Abstract
The current treatment for coronary restenosis following balloon angioplasty involves the use of a mechanical or a drug-eluting stent. Despite the high usage of commercially-available drug-eluting stents in the cardiac field, there are a number of limitations. This review will present the background of restenosis, go briefly into the molecular and cellular mechanisms of restenosis, the use of mechanical stents in coronary restenosis, and will provide an overview of the drugs and genes tested to treat restenosis. The primary focus of this article is to present a comprehensive overview on the use of nanoparticulate delivery systems in the treatment of restenosis both in-vitro and in-vivo. Nanocarriers have been tested in a variety of animal models and in human clinical trials with favorable results. Polymer-based nanoparticles, liposomes, and micelles will be discussed, in addition to the findings presented in the field of cardiovascular drug targeting. Nanocarrier-based delivery presents a viable alternative to the current stent based therapies.
Keywords: coronary restenosis, stents, nanoparticulate carriers, liposomes, polymeric nanoparticles, micelles, drug delivery, gene therapy
Introduction
In 2004, cardiovascular diseases were the number one cause of death in the United States according to the National Center for Health Statistics (Lethbridge-Çejku et al 2006). Over 24 million people in the United States have been diagnosed with some type of heart disease. (Lethbridge-Çejku et al 2006). Atherosclerosis is a condition where plaque forms on the arterial vessel wall. The plaque occludes the vessel, increasing the chance of a clot causing an obstruction to blood flow. When located in the heart, the obstruction leads to ischemia, myocardioal infarction, and can lead to death. Removal of the plaque is currently performed by invasive techniques, such as percutaneous transluminal coronary angioplasty (PTCA), atherectomy, and stenting. These techniques all can lead to a complication called restenosis, where the vessel closes upon itself again, in a different mechanism to atherosclerosis. The placement of stents has become the treatment of choice compared to PTCA due to its lower percentage of restenosis (Kastrati et al 2001). Alternatively, bypass surgery can be used to remove the occluded artery and replace it with a blood vessel from another part of the body, usually the saphenous vein from the leg. Stenting has been compared to bypass surgery and has shown similar results after a one-year follow up with significantly lower cost (Serruys et al 2001).
Coronary restenosis is caused by an injury to the arterial vessel wall that induces a series of events leading to restenosis based on the three main procedures used to remove plaque. PTCA is where a small balloon is inserted into the effected blood vessel. The balloon is then inflated and compresses the atherosclerotic plaque, this procedure is also called balloon angioplasty. Another procedure is called atherectomy; this is where the plaque is scraped, brushed, and vacuumed out. Arterial wall damage occurs during the mechanical scraping and brushing stage resulting in denudation. The final cause of restenosis is due to the insertion of a stent at the site of the atherosclerotic plaque. This fine metal wire tube was designed to reduce restenosis caused by balloon angioplasty. Restenotic lesions caused by stents and angioplasty are very different at the molecular level, though clinically the pathological outcome is the same (Scott 2006).
Definition of restenosis
Goldberg and colleagues (2001) have defined restenosis as a greater than 50% narrowing of the vessel as determined by a follow-up angiogram. Clinically, restenosis is an injury-induced effect on the arterial wall. This effect is typically classified into two distinct steps, smooth muscle cell (SMC) proliferation, typically called neointimal hyperplasia or intimal hyperplasia, and vessel remodeling (Costa and Simon 2005). As previously mentioned, restenosis caused by balloon angioplasty is distinctly different from angioplasty caused by placement of metallic stents. Restenosis caused by balloon angioplasty is attributed to three different factors: the elastic response that occurs after the overstretching of the vessel, neointimal formation, and chronic remodeling (Scott 2006). Neointimal formation associated with balloon angioplasty is primarily formed by the adventitia, with some proliferation of the media layer. Stent-induced restenosis is primarily caused by proliferation and accumulation in the intimal layer, leading to a growth of the neointima (Hoffmann et al 1996). Vascular remodeling in this case is not as defined as in balloon induced injury (Nakatani et al 2003).
Involvement of different cell types
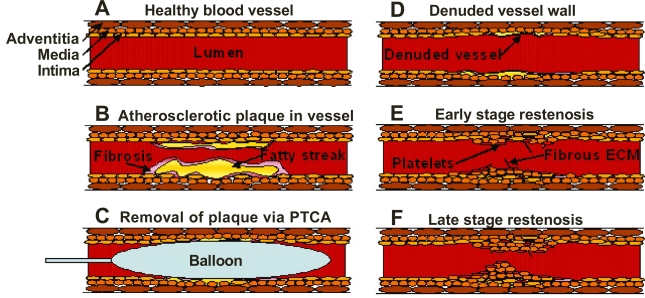
The types of cells involved in the restenotic process are well documented. There are three primary layers in a healthy blood vessel. The tunica intima or intimal layer is the innermost layer and is in contact with the blood flowing through the artery. This layer consists primarily of endothelial cells. Adjacent to the intimal layer is the tunica media or medial layer, consisting of primarily smooth muscle cells. This layer is responsible for the vascular tone. The outermost layer is the tunica adventitia or adventitial layer, consisting of primarily collagen, to provide structure and elasticity. Following vascular injury, other cells are recruited including various inflammatory cells, such as macrophages, T-cells and a small number of B-cells (Farb et al 2002). There is a degree of similarity between restenosis and wound healing. Analysis of the extracellular matrix of a restenotic lesion showed hyaluronan and collagen, which are also both involved in would healing process (Farb et al 2004; Scott 2006). Figure 1 shows an illustration of the different steps in the formation of a restenotic lesion.
Figure 1.
Schematic illustration of the processes leading to restenotic lesion development. The figures show a healthy blood vessel (A), formation of atherosclerotic plaque within the blood vessel showing a fatty streak and macrophages encapsulated within a fibrotic tissue (B), insertion of a balloon angioplasty catheter to remove the plaque (C), damage due to stripping of the endothelial cells of the vessel wall after removal of the balloon (D), platelet accumulation and activation as well as rapid growth of smooth muscle cells and fibrous extracellular matrix forming the scaffolding (E), and the late stage restenosis showing neointima protruding into the lumen causing occlusion within the vessel (F).
Molecular signals in restenosis
After stent placement, various molecular markers signals are activated in the coronary artery (Welt and Rogers 2002). Inflammatory signals play a major role in restenosis and correlates with the amount of injury at the site of stent deployment (Kornowski et al 1998). With increase in the inflammatory process in the injured arterial wall, there is greater proliferation of SMC and narrowing of the artery. As soon as 15 minutes after injury to the vessel, activated leukocytes invade the site of injury which further leads to the deposition of neutrophils, monocytes, platelets and fibrinogen (Farb et al 2002). Macrophages are recruited within the neointima as well. The recruitment of fibrinogen receptors and neutrophils up-regulate adhesion receptors on the cell surface (Kornowski et al 1998). A week after stent placement, cytokines including monocyte chemoattractant protein-1 (MCP-1), interleukin-6 (IL-6) and interleukin-8 (IL-8) are recruited depending on the type of injury causing an potent pro-inflammatory response. A leukocyte integrin class of adhesion molecules Mac-1 interacts with activated platelet receptors (P-selectin glycoprotein) leading to an accumulation of platelets on the intimal wall (Costa and Simon 2005). It was found that the blockade of CCR2 was able to reduce neointimal hyperplasia in stented vessels, while having no effect on balloon injured vessels (Horvath et al 2002). Targeting leukocyte β2-integrin MAC-1 (CD11b/CD18) was shown to reduce neointimal hyperplasia in balloon-injured models (Horvath et al 2002). In balloon-injured models, it was shown that although there was no macrophage infiltration in the neointima, there was significant neutrophil infiltration (Welt et al 2000).
There are a number of growth factors and cytokines that play a role in restenosis. Fibroblast growth factor (FGF), platelet-derived growth factor (PDGF) A and B, insulin like growth factors (IGF), and transforming growth factor β (TGF-β) are produced by SMC’s and are responsible for the SMC proliferation (Welt and Rogers 2002). Additionally PDGF A and B, released from SMC’s, platelets, and endothelial cells, are responsible for SMC migration. Vascular endothelial growth factor (VEGF) derived from endothelial cells is responsible for endothelialization and angiogenesis. The cytokines involved in restenosis are MCP-1, IL-8, and IL-6 (Welt and Rogers 2002). These cytokines, which are present early in the process, are responsible for monocyte and neutrophil recruitment and are derived from a number of cells such as macrophages, SMCs, endothelial cells, fibroblasts, T-cells and polymorphonuclear leukocytes. The cytokine signals are key indicators of an inflammatory response at the site of damage. Another molecular signal associated with inflammation and restenosis is the β-2 integrin molecule Mac-1 (CD11b/CD18), which is responsible for monocyte recruitment (Scott 2006). In addition to the inflammatory factors mentioned, other proteins or enzymes are effected by restenosis, including p27, p70, p16, TGF-β, and collagen (Costa and Simon 2005). For additional understanding on the molecular signaling and inflamation involved in restenosis, the reader is referred to excellent reviews of the subject (Libby and Tanaka 1997; Welt and Rogers 2002; Costa and Simon 2005; Gaspardone and Versaci 2005; Scott 2006).
Therapeutic strategies for restenosis
There are a variety of different ways to treat restenosis. Mechanical stents that keep the vessel open have been coated with drugs to prevent the cells from growing into the vessel lumina. A variety of different drug classes have been used to treat restenosis, including anti-cancer and anti-inflammatory agents. Modulation of genes with plasmid DNA and RNA interference have been used to create an increase or decrease in the local concentrations of specific signaling molecules used to inhibit the growth of certain cells, while promoting the growth of others. The key point for each of these therapies is to inhibit the growth of SMCs, while promoting re-endothelialization of the vessel, such as to reverse the injured vessel back into a healthy vessel.
Mechanical stents
Originally used by Dr. Charles Stent, a dentist, in the year 1856 for facial reconstruction, this device has radically changed noninvasive cardiac intervention in the early 1990’s (Schatz et al 1991; Ring 2001). Stent usage has continuously grown and is now a widely accepted treatment for occluded arteries (Topol 1998; Tung et al 2006b). The use of balloon angioplasty to remove a clot is associated with a greater than 40% restenosis rate, with greater percent restenosis in larger vessels (Elezi et al 1999). The advent of the bare metal stent reduced the incidence of restenosis to 20–53% depending on the type of stent used (Kastrati et al 2001). An important design feature of stents is the thickness of the stent strut, with the thicker struts causing greater incidence of restenosis (Pache et al 2003). The drug-eluting stent (DES) further reduced the percentage of restenosis to less than 10% in initial clinical trials (Degertekin et al 2003; Stone et al 2004). These results have lead to the current use of DES in greater than 85% of all coronary interventions (Kandzari et al 2005). The use of bare metal stents has fallen sharply since the introduction of DES, though that seems to be changing with the current studies showing that DES can lead to possible life threatening in-stent thrombosis conditions (Tung et al 2006b). In-stent thrombosis, or the formation of blood clots, occurs more frequently in DES as compared to bare metal stents since it is shown to occur prior to cell healing. In general, DES prolong the healing process and, as such, are more prone to thrombotic events (Tung et al 2006b). Despite the high usage of DES in the interventional cardiology field, there is still substantial opportunity for improvement. The polymer coating on stent surface is thought to invoke an inflammatory response at the site of injury creating a potential for restenosis (Virmani et al 2004).
Currently there are two DES on the market, the Cypher® stent and the Taxus® stent. Each stent has a different drug and coating on it. The Cypher® stent is loaded with sirolimus, which is also known as rapamycin, coated on the surface with a poly(ethylene-co-vinyl acetate) (PEVA) and poly(n-butyl methacrylate) (PBMA) nonerodable and nonthrombogenic polymers (Vishnevetsky et al 2004). Sirolimus is a cytostatic agent causing cell cycle arrest arrest in the G1 phase of cell division (Braun-Dullaeus et al 1998). The Taxus® stent has the drug paclitaxel embedded in a poly(styrene-b-isobutylene-b-styrene) (SIBS) triblock elastomeric polymer (Ranade et al 2004). Similarly to sirolimus, paclitaxel is a cytostatic agent, arresting cell division in the G1 phase (Ranade et al 2004). For reviews on the clinical performance of the Cypher® and Taxus® stents, please refer to the following reviews (Vishnevetsky et al 2004; Nawarskas and Osborn 2005).
Alternative stent designs have been tested including the use of biodegradable polymer-based stents to inhibit restenosis after initial implantation and then having them degrade in situ after the vessel has stabilized. Poly(L-lactic acid)-based biodegradable stent has been used in humans with favorable results, showing complete degradation after 6 months (Tamai et al 2000). The researchers did not evaluate the length of time the required for the stent to degrade or if the acidic bi-products of the degraded stent had unfavorable interactions with the vessel wall. A limitation of polymer based stents is that they may not be mechanically as strong as the metal-based stent. An alternative approach to using polymeric materials, is the use of a magnesium-based stent that will corrode in the body leaving no metal behind after a period of set time (Di Mario et al 2004). A follow up clinical study showed that the magnesium stent was absorbed within 3 weeks of implantation (Bose et al). More studies are necessary to show whether the residence time of the device is appropriate as vessel remodeling is likely to occur for at least a month after clearing of the vessel. A recent review by Waksman provides an excellent overview of the bioabsorable stents (Waksman 2006).
