Abstract
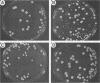
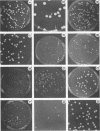
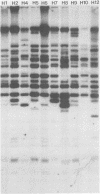
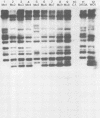
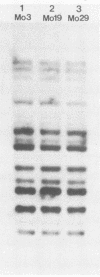
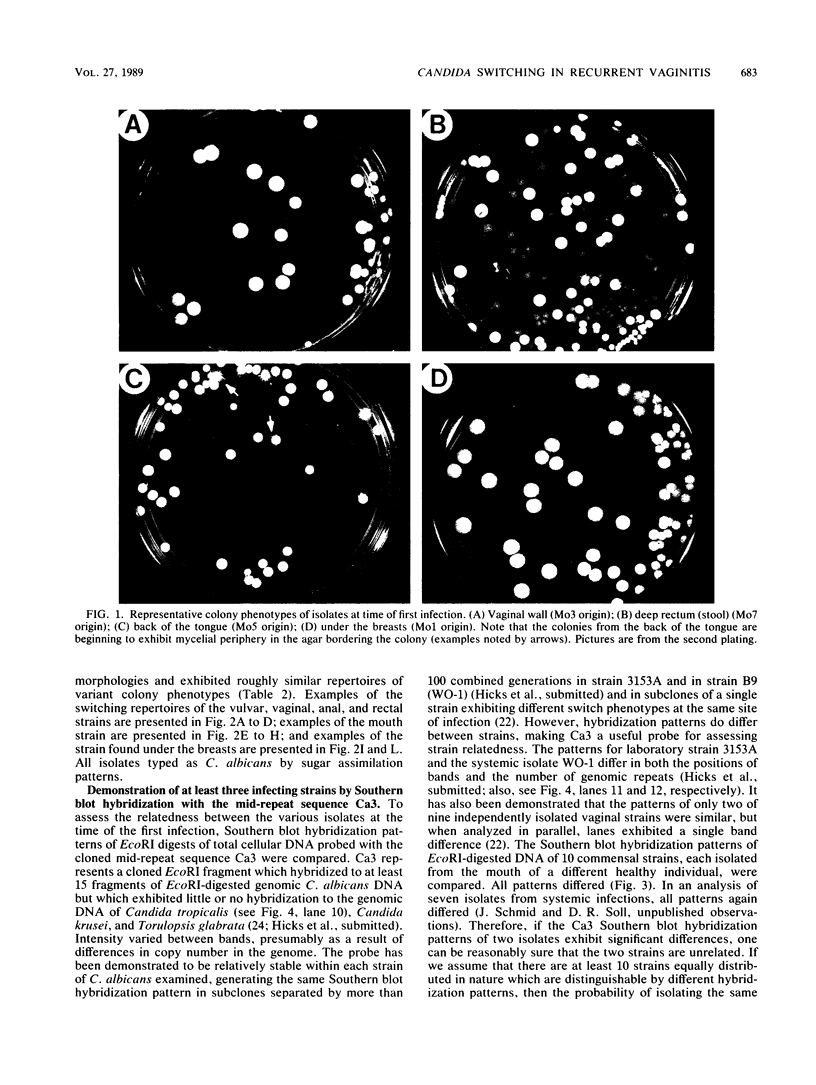
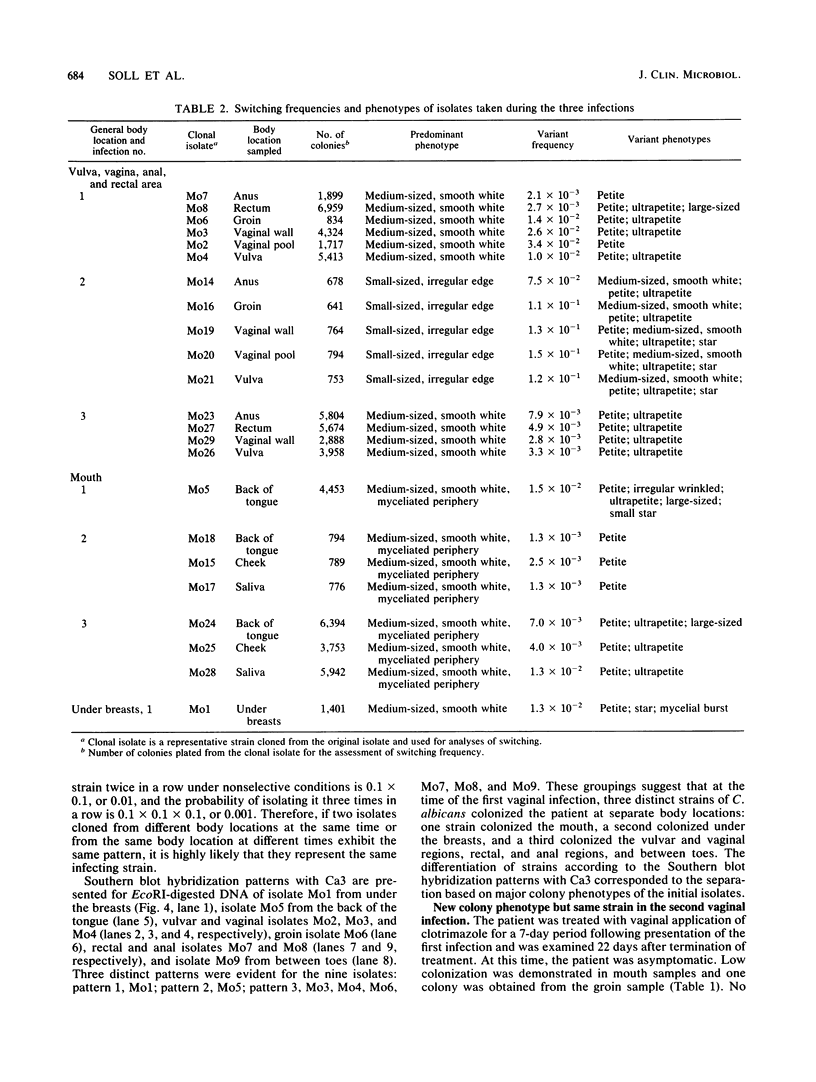
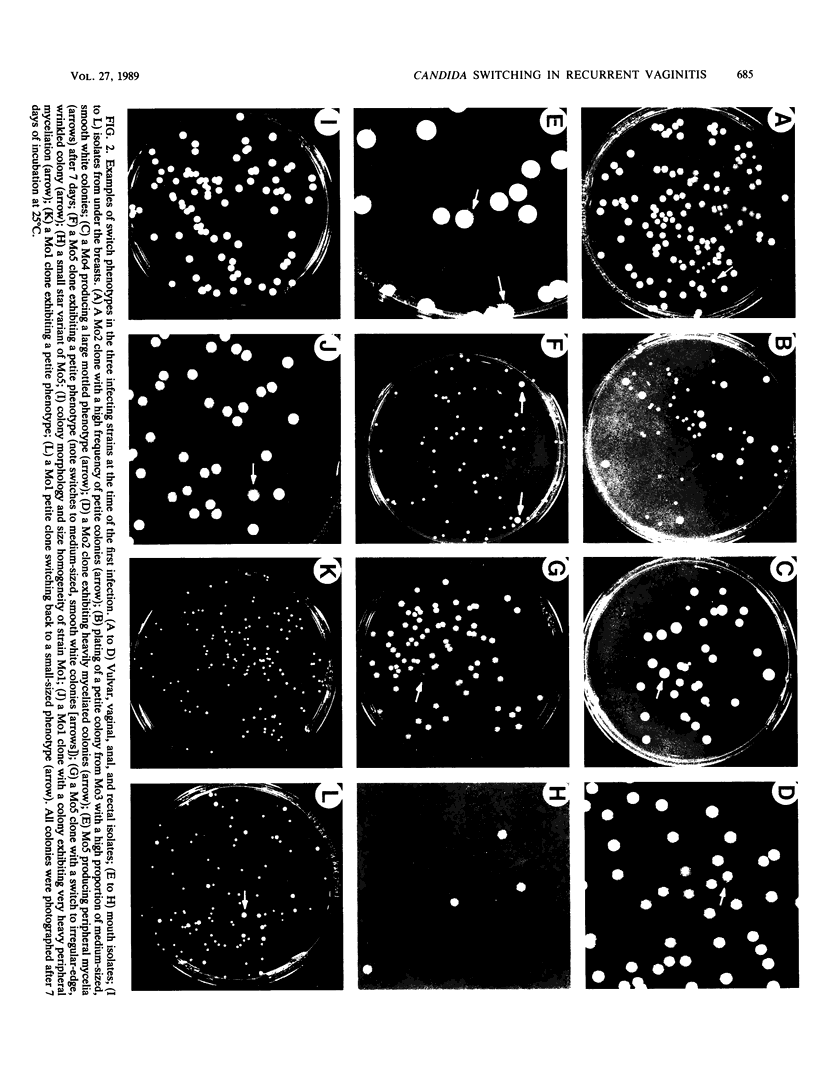
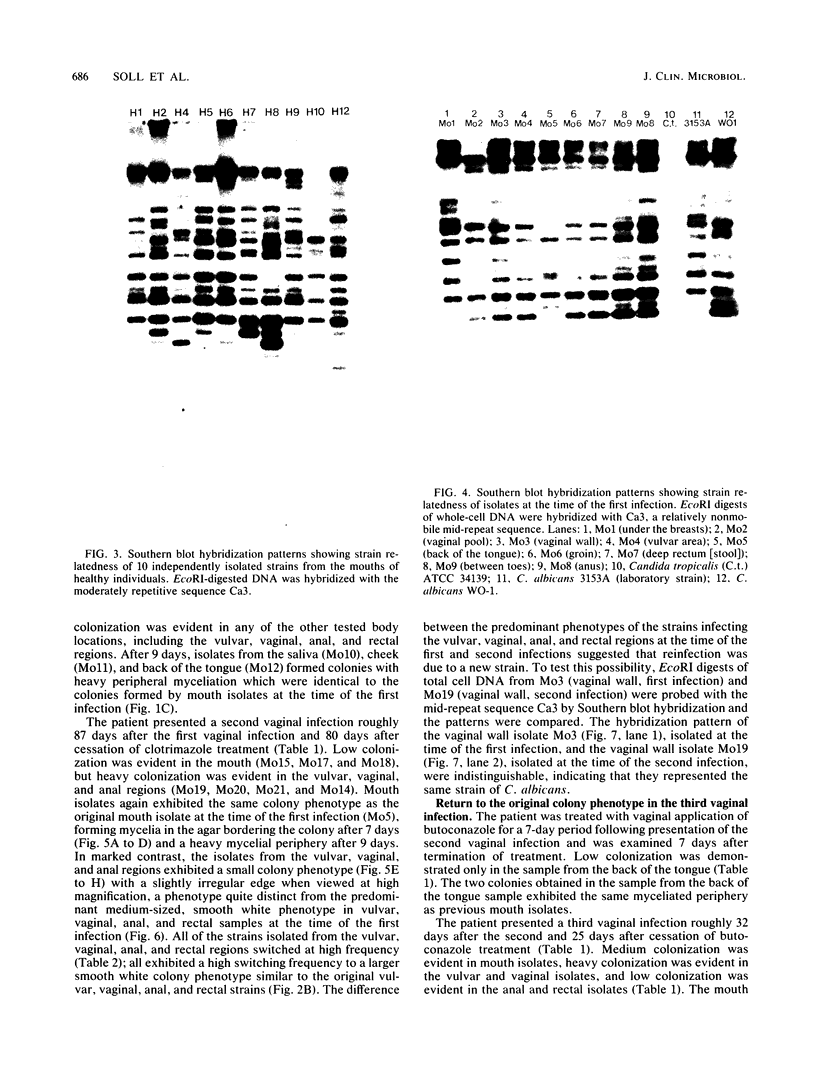
Strain relatedness and switching were monitored in Candida albicans strains isolated from different body locations through three episodes of recurrent vulvovaginal candidiasis separated by two treatment-latency periods in a single patient. Strain relatedness was assessed by comparing Southern blot hybridization patterns with the relatively immobile mid-repeat sequence Ca3. The following conclusions are demonstrated. (i) Three different strains of C. albicans colonized the mouth, the area under the breasts, and the vulvovaginal, anal, and rectal regions, respectively, at the time of the first infection. (ii) The same strain of C. albicans was responsible for the three vaginal infections. (iii) Switching of colony phenotype occurred with each new vaginal infection. (iv) Enrichment of drug-resistant switch phenotypes (assessed in vitro) was unlikely the basis for the changes in the switch phenotypes of the strain found in the vulvovaginal, anal, and rectal areas after treatment of the first infection with clotrimazole. (v) The same strain of C. albicans was responsible