Abstract
Superparamagnetic iron oxide nanoparticles (SPIONs) bound directly to luteinizing hormone releasing hormone (LHRH) have shown high efficiency for intracellular uptake to breast cancer cells, MDA-MB-435S.luc. We demonstrate in this communication that inclusion of a small spacer molecule such as glutaric acid (Glu) in between SPION and LHRH increases further receptor mediated intracellular uptake. LHRH-bound SPIONs with and without the spacer molecule were nontoxic.
Keywords: Glutaric acid, luteinizing hormone releasing hormone, superparamagnetic iron oxide nanoparticles, MDA-MB-435S.luc., intracellular uptake
Introduction
There is a growing interest in developing superparamagnetic nanoparticle-based materials that are biocompatible, biodegradable, and efficient in intracellular uptake to cancer cells for applications in cancer therapy and diagnosis (Majumdar et al 1989; Weissleder et al 1990; Josephson et al 1998; Huh et al 2005; Song et al 2005; Alexiou et al 2006; Zhou et al 2006). While there is a continuous progress in this direction in terms of excellent experimental and theoretical work, there are several challenges that need to be addressed (Soppimath et al 2001; Sapra and Allen 2003; Gabizon et al 2004; Matsumura et al 2004; Yoo and Park 2004). One of the most important challenges is to prevent uptake of nanoparticles by the reticuloendothelial system (RES) and enhance their circulation times so that they can navigate through irregularities of tumoral vasculature leading to efficient intracellular uptake preferably via endocytosis. The efficacy of a nanoparticulate system is determined by their ability for intracellular uptake in in vitro studies. Experimental studies, mathematical models, and computer simulations have already demonstrated that rapid intracellular uptake can be improved through suitable surface modifications, especially coating of superparamagnetic iron oxide nanoparticles (SPIONs) with hydrophilic polymers and surfactants (Jeon et al 1991; Lasic et al 1991; Torchlin et al 1994; Alexiou et al 2006). Several studies have demonstrated that polyethylene glycol (PEG) coatings, due to their biocompatibility, resistance to proteins, and lack of antigenicity, are the most widely utilized for increasing circulation times and enhancing intracellular uptake of SPIONs in cancer cells (Zhang and Zhang 2005). Dextran-coated SPIONs also have been demonstrated to improve intracellular uptake (Hogemann et al 2000; Berry et al 2004; Funovics et al 2004). In addition to simple coatings such as PEG and dextran, there are also investigations related to more complex coatings involving multi-step synthetic procedures to prevent RES uptake and improved cellular uptake (Kohler et al 2005, 2006; Neuberger et al 2005; Woo and Hong 2005; Koeseoglu 2006). However, to improve production and in vivo applications, it is important to avoid complicated and lengthy chemical procedures for SPION coatings. We recently demonstrated that ligands such as luteinizing hormone releasing hormone (LHRH) act as targeting agents for breast cancer cells that express receptors for LHRH (Leuschner et al 2003). We have also demonstrated that intracellular accumulation of LHRH-bound SPIONs (without any PEG or dextran coating on SPIONs) in MDA-MB-435S. luc cells in vitro was 12-fold higher than the corresponding free SPIONs uptake (Leuschner et al 2005, 2006; Zhou et al 2006). The results are not very surprising as LHRH is a decapeptide and can function as coating (in addition to being a targeting agent) thus preventing macrophage recognition, RES uptake, enhancing circulation time, and facilitating cellular uptake.
In addition to demonstrating the concept of utilizing a targeting agent to act as a coating for improved intracellular uptake, we are also currently investigating the introduction of a small spacer between SPION and LHRH to engineer SPION conjugates for further improvements in intracellular uptake. In this study, we have synthesized SPIONs and covalently bound glutaric acid (Glu) to SPIONs followed by LHRH using carbodiimide chemistry. We present below details of synthesis, characterization, intracellular uptake, and cytotoxicity of newly engineered SPIONs and compare the data with that of free SPIONs and SPION–LHRH.