Other approaches to stent design include removal of the polymer-drug reservoirs, and replacing them with nonpolymeric nanoporous reservoirs. The use of carbon-coated stents has been shown to have comparable results to current metal-based DES (Bhargava et al 2006). However, additional studies will have to be performed under the United States Food and Drug Administration guidelines to confirm these preliminary findings. Nanoporous aluminum oxide-coated stents have also been loaded with potent immunosuppressant tacromilus and shown to inhibit neointimal growth (Wieneke et al 2003). Subsequent studies seem to contradict these findings, concluding that particle debris from these stents could negate any positive inhibition of restenosis (Wieneke et al 2003; Kollum et al 2005). Additional issues such as thrombosis and inflammatory response to these implantable devices need to be well understood before they are considered clinically viable alternatives.
Drug therapy strategies
The advent of DES has brought the use of therapeutic agents and genes to treat restenosis to the forefront of cardiovascular research. A variety of different drug classes have been experimented for the prevention of SMC growth and proliferation. Antineoplastic agents, such as cytarabine, doxorubicin, and vincristine have been tested to determine their effects on SMC growth with some success (Voisard et al 1993). Voisard et al have also tested doxorubicin in addition to four other drugs – dalteparin sodium, cyclosporine A, colchicines, and etoposide – on whether the SMC proliferation was inhibited (Voisard et al 1995). Each of these agents showed some degree of SMC growth inhibition.
Sirolimus, also known as rapamycin, has been shown to inhibit SMC growth in rat cells and in human cell in cultures (Marx et al 1995). Sirolimus was later approved for use in the Cypher® brand DES developed by Cordis in conjunction with Johnson and Johnson and is now a common treatment for restenosis.
Initial studies using paclitaxel, a microtubule stabilizing chemotherapeutic agent, to prevent restenosis were performed by intravenous delivery (Sollott et al 1995). These studies showed that paclitaxel used for prevention of intimal hyperplasia can be administered at much lower concentrations than those needed for cancer therapy. Shortly following Sollott and colleagues’ study, locally-administered paclitaxel to the arterial wall using a porous balloon catheter after angioplasty was examined (Axel et al 1997). Paclitaxel administered in this fashion confirmed previous results showing significant reduction in restenosis. Recently, clinical results of paclitaxel coated onto an angioplasty balloon and delivered locally showed significant promise. The results of these studies showed that local delivery of paclitaxel could inhibit neointimal growth without the need for a metallic stent (Scheller et al 2006). Paclitaxel is the active agent in Boston Scientific’s Taxus® brand DES. The comparisons in terms of safety and efficacy between the Cypher® stent and Taxus® stent is outside the scope of this review. The readers are referred to some excellent reviews on the topic (Kittleson et al 2005; Perin 2005; Tung et al 2006a).
In addition to sirolimus and paclitaxel, membrane sphingolipids, such as ceramide, that has been shown to enhance cellular apoptosis, has also been tested for treatment of restenosis (Charles et al 2000). When ceramide was coated onto an angioplasty balloon and tested in New Zealand white rabbit carotid artery after stretch injury, the results showed significant inhibition of restenosis.
The antiplatelet drug, cilostazol, was initially tested to treat restenosis with nonfavorable results, showing that it did not significantly reduce restenosis after PTCA (Tsutsui et al 1996). Subsequent studies did show a significant difference in reduction of restenosis in patients taking cilostazol (Kunishima et al 1997; Take et al 1997). There has been several additional recent studies confirming the results of Kunishima and colleagues and Take and colleagues indicating that cilostazol does indeed have a positive effect on restenosis (Chen et al 2006).
In addition to the above drugs that modulate various cell cycle stages, researchers have recently looked at a class of drugs known as bisophosphonates to reduce the inflammatory response (Danenberg et al 2002, 2003). Clodronate, pamidronate, alendronate and a novel bisphosphonate ISA-13-1 were all tested, and showed that systemic administration lowered local concentrations of inflammatory mediators, thus reducing restenosis in a rat model (Danenberg et al 2003).
Specific molecular targets have also been used for treatment of restenosis. When PDGF-receptor specific molecules, tyrphostin, AG1295, and AGL-2043, were used to examine their effect on neointimal formation, they showed significant inhibition of restenotic effects (Banai et al 1998, 2005). For a comprehensive review on PDGF receptor specific molecules see (Levitzki 2005). Table 1 below shows a summary of the drug classes used to treat restenosis.
Table 1.
Drugs used in restenosis therapy
| Action | Drug(s) |
|---|---|
| Smooth muscle cell growth inhibition | Cytarabine, Doxorubicin, Vincristine, Dalteparin sodium, Cyclosporine A, Colchicines, Etoposide, Sirolimus, Paclitaxel, Ceramide |
| Antiplatelet | Cilostazol |
| Inflammatory response inhibition | Clodronate, Pamidronate, Alendronate, ISA-13-1 |
| PDGF-receptor specific | AG-1295, AGL-2043 |
Gene therapy strategies
A variety of different gene targets have been used to treat restenosis. Since there have been numerous reviews written discussing the use of gene therapy for the treatment of restenosis and since this review is focusing on the nanoparticles role in treating restenosis, we will only briefly touch on the subject of gene therapy to provide background information. For additional information, the readers are referred to (Rutanen et al 2002; Morishita 2004; Fishbein et al 2005a, 2005c) for more thorough reviews of this topic.
Restenosis gene therapy can be categorized by their method of action, and the cellular target (Rutanen et al 2002; Fishbein et al 2005a). The reduction of intimal hyperplasia has been the most investigated treatment option. Intimal hyperplasia can be reduced through three distinct modes of action. By transfecting cells with genes that encode for proteins known to destroy the cells as they enter the S-phase of the cell cycle such as thimidine kinase, cytosine deaminase and Fas ligand (FasL), intimal hyperplasia was inhibited (Guzman et al 1994; Ohno et al 1994; Ogata et al 1996; Simari et al 1996; Harrell et al 1997; Mano et al 2000). Alternatively, the cell cycle can be arrested by certain gene products. These gene products take part in cellular regulation and, therefore, modulation of these genes would stop the cell cycle (Sriram and Patterson 2001; Bicknell et al 2003; Koledova and Khalil 2006). Genes that encode for kinases such as CDK2, CDC3, and cyclin B as well as CDK inhibitors such as p21, p27, a fusion of p16 and p27, and p53 are all modulators of the cell cycle (Morishita et al 1994; Chen et al 1997; Yonemitsu et al 1998; Scheinman M 1999; Tanner et al 2000; Tsui et al 2001). hRAD 50 gene delivery effects p21 levels, subsequently inhibiting the cell cycle (Ahn et al 2004). Alternatively, targeting of E2F a protein involved in the G1/S transition in cells has been approached through a variety of different pathways. For example, with the use of nonphosphorylable retinoblastoma (Rb) gene, a protein that inhibits E2F, has been used to inhibit intimal hyperplasia (Chang et al 1995). An E2F decoy and a E2F-Rb chimera were used separately to successfully inhibit E2F levels (Morishita et al 1995; Kawauchi et al 2000; Wills et al 2001). Another pathway targeted for restenosis gene therapy is the protein kinase G (PKG) pathway. Truncated PKG gene has been used to increase PKG levels, a known inhibitor of neo-intimal formation (Sinnaeve et al 2002). Similarly proliferating cell nuclear antigen (PCNA) ribozymes were shown to inhibit neointimal growth by inhibiting PCNA (Frimerman et al 1999). Other pathways targeted successfully include early growth response factor (Egr-1), dominant-negative H-ras, Gax homeobox, GATA homeobox and inteferon (INF)-β (Maillard et al 1997; Stephan et al 1997; Ueno et al 1997; Mano et al 1999; Maillard et al 2000; Lowe et al 2001). Modulating local CO levels through iron metabolism with heme oxygenase-1 was found to inhibit intimal hyperplasia despite the exact lack of understanding of the mechanism (Tulis et al 2001; Kong et al 2004).
Another approach for reducing restenosis via reduction of intimal hyperplasia would be to prevent the SMCs from migrating and forming a lesion on the arterial wall. Without the SMC movement, neointimal growth is not possible. It should be noted that these genes do not promote re-endothelialization which is important in the long term health of the vessel. PDGF receptor β, a growth factor known to be involved in cell signaling and migration, has been shown to inhibit neointima formation (Cohen-Sacks et al 2002). Alternative approaches have encoded genes producing extra-cellular matrix modifying proteases such as tissue inhibitor of metalloproteinases (TIMP-1) and plasminogen activator inhibitor (PAI-1) (Carmeliet et al 1997; Furman et al 2002; Turunen et al 2002, 2006; Puhakka et al 2005a, 2005b).
The use of gene therapy that affects multiple pathways simultaneously has also been investigated. These genes protect the endothelial cells and inhibit intimal hyperplasia. The use of vascular endothelial growth factor (VEGF), a well studied regulator of endothelial cell repair, has been used with some success (Asahara et al 1996; Laitinen et al 1997; Van Belle et al 1997; Hiltunen et al 2000). Nitric oxide (NO) levels are known to play a role in the formation of intimal hyperplasia through a variety of different pathways (De Caterina et al 1995; Mooradian et al 1995). The synthesis of NO is regulated by a group of enzymes known as nitric oxide synthases (NOS). Two types of NOS have been used to reduce restenosis – inducible nitric oxide synthase (iNOS) and endothelial nitric oxide synthase (eNOS). NO is known to inhibit aggregation and adhesion of platelets, leukocytes, and to induce SMC apoptosis (Ross 1993). Local expression of nitric oxide synthase enzymes has been found to inhibit intimal hyperplasia (von der Leyen et al 1995; Chen et al 1998; Janssens et al 1998; Shears et al 1998; Varenne et al 1998; Muhs et al 2003 ; Wang et al 2003; Kong et al 2004).
The use of antithrombotic gene therapy has been investigated to reduce restenosis. A number of different approaches have been tried. For instance, local transfection with a gene encoding thrombin inhibitor (hiridun) showed inhibition of restenosis (Rade et al 1996). Other successful approaches include transfecting a gene encoding TFPI, the target of the anti-thrombotic drug heparin to inhibit restenosis (Zoldhelyi et al 2001; Yin et al 2002). Prostacyclin synthase (PGIS) gene has been used directly and indirectly by modulating cyclooxygenase-1 (COX-1) production to inhibit restenosis (Wu 1997; Todaka et al 1999; Shyue et al 2001; Numaguchi et al 2004). COX-1 has also been shown to open the arterial wall, reducing pressure, and ultimately reducing the percentage of restenosis, though it did not directly effect intimal hyperplasia growth. In combination with other genes, this could be a promising target (Shyue et al 2001; Liu et al 2005). Table 2 shows a summary of all of the gene therapy work completed for the treatment of restenosis.
Table 2.
Genes used in restenosis therapy
| Cellular target | Action | Gene Product(s) |
|---|---|---|
| Intimal Hyperplasia | Antiproliferative (cytotoxic) | Thimidine kinase, Cytosine deaminase, FasL |
| Antiproliferative (cytostatic) | cdc2, cdk2, cdc2, cyclin B, p21, hRAD 50, p27, p16–p27, p53, Nonphorphorylatable Rb, Rb/E2F chimera, E2F decoy, Truncated PKG PCNA, Egr-1, Dominant-negative H-ras, Gax homeobox, GATA homeobox
IFN-β, Heme oxygenase-1, TIMP, PAI-1 |
|
| Arterial Cytoprotection/intimal hyperplasia | Re-endothelialization
Mixed mechanism |
VEGF
eNOS, iNOS |
| Thrombosis | Antithrombotic | Hiridun, TFPI, Prostacyclin synthase, COX1 |
Nanoparticulate delivery systems
Nanoparticulate delivery systems have been used to treat a variety of disease states. In restenosis treatment, both conventional drugs- and gene-based medicines have been used in nanoparticle delivery to achieve desirable therapeutic results. There is a need for an alternative treatment since there are limitations to current therapies, such as in-stent restenosis, including the development for DES-related thrombosis and the high rate of restenosis with the use of bare metal stents and balloon angioplasty. A nanoparticle delivery system is well suited for the treatment of restenosis since local or targeted delivery can be achieved, lowering systemic toxicity, while reaching specific cell types in sufficient concentrations for the necessary period of time. Biocompatible lipids and polymers also do not create an inflammatory response. In the context of this article, liposomes, polymer-based nanoparticles, and micelles are considered as nanoparticulate carriers. The high shear pressure in the arterial blood supply leads to a very short residence time for the therapeutic agent at the target cells in the arterial wall. The use of nanoparticles allows for the rapid incorporation of the drug and gene into the cell, thus reducing the shear effects of the arterial pressure. Studies using florescent particles have shown a size dependency in arterial wall transfection using a SCIMED® REMEDY porous balloon catheter (Westedt et al 2002). Three particle sizes were tested and the data showed that the smallest size particles (~110 nm in diameter) had the greatest florescence intensity within the cell. Larger particles showed very little florescence due to short residence time on the arterial wall surface. Other studies showed an increase in inflammatory responses with particles of 5–10 μm in diameter and no therapeutic response, probably due to uptake by the macrophages and other immune cells rather than SMC or endothelial cells. (Gradus-Pizlo et al 1995; Dev et al 1997). Cells can incorporate particles varying from 50–300 nm in diameter based upon a variety of different internalization pathways including nonspecific or receptor-mediated endocytosis (Mukherjee et al 1997; Gruenberg 2001; Pelkmans and Helenius 2002; Sieczkarski and Whit-taker 2002; Mousavi et al 2004). Additionally, nanoparticles larger than 300 nm in diameter were found to accumulate in the liver, spleen and lung, rendering them unavailable for arterial delivery upon systemic administration (Wu and Wu 1988). Other known factors for cellular uptake of nanoparticles include volume, concentration, infusion pressure, and the type of infusion balloon (Tahlil et al 1997; Westedt et al 2004). Increased delivery pressure and large volumes can cause an increase in the intimal thickening, while increased particle concentration leads to effective delivery (Westedt et al 2004). In addition, the use of cell specific surface modifications, can increase the residence time of the nanoparticles at the desired site.