for the recurrent increases in mouth colonization and was distinct from the recurrent vaginal strain. The results of this case study demonstrate the need for further detailed analyses of full-body mycofloras, strain relatedness, switching repertoires, and changes in drug susceptibility during successive episodes of recurrent vulvovaginal candidiasis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M., Soll D. R. Unique phenotype of opaque cells in the white-opaque transition of Candida albicans. J Bacteriol. 1987 Dec;169(12):5579–5588. doi: 10.1128/jb.169.12.5579-5588.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedell G. W., Soll D. R. Effects of low concentrations of zinc on the growth and dimorphism of Candida albicans: evidence for zinc-resistant and -sensitive pathways for mycelium formation. Infect Immun. 1979 Oct;26(1):348–354. doi: 10.1128/iai.26.1.348-354.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buch A., Skytte Christensen E. Treatment of vaginal candidosis with natamycin and effect of treating the partner at the same time. Acta Obstet Gynecol Scand. 1982;61(5):393–396. doi: 10.3109/00016348209156578. [DOI] [PubMed] [Google Scholar]
- Cryer D. R., Eccleshall R., Marmur J. Isolation of yeast DNA. Methods Cell Biol. 1975;12:39–44. doi: 10.1016/s0091-679x(08)60950-4. [DOI] [PubMed] [Google Scholar]
