Abstract
Eph receptors play important roles in development, neural plasticity and cancer. We used an Orbitrap mass spectrometer and Stable Isotope Labeling by Amino Acids in Cell Culture (SILAC) to identify and quantify 204 proteins with significantly changed abundance in anti-phosphotyrosine immunoprecipitates after ephrinB1-Fc stimulation. More than half of all known effectors downstream of EphB receptors were identified in this study, as well as numerous novel candidates for EphB signaling.
Keywords: EphB, SILAC, mass spectrometry, phosphoproteomics, Orbitrap, IP
Introduction
Erythropoietin-producing hepatocellular carcinoma (Eph) receptors form the largest family of receptor tyrosine kinases (RTKs). There are 16 Eph genes in vertebrate genomes and 14 of them are found in mammals1, 2. Eph receptors are transmembrane proteins with conserved extra- and intracellular domains. The Eph family receptors are divided into two classes, EphA and EphB receptors, based on similarities in their extracellular domains and binding preference for either glycosylphosphatidylinositol linked ephrinA ligands or transmembrane ephrinB ligands.
Activation of Eph receptors generally leads to cytoskeleton rearrangement in the cell. However, recent studies have shown that Eph signaling can have diverse cellular effects in addition to changes in cytoskeleton dynamics depending on the cellular contexts1. First identified as key regulators of axon guidance during development, Eph receptors have been found to play important roles in tissue patterning, angiogenesis, cell morphogenesis, neural plasticity and cancer1. Despite intensive studies over the last 20 years leading to more than 2000 publications in Pubmed, Eph signaling is still of a great research interest and new effectors and mechanisms continue to be discovered frequently.
Quantitative proteomics by stable isotope labeling with amino acids in cell culture (SILAC) has quickly become a major tool for high throughput screening analysis for signaling pathways in recent years3. Particularly, in combination with anti-phosphotyrosine (pY) immunoprecipitation (IP), SILAC has shown great promise for the investigation of signaling pathways downstream of RTKs4–6. In these studies, cells are first metabolically labeled with light or heavy amino acids during cell culture. After labeling, in one cell population the RTK is activated to trigger tyrosine phosphorylation of downstream effectors. Then the lysates of the stimulated and the control cells are combined for anti-pY IP to pull down pY proteins together with their tight binding partners. The IPed proteins are then identified and quantified by MS and the relative abundance of the light and heavy versions of a protein is used to indicate whether the protein participates in the RTK pathway or not.
In a previous study, we used SILAC and quadrupole time-of-flight (QTOF) mass spectrometry (MS) to look for effectors in the EphB signaling pathway7. In that study, 46 out of 127 identified proteins were found to have changed abundance in pY IPs upon EphB receptor activation. Recently, the hybrid linear ion trap-Orbitrap (LTQ-Orbitrap™) mass spectrometer has demonstrated great utility in proteomic research8–13. With high speed and sensitivity in tandem mass spectrometry (MS/MS) mode by the linear ion trap, this instrument has been used to identify many more proteins from complex peptide mixtures than a QTOF instrument using data-dependent switching to and from MS/MS14. Moreover, the high resolution of the Orbitrap can increase the accuracy of peptide quantitation. In this study, we have repeated the SILAC experiment using an LTQ-Orbitrap with the aim of identify more candidate effectors in the EphB pathway.
Materials and Methods
Cell Culture and Cell Stimulation
Metabolic labeling and stimulation of cells were performed as previously described7. Briefly, ten 10 cm plates (approximately 108 total cells)/condition NG108 cells (mouse neuroblastoma × rat glioma hybrid) stably overexpressing EphB2 receptor 15 were differentially labeled in medium containing either normal or 13 C6 lysine and 13 C6 arginine (Cambridge Isotope Labs, Andover, MA). After five cell divisions to ensure nearly complete metabolic labeling, the cells were serum starved for 24 h. One cell population was treated with 2 μg/mL ephrinB1-Fc (Sigma-Aldrich) aggregated with anti-Fc IgG (Jackson Immunoresearch) for 45 min while the other population was treated with anti-Fc IgG aggregated Fc as a control. Cells were lysed in lysis buffer containing 1% Triton X-100, 150 mM NaCl, 20 mM Tris, pH8, 0.2mM EDTA, 2 mM Na3VO4, 2mM NaF, and protease inhibitors (Complete tablet; Roche, Mannheim, Germany).
Immunoprecipitation (IP) and SDS-PAGE
The “light” and “heavy” lysates were mixed in a 1:1 ratio (v:v) and incubated with agarose-conjugated anti-pY antibody PY99 (Santa Cruz Biotechnology, Santa Cruz, CA) for 4 h, and the beads were washed 4 times with lysis buffer. Precipitated proteins were eluted with a low pH buffer (pH2) containing 0.2% TFA/1% SDS. The eluates were neutralized with 1M NH4HCO3 and separated by SDS-PAGE on a 7.5% Tris-HCl gel (Bio-rad). The gel was stained with Coomassie Blue and the gel lanes were cut horizontally into 10 sections for in-gel tryptic digestion.
In-gel Digestion
Gel bands were cut into small pieces and destained in 25 mM NH4HCO3/50% acetonitrile, dehydrated with acetonitrile and dried. Then the gel pieces were rehydrated with 12.5 ng/μl trypsin solution in 25 mM NH4HCO3 and incubated overnight at 37 °C. Peptides were extracted twice with 5% formic acid/50% acetonitrile followed by a final extraction with acetonitrile. Samples were concentrated by vacuum centrifugation to dryness and redissolved with 2% acetonitrile in 0.1% formic acid before further analysis.
Liquid Chromatography (LC)-MS/MS
An LTQ-Orbitrap hybrid mass spectrometer (Thermo Fisher Scientific) equipped with a nano-ESI source (Jamie Hill Instrument Services) was used for all LC-MS/MS analyses. A Nano-Acquity UPLC system (Waters) equipped with a 100-μm × 15-cm reverse phase column (Symmetry C18, Waters) was coupled directly to the ion trap instrument via a 10-μm-inner diameter PicoTip™ nanoelectrospray emitter (New Objective). Samples were loaded onto a trap column (180 μm × 2 cm Symmetry C18, Waters) with 2% acetonitrile in 0.1% formic acid for 4 min at 5 μl/min. After sample loading, the flow rate was reduced to 0.4 μl/min and directed through the analytical column, and peptides were eluted by a gradient of 6–40% acetonitrile in 0.1% formic acid over 120 min. Mass spectra were acquired in data-dependent mode with one 60,000 resolution MS survey scan by the Orbitrap and up to five concurrent MS/MS scans in the LTQ for the five most intense peaks selected from each survey scan. Automatic gain control was set to 500,000 for Orbitrap survey scans and 10,000 for LTQ MS/MS scans. Survey scans were acquired in profile mode and MS/MS scans were acquired in centroid mode. Mascot generic format files were generated from the raw data using DTASuperCharge (version 1.01) and Bioworks (version 3.2, Thermo Fisher Scientific) for database searching.
Protein Identification and Quantitation
Mascot software (version 2.1.0, Matrix Science, London, UK) was used for database searching. An IPI database containing mouse and rat protein sequences (downloaded January 01, 2007) was used. Peptide mass tolerance was 20 ppm, fragment mass tolerance was 0.6 Da, trypsin specificity was applied with a maximum of one missed cleavage, and variable modifications were 13C6 Lys, 13C6 Arg, oxidation of methionine, and phosphorylation of serine, threonine and tyrosine. To estimate the false positive rate for protein identification, a decoy database was created by reversing the protein sequences of the original database. Based on the decoy database search results, three filters for protein identification were applied: (1) Peptide score threshold was 20. (2) Protein score threshold was 40. (3) Each protein was identified based on at least two unique peptide sequences. The estimated false positive rate based on the decoy database search was 0.3%.
To merge the SILAC results of multiple gel fractions from the same sample preparation, all the identified peptide sequences from different gel fractions were combined and searched against the same IPI protein database to obtain the protein matches. Proteins identified based on the same set of peptides were grouped and reported as a single protein match. Proteins that were likely introduced during sample preparation were excluded from the reported protein list. These proteins included keratins and trypsin (from in-gel digestion), immunoglobins (from the PY99 antibody and Fc fusion protein), ephrinB1 (the stimulating ligand), ferritin, and serum albumin (from cell culture media).
SILAC quantitation was carried out using the open source software MSQuant (version 1.4.2a13) developed by Peter Mortensen and Matthias Mann at the University of Southern Denmark. The XIC intensities of the heavy and light peptides were measured, with the results verified by manual inspection of the MS spectra. The SILAC ratios of proteins were calculated by comparing the summed XIC intensities of all matched light peptides with those of the heavy peptides. As a loading control, a small volume of the combined lysates was subjected to in-gel digestion, LC-MS/MS analysis and the identified proteins were also quantified. The average ratio for all quantified proteins was used as a correction for ratios of proteins identified from the IP.
Phosphorylation Analysis
Phosphopeptides were identified using Mascot. All matched MS/MS spectra were inspected manually. In cases where there were multiple possibilities for the localization of the phosphates on the peptide, a simple statistical model16 was used to calculate a score (Ascore) based on the number of matching ions for each possible localization using only the site determining ions17. We used an Ascore threshold of 19, which has been estimated to correspond to 99.5% confidence in site localization 17. In addition, the results were filtered based on the intensity of the peak corresponding to the neutral loss of phosphoric acid (98 Da) from the precursor ion: The assignment was rejected if the peptide did not contain pS or pT and the intensity of the neutral loss peak was larger than 50% of the base peak.
