Abstract
In this paper, we report the use of lead sulfide quantum dot (PbS QD) bioconjugates as near infrared (NIR) contrast agents for targeted molecular imaging with expanded emission wavelengths beyond 1000 nm. The red-shifted emission band, coupled with the small particle size, which will facilitate clearance, both afford PbS QDs unique properties for noninvasive, high resolution in vivo NIR imaging applications. We have performed imaging experiments at the molecular level using surface-modified PbS NIR QDs, together with our lab-built NIR imaging system. This novel instrumentation and fluorescent contrast agent have enabled us to study the relatively unexplored NIR biomedical imaging spectral region of 900–1200 nm. Preliminary experimental results indicate that PbS-QD/antibody bioconjugates are promising candidates for targeted NIR molecular imaging and future in vivo NIR tissue imaging applications.
Keywords: near infrared, quantum dots, bioconjugates, targeted imaging
Introduction
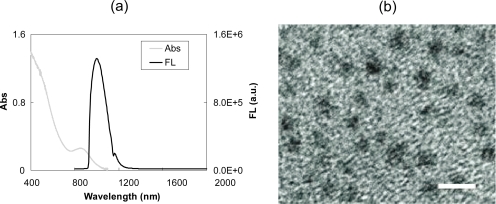
In this paper, we report the first attempt to use lead sulfide (PbS) near-infrared (NIR) quantum dot (QD) bioconjugates with imaging wavelengths beyond 1000 nm as molecular-specific contrast agents for targeted imaging applications. For example, the photoluminescent band of PbS QDs shown in Figure 1 peaks at around 1010 nm in phosphate buffered saline (PBS), and their excitation spectrum spans from the UV to NIR region (Zhu et al 2007). Before surface modification, the average size of the PbS QDs is ~4–5 nm, with no significant increase in size observed after surface coating of the particles (Zhu et al 2007). The longer emission wavelength, coupled with the small particle size, which facilitates clearance (Zimmer et al 2006), both afford PbS QDs unique properties for noninvasive, high resolution in vivo NIR imaging applications (Kim et al 2004; Chang et al 2005; Michalet et al 2005; Cai et al 2006). The “therapeutic window” of the NIR spectrum, 700–1200 nm, enables light to penetrate several centimeters deep into the tissue, thereby making PbS QDs ideal for in vivo deep-tissue imaging and diagnostics (Wilson and Jacques 1990; Weissleder 2001; Michalet et al 2005). While numerous groups have reported various fluorescent NIR imaging contrast agents, most of these agents possess the emission spectral range of 700–900 nm (Medintz et al 2005; Michalet et al 2005; Cai et al 2006). However, to date, the NIR spectral region of 900–1200 nm has not been sufficiently investigated for biomedical imaging applications, because of the lack of suitable contrast agents, as well as imaging systems, etc (Wilson and Jacques 1990; Michalet et al 2005).
Figure 1.
(a) Absorption and photoluminescent spectra of PbS QDs. (b)TEM images of PbS QDs after surface coating with a 10 nm scale bar. Adapted with permission from Zhu M-Q, Chang E, Sun J, et al. 2007. Surface modification and functionalization of semiconductor quantum dots through reactive coating of silanes in toulene. J Mater Chem, 17:800–5 Copyright © 2007.
Abbreviations: PbS, lead sulfide; QD, quantum dots; TEM, transmission electron microscopy.
To address these issues, the current study demonstrates how we have combined novel instrumentation with surface modified PbS QDs, allowing us to explore the NIR spectral region, as noted above. Specifically, we have carried out molecular level imaging experiments using our surface-modified PbS NIR QDs (Zhu et al 2007) together with our lab-built NIR imaging system. These experiments, discussed below, were conducted with a view toward future in vivo animal screening. To explain, a wide range of visible light (500–700 nm) has been used as excitation for PbS QDs imaging. The red-shifted excitation band of the NIR QDs, on the other hand, not only provides deeper penetration into tissue than the excitation band of visible QDs (Wu et al 2003; Kim et al 2004; Michalet et al 2005), but also avoids most of the absorption peaks of tissue fluorophores that usually give strong auto-fluorescence backgrounds (Richards-Kortum and Sevick-Muraca 1996). Hence, the NIR imaging method presented will potentially provide appealing results for both in vivo tissue screening and imaging applications.