- Gough P. M., Warnock D. W., Richardson M. D., Mansell N. J., King J. M. IgA and IgG antibodies to Candida albicans in the genital tract secretions of women with or without vaginal candidosis. Sabouraudia. 1984;22(4):265–271. [PubMed] [Google Scholar]
- Land G. A., Harrison B. A., Hulme K. L., Cooper B. H., Byrd J. C. Evaluation of the new API 20C strip for yeast identification against a conventional method. J Clin Microbiol. 1979 Sep;10(3):357–364. doi: 10.1128/jcm.10.3.357-364.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. L., Buckley H. R., Campbell C. C. An amino acid liquid synthetic medium for the development of mycelial and yeast forms of Candida Albicans. Sabouraudia. 1975 Jul;13(2):148–153. doi: 10.1080/00362177585190271. [DOI] [PubMed] [Google Scholar]
- Miles M. R., Olsen L., Rogers A. Recurrent vaginal candidiasis. Importance of an intestinal reservoir. JAMA. 1977 Oct 24;238(17):1836–1837. doi: 10.1001/jama.238.17.1836. [DOI] [PubMed] [Google Scholar]
- Odds F. C., Abbott A. B. A simple system for the presumptive identification of Candida albicans and differentiation of strains within the species. Sabouraudia. 1980 Dec;18(4):301–317. [PubMed] [Google Scholar]
- Odds F. C., Abbott A. B. Modification and extension of tests for differentiation of Candida species and strains. Sabouraudia. 1983 Mar;21(1):79–81. doi: 10.1080/00362178385380111. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rikkerink E. H., Magee B. B., Magee P. T. Opaque-white phenotype transition: a programmed morphological transition in Candida albicans. J Bacteriol. 