Unlike in tumor mass, where the enhanced permeability and retention effect of the tumor vasculature plays a significant role in the accumulation of nanoparticles, the vascular endothelium is a much more difficult target to reach upon systemic delivery. The vascular flow rate is much higher in the arterial blood flow compared to the capillary flow in tumors. However, some investigators, including Uwatoku and colleagues (2003) have hypothesized the presence of the enhanced permeability and retention effect in balloon injured arteries. However, it should be noted that the enhanced vascular permeability effects in restenotic vessels have not been confirmed by additional studies, especially in humans.
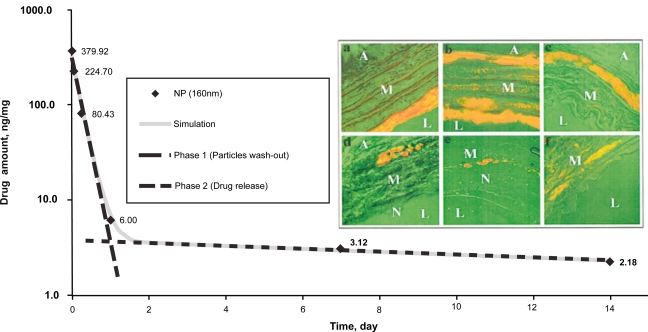
Pharmacokinetic studies were performed on the poly(L-lactide) nanoparticles to measure the local drug concentrations after site-specific arterial delivery (Fishbein et al 2001). Figure 2 shows a graph indicating two distinct first order kinetic profiles. The initial accumulation of the nanoparticles on the intima, followed by subsequent slower accumulation in the media over time is observed. This biphasic mechanism of particle internalization could be the cause of the two-phased drug pharmacokinetic profile.
Figure 2.
The effect of time on arterial AG-1295 concentrations following poly(L-lactic acid) nanoparticle-based delivery. Drug levels are depicted on a logarithmic scale. Initial washout period occurs within 24 hours of administration and the drug levels can be seen for up to 14 days. The drug concentrations were measured by a high performance liquid chromatography assay. Insert shows confocal images of rat carotid arteries following local delivery of Nile Red® dye- containing fluorescent nanoparticles. Images were acquired at 5 minutes (A), 90 minutes (B), 1 day (C), 7 days (D) and 14 days (E), as well as, after 15 minutes of intraluminal delivery and 6 hours after delivery (F). Discrete, granular, fluorescent foci of nanoparticle aggregates are clearly observed in the image F. The notations L, M, N and A indicate lumen, media, neointima, and adventitia, respectively. Copyright © 2001. Reproduced from Fishbein I, Chorny M, Banai S, et al 2001. Formulation and delivery mode affect disposition and activity of tyrphostin-loaded nanoparticles in the rat carotid model. Arterioscler Thromb Vasc Biol, 21:1434–39.
Illustrative examples of nanoparticle-based delivery in restenosis
Lipid nanoparticle-based drug delivery
Liposomes have been used to deliver a variety of drugs to the arterial endothelial cell wall. As previously outlined a number of drugs have been investigated to treat restenosis. Out of the drugs tested, only a subset has been administered through nanoparticle-based delivery systems. The bisphosphonate agent, clodronate, was delivered using a liposomal formulation consisting of 1:3 distearoyl phosphatidylglycerol (DSPG), 1, 2-distearoyl-sn-glycero-3-phosphocholine (DSPC) with an average particle size of 190 nm (Danenberg et al 2002). Results from this study showed that liposomal clodronate successfully inhibited neointima growth in balloon-injured rabbit carotid artery after systemic administration. Other bisphosphonates including, pamidronate, alendronate and ISA-13-1 were tested in a similar liposomal formulation in a balloon-injured rat model (Danenberg et al 2003). To deliver the liposomes to the target site, the researchers in these studies modified a piece of plastic tubing with a 23-gauge catheter to perform local intraluminal delivery in addition to IV and subcutaneous administration. The results of this study showed that systemic administration not local administration leads to the greatest amount of reduction of neointimal growth, which is likely due to the mechanism of action of the drugs. These drugs act on macrophages, which are important in the inflammatory mechanism of restenosis (Danenberg et al 2002, 2003). Using liposomes to incorporate the hydrophilic bisphosphonates into macrophages is a novel approach for the compound class.
The use of Zn (II)-phthalocyanine, a photoactivatable agent, in a liposomal formulation was tested to inhibit SMC growth (Magaraggia et al 2006). This system was examined in cells and the result showed significant apoptosis of vascular SMC in vitro after a short irradiation time. Cellular uptake of oxidized low density lipoproteins (LDL) is an important step in the formation of atherosclerotic plaques and inflammation. By engineering nanoparticles that block the LDL channels, researchers were able to inhibit LDL uptake, and show a reduction in inflammatory responses (Chnari et al 2006).
Polymeric nanoparticle-based drug delivery
A significant amount of work has been ongoing and completed in the field of polymer-based nanoparticle drug delivery for the treatment of restenosis. The bisphosphinate agent, alendronate, was encapsulated in a poly(D,L-lactide-co-glycolide) (PLGA) based nanoparticle system with an average size of 223 nm (Cohen-Sela et al 2006). The presence of calcium ions was found to be critical for the successful entrapment of the hydrophilic drug compound in PLGA matrices, alendronate in this formulation was delivered subcutaneously and by intravenous administration. The results showed reduction in neointimal growth in a balloon injured rabbit model using both routes of administration (Cohen-Sela et al 2006). Interestingly, unlike with the liposomal formulation, the subcutaneous PLGA nanoparticle formulation of alendronate showed a greater reduction in neointimal growth as compared to the intravenous formulation in a rabbit model (Danenberg et al 2002, 2003; Cohen-Sela et al 2006).
Paclitaxel has been used in a number of nanoparticle formulations for restenosis therapy. An alternative to paclitaxel-containing stent coating was developed by Bhargava and colleagues (2006). A cobalt chromium stent loaded with carbon-carbon nanoparticles was used in a stented injury swine model. In this study, three doses of paclitaxel in nanoparticle formulation showed inhibition of neointimal growth with less fibrosis and inflammation when compared to the Cordis Cypher® stent. A comparison to the Taxus® DES would have been more relevant to see if the nanoparticle based therapy is better than the current therapy on the market. Albumin-based nanoparticles containing paclitaxel have also been used to prevent in-stent restenosis (Kolodgie et al 2002). The paclitaxel concentrations was lower than typically used for chemotherapy in a denuded stented rabbit model. The data indicated that after 28 days, neointimal growth was inhibited with a single dose locally administered to the iliac bifurcation, while a second systemically administered dose was needed to see the effects prolonged out to 90 days. Doxorubicin an anthracycline anticancer agent, and paclitaxel were used in a tissue factor targeted lipid/perflorocarbon nano-emulsion to target vascular SMC proliferation in vitro using porcine SMC (Lanza et al 2002). Targeted nano-emulsion had greater efficacy in preventing SMC growth in these studies. The formulation containing doxorubicin and paclitaxel had a greater effect that single drug probably due to synergistic pro-apoptotic activity of these chemotherapeutic agents.
Doxorubicin has also been used in a polymeric micelle consisting of NK911, a self assembling block co-polymer, to treat restenosis in a balloon-injured rat carotid artery model (Uwatoku et al 2003). Results from this study showed that doxorubicin encapsulated within NK911 permeated the arterial wall and prevented neointimal growth. The authors claim that the formulation was able to target the damaged endothelium due to a local enhanced permeability and retention effect in the arterial wall. This effect has not been confirmed in any other reports. The small size (40 nm) of the delivery system could lead to an increased uptake in a damaged arterial wall after systemic administration. Another antiproliferative agent formulated in a PLGA based nanoparticle system was U-86, a 2-aminochromone, which is an experimental anti-proliferative compound (Labhasetwar et al 1998). A number of different surface modifications strategies to alter the charge of the nanoparticle system and to promote adhesion to the cells were tested. The results of this study showed that surface modification with a cationic surfactant, didodecyldimethylammonium bromide (DMAB), was most beneficial in producing a 7–10 fold increase in arterial drug levels due to an increase in the residence time of the nanoparticles on the endothelial surface.
In addition to the Bisphosphonates and anti-cancer drugs, tyrphostins have been tested in a variety of different nanoparticle systems for restenosis. Poly(L-lactic acid) (PLA) nanoparticles have been tested in a balloon-injured swine model (Banai et al 1998). Results showed that infusion catheter local delivery inhibited SMC growth. Similarly tyrphostin AG-1295 was encapsulated in a PLA-based nanoparticle and was administered locally using a porous balloon to a rat with denuded carotid artery (Fishbein et al 2000). Results were similar to previous work showing inhibition of SMC growth. In this formulation, the particle size was modulated and the results showed that the smaller particle sized nano-delivery systems were more efficacious. A related tyrphostin AGL-2043 was tested in a similar PLA-based nanoparticle system in balloon-injured rat carotid arteries and stented porcine arteries (Banai et al 2005). Results from this study indicated the nano-encapsulated drug was more successful in inhibiting restenosis in both animal models. Table 3 summarizes the results obtained from various studies of drug-encapsulated nanoparticle systems for restenosis therapy.
Table 3.
Illustrative examples of nanoparticulate carrier-based drug delivery in restenosis
| Drugs | Nanocarrier System | Model and Route of Administration | Conclusions | Reference(s) |
|---|---|---|---|---|
| Clodronate, Pamidronate, Alendronate, ISA-13-1 | Liposome | Balloon injured rat carotid artery, Local, IV and Sub-Q drug administration | Using bisphosphonates they were able to show that macrophage depletion would inhibit restenosis by modulating immune response reducing inflammation at site, local administration not effective systemic was (IV subcutaneous) | (Danenberg et al 2002; Danenberg et al 2003) |
| Zn(II)-phthalocyanine light therapy | Liposome | SV40LT-SMC cell line | Produced apoptosis of VSMC after light activation of phthalocyanine | (Magaraggia et al 2006) |
| Alendronate | PLGA | Balloon injured hypercholesterimic rabbit carotid artery, Sub-Q, IV administration | Inhibited macrophage build up, reducing restenosis, SC more effective than IV | (Cohen-Sela et al 2006) |
| PTX | Albumin NP loaded with PTX | Denuded iliac artery, New Zealand rabbit, stented after denuding, systemic administration | Single 5 mg/kg systemic dose good for 28 days of endothelial inhibition, second dose at 28 days holds off cell growth for 90 days. | (Kolodgie et al 2002) |
| DOX, PTX | Tissue factor antibody-targeted perflorocarbon nano-emulsion to bind to SMC | Porcine aortic smooth muscle cell line | Reduction of SMC growth seen with both DOX and PTX targeted emulsions can also be used for MRI imaging agents | (Lanza et al 2002) |
| DOX | Polymeric micelle PEG-poly-ASP | Balloon injured rat carotid artery, IV admin | Inhibited vascular proliferation, did not induce apoptosis, thought to target by EPR | (Uwatoku et al 2003) |
| AG-1295 | PLA nanoparticle | Balloon injured swine, infusion catheter used | Local delivery via infusion catheter showed inhibition of SMC growth. | (Banai et al 1998) |
| AG-1295 | PLA nanoparticle | Balloon injured rat carotid artery, Canula direct delivery | Antiproliferation observed in culture porcine SMC and rat carotid artery | (Fishbein et al 2000) |
| AGL-2043 | PLA nanoparticle | Balloon injured rat carotid artery, and stented porcine artery | Nano encapsulated drug inhibited restenosis in both animal models | (Banai et al 2005) |
| U-86 2-aminochromone (antiproliferative agent) | PLGA nanoparticle with various moieties. | Ex-vivo dog femoral artery, and in-vivo acute dog femoral and pig coronary arteries following angioplasty | Cationic surface modifying agents lead to enhanced arterial drug levels. | (Labhasetwar et al 1998) |
Abbreviations: PLGA, Poly(D,L-lactide-co-glycolide); PLA, Poly(L-lactic acid); PEG, Poly(ethylene glycol).