Compared with traditional organic NIR fluorophores, the absorption and photoluminescent spectra of semiconductor nanocrystals can be easily tailored by changing the size and shape of the particles. This provides flexibility in many biological and clinical imaging applications (Hines and Scholes 2003; Chang et al 2005; Medintz et al 2005; Cai et al 2006). Furthermore, both the chemical and physical environmental stabilities of quantum dots, as well as their high quantum yield efficiency, make them highly desirable as fluorescent imaging contrast agents (Chan and Nie 1998; Medintz et al 2005; Michalet et al 2005). PbS QDs used in the following work are synthesized (Hines and Scholes 2003) and surface modified in accordance with methods presented in previous publications (Medintz et al 2005; Zhu et al 2007).
Materials and methods
The development of molecular-specific imaging techniques remains of great interest for diagnostic applications (Wu et al 2003; Michalet et al 2005). In particular, contrast agents that specifically target disease-associated biomarkers provide a convenient means for cancer screening and detection at the onset of disease, a period of time that is critical for both effective diagnosis and treatment (Chan and Nie 1998; Wu et al 2003; Loo et al 2005; Michalet et al 2005). Our study specifically addresses the expression of human epidermal growth factor receptor 2 (HER2), which is over-expressed in living human breast carcinoma cells (Wu et al 2003; Loo et al 2005). To image the expression of these receptors, two kinds of QD/antibody bioconjugates are prepared. Both anti-HER2 and anti-immunoglobulin G (anti-IgG) antibodies are conjugated to PbS QDs, as specific and nonspecific imaging contrast agents, respectively (Wu et al 2003; Loo et al 2005).
QD/antibody conjugation
The modified PbS QDs surfaces are terminated with carboxyl groups (Zhu et al 2007), and they are attached to antibodies through EDC and Sulfo-NHS cross-linkers (Wu et al 2003; Chang et al 2005). Briefly, ~0.2 nmol surface-modified PbS QDs suspended in 1x phosphate-buffered saline (PBS) (pH 7.2) are activated with EDC (1mg) and Sulfo-NHS (1mg) (Pierce, Rockford, IL) for 30 min by gentle stirring. Following the activation, excess EDC and Sulfo-NHS are removed from the reaction with desalt spin columns (Pierce, Rockford, IL). The filtered solution is evenly split into two aliquots, ~0.1 nmol-activated QDs per aliquot. Anti-HER2 (~0.22 nmol) (Labvision, Fremont, CA) and anti-IgG (~0.22 nmol) (Sigma-Aldrich, St. Louis, MO) antibodies are added to each aliquot, respectively, and allowed to react for 0.5–1 h, with gentle stirring. Polyethylene glycol-amine (PEG-NH2) and Tris buffer (pH 7.0) are then added at the end of the conjugation to react with excess carboxyl and activated ester groups. These QD/antibody conjugates are allowed to react overnight at 4 °C and then readied for targeted imaging (Wu et al 2003; Chang et al 2005; Loo et al 2005).
Cell culture
HER2 over-expressed SK-BR-3 human breast cancer cells are cultured in McCoy’s 5A modified medium (ATCC, Manassas, VA) with 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA) and 1% antibiotics. Cells are allowed to grow on Lab-Tek 4-chambered coverglass (Nalge Nunc Inc., Rochester, NY) for 24 h at 37 °C and 5% CO2 incubation before targeting.
QD-bioconjugates targeting of cells
Culture medium from each of the cell chambers is removed, and cells are rinsed with PBS (0.6 mL/chamber). 0.3 mL paraformaldehyde (3%–4%) in PBS is added to each chamber to fix the cells, and this reaction is allowed to stand for 15 min at room temperature (r.t.) (Wu et al 2003). The fixed cells are washed 2–3 times with PBS (0.6 mL/chamber) to eliminate the influence of the fixing solution. Following the washing, 2% BSA/PBS solution is added to three chambers, with 0.3 mL/chamber, to block the nonspecific binding sites on the cell surface (Wu et al 2003). This process takes 10–15 min at room temperature. After blocking, all 3 chambers of the cells are washed with PBS. PbS-QDs/anti-HER2 conjugates in PBS 0.4 mL (~50 nM or ~35 nM) are then added to one chamber of the blocked cells. Meanwhile, the same amount and concentration of PbS-QDs/anti-IgG conjugates in PBS are added to another chamber of blocked cells. 0.4 mL PBS is added to the third chamber as a control sample. The targeting process is allowed to react for 3–4 h at r.t. on a gently moving orbital shaking plate, avoiding light. All of the cells in the three chambers are washed with PBS after the targeting process to remove free QD/bioconjugates. These QD-targeted cell samples and the control sample are fixed and washed again. Finally, 0.4 mL PBS is added to each of the three chambers to preserve the moisture, and the samples are finally ready for imaging.