Experimental
Iron II chloride (FeCl2.4H2O) 98%, iron III chloride (FeCl3) 97%, ammonium hydroxide (NH4OH) 29.05%, 1-Ethyl-3-(3-Dimethylaminopropyl)carbodiimide·hydrochloride (EDC), and Glu were purchased from Sigma Aldrich (St. Louis, MO, USA). Air-free nanopure water was made in the lab by refluxing nanopure water, made with a Barnstead Nano-Pure Water System (Barnstead International, Dubuque, IA, USA), under inert atmosphere. During the synthesis of the nanoparticles a 750D Sonicator (VWR International, Leuven, Belgium) was used, as well as a 1160A PolyScience Chiller (VWR International).
The SPIONs used in this study were prepared using the procedure similar to the one reported by Kumar and colleagues (2004). For the covalent attachment of Glu to the SPIONs, 60 mg of magnetite nanoparticles were dispersed 6 ml of water using a sonication bath at room temperature for fifteen minutes. A solution of 42 mg carbodiimide and 1.5 ml water was added. The mixture was sonicated for 10 more minutes and then cooled to 4 °C in a chiller. A solution of 3.7 mg Glu in 1.5 ml of water was added, and the reaction temperature was maintained at 4 °C for 2 h. The particles were then allowed to settle on a permanent magnet. The supernatant was removed and the particles were washed three times with water, twice with ethanol, and dried under nitrogen.
The above procedure was followed for the functionalization of the glutaric acid-bound SPIONs with LHRH. Substituting only 3.7 mg of LHRH instead of Glu and 60 mg of Glu–SPIONs instead of plain magnetite.
The supernatant of the SPION–Glu particles and of the SPION–Glu–LHRH particles was analyzed by high-performance liquid chromatography (HPLC) for the presence of unbound LHRH or Glu, according to which binding had taken place. The size and morphology of the magnetic nanoparticles were observed using a JOEL 100X (JEOL Ltd.,1–2, Musashino 3-chome Akishima Tokyo, Japan) transmission electron microscopy (TEM) at 80kV. The mean diameter was estimated from 300 particles with the aid of MetaVue software (Molecular Devices Corporation, Downingtown, PA, USA). Binding of Glu and LHRH was confirmed by Fourier transform infrared (FTIR) spectra obtained using a Thermo Nicolet Nexus 6/870 FTIR (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Samples for FTIR analysis were prepared using KBr pellets. The magnetic properties of the SPION–Glu–LHRH particles were studied with a Quantum Design MPMS-5S SQUID magnetometer (Quantum Design, San Diego, CA, USA).
MDA-MB-435S human breast cancer cells were obtained from the American Type Culture Collection (Rockville, MD) and cultured as described previously (Leuschner et al 2003). MDA-MB-435S cells were transfected with exogenous DNA by lipofection using the plasmid pRC/CMV-luc containing the Photinus pyralis luciferase gene and an antibiotic resistance gene under transcription control of the cytomegalovirus promoter (Rubio et al 1998, 2000). MDA-MB-435S.luc cells (60,000 cells/well) were grown in 24 well plates and incubated with LHRH–SPION, LHRH–Glu–SPION or SPION (Fe 1 mg/ml) at 37 °C for 3 h in the presence and absence of LHRH (50 μM). At the end of the incubations the cells were detached from the culture plates, washed, centrifuged and the cell pellets were resuspended in HCl (1 M). Iron contents were determined spectrophotometrically using a Prussian Blue reaction kit.
Toxicity studies were conducted in cultures of MDA-MB-435S.luc cells using mitochondrial dependent formazan formation (MTT) assay as described by Berridge and Tan (1993).