Lipid nanoparticle-based gene delivery
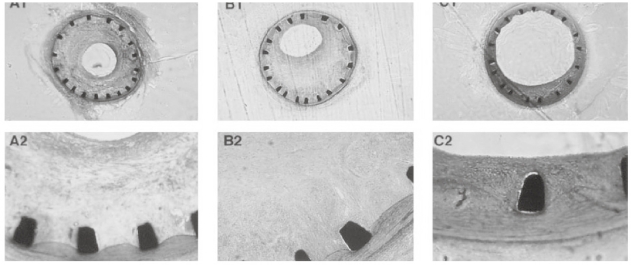
A significant amount of work has been accomplished in the field of nonviral nanoparticle-based gene delivery for restenosis. Table 4 is a summary of the studies performed to date. Most of the work on nonviral transfection has been performed with commercially-available cationic lipid transfection reagents such as Lipofectamine® or Lipofectamine Plus®. The use of Lipofectamine® or Lipofectamine Plus® in delivery of genes is not a clinically viable option due to the known toxicity of the cationic lipid transfection reagents (Armeanu et al 2000). Lipo-fectamine Plus® has been used in local delivery of proliferating cell nuclear antigen hammerhead ribozyme in a stented porcine model (Frimerman et al 1999). Results of this study showed that the ribozyme successfully inhibited intimal hyperplasia in the stented model. Figure 3 shows a cross section of the stented porcine arteries without treatment, with administration of a nonspecific ribozyme, and administration of PCNA specific ribozyme in nanoparticle formulations. The gene for eNOS was transfected in a canine ex-vivo saphenous vein graft using Lipofectamine Plus® (Kalra et al 2000). In this study, the gene was transfected through the intimal and adventetial routes. The results indicated that intimal transfection did not show an efficient gene transfection, whereas the veins treated through the adventitia did show efficient transfection. The gene encoding for prostacyclin synthase (PGIS) was delivered with Lipofectamine® into balloon-injured New Zealand white rabbits (Numaguchi et al 2004). A stent was deployed at the site of injury and a Dispatch® catheter was used for delivery of the nanoparticulate plasmid DNA formulation locally. The data from this study showed that PGIS prevented vascular SMC growth, stimulated re-endothelialization, and prevented neointimal formation.
Table 4.
Illustrative examples of nanoparticulate carrier-based gene delivery in restenosis
| Genes | Nanocarrier System | Model and Route of Administration | Conclusions | Reference(s) |
|---|---|---|---|---|
| Endothelial nitric oxide synthase (eNOS) | Cationic Liposomes (Lipofectamine plus) | Adult male mongrel dogs, ex-vivo saphenous vein transfection, both intimal and adventitial. | No efficient gene transcription with intimal transfection, adventitial transfection showed elevated mRNA levels compared to controls. | (Kalra et al 2000) |
| PCNA chimeric hammerhead ribozyme | Cationic liposomes (Lipofectamine/Lipofectin) | Balloon injured porcine coronary artery | Inhibition of intimal hyperplasia and reduction of coronary artery restenosis | (Frimerman et al 1999) |
| Prostacyclin synthase (PGIS) | Cationic liposomes (Lipofectamine) | Balloon injured and stented New Zealand White rabbits with local delivery using Dispatch® catheter. | PGIS induced PGI2 expression and inhibited VSMC growth and accelerated re-endothelialization, preventing neointimal formation | (Numaguchi et al 2004) |
| hRAD50 | Nonliposomal lipid (FuGENE 6, Boehringer Mannheim) | Coronary stented porcine model, local delivery using Dispatch® catheter | Regression of established neointimal hyperplasia after coronary stenting. | (Ahn et al 2004) |
| Inducible nitric oxide synthase (iNOS) | DAC (30% w/w) and DOPE (70% w/w) | Coronary and femoral artery stented mini pigs, local administration of lipoplex with Infiltrator® catheter. | Inhibition of intimal hyperplasia. | (Muhs et al 2003) |
| Inducible nitric oxide synthase (iNOS) | Cationic liposomes | Grafted foxhound dogs carotid artery bypass grafts, local delivery using Infiltrator® catheter | Single transfection able to show inhibition of intimal growth for up to 6 months. | (Pfeiffer et al 2006) |
| Endothelial nitric oxide synthase (eNOS) | Cationic liposomes | Human umbilical vein endothelial cell (ECV304) in vitro | Successful transfer of eNOS in-vitro, saw increase in NO. | (Qiao et al 2006) |
| Endothelial nitric oxide synthase (eNOS)) | Liposomes/cationic liposomes | New Zealand white rabbits and Stauffland rabbits heart transplants, liposomes delivered ex-vivo to aortic roots of donor heart | Despite transfection inefficiencies with liposomes, measurable NO expression was achieved, reducing endothelial activation | (Iwata et al 2000; Iwata et al 2001) |
| Chloramphenicol acetyl transferase (CAT) | Cationic liposomes | Balloon injured Yorkshire pig iliofemoral arteries, local delivery using catheter. | Saw increased CAT activity after liposome transfection in artery. | (Stephan et al 1996) |
| C-type natriuretic peptide (CNP) | Cationic liposomes DOCSPER, (1,3-dioleoyloxy-2-(N5-carbamoyl-spermine)-propane) | Porcine renal artery and pig femoral arteries, percutaneous introduced needle injector catheter used to locally deliver drug | Successful inhibition of renal artery restenosis and femoral artery restenosis. | (Kuhnl et al 2005; Pelisek et al 2006) |
| Vascular endothelial growth factor (VEGF) | Cationic liposomes | Human trial containing patients with angina and 60–99% diameter stenosis in 1–2 of main coronary arteries. Local delivery achieved using Dispatch catheter. | Did not affect post angioplasty restenosis, but significant improvement in myocardial perfusion was observed in viral vector treated patients | (Hedman et al 2003) |
| E2F oligonucleotide decoy | Hemaglutin virus of Japan (HVJ) liposomes | B10.D2 mice and Japanese monkeys hearts were treated ex-vivo prior to transplant with viral liposomes | Ex vivo liposomal transfection suppressed neointimal hyperplasia after cardiac transplant surgery | (Kawauchi et al 2000) |
| E2F oligonucleotide decoy | Hemaglutin virus of Japan (HVJ) liposomes | Balloon denuded rat carotid artery, local delivery using catheter | Neointima inhibition seen up to 8 weeks after single intraluminal injection | (Morishita et al 1995) |
| Wild type p53 | Hemaglutin virus of Japan (HVJ) liposomes | Balloon denuded Japanese white rabbits with local delivery using double-lumen catheter | p53 Gene transfer inhibits neointimal formation without apoptotic stimuli. VSMC growth inhibited. | (Yonemitsu et al 1998) |
| Tissue factor pathway inhibitor gene | Hemaglutin virus of Japan (HVJ) liposomes | High cholesterol fed rabbit model. Illiac artery, balloon angioplasty was used to open stenosis, local liposome delivery using Dispatch catheter. | Significant reduction of intimal hyperplasia compared to controls, confirmed through histology and angiography. | (Yin et al 2002) |
| Prostacyclin synthase (PGIS) | Hemaglutin virus of Japan (HVJ) liposomes | Balloon denuded Sprawly rat carotid artery, local delivery using infusion catheter. | PGIS expression in neointima, inhibiting neointimal growth. | (Todaka et al 1999) |
| Platelet derived growth factor (PDGF) receptor β-antisense | PLGA nanoparticles | Explanted rat SMC in-vitro, injured rat carotid artery in-vivo | Controlled release of oligonucleotide over 1 month, neointima growth inhibited in naked AS and PLGA NP AS, PLGA NP believed to be effective at lower concentrations, due to sustained release. | (Cohen-Sacks et al 2002) |
| Monocyte chemoattractant protein 1 (MCP-1) | PEI, PEI / PEG, PEI / mellatin | In-vitro in SMC and EC | Inhibition of SMC growth, incorporation of mellatin increased activity, while decreasing toxicity | (Lenter et al 2004) |
| Early growth response factor 1 (EGR-1) | DNAzyme | Porcine coronary stent model, local delivery with Transport catheter | Inhibition of SMC and reducing in-stent restenosis. | (Lowe et al 2001) |
Figure 3.
Low magnification (10x) images of porcine arteries 4 weeks after stent implantation. The images represent stent only (A), untreated tissue as a negative control (B), and delivery of PCN1 ribozyme therapeutic agent (C). Panels A and B show well-defined intimal hyperplasia, whereas panel C shows a significantly lesser amount of neointimal growth. Lower panel images correspond to high magnification of the above. No histological evidence of tissue inflammation is seen. Copyright © 1999. Reproduced from Frimerman A, Welch PJ, Jin X, et al 1999. “Chimeric DNA-RNA hammerhead ribozyme to proliferating cell nuclear antigen reduces stent-induced stenosis in a porcine coronary model.” Circulation, 99:697–703.
Using nonliposomal transfection reagent FuGENE 6 (Boehringer Mannheim) hRAD50 encoding plasmid DNA was delivered to stented pigs using the Dispatch® catheter (Ahn et al 2004). From this study, short term inhibition of neointimal formation was observed. However, long-term investigations are needed to determine the effect RAD50 mediated re-endothelialization.
The use of plasmid DNA encoding for inducible and endothelial nitric oxide synthase (iNOS and eNOS, respectively) has been studied extensively in other types of cationic liposomes. Using Lipofectamine® as a transfection reagent, endothelial nitric oxide synthase (eNOS) expressing plasmid DNA was administered to HUVEC cells to measure the effect of various inhibitors and inducers on eNOS production (Qiao et al 2006). The conclusion from this study was that peripheral eNOS side effects should be emphasized during clinical administration (Qiao et al 2006). In another study, the researchers examined local iNOS plasmid delivery to an injured mini pig femoral and coronary artery model (Muhs et al 2003). Administration of the complexed plasmid was performed using an Infiltrator® infusion catheter, the animals were stented to produce the injury. The lipid-DNA complex (lipoplex) contained monocationic lipid 3β-(N,N′-di-methylaminoethane)-carbamoylcholesterol (DAC) at 30% (w/w) and dioleoyl phosphatidylethanolamine (DOPE) at 70% (w/w). When administered to the injured model, the results indicated that despite the breakdown of excess NO in the bloodstream by hemoglobin, significant reduction of neointimal growth was observed with this DNA delivery system. Using a similar liposomal system, Pfiffer and colleagues (2006) used an Infiltrator® catheter to administer iNOS expressing plasmid DNA in a foxhound dog model after an arterial graft. In this study, the intimal hyperplasia was inhibited for up to 6 months with a single local plasmid DNA administration. In vivo studies of eNOS plasmid DNA formulated in cationic lipid 1-[2-(oleoyloxy)ethyl]-2-oleyl-3-(2-hydroxyethyl) imidazolinium chloride (DOTIM) and cholesterol in a 1:1 weight ratio, was performed (Iwata et al 2000). Using a Stauffland rabbit model, an in-situ transfected carotid artery was grafted into the animal. The results indicated that eNOS transfected in this manner reduced neointimal formation with effects lasting up to 21 days after transfection (Iwata et al 2000). The same formulation was also used in a heart transplanted rabbit with similar results (Iwata et al 2001).
Other genes that have been delivered to the arterial wall with cationic liposomes include chloramphenicol acetyl transferase (CAT) gene. CAT gene was delivered with two different cationic lipid formulations – (±)-N-(2-Hydroxyethyl)-N, N-dimethyl-2,3-bis(tetradecyloxy)-1-propanaminium bromide (DMRIE)/DOPE and (±)-N-(3-aminopropyl)-N,N-dimethyl-2, 3-bis(dodecyloxy)-1-propanaminium bromide) (GAP-DLRIE)/DOPE – in balloon-injured Yorkshire pigs using a catheter for local delivery (Stephan et al 1996). The results of this study indicated that GAP-DLRIE/DOPE liposomal system was more efficient in transfecting the artery as compared to the DMRIE/DOPE formulation. The cationic lipid (DOCSPER) (1,3-dioleoyloxy-2-(N5-carbamoyl-spermine)-propane) was used to transfer C-type naturetic peptide (CNP) gene in a pig femoral artery model (Pelisek et al 2006). Briefly, the CNP and CNP gene were delivered to the artery and the results showed that a single CNP gene administration inhibited neointima formation better than systemic administration of the peptide. Similar results were observed in a porcine renal model (Kuhnl et al 2005).
VEGF gene delivery was performed in human patients using a cationic mixture of DOTMA and DOPE as part of a clinical trial to induce collateral circulation following myocardial ischemia (Hedman et al 2003). Gene transfer was performed prior to stenting using a Dispatch® catheter for local tissue delivery. From this study, the results showed that cationic lipid transfer of VEGF did not affect the incidence of restenosis. When VEGF was transferred via a viral vector, myocardial perfusion was increased. A greater transfection efficiently produced by the viral vector was likely the cause for the difference in the observed activity. The gene for TIMP-1 was used in a α5β1 targeted Lipofectin® nanoparticulate delivery system (Meng et al 2006). From this study, it was observed that this type of delivery system was effective in inhibiting neointimal hyperplasia for up to 28 days in a balloon-injured rat model.