Western Blotting
Proteins were separated by SDS-PAGE and transferred to PVDF membranes. Membranes were blocked with Tris buffered saline with Tween 20 containing 2% bovine serum albumin, incubated with the corresponding primary and horseradish peroxidase (HRP)-conjugated secondary antibodies (Santa Cruz Biotechnology), and visualized with ECL (Pierce Biotechnology, Rockford, IL). Anti-Hrs18 and anti-STAM219 were kind gifts from Dr. Harald Stenmark. Anti-IRS2, anti-Erbin, PY99-HRP (Santa Cruz Biotechnology), anti-SASH1 (Abnova, Taiwan) and anti-EphB4 (R&D Systems, Minneapolis, MN) antibodies were used as indicated by the manufacturers.
Results and Discussion
Protein Identification and SILAC Quantitation
To screen for effectors in the EphB signaling pathway, we used a SILAC strategy that we employed in a previous study7. Briefly, two populations of NG108-EphB2 cells (NG108 cells that stably overexpress the EphB2 receptor) were differentially SILAC labeled with 13C6 Lys / 13C6 Arg or 12C6 Lys / 12C6 Arg. One cell population was treated with clustered ephrinB1-Fc to activate the EphB2 receptor while the other (control) population was treated with clustered Fc. After cell lysis, equal volumes of the two lysates were combined for anti-pY IP. Anti-pY Western blotting using the lysates and pY IPs indicated that several proteins were tyrosine phosphorylated after ligand stimulation (Figure 1). The IPed proteins were separated into 10 fractions using SDS-PAGE. Each fraction was digested with trypsin and analyzed by LC-MS with a hybrid LTQ-Orbitrap MS spectrometer for protein identification and quantitation. Coomassie staining of the gel before in-gel digestion revealed a faint haze of blue staining, but the gel contained insufficient protein for visualization of individual proteins (data not shown).
Figure 1.
Tyrosine phosphorylation detected by anti-pY Western blotting of proteins in NG108 cell lysates and pY immunoprecipitates after ephrinB1 treatment. NG108-EphB2 cells were treated for 45 minutes with anti-Fc IgG aggregated ephrinB1-Fc or with anti-Fc IgG aggregated Fc as a control as indicated in the Materials and Methods. Total cell lysates and pY IPs were probed with PY99-HRP. Numbers to the left of the gel show MW × 10−3 based on protein MW standards.
Two SILAC replicates (biological replicates) were carried out, in which 683 and 532 proteins were identified respectively. Figure 2A shows the number of protein identifications in the two replicates.
Figure 2.
Identification of SILAC proteins and phosphopeptides from anti-pY IPs. (A) Venn diagram depicting the overlap in proteins identified in the two SILAC replicates by LTQ-Orbitrap. (B) Venn diagram depicting the overlap in proteins quantified in this study by Orbitrap and a previous study using a QTOF Micro mass spectrometer7. (C) Venn diagram depicting the overlap in phosphopeptides identified in this study by Orbitrap and the previous study using QTOF.
Of the 804 proteins identified in the two SILAC replicates, 777 (672 from replicate 1 and 513 from replicate 2, 408 from both) were quantified and 27 were not able to be confidently quantified due to poor MS spectral quality (Figure 2B). The protein ratios from the two individual replicates were consistent (Figure 3). A list of the 777 quantified proteins is shown in Supporting Information Table 1.
Figure 3.
SILAC protein ratios from the two SILAC replicates. 672 proteins from replicate 1 and 513 from replicate 2 were quantified. In total 777 proteins were quantified, and 408 of them were quantified in both replicates.
204 proteins increased at least 1.5-fold in abundance in pY IP after ephrinB1 stimulation and 12 showed at least 1.5-fold decreased abundance. To further remove from the protein list redundancy caused by orthologous proteins from mouse and rat, proteins corresponding to the same gene name were clustered into a single entry. After clustering, 194 proteins showed at least 1.5-fold increased ratios and 10 showed decreased ratios. Table 1 lists the proteins with changed ratios.
Table 1.
Proteins with at least a 1.5 Change in Protein Abundance between Anti-phosphotyrosine Immunoprecipitates from ephrinB1-Stimulated and Unstimulated NG108-EphB2 Cells a
| No. | accession | protein name | mean ratio | Rep. 1 CV | Rep. 2 CV | CV (reps 1,2) |
Known EphB effector? |
|---|---|---|---|---|---|---|---|
| 1 | IPI00319843 | Beclin-1 | 509 | no | |||
| 2 | IPI00109667 | nicotinamide nucleotide adenylyltransferase 1 | 255 | no | |||
| 3 | IPI00222366 | sterile alpha motif domain containing 5 | 107 | 76 | 1 | 37 | no |
| 4 | IPI00556823 | protein kinase, AMP-activated, alpha 1 catalytic subunit | 106 | no | |||
| 5 | IPI00752269 | novel protein (possible orthologue of human Src homology 2 domain containing F (SHF)) | 76 | 54 | 3 | 23 | no |
| 6 | IPI00330102 | folliculin interacting protein 1 | 73 | 7 | no | ||
| 7 | IPI00115056 | trafficking protein particle complex 3 | 58 | 33 | no | ||
| 8 | IPI00309259 | partitioning-defective protein 3 homolog isoform 3 | 58 | 18 | 38 | 20 | no |
| 9 | IPI00416163 | unnamed protein product | 55 | 21 | 38 | 16 | yes |
| 10 | IPI00354665 | apoptosis-stimulating protein of p53, 1 | 52 | 51 | 6 | 57 | no |
| 11 | IPI00408219 | N-chimaerin | 49 | 33 | 19 | 24 | no |
| 12 | IPI00749688 | PREDICTED: similar to MGC114619 protein | 46 | 15 | 36 | 28 | no |
| 13 | IPI00331766 | putative C3orf6 protein | 45 | 10 | 19 | 38 | no |
| 14 | IPI00127232 | glutamate receptor interacting protein 1 isoform 1 | 39 | 8 | 31 | 36 | yes44 |
| 15 | IPI00338954 | SAM and SH3 domain-containing protein 1 | 36 | 20 | 26 | 8 | no |
| 16 | IPI00361275 | PREDICTED: similar to TPR domain, ankyrin-repeat and coiled-coil-containing | 35 | 16 | no | ||
| 17 | IPI00213347 | afadin | 33 | 22 | 22 | 0 | yes24 |
| 18 | IPI00473693 | Isoform 1 of Plakophilin-4 | 32 | 21 | no | ||
| 19 | IPI00753111 | PREDICTED: similar to Afadin (Af-6 protein) | 30 | 22 | 18 | 3 | yes24 |
| 20 | IPI00359621 | hypothetical protein LOC307833 | 30 | 13 | no | ||
| 21 | IPI00761456 | chimerin (chimaerin) 2 | 29 | 8 | 19 | 17 | no |
| 22 | IPI00454039 | Protein LAP2 (Erbb2-interacting protein) (Erbin) | 27 | 12 | 16 | 2 | no |
| 23 | IPI00125855 | protein kinase C, delta | 26 | 3 | no | ||
| 24 | IPI00468418 | signal transducing adaptor molecule2 (STAM2) | 26 | 8 | 19 | 15 | no |
| 25 | IPI00108870 | Eph receptor B2 | 26 | 11 | 17 | 13 | yes |
| 26 | IPI00565852 | PREDICTED: similar to Eph receptor B3 | 25 | 12 | 15 | 3 | yes |
| 27 | IPI00408892 | RAB7, member RAS oncogene family | 21 | 7 | 15 | 14 | no |
| 28 | IPI00421832 | dermcidin precursor | 21 | 4 | 17 | 18 | no |
| 29 | IPI00471127 | Cdc42 effector protein 1 (Binder of Rho GTPases 5) | 20 | 4 | 13 | 9 | no |
| 30 | IPI00124742 | eukaryotic translation initiation factor 4H | 19 | 7 | 6 | 4 | no |
| 31 | IPI00322033 | target of myb1-like 2 isoform a | 19 | 9 | 11 | 4 | no |
| 32 | IPI00367930 | PREDICTED: similar to Erbb2 interacting protein isoform 1 | 19 | 10 | no | ||
| 33 | IPI00125534 | Docking protein 1 (Downstream of tyrosine kinase 1) (p62(dok)) | 18 | 10 | 8 | 0 | yes15 |