Results and discussion
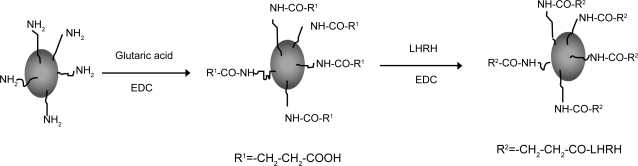
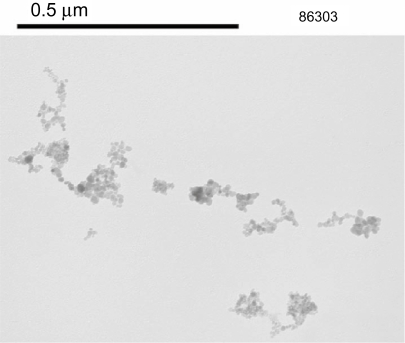
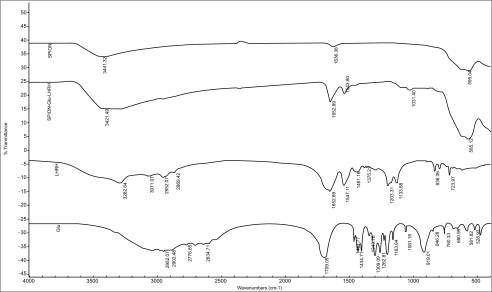
The binding of LHRH and Glu to the SPION particles is facilitated by the presence of amine groups on the surface of the SPIONs (Kumar et al 2004; Shieh et al 2005). The synthetic strategy adopted is shown in Figure 1. Analysis of the supernatant after the binding process by HPLC is one way to determine that binding has taken place. HPLC analysis did not reveal the presence of any free Glu or LHRH, confirming that the biomolecules have been bound to the SPIONs. The size and morphology of SPION–Glu–LHRH nanoparticles was investigated using TEM. A TEM micrograph of the nanoparticles is shown in Figure 2. The particles are roughly spherical in shape with a mean diameter of 9.12 nm. FTIR was also utilized to characterize SPION–Glu–LHRH nanoparticles. The particles exhibited typical amide bond signatures at around 1652 cm–1 and the absence of carboxylic carbonyl stretching of glutaric acid at 1709 cm–1 (Figure 3).
Figure 1.
Schematic representation of step-wise binding of glutaric acid and LHRH to SPIONs.
Abbreviations: EDC, N-(3-Dimethylaminopropyl)-N’-ethylcarbodiimide hydrochloride; LHRH, luteinizing hormone releasing hormone; SPIONs, superparamagnetic iron oxide nanoparticles.
Figure 2.
A TEM micrograph of SPION–Glu–LHRH nanoparticles.
Abbreviations: Glu, glutaric acid; LHRH, luteinizing hormone releasing hormone; SPIONs, superparamagnetic iron oxide nanoparticles; TEM, transmission electron microscopy.
Figure 3.
FTIR spectra of SPION, SPION–Glu, and SPION–Glu–LHRH.
Abbreviations: FTIR, Fourier transform infrared; Glu, glutaric acid; LHRH, luteinizing hormone releasing hormone; SPIONs, superparamagnetic iron oxide nanoparticles.
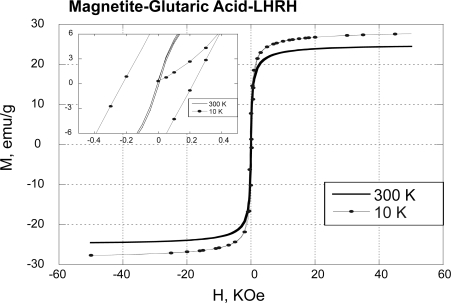
The SPION–Glu–LHRH nanoparticles are superparamagnetic as demonstrated by weak hysteresis in the plots of magnetization versus magnetic field (M-H loop) at 10K (Figure 4). Compared with free SPIONs, SPION–Glu–LHRH nanoparticles showed lower saturation magnetization (Ms = 25 Vs 72.1 emu/g). The reduction in Ms on binding Glu–LHRH to the SPIONS is not very surprising due to quenching of magnetic moment through electron exchange between organic molecules and surface atoms (Vanleeuwen et al 1994).
Figure 4.
Magnetic properties of LHRH-Glu-SPIONs.
Abbreviations: Glu, glutaric acid; LHRH, luteinizing hormone releasing hormone; SPIONs, superparamagnetic iron oxide nanoparticles.