Viral-mimicking liposomes have been extensively used to deliver genes to the arterial wall. Briefly, in this case, a liposome is made and then bound to the envelop protein of a virus, such as the hemaglutin virus of Japan (HJV). This formulation strategy allows for better transfection of the encapsulated gene into the cell. In one example, E2F oligonucleotide decoy was administered using a viral liposome system. Artificial HJV-liposomes were used to deliver E2F decoy ex-vivo in both a murine and a primate cardiac transplant model to inhibit neointimal growth (Kawauchi et al 2000). The artificial viral liposomes were shown to have increased transfection for oligonucleotides. Additionally, the results showed that SMC growth was inhibited resulting form the inhibition of neointima formation. Other investigators have used HJV-liposome delivered E2F decoy in a denuded rat carotid artery model showing inhibition of the neointima for up to 8 weeks following administration (Morishita et al 1995). Other nucleic acid therapies delivered with the HJV-liposome delivery system include wild-type p53, tissue factor pathway inhibitor gene (TFPI), and PGIS (Yonemitsu et al 1998; Todaka et al 1999; Yin et al 2002). Wild-type p53 was delivered to white rabbits using a double lumen catheter to locally administer and transfect the therapeutic gene. Results indicated that p53 gene transfer inhibited neointimal formation, with and without apoptotic stimuli (Yonemitsu et al 1998). White rabbits were also used as a model to test TFPI transfection by local administration the encapsulated plasmid (Yin et al 2002). The results of the study showed that TFPI successfully inhibits neointimal growth up to 4 weeks after administration. PGIS was delivered in a HJV-liposome in a denuded rat carotid model (Todaka et al 1999). An infusion catheter was used to deliver the encapsulated gene; results indicated that neointima growth was inhibited.
Polymeric nanoparticle-based gene delivery
Unlike lipid-based delivery, there are a small number of studies performed with polymer based nano-delivery systems to deliver genes to the arterial wall. Depending on the type of polymer used, these nanoparticulate delivery systems are able to modify the release of the genes, while sharing similar transfection efficacy as lipid nano-systems. For instance, PLGA-based nanoparticles were used to deliver PDGF receptor β antisense RNAi to an injured rat carotid artery (Cohen-Sacks et al 2002). The delivery system was made to release over a period of one month allowing for a lower dose of the therapeutic agent to be administered over a long period of time. From these studies, inhibition of the neointima growth was observed for a period of one month. When the lowest dose was used, some inhibition was observed without drug encapsulation. However, as the dose was increased, there no inhibition when the antisense was not encapsulated. Another delivery system used in the treatment of restenosis was a PEI based delivery system for monocyte chemoattractant protein 1 (MCP-1) (Lenter et al 2004). In addition to the PEI formulation, alternative formulations tested included combinations with the surface active peptide mellatin and PEG. The formulations were tested in a variety of different smooth muscle and endothelial cells, showing that delivery systems coated with PEG exhibited less toxicity and an increase in activity of the formulations when combined with mellatin.
Other therapeutic delivery systems
The use of nucleic acids therapies such as ribozymes and DNAzymes have also been investigated in the field of restenosis. DNAzymes were targeted against transcription factor EGR-1 in a stented porcine model (Lowe et al 2001). Inhibition of the neo-intimal growth was observed in this first study of DNAzymes delivery to the arterial wall.
Surface functionalization of nanoparticles for targeting restenosis
Damaged endothelial cells within the coronary artery are difficult drug targets for site-specific localization of the nanoparticles upon systemic administration. In addition, many of the drugs and genes used for the prevention and treatment of intimal hyperplasia are known to produce cytotoxic effects within the body. Systemic administration of these drugs and genes would cause unwanted effects. In order to enhance site-specificity, there are several strategies that have been tried to functionalize the nanoparticle surface in order to achieve better localization of the delivery systems at the target site.
There are very few reported studies on active targeting of damaged endothelial cells for the treatment of restenosis. A common targeting moiety for endothelial cells is the argentine-lysine-aspartic acid (RGD) tripeptide sequence that is specific to the alpha(v)-beta(3) integrins present abundantly on sprouting endothelial cells and certain tumor types (Dijkgraaf et al 2007). Another alpha(v)-beta(3) integrin specific peptide sequence is PHSRN (Mardilovich et al 2006). Other endothelial cell targeting moieties based on an amino acid sequence are YIGSR, and HWGF (Turunen et al 2002; Jun and West 2005). VCAM-1 is a potential biomarker present on atherosclerotic surface. By targeting that biomarker, the delivery system would be highly localized to the area of interest (Kelly et al 2006). A nanoparticle delivery system modified with VINP28, a peptide specific to VCAM-1, on the surface was shown to have preferential affinity to the endothelial cells as compared to macrophages or SMCs. Weissleder and colleagues (2005) produced a library of small molecule endothelial cell-specific targeting moieties. These targeting moieties have not yet been used in any restenosis studies. The use of PLGA-based nanoparticles functionalized with an ICAM antibody was recently shown to successfully target the endothelial cells in vitro (Muro et al 2006).
Alternative localized delivery
Functionalization of nanoparticles can target specific cell types. If a broad area within the vessel is the target there are other strategies available. The use of local administration using an infusion catheter as previously described with a Dispatch®, Infiltrator®, or other cardiac infusion catheters is one way to achieve local high levels of the therapeutic agent (Tahlil et al 1997; Panyam et al 2002). Local administration is also achieved through the use of drug and/or gene eluting stents such as the Cypher® and Taxus® stents (Fishbein et al 2005a, 2005b; Kittleson et al 2005; Perin 2005; Tung et al 2006b). Other drug-coated stents using different designs, such as biodegradable stents, as well as other systems are being evaluated in preclinical and clinical studies (Eisenberg and Konnyu 2006). Ex vivo application of the drug or gene in the cells is also another possible way to reduce systemic 2fects of the administered drug. However, clinical application of the ex vivo approach will be quite limited.
Conclusion and future outlook
There is a need to develop an alterative to stents and drug-eluting stents for the treatment of coronary restenosis. In this review paper, we have evaluated the current research in the field of nanoparticulate carriers for delivery of drugs and genes. Future work should focus on creating novel delivery systems that have less cytotoxic effects. Much of the liposomal work done to date, uses Lipofectamine® as the transfection agent. While this transfection reagent works well in nonclinical settings, it has shown to exhibit significant toxicity in porcine arteries upon systemic administration (Armeanu et al 2000). Few studies have utilized the advances in nanoparticle targeting for treatment of restenosis. The cytostatic drugs currently being used may not be the best option for the treatment of this disorder, as they do not promote the growth of the endothelium. The work performed in the field of active targeting has produced a number of cell-specific targeting ligands that would enhance the delivery and retention of the nanoparticles to the restenotic lesion, resulting in an increase in the efficacy of the therapeutic agent. Future studies should focus on creating biocompatible nanoparticles with targeting moieties to allow for increased tissue-specific delivery and cellular uptake with limited toxicity upon systemic administration. Gelatin-based materials, for instance, may be particularly well suited for this application, since there is abundance of collagen at the site of the lesion. Gelatin-based delivery systems also do not induce any inflammatory effect. The key to developing a successful therapy will be to promote re-endothelialization, while inhibiting any SMC growth, or vascular remodeling. Ideally, the therapy will be able to be given in a noninvasive manner or in conjunction with the initial clearing of the atherosclerotic plaque using an infusion balloon catheter. With recent observations of thrombosis upon long-term implantantation of the DES, it is clear that alternative therapeutic strategies are needed for effective treatment of coronary restenosis. This review, hopefully, will provide an impetus for further research in this area and, especially promote the development of nanoparticulate delivery systems for both drug and gene therapy of this disease.
References
- Ahn YK, Kook H, Jeong MH, et al. Local RAD50 gene delivery induces regression of preformed porcine coronary in-stent neointimal hyperplasia. The Journal of Gene Medicine. 2004;6(1):93–104. doi: 10.1002/jgm.464. [DOI] [PubMed] [Google Scholar]
- Armeanu S, Pelisek J, Krausz E, et al. Optimization of nonviral gene transfer of vascular smooth muscle cells in vitro and in vivo. Molecular Therapy. 2000;1(4):366–375. doi: 10.1006/mthe.2000.0053. [DOI] [PubMed] [Google Scholar]
- Asahara T, Chen D, Tsurumi Y, et al. Accelerated restitution of endothelial integrity and endothelium-dependent function after phVEGF165 gene transfer. Circulation. 1996;94(12):3291–3302. doi: 10.1161/01.cir.94.12.3291. [DOI] [PubMed] [Google Scholar]
- Axel DI, Kunert W, Goggelmann C, et al. Paclitaxel inhibits arterial smooth muscle cell proliferation and migration in vitro and in vivo using local drug delivery. Circulation. 1997;96(2):636–645. doi: 10.1161/01.cir.96.2.636. [DOI] [PubMed] [Google Scholar]
- Banai S, Chorny M, Gertz SD, et al. Locally delivered nanoencapsulated tyrphostin (AGL-2043) reduces neointima formation in balloon-injured rat carotid and stented porcine coronary arteries. Biomaterials. 2005;26(4):451–461. doi: 10.1016/j.biomaterials.2004.02.040. [DOI] [PubMed] [Google Scholar]
- Banai S, Wolf Y, Golomb G, et al. PDGF-receptor tyrosine kinase blocker AG1295 selectively attenuates smooth muscle cell growth in vitro and reduces neointimal formation after balloon angioplasty in swine. Circulation. 1998;97(19):1960–1969. doi: 10.1161/01.cir.97.19.1960. [DOI] [PubMed] [Google Scholar]
- Bhargava B, Reddy NK, Karthikeyan G, et al. A novel paclitaxel-eluting porous carbon-carbon nanoparticle coated, nonpolymeric cobalt-chromium stent: Evaluation in a porcine model. Catheterization and Cardiovascular Interventions. 2006;67(5):698–702. doi: 10.1002/ccd.20698. [DOI] [PubMed] [Google Scholar]
- Bicknell KA, Surry EL, Brooks G. Targeting the cell cycle machinery for the treatment of cardiovascular disease. J Pharm Pharmacol. 2003;55(5):571–91. doi: 10.1211/002235703765344487. [DOI] [PubMed] [Google Scholar]
- Bose D, Eggebrecht H, Erbel R. Absorbable metal stent in human coronary arteries: imaging with intravascular ultrasound. Heart. 2006;92(7):892. doi: 10.1136/hrt.2005.076562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun-Dullaeus RC, Mann MJ, Dzau VJ. Cell cycle progression: new therapeutic target for vascular proliferative disease. Circulation. 1998;98(1):82–9. doi: 10.1161/01.cir.98.1.82. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Moons L, Lijnen R, et al. Inhibitory role of plasminogen activator inhibitor-1 in arterial wound healing and neointima formation : A gene targeting and gene transfer study in mice. Circulation. 1997;96(9):3180–3191. doi: 10.1161/01.cir.96.9.3180. [DOI] [PubMed] [Google Scholar]
- Chang MW, Barr E, Seltzer J, et al. Cytostatic gene therapy for vascular proliferative disorders with a constitutively active form of the retinoblastoma gene product. Science. 1995;267(5197):518–22. doi: 10.1126/science.7824950. [DOI] [PubMed] [Google Scholar]