Cell imaging
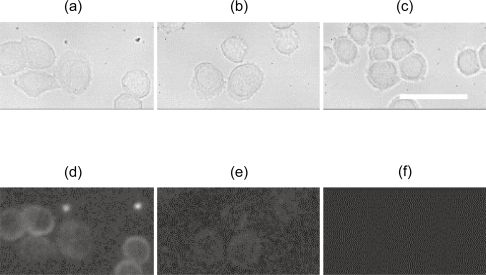
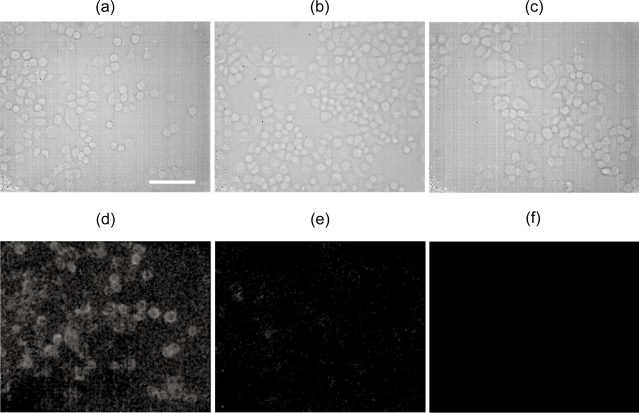
QD/bioconjugate-targeted cells and the control sample are imaged with a lab-built NIR imaging system which consists of a 2D OMA V InGaAs camera attached to an Axiovert 200 M inverted microscope. The liquid nitrogen-cooled 320 × 256 pixel 2D OMA V InGaAs focal plane array detector and the silicon multiplexer readout electronics give excellent response in the NIR region from 800 to 1700 nm (Princeton-Instruments 2005). Filtered light from a mercury arc-discharge lamp is used for excitation, with a bandpass range of 500–700 nm. An 800 nm longpass filter is used as the emission filter. An NIR-optimized dichroic mirror has been chosen to work with these filter sets (Chroma, Rockingham, VT). Photoluminescent images and their corresponding bright field images of the QD/bioconjugate-targeted cells, together with the control sample (Figure 2 and 3), are all examined with this imaging system. All of the three luminescent images in Figure 2 are taken and processed under the same experimental conditions, and the same background has been subtracted during image correction (Princeton-Instruments 2005). Following the same procedure, Figure 3 is obtained.
Figure 2.
Bright field (a–c) and photoluminescent (d–f) images of SK-BR-3 cells targeted with PbS-QD/anti-HER2 (~50 nM) (a, d) and PbS-QD/anti-IgG (~50 nM) (b, e) bioconjugates. SK-BR-3 cells incubated with PBS are used as control (c, f). All images are in the same scale with a 50 μm scale bar (40x).
Abbreviations: IgG, immunoglobulin G; PbS, lead sulfide; PBS, phosphate-buffered saline; QD, quantum dots.
Figure 3.
Bright field (a–c) and photoluminescent (d–f) images of SK-BR-3 cells targeted with PbS-QD/anti-HER2 (~35 nM) (a, d) and PbS-QD/anti-IgG (~35 nM) (b, e) bioconjugates. SK-BR-3 cells incubated with PBS are used as control (c, f). All images are in the same scale with a 100 μm scale bar (20x).
Abbreviations: IgG, immunoglobulin G; PbS, lead sulfide; PBS, phosphate-buffered saline; QD, quantum dots.
Toxicity of QD-bioconjugates to SK-BR-3 cells
The cytotoxicity introduced by our surface-modified PbS-QD/antibody conjugates in SK-BR-3 cells was studied with a LIVE/DEAD viability assay (Invitrogen). The same amount of SK-BR-3 cells are allowed to grow on Lab-Tek chambered coverglass (4 chambers) for 46 h at 37 °C and 5% CO2 incubation and thoroughly rinsed with D-PBS (at least 3 times) to eliminate dead cells. The washed cells are incubated with testing particles over a period of 4 h at 37 °C before viability stains are applied. The stained cells are examined under fluorescence and bright field microscopy with a Zeiss Axiovert 200 M inverted microscope equipped with proper filter sets.