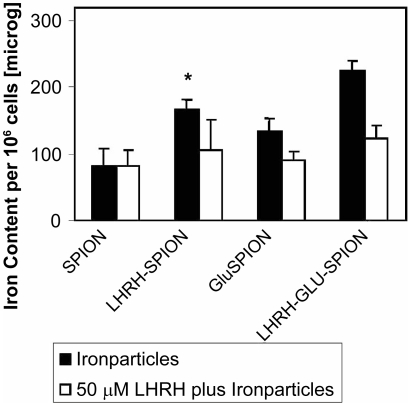
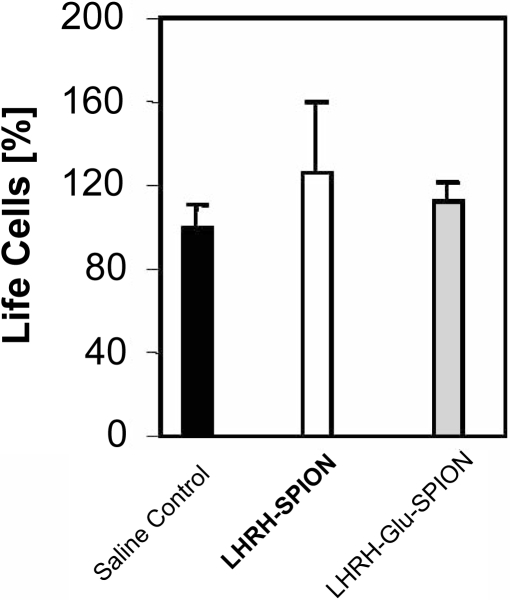
In vitro analysis of the potency for cellular uptake was carried out using breast cancer cells, MDA-MB-435S.luc that expresses LHRH-receptors. The results are shown in Figure 5. The results demonstrate that LHRH–SPION and LHRH–Glu–SPION specifically and more effectively accumulate in the breast cancer cells, reaching 82.3 ± 25 pg/cell for SPIONs, 133 ± 17 pg/cell for Glu–SPIONs (p < 0.003 vs SPION), 165 ± 16 pg/cell for LHRH–SPIONs (p < 0.003 vs SPION) and 223 ± 16 pg/cell with LHRH–Glu–SPION (p < 0.05 vs Glu–SPION, p < 0.002 vs LHRH–SPION). This accumulation is significantly reduced in the presence of the same ligand LHRH for LHRH–SPION and LHRH–Glu–SPION (106 ± 26 pg/cell, 123 ± 25 pg/cell, respectively, p < 0.001). This observation suggests that iron uptake was driven through receptor mediated endocytosis. The introduction of a spacer significantly increased the intracellular iron uptake from 165 to 223.3 pg/cell, p < 0.001. Toxicity studies over a period of 5 days revealed that none of the three iron oxide conjugates were toxic to human breast cancer cells (Figure 6).
Figure 5.
Intracellular uptake of LHRH–Glu–SPIONs.
Abbreviations: Glu, glutaric acid; LHRH, luteinizing hormone releasing hormone; SPIONs, superparamagnetic iron oxide nanoparticles.
Figure 6.
Toxicity of LHRH–Glu–SPIONs.
Abbreviations: Glu, glutaric acid; LHRH, luteinizing hormone releasing hormone; SPIONs, superparamagnetic iron oxide nanoparticles.
In summary, the work reported indicates that incorporation of a small spacer molecule such as Glu between LHRH and SPION shows significant enhancement in intracellular uptake without causing toxicity to the breast cancer cells.