1988 Feb;170(2):895–899. doi: 10.1128/jb.170.2.895-899.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer S., Stevens D. A. A Candida albicans dispersed, repeated gene family and its epidemiologic applications. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1452–1456. doi: 10.1073/pnas.85.5.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorepová M., Hauck H. Differentiation of Candida albicans biotypes by the method of Odds and Abbott. Mykosen. 1985 Jul;28(7):323–331. doi: 10.1111/j.1439-0507.1985.tb02137.x. [DOI] [PubMed] [Google Scholar]
- Slutsky B., Buffo J., Soll D. R. High-frequency switching of colony morphology in Candida albicans. Science. 1985 Nov 8;230(4726):666–669. doi: 10.1126/science.3901258. [DOI] [PubMed] [Google Scholar]
- Slutsky B., Staebell M., Anderson J., Risen L., Pfaller M., Soll D. R. "White-opaque transition": a second high-frequency switching system in Candida albicans. J Bacteriol. 1987 Jan;169(1):189–197. doi: 10.1128/jb.169.1.189-197.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel J. D. Epidemiology and pathogenesis of recurrent vulvovaginal candidiasis. Am J Obstet Gynecol. 1985 Aug 1;152(7 Pt 2):924–935. doi: 10.1016/s0002-9378(85)80003-x. [DOI] [PubMed] [Google Scholar]
- Sobel J. D. Management of recurrent vulvovaginal candidiasis with intermittent ketoconazole prophylaxis. Obstet Gynecol. 1985 Mar;65(3):435–440. [PubMed] [Google Scholar]