| 34 | IPI00316623 | catenin, delta 1 isoform 2 | 18 | 7 | no | ||
| 35 | IPI00420753 | PREDICTED: similar to SHB adaptor protein B | 18 | 12 | 8 | 2 | no |
| 36 | IPI00312067 | inositol polyphosphate phosphatase-like 1, isoform CRA_c | 18 | 9 | 6 | 1 | yes45 |
| 37 | IPI00315187 | UPF0404 protein C11orf59 homolog | 16 | 2 | 5 | 7 | no |
| 38 | IPI00343984 | coiled-coil domain containing 85B | 16 | 8 | no | ||
| 39 | IPI00187275 | Carnitine deficiency-associated gene expressed in ventricle 3 | 16 | 2 | 5 | 15 | no |
| 40 | IPI00202691 | cancer susceptibility candidate 3 | 15 | 8 | no | ||
| 41 | IPI00136475 | Leucine-rich repeats and immunoglobulin-like domains protein 1 precursor (LIG-1) | 14 | 7 | no | ||
| 42 | IPI00331568 | HGF-regulated tyrosine kinase substrate (Hrs) | 14 | 6 | 6 | 1 | no |
| 43 | IPI00153241 | vacuolar protein sorting 37C | 13 | 2 | 11 | no | |
| 44 | IPI00228877 | connector enhancer of kinase suppressor of Ras 2 | 13 | 6 | no | ||
| 45 | IPI00379844 | Insulin receptor substrate 2 (IRS-2) | 12 | 6 | 7 | 1 | no |
| 46 | IPI00132135 | midline 2 | 12 | 6 | no | ||
| 47 | IPI00124298 | Rho GTPase activating protein 5 | 12 | 6 | no | ||
| 48 | IPI00366801 | YTH domain family 2 | 12 | 4 | no | ||
| 49 | IPI00134881 | LIM domain-containing protein 1 | 11 | 4 | no | ||
| 50 | IPI00323349 | Tight junction protein ZO-2 | 11 | 4 | 4 | 4 | no |
| 51 | IPI00137731 | unnamed protein product | 11 | 6 | 4 | 3 | no |
| 52 | IPI00347255 | Protein KIAA1688 | 11 | 7 | 2 | 8 | no |
| 53 | IPI00336844 | epsin 2 | 10 | 4 | no | ||
| 54 | IPI00135971 | tight junction protein 1 | 10 | 5 | 6 | 3 | no |
| 55 | IPI00223987 | leucyl/cystinyl aminopeptidase | 10 | 4 | 4 | 4 | no |
| 56 | IPI00364933 | similar to signal transducing adaptor molecule 1 (STAM1) | 9.9 | 5.0 | 3.7 | 1.4 | no |
| 57 | IPI00130621 | RAS p21 protein activator 1 | 9.6 | 1.4 | 3.4 | 1.9 | yes15 |
| 58 | IPI00660894 | E3 ubiquitin-protein ligase CBL-B | 9.5 | 3.8 | no | ||
| 59 | IPI00229955 | Ras association (RalGDS/AF-6) domain family 8 | 9.3 | 5.8 | no | ||
| 60 | IPI00110435 | nischarin | 9.3 | 2.6 | 4.2 | 1.6 | no |
| 61 | IPI00120433 | SH2B adapter protein 2 | 8.9 | 2.7 | 2.0 | 1.1 | no |
| 62 | IPI00480842 | hypothetical protein LOC684097 | 8.6 | 2.4 | no | ||
| 63 | IPI00221581 | Eukaryotic translation initiation factor 4B (eIF-4B) | 8.5 | 2.7 | 4.6 | 2.4 | no |
| 64 | IPI00765594 | non-catalytic region of tyrosine kinase adaptor protein 1 (predicted), isoform CRA_a | 8.5 | 4.0 | 3.7 | 1.7 | yes15 |
| 65 | IPI00119809 | lectin, galactoside-binding, soluble, 3 binding protein | 8.3 | 3.9 | no | ||
| 66 | IPI00363834 | PREDICTED: similar to pleckstrin homology domain containing, family A member 6 | 8.2 | 3.0 | 2.8 | 2.9 | no |
| 67 | IPI00121319 | LIM only protein HLP | 7.6 | 1.1 | 2.4 | 0.0 | no |
| 68 | IPI00117375 | syndecan binding protein isoform 1 (syntenin) | 7.4 | 0.9 | 4.7 | 4.3 | yes43 |
| 69 | IPI00123505 | Synaptophysin | 6.9 | 0.6 | no | ||
| 70 | IPI00116554 | protein tyrosine phosphatase, non-receptor type 11 | 6.6 | 3.8 | 1.6 | 4.1 | yes46 |
| 71 | IPI00154012 | ubiquitin specific peptidase 15 | 6.5 | 3.0 | 1.4 | 2.9 | no |
| 72 | IPI00128454 | seizure related 6 homolog like 2 | 6.5 | 0.3 | 1.2 | 2.1 | no |
| 73 | IPI00130185 | protein phosphatase 1, catalytic subunit, alpha | 6.4 | 1.4 | 2.5 | 1.8 | no |
| 74 | IPI00133679 | hypothetical protein LOC73711 | 6.2 | 0.5 | 0.9 | 0.0 | no |
| 75 | IPI00117944 | tumor susceptibility gene 101 protein | 6.2 | 1.8 | 0.7 | 2.2 | no |
| 76 | IPI00205566 | calponin 3, acidic | 6.0 | no | |||
| 77 | IPI00229392 | Ras-related GTP binding A | 6.0 | 0.5 | 1.3 | 2.6 | no |
| 78 | IPI00229434 | tumor protein p53 binding protein, 2 | 5.9 | 3.5 | no | ||
| 79 | IPI00323590 | E3 ubiquitin-protein ligase CBL | 5.8 | 1.4 | 1.3 | 0.2 | yes42 |
| 80 | IPI00136618 | toll interacting protein | 5.7 | 1.1 | 1.1 | no | |
| 81 | IPI00272559 | Vav2 protein | 5.6 | 1.1 | yes41 | ||
| 82 | IPI00381394 | filamin C, gamma | 5.5 | 1.9 | 2.4 | 0.5 | no |
| 83 | IPI00133591 | vacuolar protein sorting 28 | 5.5 | 0.8 | no | ||
| 84 | IPI00308222 | drebrin-like | 5.5 | 1.0 | 2.0 | 0.1 | no |
| 85 | IPI00227149 | YTH domain family protein 3 | 5.4 | 1.7 | 1.3 | 0.2 | no |
| 86 | IPI00272148 | Cytohesin-3 | 5.4 | 1.0 | 1.2 | 0.4 | no |
| 87 | IPI00132604 | unnamed protein product | 5.2 | 2.1 | 0.6 | 3.0 | no |
| 88 | IPI00153207 | unnamed protein product | 5.2 | 0.1 | 2.8 | 2.2 | no |
| 89 | IPI00325146 | annexin A2 | 5.2 | 3.2 | no | ||
| 90 | IPI00130883 | Putative RNA-binding protein 3 (RNA-binding motif protein 3) | 5.1 | 0.9 | no | ||
| 91 | IPI00323483 | programmed cell death 6 interacting protein | 4.9 | 1.8 | 0.6 | 0.6 | no |
| 92 | IPI00454019 | unnamed protein product | 4.9 | 1.5 | no | ||
| 93 | IPI00458001 | ataxin 2-like, isoform CRA_g | 4.8 | 0.9 | 1.3 | 0.3 | no |
| 94 | IPI00231715 | protein phosphatase 1 gamma2 | 4.8 | 2.0 | no | ||
| 95 | IPI00130115 | Vesicle transport through interaction with t-SNAREs homolog 1B | 4.8 | 2.4 | 0.6 | 1.6 | no |
| 96 | IPI00626620 | SEC24 related gene family, member C (S. cerevisiae), isoform CRA_b | 4.8 | 1.3 | no | ||
| 97 | IPI00230035 | ATP-dependent RNA helicase DDX3X | 4.4 | 0.7 | 1.9 | 1.4 | no |
| 98 | IPI00553792 | Isoform 2 of Caskin-1 | 4.4 | 2.0 | no | ||
| 99 | IPI00114332 | ribosomal protein S6 kinase polypeptide 1 | 4.3 | 1.2 | no | ||
| 100 | IPI00132322 | Trafficking protein particle complex subunit 5 | 4.1 | 1.0 | no | ||
| 101 | IPI00402900 | Isoform 1 of Engulfment and cell motility protein 2 | 4.1 | 0.5 | no | ||
| 102 | IPI00222107 | FERM domain containing 4A | 4.0 | 0.5 | 0.4 | 0.3 | no |
| 103 | IPI00264501 | phosphatidylinositol-binding clathrin assembly protein | 3.9 | 0.9 | no | ||
| 104 | IPI00114613 | Cdc42 binding protein kinase beta | 3.9 | 1.2 | 0.3 | 0.8 | no |
| 105 | IPI00331016 | SEC24 related gene family, member B | 3.8 | 0.6 | 1.0 | 1.1 | no |
| 106 | IPI00420553 | Serine/threonine-protein kinase TAO2 | 3.8 | no | |||
| 107 | IPI00330862 | Ezrin | 3.7 | 1.0 | 1.1 | 0.7 | no |
| 108 | IPI00221494 | Lipoma-preferred partner homolog | 3.7 | 0.1 | no | ||
| 109 | IPI00313841 | ATPase, H+ transporting, V0 subunit D isoform 1 | 3.7 | 0.6 | 0.9 | no | |
| 110 | IPI00118899 | actinin alpha 4 | 3.7 | 0.6 | 1.0 | 0.3 | no |
| 111 | IPI00380817 | breakpoint cluster region homolog | 3.6 | 0.0 | no | ||
| 112 | IPI00223070 | dedicator of cytokinesis 4 | 3.6 | 1.0 | 0.9 | 0.1 | no |
| 113 | IPI00458995 | polyubiquitin C | 3.5 | 0.1 | 1.7 | 0.6 | no |
| 114 | IPI00313275 | Sorting nexin-9 | 3.5 | 0.7 | 0.4 | 0.3 | no |
| 115 | IPI00132462 | cytotoxic granule-associated RNA binding protein 1 | 3.3 | 0.0 | 0.8 | 0.6 | no |
| 116 | IPI00206710 | pleckstrin homology, Sec7 and coiled/coil domains 2 | 3.2 | 0.9 | no | ||
| 117 | IPI00154057 | protocadherin 1 | 3.2 | 0.7 | 0.8 | 0.2 | no |
| 118 | IPI00380436 | actinin, alpha 1 | 3.2 | 0.6 | 0.6 | 0.3 | no |
| 119 | IPI00110247 | TBC1 domain family member 15 | 3.0 | 0.5 | no | ||
| 120 | IPI00464282 | Hbs1-like (S. cerevisiae), isoform CRA_b | 2.9 | 1.0 | no | ||
| 121 | IPI00368041 | similar to DNA-directed RNA polymerase II largest subunit | 2.8 | 0.1 | no | ||
