Abstract
Alginates from nine mucoid Pseudomonas aeruginosa isolates from patients with cystic fibrosis were purified by repeated ethanol precipitation, nuclease digestion, anion-exchange chromatography, dialysis, and lyophilization. Uronic acid constituted 72% of the dry weight when mannuronolactone was used as the internal standard in the carbazole-borate assay for uronic acids. The average degree of acetylation was 16%, and the ratio of mannuronic acid to gluluronic acid was 4.7. No homopolymeric blocks of guluronic acid were found when analyzed by nuclear magnetic resonance spectroscopy. Contaminating proteins were denatured by heating, and during purification the content of protein relative to alginate fell from 566 to 0.9%. The content of lipopolysaccharide was 0.012%. No immunological or biological activity was attributable to the protein or lipopolysaccharide content as estimated by immunoblotting, enzyme-linked immunosorbent assay (ELISA), and a neutrophil chemotaxis assay. Rabbits were hyperimmunized with P. aeruginosa alginates and alginate from the seaweed Laminaria hyperborea, and an ELISA that detected alginate-specific antibodies was developed. Antibodies to P. aeruginosa alginate were detected by ELISA in 1:4,000 dilutions of serum from patients with cystic fibrosis with chronic P. aeruginosa lung infection. The serological cross-reactions between serum from the nine patients with cystic fibrosis and the corresponding P. aeruginosa alginates were investigated and showed considerable heterogeneity. This finding indicates that P. aeruginosa alginate from more than one P. aeruginosa strain should be used in serological tests. There was no serological cross-reactivity between P. aeruginosa and Laminaria hyperborea alginate in either rabbits or patients with cystic fibrosis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baek L., Høiby N., Hertz J. B., Espersen F. Interaction between limulus amoebocyte lysate and soluble antigens from Pseudomonas aeruginosa and Staphylococcus aureus studied by quantitative immunoelectrophoresis. J Clin Microbiol. 1985 Aug;22(2):229–237. doi: 10.1128/jcm.22.2.229-237.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek L. New, sensitive rocket immunoelectrophoretic assay for measurement of the reaction between endotoxin and Limulus amoebocyte lysate. J Clin Microbiol. 1983 Jun;17(6):1013–1020. doi: 10.1128/jcm.17.6.1013-1020.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore R. S., Fick R. B., Jr, Fino L. Antibody to multiple mucoid strains of Pseudomonas aeruginosa in patients with cystic fibrosis, measured by an enzyme-linked immunosorbent assay. Pediatr Res. 1986 Nov;20(11):1085–1090. doi: 10.1203/00006450-198611000-00005. [DOI] [PubMed] [Google Scholar]