References
- Alexiou C, Schmid RJ, Jurgons R, et al. Targeting cancer cells: magnetic nanoparticles as drug carriers. Eur Biophys J. 2006;35:446–50. doi: 10.1007/s00249-006-0042-1. [DOI] [PubMed] [Google Scholar]
- Berridge MV, Tan AS. Characterization of the cellular reduction of 3–(4,5-dimethylthiazol-2-yl)–2,5-diphenyltetrazolium bromide (MTT): subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Arch Biochem Biophys. 1993;303:474–82. doi: 10.1006/abbi.1993.1311. [DOI] [PubMed] [Google Scholar]
- Berry CC, Charles S, Wells S, et al. The influence of transferrin stabilised magnetic nanoparticles on human dermal fibroblasts in culture. Int J Pharm. 2004;269:211–25. doi: 10.1016/j.ijpharm.2003.09.042. [DOI] [PubMed] [Google Scholar]
- Funovics MA, Kapeller B, Hoeller C, et al. MR imaging of the her2/neu and 9.2.27 tumor antigens using immunospecific contrast agents. Magn Reson Imaging. 2004;22:843. doi: 10.1016/j.mri.2004.01.050. [DOI] [PubMed] [Google Scholar]
- Gabizon A, Shmeeda H, Horowitz AT, et al. Tumor cell targeting of liposome-entrapped drugs with phospholipid-anchored folic acid-PEG conjugates. Adv Drug Deliv Rev. 2004;56:1177–92. doi: 10.1016/j.addr.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Hogemann D, Josephson L, Weissleder R, et al. Improvement of MRI probes to allow efficient detection of gene expression. Bioconjugate Chem. 2000;11:941–6. doi: 10.1021/bc000079x. [DOI] [PubMed] [Google Scholar]
- Huh YM, Jun YW, Song HT, et al. In vivo magnetic resonance detection of cancer by using multifunctional magnetic nanocrystals. J Am Chem Soc. 2005;127:12387–91. doi: 10.1021/ja052337c. [DOI] [PubMed] [Google Scholar]
- Jeon SI, Lee JH, Andrade JD, et al. Protein-surface interactions in the presence of polyethylene oxide, I: Simplified theory. J Colloid Interface Sci. 1991;142:149–58. [Google Scholar]
- Josephson L, Lewis J, Jacobs P, et al. The effects of iron-oxides on proton relaxivity. Magn Reson Imaging. 1998;6:647–53. doi: 10.1016/0730-725x(88)90088-4. [DOI] [PubMed] [Google Scholar]
- Koeseoglu Y. Effect of surfactant coating on magnetic properties of Fe3O4 nanoparticles: ESR study. J Magn Magn Mater. 2006;300:e327–e330. [Google Scholar]
- Kohler N, Sun C, Fichtenholtz A, et al. Methotrexate-immobilized poly(ethylene glycol) magnetic nanoparticles for MR imaging and drug delivery. Small. 2006;2:785–92. doi: 10.1002/smll.200600009. [DOI] [PubMed] [Google Scholar]
- Kohler N, Sun C, Wang J, et al. Methotrexate-modified superparamagnetic nanoparticles and their intracellular uptake into human cancer cells. Langmuir. 2005;21:8858–64. doi: 10.1021/la0503451. [DOI] [PubMed] [Google Scholar]
- Kumar C, Leuschner C, Doomes EE, et al. Efficacy of lytic peptide bound magnetite nanoparticles in destroying breast cancer cells. J Nanosci Nanotechnol. 2004;4:245–9. [PubMed] [Google Scholar]
- Lasic DD, Martin FJ, Gabizon A, et al. Sterically stabilized liposomes: A hypothesis on the molecular origin of the extended circulation times. Biochim Biophys Acta. 1991;1070:187–92. doi: 10.1016/0005-2736(91)90162-2. [DOI] [PubMed] [Google Scholar]
- Leuschner C, Enright F, Gawronska B, et al. Membrane disrupting lytic peptide conjugates destroy hormone dependent and independent breast cancer cells in vitro and in vivo. Breast Cancer Res Treat. 2003;78:17–27. doi: 10.1023/a:1022169525521. [DOI] [PubMed] [Google Scholar]
- Leuschner C, Kumar C, Hansel W, et al. Targeting breast cancer cells and their metastases through luteinizing hormone releasing hormone (LHRH) receptors using magnetic nanoparticles. J Biomed Nanotech. 2005;1:229–33. [Google Scholar]
- Leuschner C, Kumar C, Zhou J, et al. LHRH-conjugated magnetic iron oxide nanoparticles for detection of breast cancer metastases. Breast Cancer Res Treat. 2006;99:163–76. doi: 10.1007/s10549-006-9199-7. [DOI] [PubMed] [Google Scholar]