| 122 | IPI00109334 | Proto-oncogene tyrosine-protein kinase FER (p94-FER) (c-FER) | 2.8 | 0.2 | 0.0 | no | |
| 123 | IPI00226727 | Isoform 2 of Discs large homolog 2 | 2.8 | 0.6 | 0.9 | 0.0 | no |
| 124 | IPI00189519 | Histone H3.3 | 2.7 | no | |||
| 125 | IPI00108150 | Rho-associated protein kinase 2 (p164 ROCK-2) | 2.7 | 0.6 | no | ||
| 126 | IPI00117159 | phosphatidylinositol 3-kinase, regulatory subunit, polypeptide | 2.7 | 0.8 | 0.4 | 0.3 | yes39 |
| 127 | IPI00120923 | Vacuolar protein sorting 16 | 2.6 | no | |||
| 128 | IPI00380814 | target of myb1 homolog | 2.6 | 0.4 | no | ||
| 129 | IPI00124753 | misshapen-like kinase 1 isoform 2 | 2.6 | 0.5 | 0.8 | 0.0 | no |
| 130 | IPI00380108 | transmembrane protein 1 | 2.6 | 0.0 | no | ||
| 131 | IPI00136498 | lin 7 homolog c | 2.6 | 0.3 | no | ||
| 132 | IPI00558156 | 61 kDa protein | 2.6 | 0.3 | 0.4 | 0.0 | no |
| 133 | IPI00204923 | ubiquitin specific peptidase 9, X chromosome | 2.5 | 1.0 | no | ||
| 134 | IPI00109932 | DEAD (Asp-Glu-Ala-Asp) box polypeptide 6 | 2.5 | 0.4 | no | ||
| 135 | IPI00123313 | ubiquitin-activating enzyme E1, Chr X | 2.5 | 0.6 | no | ||
| 136 | IPI00123349 | SEC23A | 2.4 | 0.3 | 0.3 | 0.2 | no |
| 137 | IPI00130423 | Growth factor receptor bound protein 2-associated protein 2 | 2.4 | 0.3 | no | ||
| 138 | IPI00462445 | E3 ubiquitin-protein ligase NEDD4 | 2.3 | 0.9 | 0.1 | 0.2 | no |
| 139 | IPI00133024 | 1110059P08Rik protein | 2.2 | 0.6 | no | ||
| 140 | IPI00114948 | interferon induced transmembrane protein 2 | 2.2 | 0.3 | 0.4 | 0.3 | no |
| 141 | IPI00457533 | ubiquitin-associated protein 2 | 2.2 | 0.2 | 0.1 | 0.2 | no |
| 142 | IPI00229895 | dispatched homolog 2 | 2.2 | no | |||
| 143 | IPI00331579 | synaptogyrin 3 | 2.2 | 0.1 | no | ||
| 144 | IPI00123292 | Isoform 2 of Far upstream element-binding protein 1 | 2.2 | 0.3 | no | ||
| 145 | IPI00226563 | tweety homolog 3 (Drosophila), isoform CRA_a | 2.1 | 0.5 | 0.3 | 0.2 | no |
| 146 | IPI00329998 | Histone cluster 1, H4h | 2.1 | 0.2 | 0.2 | 0.0 | no |
| 147 | IPI00116697 | RAB6A, member RAS oncogene family | 2.1 | 0.7 | 0.7 | 0.2 | no |
| 148 | IPI00676192 | latrophilin 2 | 2.1 | no | |||
| 149 | IPI00111416 | syntaxin 12 | 2.1 | 0.3 | 0.4 | 0.5 | no |
| 150 | IPI00223253 | heterogeneous nuclear ribonucleoprotein K | 2.1 | 0.2 | 1.0 | 0.6 | no |
| 151 | IPI00380824 | MKIAA0144 protein | 2.0 | 0.1 | 0.4 | 0.1 | no |
| 152 | IPI00408378 | Isoform 2 of 14-3-3 protein theta | 2.0 | 0.3 | 0.4 | 0.0 | no |
| 153 | IPI00762547 | Isoform 1 of Intersectin-2 | 2.0 | 0.2 | 0.6 | 0.2 | yes31 |
| 154 | IPI00322492 | Ewing sarcoma homolog | 2.0 | 0.1 | 0.3 | 0.5 | no |
| 155 | IPI00360064 | PREDICTED: similar to Son of sevenless homolog 1 | 2.0 | 0.5 | yes20 | ||
| 156 | IPI00133428 | protease (prosome, macropain) 26S subunit, ATPase 1 | 2.0 | no | |||
| 157 | IPI00312527 | Crmp1 protein | 1.9 | 0.5 | 0.4 | 0.1 | no |
| 158 | IPI00109375 | Poliovirus receptor-related protein 2 precursor | 1.9 | 0.2 | no | ||
| 159 | IPI00125778 | Transgelin-2 | 1.9 | 0.2 | no | ||
| 160 | IPI00117039 | Tyrosine-protein kinase ABL2 | 1.9 | 0.2 | 0.1 | 0.0 | no |
| 161 | IPI00116112 | Dynactin subunit 2 | 1.9 | 0.2 | no | ||
| 162 | IPI00515195 | eukaryotic translation initiation factor 4, gamma 1 isoform b | 1.9 | 0.4 | no | ||
| 163 | IPI00309413 | non-catalytic region of tyrosine kinase adaptor protein 2 | 1.9 | 0.4 | 0.3 | 0.1 | no |
| 164 | IPI00622847 | Heterogeneous nuclear ribonucleoproteins A2/B1 | 1.9 | 0.2 | 0.2 | 0.4 | no |
| 165 | IPI00221796 | poly(rC) binding protein 2, isoform CRA_a | 1.9 | 0.5 | no | ||
| 166 | IPI00372054 | PEF protein with a long N-terminal hydrophobic domain | 1.9 | no | |||
| 167 | IPI00135887 | transmembrane protein 106B, isoform CRA_b | 1.8 | 0.2 | no | ||
| 168 | IPI00136917 | Tyrosine-protein kinase-protein kinase SgK269 | 1.8 | 0.3 | no | ||
| 169 | IPI00365284 | eukaryotic translation initiation factor 4 gamma, 3 | 1.8 | no | |||
| 170 | IPI00125298 | SHC-transforming protein 1 | 1.8 | 0.3 | 0.2 | 0.0 | yes40 |
| 171 | IPI00420185 | Epidermal growth factor receptor substrate 15-like 1 | 1.8 | 0.4 | 0.1 | 0.2 | no |
| 172 | IPI00415402 | Syntaxin-binding protein 1 | 1.8 | 0.4 | no | ||
| 173 | IPI00463573 | eukaryotic translation initiation factor 3 subunit 6 interacting protein | 1.8 | 0.2 | no | ||
| 174 | IPI00116966 | Asparagine synthetase | 1.8 | 0.3 | no | ||
| 175 | IPI00226275 | WD repeat domain 26 | 1.7 | 0.1 | no | ||
| 176 | IPI00406118 | NS1-associated protein 1 isoform 2 | 1.7 | 0.3 | 0.0 | 0.1 | no |
| 177 | IPI00553633 | cullin 7 | 1.7 | 0.3 | no | ||
| 178 | IPI00129417 | heterogeneous nuclear ribonucleoprotein D-like | 1.7 | 0.1 | 0.2 | no | |
| 179 | IPI00227392 | 14-3-3 protein eta | 1.7 | 0.2 | 0.5 | 0.3 | no |
| 180 | IPI00470095 | G protein-coupled receptor kinase-interactor 1 | 1.7 | 0.3 | 0.1 | 0.1 | no |
| 181 | IPI00114401 | emerin | 1.6 | 0.3 | 0.5 | no | |
| 182 | IPI00128202 | eukaryotic translation initiation factor 3, subunit 3 (gamma) | 1.6 | 0.1 | no | ||
| 183 | IPI00308162 | solute carrier family 25 (mitochondrial carrier, Aralar), member 12 | 1.6 | 0.4 | no | ||
| 184 | IPI00280250 | SH3 and PX domains 2A | 1.6 | no | |||
| 185 | IPI00321647 | eukaryotic translation initiation factor 3, subunit 8 | 1.6 | 0.1 | 0.1 | 0.1 | no |
| 186 | IPI00118384 | 14-3-3 protein epsilon (14-3-3E) | 1.5 | 0.1 | 0.2 | 0.1 | no |
| 187 | IPI00230704 | betaPix-c | 1.5 | 0.1 | no | ||
| 188 | IPI00229548 | Solute carrier family 1 (neutral amino acid transporter), member 5 | 1.5 | 0.1 | no | ||
| 189 | IPI00331334 | Bcl-2-binding protein Bis | 1.5 | 0.0 | 0.0 | 0.1 | no |
| 190 | IPI00230707 | 14-3-3 protein gamma | 1.5 | 0.2 | 0.1 | 0.0 | no |
| 191 | IPI00114560 | Ras-related protein Rab-1A | 1.5 | 0.0 | 0.1 | 0.2 | no |
| 192 | IPI00131329 | Sorting nexin-18 | 1.5 | 0.1 | no | ||
| 193 | IPI00116498 | tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta polypeptide | 1.5 | 0.1 | 0.1 | 0.0 | no |
| 194 | IPI00362014 | Tln1 protein | 1.5 | no | |||
| 195 | IPI00230445 | peripherin | 0.68 | 0.04 | 0.09 | 0.13 | no |
| 196 | IPI00331738 | 52 kDa Ro protein | 0.67 | 0.03 | 0.07 | 0.08 | no |
| 197 | IPI00222801 | Neuronal proto-oncogene tyrosine-protein kinase Src | 0.67 | 0.12 | no | ||
| 198 | IPI00117821 | Breast cancer anti-estrogen resistance protein 1 (p130cas) | 0.66 | 0.05 | 0.07 | 0.02 | yes47 |
| 199 | IPI00113563 | Focal adhesion kinase 1 (FADK 1) (pp125FAK) | 0.64 | 0.06 | 0.05 | 0.02 | yes47, 48 |
| 200 | IPI00135965 | Alpha-internexin | 0.57 | 0.04 | no | ||
| 201 | IPI00137970 | SH2 domain containing 3C | 0.55 | 0.05 | 0.04 | 0.01 | yes49 |
| 202 | IPI00223751 | Rho GTPase activating protein 12 isoform 1 | 0.52 | 0.05 | 0.05 | 0.01 | no |
| 203 | IPI00330231 | Rap guanine nucleotide exchange factor (GEF) 1 isoform 3 | 0.52 | 0.05 | no | ||
| 204 | IPI00111258 | unnamed protein product | 0.46 | 0.04 | 0.04 | 0.01 | no |
for proteins identified based on the same set of peptides, only one protein is shown in this table for each protein group. More detailed information about each protein group is included in Supporting Information Table 1.