- Bryan L. E., Kureishi A., Rabin H. R. Detection of antibodies to Pseudomonas aeruginosa alginate extracellular polysaccharide in animals and cystic fibrosis patients by enzyme-linked immunosorbent assay. J Clin Microbiol. 1983 Aug;18(2):276–282. doi: 10.1128/jcm.18.2.276-282.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucke C. Polyacrylamide gel electrophoresis of alginic acid. J Chromatogr. 1974 Feb 13;89(1):99–102. doi: 10.1016/s0021-9673(01)84167-4. [DOI] [PubMed] [Google Scholar]
- Bussolati G., Gugliotta P. Nonspecific staining of mast cells by avidin-biotin-peroxidase complexes (ABC). J Histochem Cytochem. 1983 Dec;31(12):1419–1421. doi: 10.1177/31.12.6195216. [DOI] [PubMed] [Google Scholar]
- Callahan L. T., 3rd, Woodhour A. F., Meeker J. B., Hilleman M. R. Enzyme-linked immunosorbent assay for measurement of antibodies against pneumococcal polysaccharide antigens: comparison with radioimmunoassay. J Clin Microbiol. 1979 Oct;10(4):459–463. doi: 10.1128/jcm.10.4.459-463.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
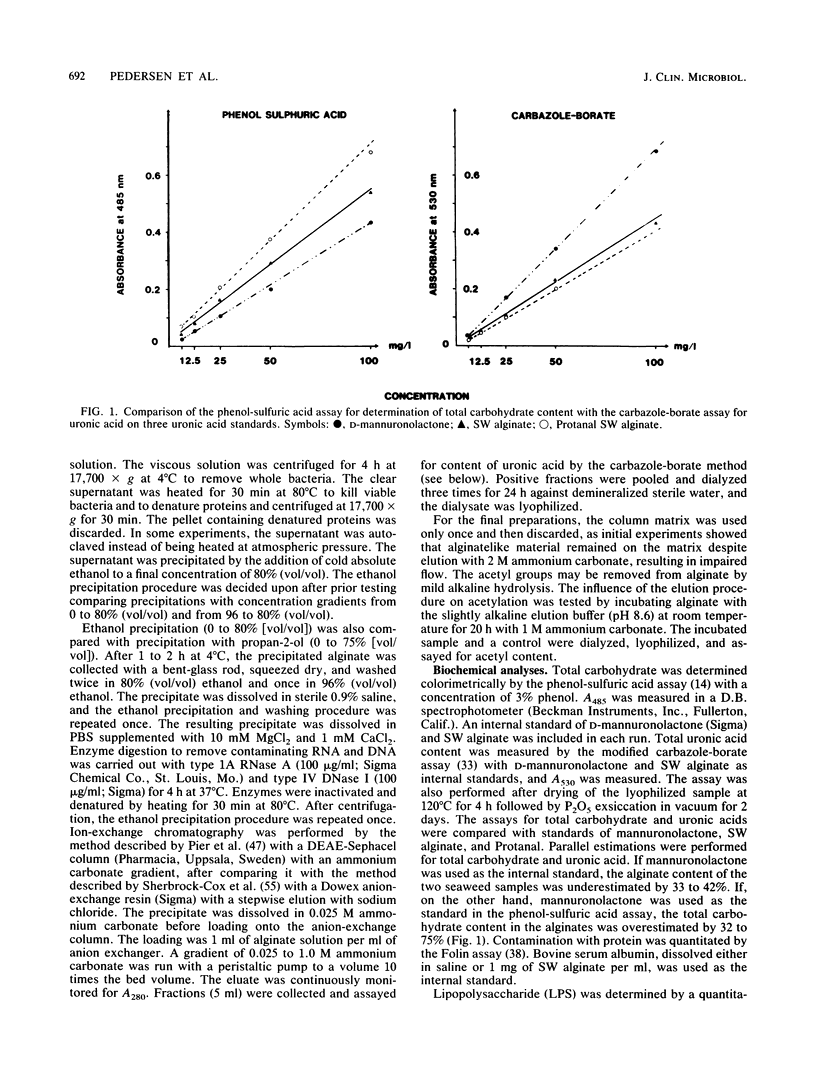
- Carlson D. M., Matthews L. W. Polyuronic acids produced by Pseudomonas aeruginosa. Biochemistry. 1966 Sep;5(9):2817–2822. doi: 10.1021/bi00873a006. [DOI] [PubMed] [Google Scholar]
- Ceri H., McArthur H. A., Whitfield C. Association of alginate from Pseudomonas aeruginosa with two forms of heparin-binding lectin isolated from rat lung. Infect Immun. 1986 Jan;51(1):1–5. doi: 10.1128/iai.51.1.1-5.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley L., Pier G. B., Liporace J. D., Eardley D. D. Polyclonal B cell stimulation and interleukin 1 induction by the mucoid exopolysaccharide of Pseudomonas aeruginosa associated with cystic fibrosis. J Immunol. 1985 May;134(5):3089–3093. [PubMed] [Google Scholar]
- Evans L. R., Linker A. Production and characterization of the slime polysaccharide of Pseudomonas aeruginosa. J Bacteriol. 1973 Nov;116(2):915–924. doi: 10.1128/jb.116.2.915-924.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
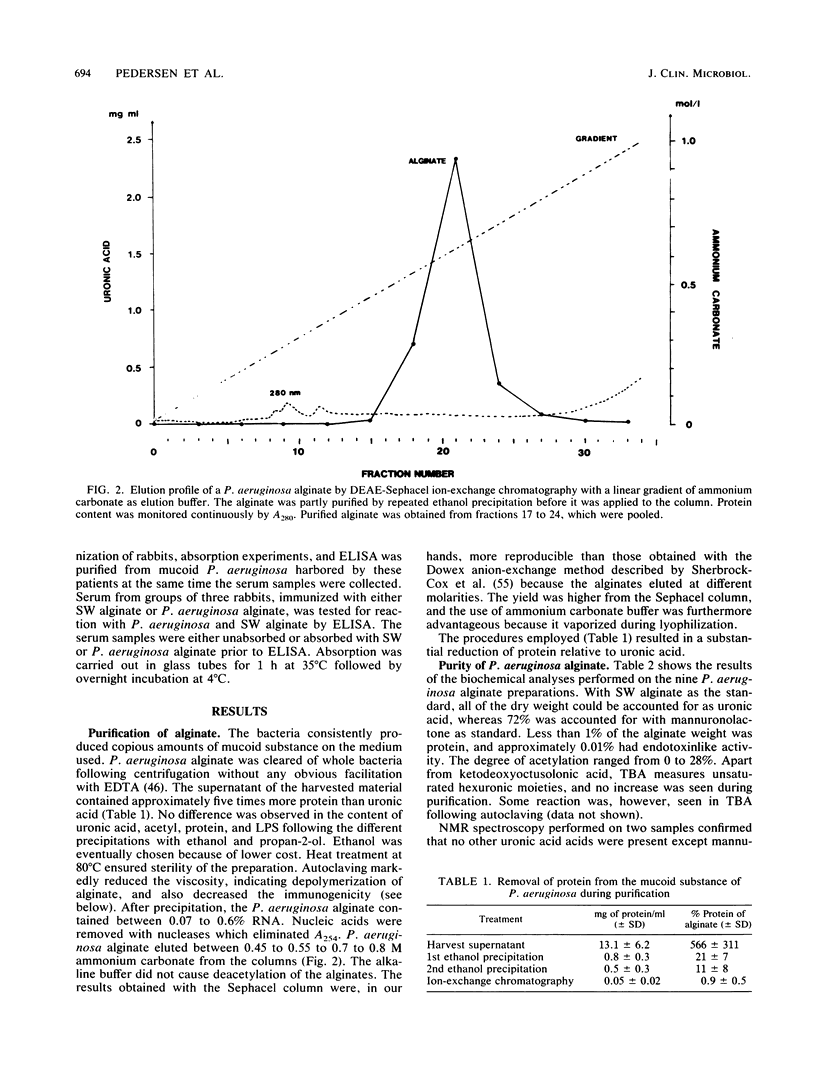