Results and discussion
Qualitatively, as shown in Figure 2 (taken with 40x objective) and Figure 3 (taken with 20x objective), PbS-QD/anti-HER2-targeted cells have the brightest luminescent images with the best contrast when compared with either cells targeted by PbS-QD/anti-IgG conjugates or control cells incubated with PBS. These results agree with previous reports (Chan and Nie 1998; Wu et al 2003; Loo et al 2005). Since SK-BR-3 human breast cancer cells are HER2 over-expressed, they demonstrate more favorable binding efficiency with the anti-HER2-conjugated QDs than with anti-IgG-conjugated QDs (Wu et al 2003; Loo et al 2005). Therefore, the significantly increased luminescent signal and image contrast in the NIR range may be particularly ascribed to the PbS QDs labeling of the HER2 receptors (Chan and Nie 1998; Wu et al 2003; Loo et al 2005). It is worthwhile noting that both the luminescent intensities and contrast of PbS-QD/anti-IgG-targeted cells are much lower than those of the PbS-QD/anti-HER2-labeled cells. These same measurements are, however, still higher than those of the control sample, which has no detectable luminescent signal in the NIR (>800 nm) region (Figure 2 and 3). The difference between the QDs/anti-IgG-labeled cells and the control cells likely results from the nonspecific binding of anti-IgG antibodies to the cell surfaces (Wu et al 2003; Loo et al 2005). It is also important to consider that improved image quality is expected after further optimization of the NIR imaging system.
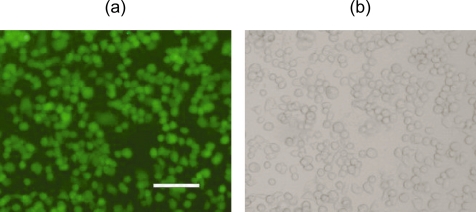
Aside from the development of effective contrast agents, still another important issue for in vivo imaging applications of quantum dots derives from their toxicity, which varies with different surface coatings (Medintz et al 2005; Delehanty et al 2006; Zhu et al 2007). Preliminary results show that, within an acute incubation range of 4 h, cells incubated with anti-HER2-conjugated PbS QDs (~35 nM) (Figure 4), anti-IgG-conjugated PbS QDs (~35 nM) and unconjugated PbS QDs (~35 nM), respectively, show no significant statistical difference in viability when compared with the control cells that are incubated with D-PBS. Notwithstanding these results, further tests are in progress to fully assess the toxicity of these QD bioconjugates over a longer period of time.
Figure 4.
LIVE/DEAD test of SK-BR-3 cells incubated with PbS-QD/anti-HER2 bioconjugates. Calcein fluorescence (a) and bright field (b) images with a 100 μm scale bar (10x).
Abbreviations: PbS, lead sulfide; QD, quantum dots.
Moreover, the presented technique has unique advantages for future in vivo pre-cancer/cancer screening and imaging implementations particularly for the large cohort of breast cancer patients who are HER2 positive (Ross and Fletcher 1998; Horton 2001). To explain, both the red-shifted excitation and emission bands of these PbS NIR QDs allow for deep-tissue diagnosis. The relatively small size of these particles is believed to facilitate body clearance which is crucial for in vivo implementations (Zimmer et al 2006). Additionally, the general tunability of the emission peak of these nanoparticles offers flexibility in clinical imaging applications (Hines and Scholes 2003; Medintz et al 2005; Cai et al 2006). Although the relatively high cost of the current lab-built NIR imaging system might be one of the disadvantages of this technique, the total equipment expenses are expected to decrease dramatically with breakthroughs in sensor fabrication technologies and system optimization.
Conclusions
In conclusion, the main objective of this work is to study PbS QD bioconjugates with expanded emission wavelengths beyond 1000 nm as NIR contrast agents for targeted molecular imaging. We are able to study the relatively unexplored NIR biomedical imaging region of 900–1200 nm with the help of PbS QDs and the latest lab-built NIR imaging system. The proof-of-concept imaging results and the preliminary cytotoxicity tests all indicate that PbS-QD/anti-HER2 bioconjugates are promising candidates for targeted NIR molecular imaging with SK-BR-3 breast cancer cells and future in vivo NIR tissue imaging applications.