- Majumdar S, Zoghbi S, Pope CF, et al. A quantitative study of relaxation rate enhancement produced by iron-oxide particles in poly-acrylamide gels and tissue. Magn Reson Med. 1989;9:185–202. doi: 10.1002/mrm.1910090205. [DOI] [PubMed] [Google Scholar]
- Matsumura Y, Gotoh M, Muro K, et al. Phase I and pharmacokinetic study of MCC-465, a doxorubicin (DXR) encapsulated in PEG immunoliposome, in patients with metastatic stomach cancer. Ann Oncol. 2004;15:517–25. doi: 10.1093/annonc/mdh092. [DOI] [PubMed] [Google Scholar]
- Neuberger T, Schoepf B, Hofmann H, et al. Superparamagnetic nanoparticles for biomedical applications: possibilities and limitations of a new drug delivery system. J Magn Magn Mater. 2005;293:483–96. [Google Scholar]
- Rubio N, Villacampa MM, Blanco J. Traffic to lymph nodes of PC-3 tumor cells in nude mice visualized using the luciferase gene as a tumor cell marker. Lab Invest. 1998;78:1315–25. [PubMed] [Google Scholar]
- Rubio N, Villacampa MM, Hilali NE, et al. Metastatic burden in nude mice organs measured using prostate tumor PC-3 cells expressing the luciferase gene as a quantifiable tumor cell marker. Prostate. 2000;44:133–43. doi: 10.1002/1097-0045(20000701)44:2<133::aid-pros6>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Sapra P, Allen TM. Ligand-targeted liposomal anticancer drugs. Prog Lipid Res. 2003;42:439–62. doi: 10.1016/s0163-7827(03)00032-8. [DOI] [PubMed] [Google Scholar]
- Shieh DB, Cheng FF, Su CH, et al. Aqueous dispersions of magnetite nanoparticles with NH3+ surfaces for magnetic manipulations of bio-molecules and MRI contrast agents. Biomaterials. 2005;26:7183–7191. doi: 10.1016/j.biomaterials.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Song HT, Choi JS, Huh YM, et al. Surface modulation of magnetic nanocrystals in the development of highly efficient magnetic resonance probes for intracellular labeling. J Am Chem. 2005;127:9992–3. doi: 10.1021/ja051833y. [DOI] [PubMed] [Google Scholar]
- Soppimath KS, Aminabhavi TM, Kulkarni AR, et al. Biodegradable poly-meric nanoparticles as drug delivery devices. J Control Release. 2001;70:1–20. doi: 10.1016/s0168-3659(00)00339-4. [DOI] [PubMed] [Google Scholar]
- Torchilin VP, Omelyanenko VG, Papisov MI, et al. Poly(ethylene glycol) on the liposome surface: on the mechanism of polymer-coated liposome longevity. Biochim Biophys Acta. 1994;1195:11–20. doi: 10.1016/0005-2736(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Vanleeuwen DA, Vanruitenbeek JM, Dejongh LJ, et al. Quenching of magnetic moments by ligand-metal interactions in nanosized magnetic metal clusters. Phys Rev Lett. 1994;73:1432–5. doi: 10.1103/PhysRevLett.73.1432. [DOI] [PubMed] [Google Scholar]
- Weissleder R, Elizondo G, Wittenberg J, et al. Ultrasmall superparamagnetic iron-oxide - characterization of a new class of contrast agents for MR imaging. Radiology. 1990;175:489–93. doi: 10.1148/radiology.175.2.2326474. [DOI] [PubMed] [Google Scholar]
- Woo K, Hong J. Surface modification of hydrophobic iron oxide nanoparticles for clinical applications. IEEE Transact Magn. 2005;41:4137–39. [Google Scholar]
- Yoo HS, Park TG. Folate-receptor-targeted delivery of doxorubicin nano-aggregates stabilized by doxorubicin-PEG-folate conjugate. J Control Release. 2004;100:247–56. doi: 10.1016/j.jconrel.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang J. Surface modification of monodisperse magnetite nanoparticles for improved intracellular uptake to breast cancer cells. J Colloid Interface Sci. 2005;283:352–7. doi: 10.1016/j.jcis.2004.09.042. [DOI] [PubMed] [Google Scholar]
- Zhou J, Leuschner C, Kumar C, et al. Sub-cellular accumulation of magnetic nanoparticles in breast tumors and metastases. Biomaterials. 2006;27:2001–8. doi: 10.1016/j.biomaterials.2005.10.013. [DOI] [PubMed] [Google Scholar]