- Fett W. F., Osman S. F., Fishman M. L., Siebles T. S. Alginate production by plant-pathogenic pseudomonads. Appl Environ Microbiol. 1986 Sep;52(3):466–473. doi: 10.1128/aem.52.3.466-473.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fomsgaard A., Conrad R. S., Galanos C., Shand G. H., Høiby N. Comparative immunochemistry of lipopolysaccharides from typable and polyagglutinable Pseudomonas aeruginosa strains isolated from patients with cystic fibrosis. J Clin Microbiol. 1988 May;26(5):821–826. doi: 10.1128/jcm.26.5.821-826.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govan J. R., Fyfe J. A., Jarman T. R. Isolation of alginate-producing mutants of Pseudomonas fluorescens, Pseudomonas putida and Pseudomonas mendocina. J Gen Microbiol. 1981 Jul;125(1):217–220. doi: 10.1099/00221287-125-1-217. [DOI] [PubMed] [Google Scholar]
- Gray B. M. ELISA methodology for polysaccharide antigens: protein coupling of polysaccharides for adsorption to plastic tubes. J Immunol Methods. 1979;28(1-2):187–192. doi: 10.1016/0022-1759(79)90340-5. [DOI] [PubMed] [Google Scholar]
- Hoiby N., Flensborg E. W., Beck B., Friis B., Jacobsen S. V., Jacobsen L. Pseudomonas aeruginosa infection in cystic fibrosis. Diagnostic and prognostic significance of Pseudomonas aeruginosa precipitins determined by means of crossed immunoelectrophoresis. Scand J Respir Dis. 1977 Apr;58(2):65–79. [PubMed] [Google Scholar]
- Hoiby N. Prevalence of mucoid strains of Pseudomonas aeruginosa in bacteriological specimens from patients with cystic fibrosis and patients with other diseases. Acta Pathol Microbiol Scand Suppl. 1975 Dec;83(6):549–552. [PubMed] [Google Scholar]
- Holby N. Antibodies against Pseudomonas aeruginosa in serum from normal persons and patients colonized with mucoid or non-mucoid Pseudomonas aeruginosa: Results obtained by crossed immunoelectrophoresis. Acta Pathol Microbiol Scand C. 1977 Apr;85(2):142–148. [PubMed] [Google Scholar]
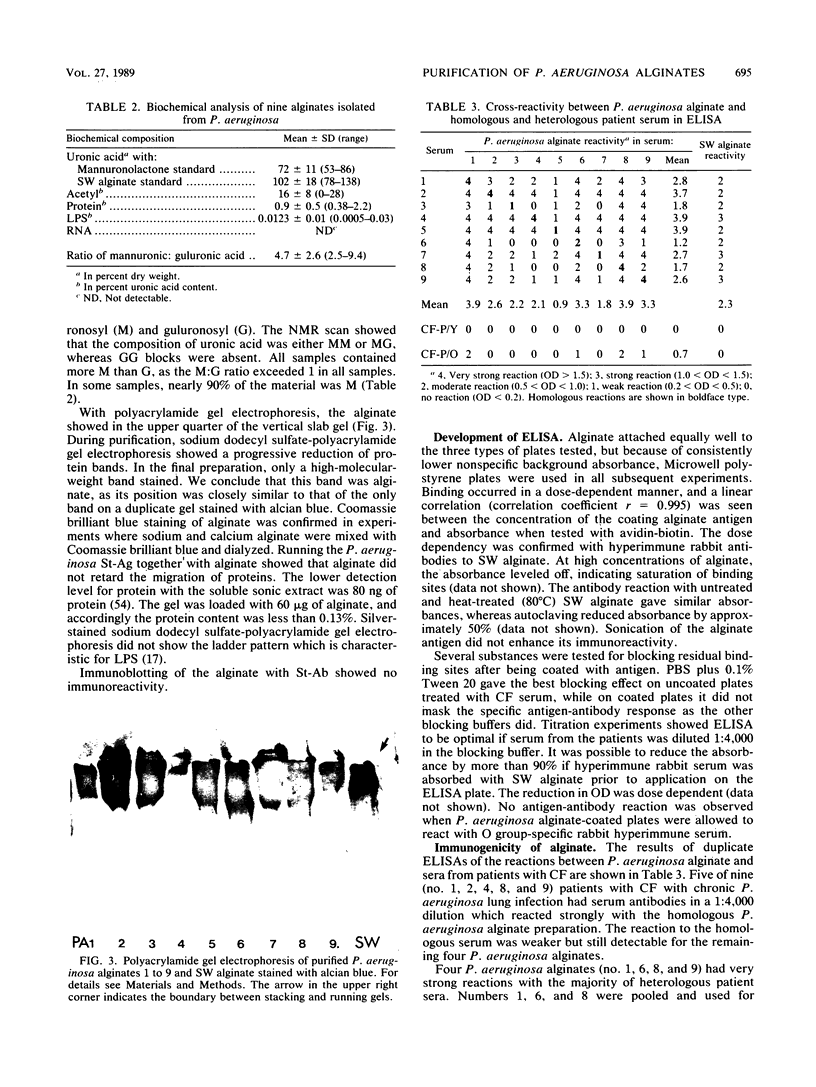
- Høiby N., Döring G., Schiøtz P. O. The role of immune complexes in the pathogenesis of bacterial infections. Annu Rev Microbiol. 1986;40:29–53. doi: 10.1146/annurev.mi.40.100186.000333. [DOI] [PubMed] [Google Scholar]
- Irvin R. T., Ceri H. Immunochemical examination of the Pseudomonas aeruginosa glycocalyx: a monoclonal antibody which recognizes L-guluronic acid residues of alginic acid. Can J Microbiol. 1985 Mar;31(3):268–275. doi: 10.1139/m85-050. [DOI] [PubMed] [Google Scholar]