Acknowledgments
The authors would like to acknowledge the financial support for this project provided by NSF (EEC-0118007) and the Beckman Foundation. We also want to thank Mr. David Martin for his editing assistance.
References
- Cai W, Shin D-W, Chen K, et al. Peptide-labeled near-infrared quantum dots for imaging tumor vasculature in living subjects. Nano Lett. 2006;6:669–76. doi: 10.1021/nl052405t. [DOI] [PubMed] [Google Scholar]
- Chan WCW, Nie S. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science. 1998;281:2016–18. doi: 10.1126/science.281.5385.2016. [DOI] [PubMed] [Google Scholar]
- Chang E, Miller JS, Sun J, et al. Protease-activated quantum dot probes. Biochem Biophys Res Commun. 2005;334:1317–21. doi: 10.1016/j.bbrc.2005.07.028. [DOI] [PubMed] [Google Scholar]
- Delehanty JB, Medintz IL, Pons T, et al. Self-assembled quantum dot-peptide bioconjugates for selective intracellular delivery. Bioconjugate Chem. 2006;17:920–7. doi: 10.1021/bc060044i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines MA, Scholes GD. Colloidal PbS nanocrystals with size-tunable near-infrared emission: observation of post-synthesis self-narrowing of the particle size distribution. Adv Mater. 2003;15:1844–9. [Google Scholar]
- Horton J. HER2 and Trastuzumab in breast cancer. Cancer Control. 2001;8:103–10. doi: 10.1177/107327480100800113. [DOI] [PubMed] [Google Scholar]
- Kim S, Lim YT, Soltesz EG, et al. Near-infrared fluorescent type II quantum dots for sentinel lymph node mapping. Nature Biotechnol. 2004;22:93–7. doi: 10.1038/nbt920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo C, Hirsch L, Lee M-H, et al. Gold nanoshell bioconjugates for molecular imaging in living cells. Opt Lett. 2005;30:1012–14. doi: 10.1364/ol.30.001012. [DOI] [PubMed] [Google Scholar]
- Medintz IL, Uyeda HT, Goldman ER, et al. Quantum dot bioconjugates for imaging, labeling and sensing. Nature Mater. 2005;4:435–46. doi: 10.1038/nmat1390. [DOI] [PubMed] [Google Scholar]
- Michalet X, Pinaud FF, Bentolila LA, et al. Quantum dots for live cells, in vivo imaging, and diagnostics. Science. 2005;307:538–44. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Princeton-Instruments OMA V InGaAs system user manual. Richards-Kortum R, Sevick-Muraca E. 1996. Quantitative optical spectroscopy for tissue diagnosis. Annu Rev Phys Chem. 2005;47:555–606. doi: 10.1146/annurev.physchem.47.1.555. [DOI] [PubMed] [Google Scholar]
- Ross JS, Fletcher JA. The Her2/neu oncogene in breast cancer: prognostic factor, predictive factor, and target for therapy. Oncologist. 1998;3:237–52. [PubMed] [Google Scholar]
- Weissleder R. A clearer vision for in vivo imaging. Nature Biotechnol. 2001;19:316–17. doi: 10.1038/86684. [DOI] [PubMed] [Google Scholar]
- Wilson BC, Jacques SL. Optical reflectance and transmittance of tissue: principles and application. IEEE J Quantum Electron. 1990;26:2186–99. [Google Scholar]
- Wu X, Liu H, Liu J, et al. Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots. Nat Biotechnol. 2003;21:41–6. doi: 10.1038/nbt764. [DOI] [PubMed] [Google Scholar]
- Zhu M-Q, Chang E, Sun J, et al. Surface modification and functionalization of semiconductor quantum dots through reactive coating of silanes in toluene. J Mater Chem. 2007;17:800–5. [Google Scholar]
- Zimmer JP, Kim S-W, Ohnishi S, et al. Size series of small indium arsenide-zinc selenide core-shell nanocrystals and their application to in vivo imaging. J Am Chem Soc. 2006;128:2526–7. doi: 10.1021/ja0579816. [DOI] [PMC free article] [PubMed] [Google Scholar]