- Charles R, Sandirasegarane L, Yun J, et al. Ceramide-coated balloon catheters limit neointimal hyperplasia after stretch injury in carotid arteries. Circ Res. 2000;87(4):282–288. doi: 10.1161/01.res.87.4.282. [DOI] [PubMed] [Google Scholar]
- Chen D, Krasinski K, Chen D, et al. Downregulation of cyclin-dependent kinase 2 activity and cyclin a promoter activity in vascular smooth muscle cells by p27KIP1, an inhibitor of neointima formation in the rat carotid artery. J. Clin. Invest. 1997;99(10):2334–2341. doi: 10.1172/JCI119414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Daum G, Forough R, et al. Overexpression of human endothelial nitric oxide synthase in rat vascular smooth muscle cells and in balloon-injured carotid artery. Circ Res. 1998;82(8):862–870. doi: 10.1161/01.res.82.8.862. [DOI] [PubMed] [Google Scholar]
- Chen YD, Lu YL, Jin ZN, et al. A prospective randomized antiplatelet trial of cilostazol versus clopidogrel in patients with bare metal stent. Chinese Medical Journal. 2006;119(5):360–366. [PubMed] [Google Scholar]
- Chnari E, Nikitczuk JS, Wang J, et al. Engineered polymeric nanoparticles for receptor-targeted blockage of oxidized low density lipoprotein uptake and atherogenesis in macrophages. Biomacromolecules. 2006;7(6):1796–1805. doi: 10.1021/bm0600872. [DOI] [PubMed] [Google Scholar]
- Cohen-Sacks H, Najajreh Y, Tchaikovski V, et al. Novel PDGFbetaR antisense encapsulated in polymeric nanospheres for the treatment of restenosis. Gene Therapy. 2002;9(23):1607–16. doi: 10.1038/sj.gt.3301830. [DOI] [PubMed] [Google Scholar]
- Cohen-Sela E, Rosenzweig O, Gao J, et al. Alendronate-loaded nanoparticles deplete monocytes and attenuate restenosis. Journal of Controlled Release. 2006;113(1):23–30. doi: 10.1016/j.jconrel.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Costa MA, Simon DI. Molecular basis of restenosis and drug-eluting stents. Circulation. 2005;111(17):2257–2273. doi: 10.1161/01.CIR.0000163587.36485.A7. [DOI] [PubMed] [Google Scholar]
- Danenberg HD, Fishbein I, Epstein H, et al. Systemic depletion of macrophages by liposomal bisphosphonates reduces neointimal formation following balloon-injury in the rat carotid artery. Journal of Cardiovascular Pharmacology. 2003;42(5):671–679. doi: 10.1097/00005344-200311000-00014. [DOI] [PubMed] [Google Scholar]
- Danenberg HD, Fishbein I, Gao J, et al. Macrophage depletion by clodronate-containing liposomes reduces neointimal formation after balloon injury in rats and rabbits. Circulation. 2002;106(5):599–605. doi: 10.1161/01.cir.0000023532.98469.48. [DOI] [PubMed] [Google Scholar]
- De Caterina R, Libby P, Peng HB, et al. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest. 1995;96(1):60–68. doi: 10.1172/JCI118074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degertekin M, Regar E, Tanabe K, et al. Sirolimus-eluting stent for treatment of complex in-stent restenosis: The first clinical experience. Journal of the American College of Cardiology. 2003;41(2):184–189. doi: 10.1016/s0735-1097(02)02704-3. [DOI] [PubMed] [Google Scholar]
- Dev V, Eigler N, Fishbein MC, et al. Sustained local drug delivery to the arterial wall via biodegradable microspheres. Catheterization and Cardiovascular Diagnosis. 1997;41(3):324–332. doi: 10.1002/(sici)1097-0304(199707)41:3<324::aid-ccd14>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Di Mario C, Griffiths HUW, Goktekin O, et al. Drug-Eluting Bioabsorbable magnesium stent. Journal of Interventional Cardiology. 2004;17(6):391–395. doi: 10.1111/j.1540-8183.2004.04081.x. [DOI] [PubMed] [Google Scholar]
- Dijkgraaf I, Kruijtzer JA, Liu S, et al. Improved targeting of the αvβ 3 integrin by multimerisation of RGD peptides. Eur J Nucl Med Mol Imaging. 2007;34(2):267–273. doi: 10.1007/s00259-006-0180-9. [DOI] [PubMed] [Google Scholar]
- Eisenberg MJ, Konnyu KJ. Review of randomized clinical trials of drug-eluting stents for the prevention of in-stent restenosis. The American Journal of Cardiology. 2006;98(3):375–382. doi: 10.1016/j.amjcard.2006.02.042. [DOI] [PubMed] [Google Scholar]
- Elezi S, Kastrati A, Hadamitzky M, et al. Clinical and angiographic follow-up after balloon angioplasty with provisional stenting for coronary in-stent restenosis. Catheterization and Cardiovascular Interventions. 1999;48(2):151–156. doi: 10.1002/(sici)1522-726x(199910)48:2<151::aid-ccd6>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Farb A, Kolodgie FD, Hwang JY, et al. Extracellular matrix changes in stented human coronary arteries. Circulation. 2004;110(8):940–947. doi: 10.1161/01.CIR.0000139337.56084.30. [DOI] [PubMed] [Google Scholar]
- Farb A, Weber DK, Kolodgie FD, et al. Morphological predictors of restenosis after coronary stenting in humans. Circulation. 2002;105(25):2974–2980. doi: 10.1161/01.cir.0000019071.72887.bd. [DOI] [PubMed] [Google Scholar]
- Fishbein I, Chorny M, Banai S, et al. Formulation and delivery mode affect disposition and activity of tyrphostin-loaded nanoparticles in the rat carotid model. Arterioscler Thromb Vasc Biol. 2001;21(9):1434–1439. doi: 10.1161/hq0901.095567. [DOI] [PubMed] [Google Scholar]
- Fishbein I, Chorny M, Rabinovich L, et al. Nanoparticulate delivery system of a tyrphostin for the treatment of restenosis. Journal of Controlled Release. 2000;65(1–2):221–229. doi: 10.1016/s0168-3659(99)00244-8. [DOI] [PubMed] [Google Scholar]
- Fishbein I, Perlstein I, Levy R. Gene therapy for in-stent restenosis polymeric gene delivery: principles and applications M Amiji. New York: CRC Press; 2005a. [Google Scholar]
- Fishbein I, Stachelek SJ, Connolly JM, et al. Site specific gene delivery in the cardiovascular system. J Control Release. 2005b;109(1–3):37–48. doi: 10.1016/j.jconrel.2005.09.031. [DOI] [PubMed] [Google Scholar]
- Fishbein I, Stachelek SJ, Connolly JM, et al. Site specific gene delivery in the cardiovascular system. Journal of Controlled Release. 2005c;109(1–3):37–48. doi: 10.1016/j.jconrel.2005.09.031. [DOI] [PubMed] [Google Scholar]
- Frimerman A, Welch PJ, Jin X, et al. Chimeric DNA-RNA hammer-head ribozyme to proliferating cell nuclear antigen reduces stent-induced stenosis in a porcine coronary model. Circulation. 1999;99(5):697–703. doi: 10.1161/01.cir.99.5.697. [DOI] [PubMed] [Google Scholar]
- Furman C, Luo Z, Walsh K, et al. Systemic tissue inhibitor of metal-loproteinase-1 gene delivery reduces neointimal hyperplasia in balloon-injured rat carotid artery. FEBS Letters. 2002;531(2):122–126. doi: 10.1016/s0014-5793(02)03388-4. [DOI] [PubMed] [Google Scholar]
- Gaspardone A, Versaci F. Coronary stenting and inflammation. Am J Cardiol. 2005;96(12A):65L–70L. doi: 10.1016/j.amjcard.2005.09.064. [DOI] [PubMed] [Google Scholar]
- Goldberg SL, Loussararian A, De Gregorio J, et al. Predictors of diffuse and aggressive intra-stent restenosis. J Am Coll Cardiol. 2001;37(4):1019–25. doi: 10.1016/s0735-1097(01)01107-x. [DOI] [PubMed] [Google Scholar]
- Gradus-Pizlo I, Wilensky RL, March KL, et al. Local delivery of biodegradable microparticles containing colchicine or a colchicine analogue: Effects on restenosis and implications for catheter-based drug delivery. Journal of the American College of Cardiology. 1995;26(6):1549–1557. doi: 10.1016/0735-1097(95)00345-2. [DOI] [PubMed] [Google Scholar]
- Gruenberg J. The endocytic pathway: a mosaic of domains. Nature Reviews. Molecular Cell Biology. 2001;2(10):721–730. doi: 10.1038/35096054. [DOI] [PubMed] [Google Scholar]
- Guzman RJ, Hirschowitz EA, Brody SL, et al. In vivo suppression of injury-induced vascular smooth muscle cell accumulation using adenovirus-mediated transfer of the herpes simplex virus thymidine kinase gene. PNAS. 1994;91(22):10732–10736. doi: 10.1073/pnas.91.22.10732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell RL, Rajanayagam MAS, Doanes AM, et al. Inhibition of vascular smooth muscle cell proliferation and neointimal accumulation by adenovirus-mediated gene transfer of cytosine deaminase. Circulation. 1997;96(2):621–627. doi: 10.1161/01.cir.96.2.621. [DOI] [PubMed] [Google Scholar]
- Hedman M, Hartikainen J, Syvanne M, et al. Safety and feasibility of catheter-based local intracoronary vascular endothelial growth factor gene transfer in the prevention of postangioplasty and in-stent restenosis and in the treatment of chronic myocardial ischemia: Phase II results of the kuopio angiogenesis trial (KAT) Circulation. 2003;107(21):2677–2683. doi: 10.1161/01.CIR.0000070540.80780.92. [DOI] [PubMed] [Google Scholar]
- Hiltunen MO, Laitinen M, Turunen MP, et al. Intravascular adeno-virus-mediated vegf-c gene transfer reduces neointima formation in balloon-denuded rabbit aorta. Circulation. 2000;102(18):2262–2268. doi: 10.1161/01.cir.102.18.2262. [DOI] [PubMed] [Google Scholar]
- Hoffmann R, Mintz GS, Dussaillant GR, et al. Patterns and mechanisms of in-stent restenosis. A serial intravascular ultrasound study. Circulation. 1996;94(6):1247–54. doi: 10.1161/01.cir.94.6.1247. [DOI] [PubMed] [Google Scholar]
- Horvath C, Welt FGP, Nedelman M, et al. Targeting CCR2 or CD18 inhibits experimental in-stent restenosis in primates: Inhibitory potential depends on type of injury and leukocytes targeted. Circ Res. 2002;90(4):488–494. doi: 10.1161/hh0402.105956. [DOI] [PubMed] [Google Scholar]
- Iwata A, Sai S, Moore M, et al. Gene therapy of transplant arteriopathy by liposome-mediated transfection of endothelial nitric oxide synthase. The Journal of Heart and Lung Transplantation. 2000;19(11):1017–1028. doi: 10.1016/s1053-2498(00)00200-x. [DOI] [PubMed] [Google Scholar]
- Iwata A, Sai S, Nitta Y, et al. Liposome-mediated gene transfection of endothelial nitric oxide synthase reduces endothelial activation and leukocyte infiltration in transplanted hearts. Circulation. 2001;103(22):2753–2759. doi: 10.1161/01.cir.103.22.2753. [DOI] [PubMed] [Google Scholar]
- Janssens S, Flaherty D, Nong Z, et al. Human endothelial nitric oxide synthase gene transfer inhibits vascular smooth muscle cell proliferation and neointima formation after balloon injury in rats. Circulation. 1998;97(13):1274–1281. doi: 10.1161/01.cir.97.13.1274. [DOI] [PubMed] [Google Scholar]
- Jun HW, West JL. Endothelialization of microporous YIGSR/PEG-modified polyurethaneurea. Tissue Engineering. 2005;11(7–8):1133–1140. doi: 10.1089/ten.2005.11.1133. [DOI] [PubMed] [Google Scholar]
- Kalra M, Jost CJ, Severson SR, et al. Adventitial versus intimal liposome-mediated ex vivo transfection of canine saphenous vein grafts with endothelial nitric oxide synthase gene. Journal of Vascular Surgery. 2000;32(6):1190–1200. doi: 10.1067/mva.2000.109211. [DOI] [PubMed] [Google Scholar]
- Kandzari DE, Roe MT, Ohman EM, et al. Frequency, predictors, and outcomes of drug-eluting stent utilization in patients with high-risk Non-ST-Segment elevation acute coronary syndromes. The American Journal of Cardiology. 2005;96(6):750–755. doi: 10.1016/j.amjcard.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Kastrati A, Mehilli J, Dirschinger J, et al. Restenosis after coronary placement of various stent types. The American Journal of Cardiology. 2001;87(1):34–39. doi: 10.1016/s0002-9149(00)01268-6. [DOI] [PubMed] [Google Scholar]
- Kawauchi M, Suzuki JI, Morishita R, et al. Gene therapy for attenuating cardiac allograft arteriopathy using ex vivo E2F Decoy transfection by HVJ-AVE-Liposome method in mice and nonhuman primates. Circ Res. 2000;87(11):1063–1068. doi: 10.1161/01.res.87.11.1063. [DOI] [PubMed] [Google Scholar]
- Kelly K, Nahrendorf M, Yu A, et al. In vivo phage display selection yields atherosclerotic plaque targeted peptides for imaging. Molecular Imaging and Biology. 2006;8(4):201–207. doi: 10.1007/s11307-006-0043-6. [DOI] [PubMed] [Google Scholar]
- Kittleson MM, Needham DM, Kim SJ, et al. The efficacy of sirolimus- and paclitaxeleluting stents: a meta-analysis of randomized controlled trials. Can J Cardiol. 2005;21(7):581–7. [PubMed] [Google Scholar]