- Sobel J. D. Vulvovaginal candidiasis--what we do and do not know. Ann Intern Med. 1984 Sep;101(3):390–392. doi: 10.7326/0003-4819-101-3-390. [DOI] [PubMed] [Google Scholar]
- Soll D. R. High-frequency switching in Candida albicans and its relations to vaginal candidiasis. Am J Obstet Gynecol. 1988 Apr;158(4):997–1001. doi: 10.1016/0002-9378(88)90113-5. [DOI] [PubMed] [Google Scholar]
- Soll D. R., Langtimm C. J., McDowell J., Hicks J., Galask R. High-frequency switching in Candida strains isolated from vaginitis patients. J Clin Microbiol. 1987 Sep;25(9):1611–1622. doi: 10.1128/jcm.25.9.1611-1622.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
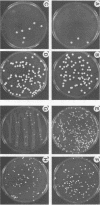
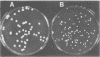
- Soll D. R., Staebell M., Langtimm C., Pfaller M., Hicks J., Rao T. V. Multiple Candida strains in the course of a single systemic infection. J Clin Microbiol. 1988 Aug;26(8):1448–1459. doi: 10.1128/jcm.26.8.1448-1459.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumbore D. J., Sobel J. D. Recurrent vulvovaginal candidiasis: vaginal epithelial cell susceptibility to Candida albicans adherence. Obstet Gynecol. 1986 Jun;67(6):810–812. [PubMed] [Google Scholar]
- Witkin S. S., Hirsch J., Ledger W. J. A macrophage defect in women with recurrent Candida vaginitis and its reversal in vitro by prostaglandin inhibitors. Am J Obstet Gynecol. 1986 Oct;155(4):790–795. doi: 10.1016/s0002-9378(86)80022-9. [DOI] [PubMed] [Google Scholar]