequency distribution demonstrated that about 35% of the vesicles having sizes within 100 nm and all the niosomes were in nano-dimension.
Keeping the amount of soya-lecithin fixed (100 mg), different formulations (L1, L2, and L3) were prepared by varying the amount of cholesterol. It was found that drug loading in L1 was about 8% (Table 1). When the amount of cholesterol was decreased in L2 as compared with L1, the drug loadings did not vary a lot. But there seemed to be an influence of cholesterol content on drug loading in case of the formulations with cholesterol and span 20. With the decreasing amount of cholesterol, drug loading substantially decreased in those formulations. One important observation was that with a fixed amount of cholesterol, addition of PEG reduced the drug loading too. The maximum drug loading was noticed in N1 among the prepared formulations.
When zeta potential was studied to understand the surface charges of the vesicles (Table 2), it was noticed that acyclovir reduces the highly negative surface charges to less negative values and the addition of nonionic surfactant made it positive. This may be due to the cationic charge present with the drug and the drug in presence of Span 20 might have interacted and neutralized the surface charges existed on the formulation surface. Extra positive charge generated on the formulation surface in the process was detected.
Nonlyophilized samples were found to be degraded and conglomerated along with the lyophilized samples without sucrose. But the samples with sucrose-lipid ratio 2.5:1, were found to maintain the physicochemical stabilities at −20 °C and in some cases (niosomes) at 4 °C. The formulation with sucrose to lipid ratio by weight 2.5:1 showed persistent result in terms of stability, preventing conglomeration and maintaining size and shape at the temperatures mentioned.
Lipid peroxidation causes the instability of the constituent phospholipid, which ultimately leads to the degradation of the formulations. When the reconstituted lyophilized and lyophilized formulations were compared, it was noticed that all the formulations stored at −20 °C and lyophilized niosomes stored at 4 °C did not vary LP considerably as compared with the fresh preparations. This indicates the stability of the formulations from lipid degradation also.
When the in vitro release of acyclovir was studied from the liposomes and the niosomes, it was found that about 90% release of drug was obtained from the first type formulation (L1) in 150 min whereas about 50% drug release was achieved in case of the niosomal formulation (N1) in about 200 min. The trend was similar in case of the other liposomal and niosomal formulations. This could be because niosomes contain span 20, which has been reported to interact with amino group (Hao et al 2002), which is present in acyclovir. Thus, this interaction might hold a substantial amount of drug (ie, about 50%) and released the drug molecules slowly from the niosomal formulations. About 95% drug release was monitored from N1 only after 7 h.
Conclusion
The results suggest that acyclovir released for a prolonged period of time from the niosomal formulation (N1). Since they had nano-dimensions, longer residence time in systemic circulation could help them reaching the target tissues. However, further studies regarding in vivo experiments are warranted.
Acknowledgments
The work was supported by the grant no. P-1/RS/152/03 of the SGSF Scheme, Government of West Bengal, India.
References
- Brown RJ, Mc Crary M, Tyring SK. Antiviral agents, nonantiviral drugs. J Am Acad Dermatol. 2002;47:581–99. doi: 10.1067/mjd.2002.121033. [DOI] [PubMed] [Google Scholar]
- Bundgared H, Jensen E, Falch E. Water soluble and solution stable and biolabile N-substituted (aminomethyl) benzoate ester prodrug of acyclovir. Pharm Res. 1991;8:1087–93. doi: 10.1023/a:1015837931256. [DOI] [PubMed] [Google Scholar]
- Chetoni P, Rossi S, Burgalassi S, et al. Comparison of liposome-encapsulated acyclovir with acyclovir ointment: ocular pharmacokinetics in rabbits. J Ocul Pharmacol Ther. 2004;20:169–77. doi: 10.1089/108076804773710849. [DOI] [PubMed] [Google Scholar]
- Chikhale PJ, Venkatraghavan V, Bodor NS. Improved delivery through biological membranes: Intradermal targeting of acyclovir using redox based chemical drug delivery systems. Drug Del. 1996;3:17–26. [Google Scholar]
- Fang JY, Hong CT, Chiu WT, et al. Effect of liposomes and niosomes on skin permeation of enoxacin. Int J Pharm. 2001;219:61–72. doi: 10.1016/s0378-5173(01)00627-5. [DOI] [PubMed] [Google Scholar]
- Freeman DJ, Sheth NV, Spruance SL. Failure of topical acyclovir ointment to penetrate human skin. J Antimicrob Agents Chemother. 1986;29:730–2. doi: 10.1128/aac.29.5.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallova J, Szalayova S. The effect of stobadine on the copper-induced peroxidation of egg yolk phosphatidylcholine in mutilayer liposomes. Gen Physiol Biophys. 2004;23:297–306. [PubMed] [Google Scholar]
- Hayden FG. Antimicrobial agents: Antiviral agents (non retroviral) In: Sweetman SC, editor. Martindale: the complete drug reference. 33rd ed. London: Pharmaceutical Pr; 1996. pp. 1313–21. [Google Scholar]
- Hao Y, Zhao F, Li N, et al. Studies on a high encapsulation of colchicines by a niosome system. Int J Pharm. 2002;4:73–80. doi: 10.1016/s0378-5173(02)00301-0. [DOI] [PubMed] [Google Scholar]
- Jain SK, Jain RK, Chourasia MK, et al. Design and development of multivesicular liposomal depot delivery system for controlled systemic delivery of acyclovir sodium. AAPS Pharm Sci Tech. 2005;6:E35–41. doi: 10.1208/pt060108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee B, Kanupriya, Mahapatra S, et al. Sorbitan monolaurate 20 as a potential skin permeation enhancer in transdermal patches. J Appl Res. 2005;5:96–108. [Google Scholar]
- Pavelic Z, Skalko-Basnet N, Filipovic-Grcic J, et al. Characterization and in vitro evaluation of bioasdhesive liposome gels for local therapy of vaginitis. J Control Rel. 2005;106:34–43. doi: 10.1016/j.ijpharm.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Shao Z, Park G, Krishnamoorthy R, et al. The physicochemical properties, plasma enzymatic hydrolysis and nasal absorption of acyclovir and its 2/-ester prodrug. Phar Res. 1994;11:237–42. doi: 10.1023/a:1018903407592. [DOI] [PubMed] [Google Scholar]
- Thummel KE, Shen DD. Design and optimization of dosage regimens: Pharmacokinetic data. In: Hardman JG, Limbird LE, editors. Goodman & Gilman’s the pharmacological basis of therapeutics. 10th ed. New York: McGraw-Hill Co Inc; 2001. 1925. [Google Scholar]
- Wagstaff AJ, Faulds D, Goa KL. Acyclovir: a reappraisal of its antiviral activity, pharmacokinetic properties and therapeutic efficacy. Drugs. 1994;47:153–205. doi: 10.2165/00003495-199447010-00009. [DOI] [PubMed] [Google Scholar]
- Zhang ZA, Anyarambhatla G, Ma L, et al. Development and characterization of a novel Cremophor® EL free liposome based Paclitaxel (LEP-ETU) formulation. Eur J Pharm Biopharm. 2005;54:177–87. doi: 10.1016/j.ejpb.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Zhao L, Feng S. Effects of lipid chain unsaturation and head group type on molecular interactions between paclitaxel and phospholipids within model biomembrane. J Coll Interfac Sci. 2005;285:326–35. doi: 10.1016/j.jcis.2004.11.032. [DOI] [PubMed] [Google Scholar]