Western Blotting Verification
To further verify the SILAC result from the MS analysis, Western Blotting was carried out for five proteins with changed ratios whose antibodies were available. These proteins include: Hrs, STAM2, Erbin, IRS-2 and SASH1. None of these proteins have been previously reported to participate in Eph signaling except in our previous SILAC study7 that found Hrs, STAM2, IRS-2 and SASH1. As shown in Figure 4, the Western blotting results for all the selected proteins were consistent with the corresponding SILAC ratios.
Figure 4. Western blotting analysis of selected candidate effector proteins.
Cells were cultured and stimulated with ephrinB1 in the same way as in the SILAC experiments. The whole cell lysates and anti-pY IPs were probed with the indicated antibodies. For the EphB4 receptor, wild type NG108 cells were used. For all other proteins, NG108-EphB2 cells were used.
The NG108-EphB2 cell line has been used frequently in previous studies of ephrin/Eph signaling 15, 20–25. We have observed from our previous SILAC study7 that the NG108-EphB2 cells express endogenous EphB4 and EphB3 receptors and upon ephrinB1 stimulation these receptors are activated along with EphB2. This observation was confirmed in this study. To verify that EphB receptors other than EphB2 are expressed in NG108 cells, wild type NG108 cells were treated with ephrinB1 using the same procedure as the SILAC experiment. Lysates and pY IPs were probed with anti-EphB4 and anti-pY antibodies. The anti-EphB4 blot (Figure 4) indicates that EphB4 is expressed in wild type NG108 cells and can be tyrosine phosphorylated upon ligand treatment. The anti-pY blot on the lysates and pY IPs did not show detectable difference between the control and stimulated cells due to interference from basal signals (data not shown), suggesting the effect of Eph signaling in wile type NG108 cells is much subtler than in the NG108-EphB2 cells. Based on these new findings, care should be taken when using NG108 cell lines for Eph signaling studies as well as interpreting results from previous studies in which these cell lines were used.
Phosphorylation Sites
The SILAC experiment identified 128 unique phosphopeptide sequences using Mascot. Because most of these phosphopeptides contain multiple serine/threonine/tyrosine residues, we tried to localize the phosphorylation sites using a simple statistical model for matching site-determining ions in the MS/MS spectra16, 17.
Using this model, we localized 116 phosphorylation sites (38 pS, 12 pT and 66 pY) on 115 peptides. To determine how many of the identified phosphorylation sites are novel, the Swiss-prot knowledgebase (downloaded September 03, 2007) and datasets of phosphorylation sites from several major large scale proteomics studies26–30 were searched using in-house written Perl scripts. 67 of the 116 localized phosphorylation sites were not found in these databases (Supporting Information Table 2). The annotated MS/MS spectra of all identified phosphopeptides are included in Supporting Information File1 and File2. File1 contains spectra of phosphopeptides whose phosphorylation sites were localized by Ascores. File2 contains spectra of phosphopeptides whose phosphorylation sites could not be localized by Ascores. For these peptides, the phosphorylation sites corresponding to the best Ascores were used to annotate the fragment ions in the spectra. All the identified phosphopeptides together with their Ascores are listed in Supporting Information Table 2. It is possible that the phosphotyrosine sites on proteins with ratio changes are regulated by the EphB activity, but due to the fact that almost all phosphoproteins have multiple phosphorylation sites, a protein ratio change from anti-pY IP cannot be attributed to the change of a specific pY site. A quantitative experiment that does not involve pY protein IP (for example, phosphopeptide enrichment after digestion of the whole cell lysate) would be needed to confirm the link between specific pY sites and EphB signaling.
Comparison between the LTQ-Orbitrap results and previous QTOF results
Previously we have carried out a similar SILAC study using an older QTOF instrument (Micromass, QTOF-Micro, installed in 2003)7. Figure 2B and 2C show a comparison of the numbers of protein identifications and phosphopeptide identifications in the two studies. Using approximately the same number of cells, the LTQ-Orbitrap analysis identified 5 times more proteins and 10 times more phosphopeptides than the QTOF Micro. This is largely attributed to the high sensitivity and sequencing speed afforded by the LTQ-Orbitrap as has been documented by previous studies10, 14. When considering these results, it should be kept in mind that the Orbitrap SILAC experiment was performed twice, while the QTOF experiment was performed once, which would slightly exaggerate the difference in number of proteins quantified. 95 of the 127 proteins that were identified in the QTOF experiment were identified and quantified in the Orbitrap study. Supporting Information Figure 1 shows the protein ratios measured by the QTOF and the Orbitrap. While the majority of these proteins have consistent ratios in the two studies, we did observe that 13 of the 95 proteins changed by more than 1.5-fold in one experiment but not the other. These proteins are listed in Supporting Information Table 3. However for most of these proteins the two ratios are close to the cutoff ratio of 1.5. A summary comparison of the proteins identified in both Orbitrap experiments as well as the previous QTOF study is shown in Supporting Information Table 4.
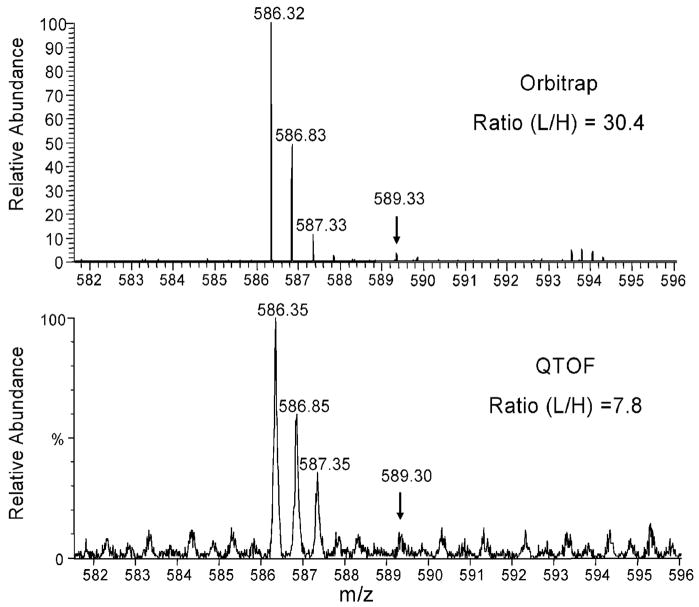
Differences in SILAC ratios between the two studies can be attributed to two major reasons. First, although the same protocol for cell culture and treatment was used, the two experiments were performed more than two years apart and the intensities of Eph activation may have been slightly different in the two studies (biological variation). For example the protein Intersectin, which is a known effector downstream of EphB 31, has a SILAC ratio of 0.99 in the QTOF study and 2.0 in the Orbitrap study. Second, the QTOF Micro and Orbitrap instruments have different dynamic ranges for quantitation. It was observed that the SILAC ratios measured by the Orbitrap were generally more dramatic than the ratio from the QTOF (Supporting Information Figure 1). We attribute this to the high dynamic range of Orbitrap 32. Due to the different detection principles employed, the two instruments have quite different characteristics of spectral noise. As shown in Figure 5, the Orbitrap spectrum has lower background signal than the QTOF spectrum. When the peak intensity is low the background can contribute considerably to the ratio obtained by automated SILAC quantitation.