- Koledova VV, Khalil RA. Ca2+, Calmodulin, and cyclins in vascular smooth muscle cell cycle. Circ Res. 2006;98(10):1240–1243. doi: 10.1161/01.RES.0000225860.41648.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollum M, Farb A, Schreiber R, et al. Particle debris from a nanoporous stent coating obscures potential antiproliferative effects of tacrolimus-eluting stents in a porcine model of restenosis. Catheterization and Cardiovascular Interventions. 2005;64(1):85–90. doi: 10.1002/ccd.20213. [DOI] [PubMed] [Google Scholar]
- Kolodgie FD, John M, Khurana C, et al. Sustained reduction of in-stent neointimal growth with the use of a novel systemic nanoparticle paclitaxel. Circulation. 2002;106(10):1195–1198. doi: 10.1161/01.cir.0000032141.31476.15. [DOI] [PubMed] [Google Scholar]
- Kong D, Melo LG, Mangi AA, et al. Enhanced inhibition of neointimal hyperplasia by genetically engineered endothelial progenitor cells. Circulation. 2004;109(14):1769–1775. doi: 10.1161/01.CIR.0000121732.85572.6F. [DOI] [PubMed] [Google Scholar]
- Kornowski R, Hong MK, Tio FO, et al. In-stent restenosis: contributions of inflammatory responses and arterial injury to neointimal hyperplasia. J Am Coll Cardiol. 1998;31(1):224–30. doi: 10.1016/s0735-1097(97)00450-6. [DOI] [PubMed] [Google Scholar]
- Kuhnl A, Pelisek J, Tian W, et al. C-type natriuretic peptide inhibits constrictive remodeling without compromising re-endothelialization in balloon-dilated renal arteries. Journal of Endovascular Therapy. 2005;12(2):171–182. doi: 10.1583/1384R.1. [DOI] [PubMed] [Google Scholar]
- Kunishima T, Musha H, Eto F, et al. A randomized trial of aspirin versus cilostazol therapy after successful coronary stent implantation. Clinical Therapeutics. 1997;19(5):1058–1066. doi: 10.1016/s0149-2918(97)80058-6. [DOI] [PubMed] [Google Scholar]
- Labhasetwar V, Song C, Humphrey W, et al. Arterial uptake of biodegradable nanoparticles: Effect of surface modifications. Journal of Pharmaceutical Sciences. 1998;87(10):1229–1234. doi: 10.1021/js980021f. [DOI] [PubMed] [Google Scholar]
- Laitinen M, Zachary I, Breier G, et al. VEGF gene transfer reduces intimal thickening via increased production of nitric oxide in carotid arteries. Hum Gene Ther. 1997;10(8):1737–44. doi: 10.1089/hum.1997.8.15-1737. [DOI] [PubMed] [Google Scholar]
- Lanza GM, Yu X, Winter PM, et al. Targeted antiproliferative drug delivery to vascular smooth muscle cells with a magnetic resonance imaging nanoparticle contrast agent: Implications for rational therapy of restenosis. Circulation. 2002;106(22):2842–2847. doi: 10.1161/01.cir.0000044020.27990.32. [DOI] [PubMed] [Google Scholar]
- Lenter M, Garidel P, Pelisek J, et al. Stabilized nonviral formulations for the delivery of MCP-1 gene into cells of the vasculoendothelial system. Pharmaceutical Research. 2004;21(4):683–691. doi: 10.1023/b:pham.0000022416.33048.81. [DOI] [PubMed] [Google Scholar]
- Lethbridge-Çejku M, Rose D, Vickerie J. Summary health statistics for US Adults: National Health Interview Survey, 2004. National Center for Health Statistics. Vital Health Stat. 2006;10(228) [PubMed] [Google Scholar]
- Levitzki A. PDGF receptor kinase inhibitors for the treatment of restenosis. Cardiovascular Research. 2005;65(3):581–586. doi: 10.1016/j.cardiores.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Libby P, Tanaka H. The molecular bases of restenosis. Prog Cardiovasc Dis. 1997;40(2):97–106. doi: 10.1016/s0033-0620(97)80002-3. [DOI] [PubMed] [Google Scholar]
- Liu Q, Chen ZQ, Bobustuc GC, et al. Local gene transduction of cyclooxygenase-1 increases blood flow in injured atherosclerotic rabbit arteries. Circulation. 2005;111(14):1833–1840. doi: 10.1161/01.CIR.0000158480.28733.89. [DOI] [PubMed] [Google Scholar]
- Lowe HC, Fahmy RG, Kavurma MM, et al. Catalytic oligodeoxy-nucleotides define a key regulatory role for early growth response factor-1 in the porcine model of coronary in-stent restenosis. Circ Res. 2001;89(8):670–677. doi: 10.1161/hh2001.097867. [DOI] [PubMed] [Google Scholar]
- Magaraggia M, Visona A, Furlan A, et al. Inactivation of vascular smooth muscle cells photosensitised by liposome-delivered Zn(II)-phthalocyanine. Journal of Photochemistry and Photobiology B: Biology. 2006;82(1):53–58. doi: 10.1016/j.jphotobiol.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Maillard L, Van Belle E, Smith RC, et al. Percutaneous delivery of the gax gene inhibits vessel stenosis in a rabbit model of balloon angioplasty. Cardiovascular Research. 1997;35(3):536–546. doi: 10.1016/s0008-6363(97)00147-8. [DOI] [PubMed] [Google Scholar]
- Maillard L, Van Belle E, Tio FO, et al. Effect of percutaneous adenovirus-mediated Gax gene delivery to the arterial wall in double-injured atheromatous stented rabbit iliac arteries. Gene Therapy. 2000;7(16):1353–61. doi: 10.1038/sj.gt.3301255. [DOI] [PubMed] [Google Scholar]
- Mano T, Luo Z, Malendowicz SL, et al. Reversal of GATA-6 downregulation promotes smooth muscle differentiation and inhibits intimal hyperplasia in balloon-injured rat carotid artery. Circ Res. 1999;84(6):647–654. doi: 10.1161/01.res.84.6.647. [DOI] [PubMed] [Google Scholar]
- Mano T, Luo Z, Suhara T, et al. Expression of wild-type and non-cleavable fas ligand by tetracycline-regulated adenoviral vectors to limit intimal hyperplasia in vascular lesions. Human Gene Therapy. 2000;11(12):1625–1635. doi: 10.1089/10430340050111287. [DOI] [PubMed] [Google Scholar]
- Mardilovich A, Craig JA, McCammon MQ, et al. Design of a novel fibronectin-mimetic peptide-amphiphile for functionalized biomaterials. Langmuir. 2006;22(7):3259–3264. doi: 10.1021/la052756n. [DOI] [PubMed] [Google Scholar]
- Marx SO, Jayaraman T, Go LO, et al. Rapamycin-FKBP inhibits cell cycle regulators of proliferation in vascular smooth muscle cells. Circ Res. 1995;76(3):412–417. doi: 10.1161/01.res.76.3.412. [DOI] [PubMed] [Google Scholar]
- Meng QH, Jamal W, Hart SL, et al. Application to vascular adventitia of a nonviral vector for TIMP-1 gene therapy to prevent intimal hyperplasia. Human Gene Therapy. 2006;17:1–11. doi: 10.1089/hum.2006.17.717. [DOI] [PubMed] [Google Scholar]
- Mooradian DL, Hutsell TC, Keefer LK. Nitric oxide (NO) donor molecules: effect of NO release rate on vascular smooth muscle cell proliferation in vitro. J Cardiovasc Pharmacol. 1995;25(4):674–8. [PubMed] [Google Scholar]
- Morishita R. Perspective in progress of cardiovascular gene therapy. J Pharmacol Sci. 2004;95(1):1–8. doi: 10.1254/jphs.95.1. [DOI] [PubMed] [Google Scholar]
- Morishita R, Gibbons GH, Ellison KE, et al. Evidence for direct local effect of angiotensin in vascular hypertrophy. In vivo gene transfer of angiotensin converting enzyme. J Clin Invest. 1994;94(3):978–984. doi: 10.1172/JCI117464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita R, Gibbons GH, Horiuchi M, et al. A gene therapy strategy using a transcription factor decoy of the e2f binding site inhibits smooth muscle proliferation in vivo. PNAS. 1995;92(13):5855–5859. doi: 10.1073/pnas.92.13.5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi SA, Malerod L, Berg T, et al. 2004Clathrin-dependent endocytosis J Biochem 377(Pt 1)1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhs A, Heublein B, Schletter J, et al. Preclinical evaluation of inducible nitric oxide synthase lipoplex gene therapy for inhibition of stent-induced vascular neointimal lesion formation. Human Gene Therapy. 2003;14(4):375–383. doi: 10.1089/104303403321208970. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Ghosh RN, Maxfield FR. Endocytosis. Physiological Reviews. 1997;77(3):759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- Muro S, Dziubla T, Qiu W, et al. Endothelial targeting of high-affinity multivalent polymer nanocarriers directed to intercellular adhesion molecule 1. J Pharmacol Exp Ther. 2006;317(3):1161–1169. doi: 10.1124/jpet.105.098970. [DOI] [PubMed] [Google Scholar]
- Nakatani M, Takeyama Y, Shibata M, et al. Mechanisms of restenosis after coronary intervention: difference between plain old balloon angioplasty and stenting. Cardiovasc Pathol. 2003;12(1):40–8. doi: 10.1016/s1054-8807(02)00135-7. [DOI] [PubMed] [Google Scholar]
- Nawarskas JJ, Osborn LA. Paclitaxel-eluting stents in coronary artery disease. Am J Health Syst Pharm. 2005;62(21):2241–51. doi: 10.2146/ajhp040621. [DOI] [PubMed] [Google Scholar]
- Numaguchi Y, Okumura K, Harada M, et al. Catheter-based prostacyclin synthase gene transfer prevents in-stent restenosis in rabbit atheromatous arteries. Cardiovascular Research. 2004;61(1):177–185. doi: 10.1016/j.cardiores.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Ogata H, Mishio M, Luo XX, et al. Significance of elevated cytochrome aa3 in a state of endotoxemia in dogs. Resuscitation. 1996;33(1):63–68. doi: 10.1016/s0300-9572(96)00984-7. [DOI] [PubMed] [Google Scholar]
- Ohno T, Gordon D, San H, et al. Gene therapy for vascular smooth muscle cell proliferation after arterial injury. Science. 1994;265(5173):781–784. doi: 10.1126/science.8047883. [DOI] [PubMed] [Google Scholar]
- Pache J, Kastrati A, Mehilli J, et al. Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO-2) trial. J Am Coll Cardiol. 2003;41(8):1283–8. doi: 10.1016/s0735-1097(03)00119-0. [DOI] [PubMed] [Google Scholar]
- Panyam J, Lof J, O’Leary E, et al. Efficiency of Dispatch® and Infiltrator® Cardiac Infusion Catheters in Arterial Localization of Nanoparticles in a Porcine Coronary Model of Restenosis. Journal of Drug Targeting. 2002;10(6):515–523. doi: 10.1080/1061186021000038391. [DOI] [PubMed] [Google Scholar]
- Pelisek J, Fuchs AT, Kuehnl A, et al. C-type natriuretic peptide for reduction of restenosis:gene transfer is superior over single peptide administration. The Journal of Gene Medicine. 2006;8(7):835–844. doi: 10.1002/jgm.905. [DOI] [PubMed] [Google Scholar]
- Pelkmans L, Helenius A. Endocytosis via caveolae. Traffic. 2002;3(5):311–320. doi: 10.1034/j.1600-0854.2002.30501.x. [DOI] [PubMed] [Google Scholar]
- Perin EC. Choosing a drug-eluting stent: a comparison between CYPHER and TAXUS. Rev Cardiovasc Med. 2005;6(Suppl 1):S13–21. [PubMed] [Google Scholar]
- Pfeiffer T, Wallich M, Sandmann W, et al. Lipoplex gene transfer of inducible nitric oxide synthase inhibits the reactive intimal hyperplasia after expanded polytetrafluoroethylene bypass grafting. Journal of Vascular Surgery. 2006;43(5):1021–1027. doi: 10.1016/j.jvs.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Puhakka HL, Turunen P, Gruchala M, et al. Effects of vaccinia virus anti-inflammatory protein 35K and TIMP-1 gene transfers on vein graft stenosis in rabbits. In Vivo. 2005a;19(3):515–21. [PubMed] [Google Scholar]
- Puhakka HL, Turunen P, Rutanen J, et al. Tissue Inhibitor of Metalloproteinase 1 adenoviral gene therapy alone is equally effective in reducing restenosis as combination gene therapy in a rabbit restenosis model. Journal of Vascular Research. 2005b;42(5):361–367. doi: 10.1159/000087120. [DOI] [PubMed] [Google Scholar]
- Qiao T, Liu CJ, Ran F, et al. Experimental study of recombinant eukaryotic expression vector of human eNOS in ECV304. Swiss Med Wkly. 2006;136(1–2):19–25. doi: 10.4414/smw.2006.10910. [DOI] [PubMed] [Google Scholar]
- Rade J, Schulick A, Virmani R, et al. Local adenoviral-mediated expression of recombinant hirudin reduces neointima formation after arterial injury. Nat Med. 1996;2(3):293–8. doi: 10.1038/nm0396-293. [DOI] [PubMed] [Google Scholar]
- Ranade SV, Miller KM, Richard RE, et al. Physical characterization of controlled release of paclitaxel from the TAXUS Express2 drug-eluting stent. J Biomed Mater Res, A. 2004;71(4):625–34. doi: 10.1002/jbm.a.30188. [DOI] [PubMed] [Google Scholar]
- Ring ME. How a dentist’s name became a synonym for a life-saving device: the story of Dr. Charles Stent. J Hist Dent. 2001;49(2):77–80. [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362(6423):801–9. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Rutanen J, Markkanen J, Yla-Herttuala S. Gene therapy for restenosis: current status. Drugs. 2002;62(11):1575–85. doi: 10.2165/00003495-200262110-00001. [DOI] [PubMed] [Google Scholar]
- Schatz RA, Baim DS, Leon M, et al. Clinical experience with the Palmaz-Schatz coronary stent. Initial results of a multicenter study. Circulation. 1991;83(1):148–161. doi: 10.1161/01.cir.83.1.148. [DOI] [PubMed] [Google Scholar]
- Scheinman MAE, Kallakuri S, Hingorani A, et al. P53 gene transfer to the injured rat carotid artery promotes apoptosis. Surgery. 1999;126(5):863–868. [PubMed] [Google Scholar]