- Karkhanis Y. D., Zeltner J. Y., Jackson J. J., Carlo D. J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem. 1978 Apr;85(2):595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- Knutson C. A., Jeanes A. A new modification of the carbazole analysis: application to heteropolysaccharides. Anal Biochem. 1968 Sep;24(3):470–481. doi: 10.1016/0003-2697(68)90154-1. [DOI] [PubMed] [Google Scholar]
- LINKER A., JONES R. S. A POLYSACCHARIDE RESEMBLING ALGINIC ACID FROM A PSEUDOMONAS MICRO-ORGANISM. Nature. 1964 Oct 10;204:187–188. doi: 10.1038/204187a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Learn D. B., Brestel E. P., Seetharama S. Hypochlorite scavenging by Pseudomonas aeruginosa alginate. Infect Immun. 1987 Aug;55(8):1813–1818. doi: 10.1128/iai.55.8.1813-1818.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linker A., Jones R. S. A new polysaccharide resembling alginic acid isolated from pseudomonads. J Biol Chem. 1966 Aug 25;241(16):3845–3851. [PubMed] [Google Scholar]
- Martínez-Maza O., Fehniger T. E., Ashman R. F. Antibody-secreting cell precursor frequencies among the sheep-erythrocyte-binding cells after immunization. Scand J Immunol. 1983 Apr;17(4):345–354. doi: 10.1111/j.1365-3083.1983.tb00799.x. [DOI] [PubMed] [Google Scholar]
- McCarthy V. P., Rosenberg G., Rosenstein B. J., Hubbard V. S. Mucoid Pseudomonas aeruginosa from a patient without cystic fibrosis: implications and review of the literature. Pediatr Infect Dis. 1986 Mar-Apr;5(2):256–258. doi: 10.1097/00006454-198603000-00019. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Ulevitch R. J. The effects of bacterial endotoxins on host mediation systems. A review. Am J Pathol. 1978 Nov;93(2):526–618. [PMC free article] [PubMed] [Google Scholar]
- PREISS J., ASHWELL G. Alginic acid metabolism in bacteria. I. Enzymatic formation of unsaturated oligosac-charides and 4-deoxy-L-erythro-5-hexoseulose uronic acid. J Biol Chem. 1962 Feb;237:309–316. [PubMed] [Google Scholar]
- Pedersen S. S., Jensen T., Pressler T., Høiby N., Rosendal K. Does centralized treatment of cystic fibrosis increase the risk of Pseudomonas aeruginosa infection? Acta Paediatr Scand. 1986 Sep;75(5):840–845. doi: 10.1111/j.1651-2227.1986.tb10299.x. [DOI] [PubMed] [Google Scholar]
- Pier G. B., Elcock M. E. Nonspecific immunoglobulin synthesis and elevated IgG levels in rabbits immunized with mucoid exopolysaccharide from cystic fibrosis isolates of Pseudomonas aeruginosa. J Immunol. 1984 Aug;133(2):734–739. [PubMed] [Google Scholar]
- Pier G. B., Matthews W. J., Jr, Eardley D. D. Immunochemical characterization of the mucoid exopolysaccharide of Pseudomonas aeruginosa. J Infect Dis. 1983 Mar;147(3):494–503. doi: 10.1093/infdis/147.3.494. [DOI] [PubMed] [Google Scholar]