Figure 5.
Measurement of SILAC peptide ratios using LTQ-Orbitrap and QTOF Micro mass spectrometers. The identified peptide (LLVDNQGLSGR) was from SAM and SH3 domain-containing protein 1 (SASH1, IPI00338954). The peptide ratio was calculated as the sum of intensities of the first three isotopic peaks of the light peptide over the sum of the heavy peptide peak intensities. The arrow in each panel indicates the monoisotopic peak for the heavy peptide.
Comprehensiveness of the Screen
An extensive literature search using Pubmed found 42 key signaling proteins that are known to be close EphB binding partners or regulated by activation of EphB receptors (Supporting Information Table 5). 24 of these were identified in this study, and 17 of the 24 proteins had SILAC pY IP abundance changes of 1.5 or more. Seven proteins had pY IP abundance changes upon ephrin stimulation of less than 1.5-fold, but for most of them the directions of the subtle ratio changes were consistent with previous studies, such as PI3K, Grb2 and MAPK. The fact that more than half of all known effectors in EphB signaling were identified in this single study suggested the power of the strategy and the comprehensiveness of the screen. These known effectors only account for a small proportion (~10%) of the proteins with changed ratios identified in this study, suggesting many more novel candidates may participate in EphB signaling with their roles yet to be characterized.
The reasons why many known effectors were not identified in this screen may include: (1) Eph signaling has versatile functions. Many known effectors and signaling effects are only observed in specific cell types and cellular contexts, for example, the NMDA receptor in neurons 33 and ZAP70 in T cells 34. (2) The known effectors are based on studies on any of the 6 EphB receptors while only three of them (EphB2, EphB3 and EphB4) are known to be expressed in the cell line that was used in this study. (3) It has been shown that different intensities of Eph signaling can produce very different cellular effects1. Therefore regulation of effectors is dependent on intensity of Eph receptor activation, which is in turn dependent on level of receptor expression and concentration/affinity of stimulating ligands etc. (4) Different effectors may have different time courses of activation and the activation of specific effectors may only be observed at a specific time point. (5) Some effectors may not contain tyrosine phosphorylation sites or have low binding affinity/stoichiometry to other effectors. (6) Some effectors, for example, Src family kinases including Src, Fyn and Yes, are tyrosine phosphorylated at different sites both when activated and inhibited 35. In this case the overall protein phosphorylation level, i.e. the SILAC ratio, might not reflect the level of activation. (7) The amounts of the effector proteins, in the context of the other proteins being analyzed, were below the detection limit of the mass spectrometer.
Gene Ontology (GO) analysis
We performed a GO analysis of the quantified proteins using a commercial tool from ProteinCenter™ (Proxeon) for annotating and comparing protein datasets. The quantified proteins were first classified into two groups: proteins with ratio changes and proteins without ratio changes. GO analysis was performed for both groups using biological processes, cellular components and molecular functions classifications (Figure 6).
Figure 6.
GO analysis of proteins with changed and unchanged SILAC ratios. All quantified proteins were classified into two groups: proteins with ratio changes less than 1.5 fold (unchanged) and proteins without ratio changes greater 1.5 (changed). GO analysis was performed for both groups on cellular components (A), molecular functions (B) and biological processes (C).
In theory, all proteins with changed ratios were pulled down in pY IPs due to specific binding and are specific to EphB signaling. Pulldown of proteins with no ratio changes could be due to either specific (some tyrosine phosphorylated proteins do not change their phosphorylation status after Eph activation) or non-specific binding during IP. Therefore enrichment of a specific GO annotation in one group can indicate a general difference between the Eph signaling specific/non-specific proteins or pY IP specific/non-specific proteins.
The GO cellular components analysis (Figure 6A) shows that proteins from ribosome, nucleus, mitochondrion, and endoplasmic reticulum (ER) are enriched in the unchanged protein group, suggesting that a considerable number of non-specific binding proteins from the pY IP came from these organelles. GO molecular functions analysis (Figure 6B) shows more nucleic acid/nucleotide binding proteins in the unchanged protein group, which also suggests the nucleus and ribosome as major sources of non-specific binding proteins. This implies that more vigorous clarification of lysate before pY IP, e.g. centrifugation at higher speed or for longer time, which can better remove these organelles, may reduce IP background and promote identification of more specific target proteins. Another observation is that membrane proteins are enriched in the changed groups, suggesting significant Eph signaling occurs on the cellular membrane.
From the GO molecular functions/biological processes analysis (Figure 6B and 6C), proteins in the categories of cell communication and signal transduction are enriched in the changed proteins, while proteins in the categories of metabolism and structural activity are enriched in the non-changed proteins. This is consistent with the established notion that the SILAC strategy we employed is an effective way to identify specific signaling target proteins from a background of non-specific interactions, which might be expected to be dominated by highly abundant housekeeping/structural proteins.
In all three GO analyses, the unannotated proteins are more enriched in the regulated proteins category, suggesting that many of the proteins with changed SILAC ratios have not been well studied.
Domain Analysis
The presence of conserved structural domains in a protein can suggest particular functions for the protein. Therefore domain analysis can be used as a preliminary search for potential protein groups with specific functions or interactions in a signaling pathway.
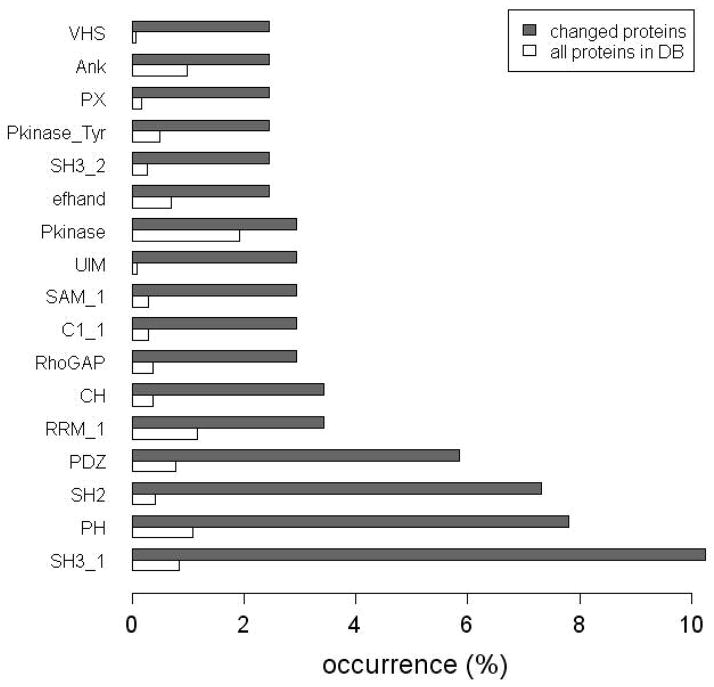
The cross-reference files for the IPI mouse and rat protein databases were searched to obtain the Pfam domain annotations for the all quantified proteins (Supporting Information Table 1). For the proteins with changed SILAC ratios upon ephrinB1 stimulation (shown in Figure 7), it is clear that several specific domains are overrepresented: (1) SH3 (including SH3_1 and SH3_2), PH, CH and RhoGAP domains are known to be indicative of a protein involved in signal transduction related to cytoskeletal organization, which is consistent with the consensus that cytoskeleton rearrangement is one major outcome of EphB signaling. (2) SH2 domains, which are important regulatory modules of tyrosine phosphorylation-dependent signaling cascades, are also overrepresented. (3) The EphB receptor has a PDZ binding motif at the C-terminus and is known to bind to PDZ-domain containing proteins including Syntenin, Afadin, and Grip1. All these proteins were identified in this SILAC study as proteins with changed ratios. In addition, nine proteins with changed SILAC ratios were found to contain PDZ domains, which are possible novel binding partners of EphB receptors through their PDZ domains. (4) UIM and VHS domains are also overrepresented, suggesting many effectors may be involved in vesicular trafficking / protein degradation triggered by ubiquitination of Eph receptors.
Figure 7. Domain analysis for proteins with SILAC ratio changes.
Domain information was obtained from the Pfam annotation in the IPI cross-reference files. Domains that occur in at least 5 (out of 204) proteins are included in the figure. The occurrence of all domains in the combined IPI mouse and rat database is used as a control.