- Scheller B, Hehrlein C, Bocksch W, et al. Treatment of coronary in-stent restenosis with a paclitaxel-coated balloon catheter. N Engl J Med. 2006;355(20):2113–2124. doi: 10.1056/NEJMoa061254. [DOI] [PubMed] [Google Scholar]
- Scott NA. Restenosis following implantation of bare metal coronary stents: Pathophysiology and pathways involved in the vascular response to injury. Advanced Drug Delivery Reviews. 2006;58(3):358–376. doi: 10.1016/j.addr.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Serruys PW, Unger F, Sousa JE, et al. Comparison of coronary-artery bypass surgery and stenting for the treatment of multivessel disease. N Engl J Med. 2001;344(15):1117–1124. doi: 10.1056/NEJM200104123441502. [DOI] [PubMed] [Google Scholar]
- Shears LL, 2nd, Kibbe MR, Murdock AD, et al. Efficient inhibition of intimal hyperplasia by adenovirus-mediated inducible nitric oxide synthase gene transfer to rats and pigs in vivo. J Am Coll Surg. 1998;187(3):295–306. doi: 10.1016/s1072-7515(98)00163-x. [DOI] [PubMed] [Google Scholar]
- Shyue S-K, Tsai M-J, Liou J-Y, et al. Selective augmentation of prostacyclin production by combined prostacyclin synthase and cyclooxygenase-1 gene transfer. Circulation. 2001;103(16):2090–2095. doi: 10.1161/01.cir.103.16.2090. [DOI] [PubMed] [Google Scholar]
- Sieczkarski SB, Whittaker GR.2002Dissecting virus entry via endocytosis The Journal Of General Virology 83(Part 7)1535–1545. [DOI] [PubMed] [Google Scholar]
- Simari RD, San H, Rekhter M, et al. Regulation of cellular proliferation and intimal formation following balloon injury in atherosclerotic rabbit arteries. J Clin Invest. 1996;98(1):225–235. doi: 10.1172/JCI118770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnaeve P, Chiche J-D, Gillijns H, et al. Overexpression of a constitutively active protein kinase g mutant reduces neointima formation and in-stent restenosis. Circulation. 2002;105(24):2911–2916. doi: 10.1161/01.cir.0000018169.59205.ca. [DOI] [PubMed] [Google Scholar]
- Sollott SJ, Cheng L, Pauly RR, et al. Taxol inhibits neointimal smooth muscle cell accumulation after angioplasty in the rat. J Clin Invest. 1995;95(4):1869–1876. doi: 10.1172/JCI117867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram V, Patterson C. Cell cycle in vasculoproliferative diseases: Potential interventions and routes of delivery. Circulation. 2001;103(19):2414–2419. doi: 10.1161/01.cir.103.19.2414. [DOI] [PubMed] [Google Scholar]
- Stephan DJ, San H, Yang ZY, et al. Inhibition of vascular smooth muscle cell proliferation and intimal hyperplasia by gene transfer of beta-interferon. Molecular Medicine. 1997;3(9):593–9. [PMC free article] [PubMed] [Google Scholar]
- Stephan DJ, Yang ZY, San H, et al. A new cationic liposome DNA complex enhances the efficiency of arterial gene transfer in vivo. Hum Gene Ther. 1996;7(15):1803–12. doi: 10.1089/hum.1996.7.15-1803. [DOI] [PubMed] [Google Scholar]
- Stone GW, Ellis SG, Cox DA, et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med. 2004;350(3):221–231. doi: 10.1056/NEJMoa032441. [DOI] [PubMed] [Google Scholar]
- Tahlil O, Brami M, Feldman LJ, et al. The dispatchTM catheter as a delivery tool for arterial gene transfer. Cardiovascular Research. 1997;33(1):181–187. doi: 10.1016/s0008-6363(96)00188-5. [DOI] [PubMed] [Google Scholar]
- Take S, Matsutani M, Ueda H, et al. Effect of cilostazol in preventing restenosis after percutaneous transluminal coronary angioplasty. The American Journal of Cardiology. 1997;79(8):1097–1099. doi: 10.1016/s0002-9149(97)00052-0. [DOI] [PubMed] [Google Scholar]
- Tamai H, Igaki K, Kyo E, et al. Initial and 6-Month Results of Biodegradable poly-l-lactic acid coronary stents in humans. Circulation. 2000;102(4):399–404. doi: 10.1161/01.cir.102.4.399. [DOI] [PubMed] [Google Scholar]
- Tanner FC, Boehm M, Akyurek LM, et al. Differential effects of the cyclin-dependent kinase inhibitors p27Kip1, p21Cip1, and p16Ink4 on Vascular Smooth Muscle Cell Proliferation. Circulation. 2000;101(17):2022–2025. doi: 10.1161/01.cir.101.17.2022. [DOI] [PubMed] [Google Scholar]
- Todaka T, Yokoyama C, Yanamoto H, et al. Gene transfer of human prostacyclin synthase prevents neointimal formation after carotid balloon injury in rats editorial Comment. Stroke. 1999;30(2):419–426. doi: 10.1161/01.str.30.2.419. [DOI] [PubMed] [Google Scholar]
- Topol EJ. Coronary-artery stents—gauging, gorging, and gouging. N Engl J Med. 1998;339(23):1702–1704. doi: 10.1056/NEJM199812033392311. [DOI] [PubMed] [Google Scholar]
- Tsui LV, Camrud A, Mondesire J, et al. p27-p16 Fusion gene inhibits angioplasty-induced neointimal hyperplasia and coronary artery occlusion. Circ Res. 2001;89(4):323–328. doi: 10.1161/hh1601.094482. [DOI] [PubMed] [Google Scholar]
- Tsutsui M, Shimokawa H, Higuchi S, et al. Effect of cilostazol, a novel anti-platelet drug, on restenosis after percutaneous transluminal coronary angioplasty. Jpn Circ J. 1996;60(4):207–15. doi: 10.1253/jcj.60.207. [DOI] [PubMed] [Google Scholar]
- Tulis DA, Durante W, Liu X, et al. Adenovirus-Mediated Heme Oxygenase-1 Gene Delivery Inhibits Injury-Induced Vascular Neointima Formation. Circulation. 2001;104(22):2710–2715. doi: 10.1161/hc4701.099585. [DOI] [PubMed] [Google Scholar]
- Tung R, Kaul S, Diamond GA, et al. Narrative review: drug-eluting stents for the management of restenosis: A Critical appraisal of the evidence. Ann Intern Med. 2006a;144(12):913–919. doi: 10.7326/0003-4819-144-12-200606200-00009. [DOI] [PubMed] [Google Scholar]
- Tung R, Kaul S, Diamond GA, et al. Narrative review: Drug-eluting stents for the management of restenosis: a critical appraisal of the evidence. Annals of Internal Medicine. 2006b;144(12):913–W215. doi: 10.7326/0003-4819-144-12-200606200-00009. [DOI] [PubMed] [Google Scholar]
- Turunen MP, Puhakka HL, Koponen JK, et al. Peptide-retargeted adenovirus encoding a tissue inhibitor of metalloproteinase-1 decreases restenosis after intravascular gene transfer. Mol Ther. 2002;6(3):306–12. doi: 10.1006/mthe.2002.0668. [DOI] [PubMed] [Google Scholar]
- Turunen P, Puhakka HL, Heikura T, et al. Extracellular superoxide dismutase with vaccinia virus anti-inflammatory protein 35K or tissue inhibitor of metalloproteinase-1: Combination gene therapy in the treatment of vein graft stenosis in rabbits. Hum Gene Ther. 2006;17(4):405–14. doi: 10.1089/hum.2006.17.405. [DOI] [PubMed] [Google Scholar]
- Ueno H, Yamamoto H, Ito SI, et al. Adenovirus-mediated transfer of a dominant-negative h-ras suppresses neointimal formation in balloon-injured arteries in vivo. Arterioscler Thromb Vasc Biol. 1997;17(5):898–904. doi: 10.1161/01.atv.17.5.898. [DOI] [PubMed] [Google Scholar]
- Uwatoku T, Shimokawa H, Abe K, et al. Application of nanoparticle technology for the prevention of restenosis after balloon injury in rats. Circ Res. 2003;92(7):62e–69. doi: 10.1161/01.RES.0000069021.56380.E2. [DOI] [PubMed] [Google Scholar]
- Van Belle E, Tio FO, Chen D, et al. Passivation of metallic stents after arterial gene transfer of phVEGF165 inhibits thrombus formation and intimal thickening. Journal of the American College of Cardiology. 1997;29(6):1371–1379. doi: 10.1016/s0735-1097(97)00049-1. [DOI] [PubMed] [Google Scholar]
- Varenne O, Pislaru S, Gillijns H, et al. Local adenovirus-mediated transfer of human endothelial nitric oxide synthase reduces luminal narrowing after coronary angioplasty in pigs. Circulation. 1998;98(9):919–926. doi: 10.1161/01.cir.98.9.919. [DOI] [PubMed] [Google Scholar]
- Virmani R, Farb A, Guagliumi G, et al. Drug-eluting stents: caution and concerns for long-term outcome. Coron Artery Dis. 2004;15(6):313–8. doi: 10.1097/00019501-200409000-00003. [DOI] [PubMed] [Google Scholar]
- Vishnevetsky D, Patel P, Tijerino H, et al. Sirolimus-eluting coronary stent. Am J Health Syst Pharm. 2004;61(5):449–56. doi: 10.1093/ajhp/61.5.449. [DOI] [PubMed] [Google Scholar]
- Voisard R, Dartsch PC, Seitzer U, et al. The in-vitro effect of antineoplastic agents on proliferative activity and cytoskeletal components of plaque-derived smooth-muscle cells from human coronary arteries. Coron Artery Dis. 1993;4(10):935–42. doi: 10.1097/00019501-199310000-00014. [DOI] [PubMed] [Google Scholar]
- Voisard R, Seitzer U, Baur R, et al. A prescreening system for potential antiproliferative agents: implications for local treatment strategies of postangioplasty restenosis. International Journal of Cardiology. 1995;51(1):15–28. doi: 10.1016/0167-5273(95)02377-9. [DOI] [PubMed] [Google Scholar]
- von der Leyen HE, Gibbons GH, Morishita R, et al. Gene therapy inhibiting neointimal vascular lesion: in vivo transfer of endothelial cell nitric oxide synthase gene. PNAS. 1995;92(4):1137–1141. doi: 10.1073/pnas.92.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waksman R. Biodegradable stents: they do their job and disappear. J Invasive Cardiol. 2006;18(2):70–4. [PubMed] [Google Scholar]
- Wang K, Kessler PD, Zhou Z, et al. Local adenoviral-mediated inducible nitric oxide synthase gene transfer inhibits neointimal formation in the porcine coronary stented model. Molecular Therapy. 2003;7(5):597–603. doi: 10.1016/s1525-0016(03)00061-3. [DOI] [PubMed] [Google Scholar]
- Weissleder R, Kelly K, Sun EY, et al. Cell-specific targeting of nanoparticles by multivalent attachment of small molecules. Nat Biotech. 2005;23(11):1418–1423. doi: 10.1038/nbt1159. [DOI] [PubMed] [Google Scholar]
- Welt FGP, Edelman ER, Simon DI, et al. Neutrophil, not macrophage, infiltration precedes neointimal thickening in balloon-injured arteries. Arterioscler Thromb Vasc Biol. 2000;20(12):2553–2558. doi: 10.1161/01.atv.20.12.2553. [DOI] [PubMed] [Google Scholar]
- Welt FGP, Rogers C. Inflammation and restenosis in the stent era. Arterioscler Thromb Vasc Biol. 2002;22(11):1769–1776. doi: 10.1161/01.atv.0000037100.44766.5b. [DOI] [PubMed] [Google Scholar]
- Westedt U, Barbu-Tudoran L, Schaper AK, et al. 2002Deposition of nanoparticles in the arterial vessel by porous balloon catheters: Localization by confocal laser scanning microscopy and transmission electron microscopy AAPS PharmSci 44Article 41 pages 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westedt U, Barbu-Tudoran L, Schaper AK, et al. Effects of different application parameters on penetration characteristics and arterial vessel wall integrity after local nanoparticle delivery using a porous balloon catheter. Eur J Pharm Biopharm. 2004;58(1):161–8. doi: 10.1016/j.ejpb.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Wieneke H, Dirsch O, Sawitowski T, et al. Synergistic effects of a novel nanoporous stent coating and tacrolimus on intima proliferation in rabbits. Catheterization and Cardiovascular Interventions. 2003;60(3):399–407. doi: 10.1002/ccd.10664. [DOI] [PubMed] [Google Scholar]
- Wills KN, Mano T, Avanzini JB, et al. Tissue-specific expression of an anti-proliferative hybrid transgene from the human smooth muscle alpha-actin promoter suppresses smooth muscle cell proliferation and neointima formation. Gene Therapy. 2001;8(24):1847–54. doi: 10.1038/sj.gt.3301603. [DOI] [PubMed] [Google Scholar]
- Wu GY, Wu CH. Receptor-mediated gene delivery and expression in vivo. J Biol Chem. 1988;263(29):14621–14624. [PubMed] [Google Scholar]
- Wu KK. Prostacyclin and nitric oxide-related gene transfer in preventing arterial thrombosis and restenosis. Agents Actions Suppl. 1997;48:107–23. doi: 10.1007/978-3-0348-7352-9_6. [DOI] [PubMed] [Google Scholar]
- Yin X, Yutani C, Ikeda Y, et al. Tissue factor pathway inhibitor gene delivery using HVJ-AVE liposomes markedly reduces restenosis in atherosclerotic arteries. Cardiovascular Research. 2002;56(3):454–463. doi: 10.1016/s0008-6363(02)00595-3. [DOI] [PubMed] [Google Scholar]
- Yonemitsu Y, Kaneda Y, Tanaka S, et al. Transfer of wild-type p53 gene effectively inhibits vascular smooth muscle cell proliferation in vitro and in vivo. Circ Res. 1998;82(2):147–156. doi: 10.1161/01.res.82.2.147. [DOI] [PubMed] [Google Scholar]
- Zoldhelyi P, Chen ZQ, Shelat HS, et al. Local gene transfer of tissue factor pathway inhibitor regulates intimal hyperplasia in atherosclerotic arteries. PNAS. 2001;98(7):4078–4083. doi: 10.1073/pnas.061004098. [DOI] [PMC free article] [PubMed] [Google Scholar]