- Pier G. B., Saunders J. M., Ames P., Edwards M. S., Auerbach H., Goldfarb J., Speert D. P., Hurwitch S. Opsonophagocytic killing antibody to Pseudomonas aeruginosa mucoid exopolysaccharide in older noncolonized patients with cystic fibrosis. N Engl J Med. 1987 Sep 24;317(13):793–798. doi: 10.1056/NEJM198709243171303. [DOI] [PubMed] [Google Scholar]
- Pier G. B., Sidberry H. F., Sadoff J. C. High-molecular-weight polysaccharide antigen from Pseudomonas aeruginosa immunotype 2. Infect Immun. 1981 Nov;34(2):461–468. doi: 10.1128/iai.34.2.461-468.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell N. J., Gacesa P. Chemistry and biology of the alginate of mucoid strains of Pseudomonas aeruginosa in cystic fibrosis. Mol Aspects Med. 1988;10(1):1–91. doi: 10.1016/0098-2997(88)90002-7. [DOI] [PubMed] [Google Scholar]
- Schwarzmann S., Boring J. R. Antiphagocytic Effect of Slime from a Mucoid Strain of Pseudomonas aeruginosa. Infect Immun. 1971 Jun;3(6):762–767. doi: 10.1128/iai.3.6.762-767.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shand G. H., Pedersen S. S., Tilling R., Brown M. R., Høiby N. Use of immunoblot detection of serum antibodies in the diagnosis of chronic Pseudomonas aeruginosa lung infection in cystic fibrosis. J Med Microbiol. 1988 Nov;27(3):169–177. doi: 10.1099/00222615-27-3-169. [DOI] [PubMed] [Google Scholar]
- Sherbrock-Cox V., Russell N. J., Gacesa P. The purification and chemical characterisation of the alginate present in extracellular material produced by mucoid strains of Pseudomonas aeruginosa. Carbohydr Res. 1984 Dec 15;135(1):147–154. doi: 10.1016/0008-6215(84)85012-0. [DOI] [PubMed] [Google Scholar]
- Skjåk-Braek G., Grasdalen H., Larsen B. Monomer sequence and acetylation pattern in some bacterial alginates. Carbohydr Res. 1986 Oct 15;154:239–250. doi: 10.1016/s0008-6215(00)90036-3. [DOI] [PubMed] [Google Scholar]
- Speert D. P., Lawton D., Mutharia L. M. Antibody to Pseudomonas aeruginosa mucoid exopolysaccharide and to sodium alginate in cystic fibrosis serum. Pediatr Res. 1984 May;18(5):431–433. doi: 10.1203/00006450-198405000-00008. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Vadas L., Prihar H. S., Pugashetti B. K., Feingold D. S. A gas chromatographic method for the quantitative determination of hexuronic acids in alginic acid. Anal Biochem. 1981 Jul 1;114(2):294–298. doi: 10.1016/0003-2697(81)90484-x. [DOI] [PubMed] [Google Scholar]