Biological Implications of the Novel Candidate Effectors
Of the proteins found to be involved in EphB signaling in this study but not our previous study, we validated Erbin by Western blotting (Fig. 4). Lending further support to the validity of our results, nine of the proteins found in this but not our previous study have been reported by others to be involved in ephrin signaling. These proteins include Intersectin 31, PI3K 36–39, Shc 40, Vav2 41, Cbl 42, Syntenin 43, Grip1 44, Grb2 40, and Sos1 20. In addition, in-silico protein function and interaction analysis was performed by feeding the list of proteins of changed SILAC ratios into Ingenuity Pathways Analysis software (Ingenuity Systems, http://www.ingenuity.com), which searches existing literature and interaction databases (including Ingenuity curated findings, BIND, BIOGRID, DIP, INTACT, Interactome studies, MINT and MIPS, all downloaded 07/08/2008) for protein interaction and regulation networks. 98 of the 204 proteins with changed SILAC ratios could be assigned to a single interaction network based on previously reported direct protein-protein binding (Supporting Information Figure 2), thus supporting the ability of our screen to find functionally related proteins. The software was also able to classify the proteins into eleven major signaling networks (each network included a minimum of 10 proteins from our list of proteins that change in response to ephrinB addition) with relatively independent functions (Supporting Information Figure 3). These networks are mainly involved in regulation of cell morphology, cellular assembly and organization as well as development, which are consistent with known functions of EphB signaling. The same analysis was also carried out for the 46 proteins with changed SILAC ratios found in the QTOF analysis, which resulted in only two networks with 10 or more members from the list of changing proteins (Supporting Information Figure 3). It was noted that a considerable number of candidate effectors from the current study, which were missing in the previous QTOF analysis, were assigned into networks that participate in protein synthesis, indicating gene translation is quickly activated in response to EphB receptor activation. Another novel group of networks are involved in cell death and growth/proliferation, which is in line with the emerging discovery that Eph receptors play important roles in cancer.1 Compared to the QTOF study, the much improved pY proteome coverage by the Orbitrap analysis greatly facilitated pathway analysis of EphB signaling.
Conclusions
We have used SILAC and LTQ-Orbitrap mass spectrometry to screen for novel effector proteins in the EphB signaling pathway. A considerable proportion of the tyrosine phosphoproteome was identified and quantified, allowing for a global view of the changes of a huge signaling network in response to EphB receptor activation. This study revealed an unprecedented large number of candidate effectors, which will greatly accelerate the achievement of our goal of a more complete understanding of the EphB signaling pathway.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants P30 NS050276 from NINDS and Shared Instrumentation Grant S10 RR 017990-01 to T. A. N. We thank Dr. Harald Stenmark for the Hrs and STAM2 antibodies, Dr. Stevan Hubbard for the IRS-2 antibody and Drs. Moses Chao and Tony Pawson for NG108 cell lines. We thank Proxeon for the use of ProteinCenter.
Abbreviations
- IP
immunoprecipitation
- pY
phosphotyrosine
- SILAC
stable isotope labeling with amino acids in cell culture
- RTK
receptor tyrosine kinase
- GO
gene ontology
- IPI
international protein index
- QTOF
quadrupole time-of-flight
- LC
liquid chromatography
- MS
mass spectrometry
- MS/MS
tandem mass spectrometry
- HRP
horseradish peroxidase
Footnotes
Supporting Information Available: Supporting Information Table 1 shows SILAC ratios and Pfam domain annotations for identified proteins. Supporting Information Table 2 shows the identified phosphopeptides and localization of phosphorylation sites using Ascore. Supporting Information Table 3 shows proteins that showed significantly different SILAC ratios in this study and a previous study that used QTOF mass spectrometry. Supporting Information Table 4 contains a list of all proteins with SILAC ratios showing more that 1.5-fold change after ephrin signaling from both replicates of the Orbitrap analysis (reported here) as well as those found in our previous QTOF study. Supporting Information Table 5 shows known effector proteins in EphB signaling. Supporting Information Figure 1 shows SILAC ratios measured by the QTOF and the Orbitrap of the proteins that were identified in both studies. Supporting Information Figure 2 and Figure 3 show the protein networks from the Ingenuity Pathways Analysis software. Supporting Information File1 and File2 show the annotated MS/MS spectra of identified phosphopeptides. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol. 2005;6 (6):462–75. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- 2.Murai KK, Pasquale EB. ‘Eph’ective signaling: forward, reverse and crosstalk. J Cell Sci. 2003;116 (Pt 14):2823–32. doi: 10.1242/jcs.00625. [DOI] [PubMed] [Google Scholar]
- 3.Ong SE, Blagoev B, Kratchmarova I, Kristensen DB, Steen H, Pandey A, Mann M. Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol Cell Proteomics. 2002;1 (5):376–86. doi: 10.1074/mcp.m200025-mcp200. [DOI] [PubMed] [Google Scholar]
- 4.Blagoev B, Mann M. Quantitative proteomics to study mitogen-activated protein kinases. Methods. 2006;40 (3):243–50. doi: 10.1016/j.ymeth.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Kratchmarova I, Blagoev B, Haack-Sorensen M, Kassem M, Mann M. Mechanism of divergent growth factor effects in mesenchymal stem cell differentiation. Science. 2005;308 (5727):1472–7. doi: 10.1126/science.1107627. [DOI] [PubMed] [Google Scholar]
- 6.Blagoev B, Ong SE, Kratchmarova I, Mann M. Temporal analysis of phosphotyrosine-dependent signaling networks by quantitative proteomics. Nat Biotechnol. 2004;22 (9):1139–45. doi: 10.1038/nbt1005. [DOI] [PubMed] [Google Scholar]
- 7.Zhang G, Spellman DS, Skolnik EY, Neubert TA. Quantitative phosphotyrosine proteomics of EphB2 signaling by stable isotope labeling with amino acids in cell culture (SILAC) J Proteome Res. 2006;5 (3):581–8. doi: 10.1021/pr050362b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hardman M, Makarov AA. Interfacing the orbitrap mass analyzer to an electrospray ion source. Anal Chem. 2003;75 (7):1699–705. doi: 10.1021/ac0258047. [DOI] [PubMed] [Google Scholar]
- 9.Hu Q, Noll RJ, Li H, Makarov A, Hardman M, Graham Cooks R. The Orbitrap: a new mass spectrometer. J Mass Spectrom. 2005;40 (4):430–43. doi: 10.1002/jms.856. [DOI] [PubMed] [Google Scholar]
- 10.Yates JR, Cociorva D, Liao L, Zabrouskov V. Performance of a linear ion trap-Orbitrap hybrid for peptide analysis. Anal Chem. 2006;78 (2):493–500. doi: 10.1021/ac0514624. [DOI] [PubMed] [Google Scholar]
- 11.Makarov A, Denisov E, Kholomeev A, Balschun W, Lange O, Strupat K, Horning S. Performance evaluation of a hybrid linear ion trap/orbitrap mass spectrometer. Anal Chem. 2006;78 (7):2113–20. doi: 10.1021/ac0518811. [DOI] [PubMed] [Google Scholar]
- 12.Erickson B. Linear ion trap/Orbitrap mass spectrometer. Anal Chem. 2006;78 (7):2089. doi: 10.1021/ac069390j. [DOI] [PubMed] [Google Scholar]
- 13.Scigelova M, Makarov A. Orbitrap mass analyzer - overview and applications in proteomics. Proteomics. 2006;6 Suppl 2:16–21. doi: 10.1002/pmic.200600528. [DOI] [PubMed] [Google Scholar]
- 14.Elias JE, Haas W, Faherty BK, Gygi SP. Comparative evaluation of mass spectrometry platforms used in large-scale proteomics investigations. Nat Methods. 2005;2 (9):667–75. doi: 10.1038/nmeth785. [DOI] [PubMed] [Google Scholar]
- 15.Holland SJ, Gale NW, Gish GD, Roth RA, Songyang Z, Cantley LC, Henkemeyer M, Yancopoulos GD, Pawson T. Juxtamembrane tyrosine residues couple the Eph family receptor EphB2/Nuk to specific SH2 domain proteins in neuronal cells. Embo J. 1997;16 (13):3877–88. doi: 10.1093/emboj/16.13.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olsen JV, Mann M. Improved peptide identification in proteomics by two consecutive stages of mass spectrometric fragmentation. Proc Natl Acad Sci U S A. 2004;101 (37):13417–22. doi: 10.1073/pnas.0405549101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beausoleil SA, Villen J, Gerber SA, Rush J, Gygi SP. A probability-based approach for high-throughput protein phosphorylation analysis and site localization. Nat Biotechnol. 2006;24 (10):1285–92. doi: 10.1038/nbt1240. [DOI] [PubMed] [Google Scholar]
- 18.Raiborg C, Bache KG, Mehlum A, Stang E, Stenmark H. Hrs recruits clathrin to early endosomes. Embo J. 2001;20 (17):5008–21. doi: 10.1093/emboj/20.17.5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bache KG, Raiborg C, Mehlum A, Stenmark H. STAM and Hrs are subunits of a multivalent ubiquitin-binding complex on early endosomes. J Biol Chem. 2003;278 (14):12513–21. doi: 10.1074/jbc.M210843200. [DOI] [PubMed] [Google Scholar]
- 20.Tong J, Elowe S, Nash P, Pawson T. Manipulation of EphB2 regulatory motifs and SH2 binding sites switches MAPK signaling and biological activity. J Biol Chem. 2003;278 (8):6111–9. doi: 10.1074/jbc.M208972200. [DOI] [PubMed] [Google Scholar]
- 21.Kong H, Boulter J, Weber JL, Lai C, Chao MV. An evolutionarily conserved transmembrane protein that is a novel downstream target of neurotrophin and ephrin receptors. J Neurosci. 2001;21 (1):176–85. doi: 10.1523/JNEUROSCI.21-01-00176.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Binns KL, Taylor PP, Sicheri F, Pawson T, Holland SJ. Phosphorylation of tyrosine residues in the kinase domain and juxtamembrane region regulates the biological and catalytic activities of Eph receptors. Mol Cell Biol. 2000;20 (13):4791–805. doi: 10.1128/mcb.20.13.4791-4805.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elowe S, Holland SJ, Kulkarni S, Pawson T. Downregulation of the Ras-mitogen-activated protein kinase pathway by the EphB2 receptor tyrosine kinase is required for ephrin-induced neurite retraction. Mol Cell Biol. 2001;21 (21):7429–41. doi: 10.1128/MCB.21.21.7429-7441.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hock B, Bohme B, Karn T, Yamamoto T, Kaibuchi K, Holtrich U, Holland S, Pawson T, Rubsamen-Waigmann H, Strebhardt K. PDZ-domain-mediated interaction of the Eph-related receptor tyrosine kinase EphB3 and the ras-binding protein AF6 depends on the kinase activity of the receptor. Proc Natl Acad Sci U S A. 1998;95 (17):9779–84. doi: 10.1073/pnas.95.17.9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Becker E, Huynh-Do U, Holland S, Pawson T, Daniel TO, Skolnik EY. Nck-interacting Ste20 kinase couples Eph receptors to c-Jun N-terminal kinase and integrin activation. Mol Cell Biol. 2000;20 (5):1537–45. doi: 10.1128/mcb.20.5.1537-1545.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collins MO, Yu L, Coba MP, Husi H, Campuzano I, Blackstock WP, Choudhary JS, Grant SG. Proteomic analysis of in vivo phosphorylated synaptic proteins. J Biol Chem. 2005;280 (7):5972–82. doi: 10.1074/jbc.M411220200. [DOI] [PubMed] [Google Scholar]
- 27.Rush J, Moritz A, Lee KA, Guo A, Goss VL, Spek EJ, Zhang H, Zha XM, Polakiewicz RD, Comb MJ. Immunoaffinity profiling of tyrosine phosphorylation in cancer cells. Nat Biotechnol. 2005;23 (1):94–101. doi: 10.1038/nbt1046. [DOI] [PubMed] [Google Scholar]
- 28.Beausoleil SA, Jedrychowski M, Schwartz D, Elias JE, Villen J, Li J, Cohn MA, Cantley LC, Gygi SP. Large-scale characterization of HeLa cell nuclear phosphoproteins. Proc Natl Acad Sci U S A. 2004;101 (33):12130–5. doi: 10.1073/pnas.0404720101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trinidad JC, Specht CG, Thalhammer A, Schoepfer R, Burlingame AL. Comprehensive identification of phosphorylation sites in postsynaptic density preparations. Mol Cell Proteomics. 2006;5 (5):914–22. doi: 10.1074/mcp.T500041-MCP200. [DOI] [PubMed] [Google Scholar]
- 30.Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P, Mann M. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell. 2006;127 (3):635–48. doi: 10.1016/j.cell.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 31.Irie F, Yamaguchi Y. EphB receptors regulate dendritic spine development via intersectin, Cdc42 and N-WASP. Nat Neurosci. 2002;5 (11):1117 –8. doi: 10.1038/nn964. [DOI] [PubMed] [Google Scholar]
- 32.Venable JD, Wohlschlegel J, McClatchy DB, Park SK, Yates JR. 3rd, Relative quantification of stable isotope labeled peptides using a linear ion trap-Orbitrap hybrid mass spectrometer. Anal Chem. 2007;79 (8):3056–64. doi: 10.1021/ac062054i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takasu MA, Dalva MB, Zigmond RE, Greenberg ME. Modulation of NMDA receptor-dependent calcium influx and gene expression through EphB receptors. Science. 2002;295 (5554):491–5. doi: 10.1126/science.1065983. [DOI] [PubMed] [Google Scholar]
- 34.Luo H, Yu G, Tremblay J, Wu J. EphB6-null mutation results in compromised T cell function. J Clin Invest. 2004;114 (12):1762–73. doi: 10.1172/JCI21846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu W, Doshi A, Lei M, Eck MJ, Harrison SC. Crystal structures of c-Src reveal features of its autoinhibitory mechanism. Mol Cell. 1999;3 (5):629–38. doi: 10.1016/s1097-2765(00)80356-1. [DOI] [PubMed] [Google Scholar]
- 36.Wong EV, Kerner JA, Jay DG. Convergent and divergent signaling mechanisms of growth cone collapse by ephrinA5 and slit2. J Neurobiol. 2004;59 (1):66–81. doi: 10.1002/neu.10342. [DOI] [PubMed] [Google Scholar]
- 37.Maekawa H, Oike Y, Kanda S, Ito Y, Yamada Y, Kurihara H, Nagai R, Suda T. Ephrin-B2 induces migration of endothelial cells through the phosphatidylinositol-3 kinase pathway and promotes angiogenesis in adult vasculature. Arterioscler Thromb Vasc Biol. 2003;23 (11):2008–14. doi: 10.1161/01.ATV.0000096655.56262.56. [DOI] [PubMed] [Google Scholar]
- 38.Brantley-Sieders DM, Caughron J, Hicks D, Pozzi A, Ruiz JC, Chen J. EphA2 receptor tyrosine kinase regulates endothelial cell migration and vascular assembly through phosphoinositide 3-kinase-mediated Rac1 GTPase activation. J Cell Sci. 2004;117 (Pt 10):2037–49. doi: 10.1242/jcs.01061. [DOI] [PubMed] [Google Scholar]
- 39.Steinle JJ, Meininger CJ, Forough R, Wu G, Wu MH, Granger HJ. Eph B4 receptor signaling mediates endothelial cell migration and proliferation via the phosphatidylinositol 3-kinase pathway. J Biol Chem. 2002;277 (46):43830–5. doi: 10.1074/jbc.M207221200. [DOI] [PubMed] [Google Scholar]
- 40.Vindis C, Cerretti DP, Daniel TO, Huynh-Do U. EphB1 recruits c-Src and p52Shc to activate MAPK/ERK and promote chemotaxis. J Cell Biol. 2003;162 (4):661–71. doi: 10.1083/jcb.200302073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cowan CW, Shao YR, Sahin M, Shamah SM, Lin MZ, Greer PL, Gao S, Griffith EC, Brugge JS, Greenberg ME. Vav family GEFs link activated Ephs to endocytosis and axon guidance. Neuron. 2005;46 (2):205–17. doi: 10.1016/j.neuron.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 42.Walker-Daniels J, Riese DJ, 2nd, Kinch MS. c-Cbl-dependent EphA2 protein degradation is induced by ligand binding. Mol Cancer Res. 2002;1 (1):79–87. [PubMed] [Google Scholar]
- 43.Torres R, Firestein BL, Dong H, Staudinger J, Olson EN, Huganir RL, Bredt DS, Gale NW, Yancopoulos GD. PDZ proteins bind, cluster, and synaptically colocalize with Eph receptors and their ephrin ligands. Neuron. 1998;21 (6):1453–63. doi: 10.1016/s0896-6273(00)80663-7. [DOI] [PubMed] [Google Scholar]
- 44.Contractor A, Rogers C, Maron C, Henkemeyer M, Swanson GT, Heinemann SF. Trans-synaptic Eph receptor-ephrin signaling in hippocampal mossy fiber LTP. Science. 2002;296 (5574):1864–9. doi: 10.1126/science.1069081. [DOI] [PubMed] [Google Scholar]
- 45.Zhuang G, Hunter S, Hwang Y, Chen J. Regulation of EphA2 receptor endocytosis by SHIP2 lipid phosphatase via phosphatidylinositol 3-Kinase-dependent Rac1 activation. J Biol Chem. 2007;282 (4):2683–94. doi: 10.1074/jbc.M608509200. [DOI] [PubMed] [Google Scholar]
- 46.Miao H, Burnett E, Kinch M, Simon E, Wang B. Activation of EphA2 kinase suppresses integrin function and causes focal-adhesion-kinase dephosphorylation. Nat Cell Biol. 2000;2 (2):62–9. doi: 10.1038/35000008. [DOI] [PubMed] [Google Scholar]
- 47.Carter N, Nakamoto T, Hirai H, Hunter T. EphrinA1-induced cytoskeletal re-organization requires FAK and p130(cas) Nat Cell Biol. 2002;4 (8):565–73. doi: 10.1038/ncb823. [DOI] [PubMed] [Google Scholar]
- 48.Moeller ML, Shi Y, Reichardt LF, Ethell IM. EphB receptors regulate dendritic spine morphogenesis through the recruitment/phosphorylation of focal adhesion kinase and RhoA activation. J Biol Chem. 2006;281 (3):1587–98. doi: 10.1074/jbc.M511756200. [DOI] [PubMed] [Google Scholar]
- 49.Dodelet VC, Pazzagli C, Zisch AH, Hauser CA, Pasquale EB. A novel signaling intermediate, SHEP1, directly couples Eph receptors to R-Ras and Rap1A. J Biol Chem. 1999;274 (45):31941–6. doi: 10.1074/jbc.274.45.31941. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.