Gap junctions mediate the direct interchange of small molecules (<1000 daltons) between the cytoplasm of neighboring cells and play important roles in the normal function of numerous organs, in tissue homeostasis, and in development (1-3). Mutations in the genes that encode connexins, the gap junction protein subunits, have been implicated in human peripheral nerve and skin disorders, deafness, and cataract formation (4). Functional gap junctions are assembled by the “docking” of hemichannels in the membranes of adjacent cells, with each hemichannel containing six connexin protein subunits (1-3). Connexins are a family of proteins that traverse the plasma membrane four times, creating two extracellular domains responsible for the docking of the hemichannels across the extracellular space and three intracellular regions, consisting of the N- and C-terminal domains and a loop located between transmembrane regions 2 and 3 (1-3). The three intracellular domains appear to regulate gap junction channel assembly and activity (1-3). Mechanisms involving the phosphorylation of the connexin proteins or the interaction of connexins with non-connexin cellular proteins (or both) regulate the trafficking and assembly of connexins into functional gap junctions, channel gating, and the subsequent degradation of connexins (5-8).
A recent paper by Li et al. (9) provides evidence that the disruption of gap junctional communication in ischemic or hypoxic astrocytes is associated with both the altered phosphorylation of connexin43 (Cx43) and the association with gap junctions of several signal transducing proteins, including the c-Src tyrosine kinase and a novel interacting protein, mitogen-activated protein kinase (MAPK) phosphatase 1 (MKP-1). These intriguing data suggest that the closure of gap junction channels in response to divergent stimuli such as ischemia-hypoxia or occupancy of ligand-activated receptors may result from a common mechanism involving the phosphorylation of connexin, its interaction with regulatory proteins, or both. This work merges three distinct areas of research pertinent to the regulation of connexins: protein phosphorylation, connexin-interacting proteins, and intracellular acidification.
Regulation of Gap Junction Permeability by Src-Mediated Phosphorylation
There has been renewed interest in understanding the precise functions of activated Src, the first oncoprotein to be discovered, and its substrates involved in cell proliferation, invasion, and motility (10, 11). The phosphorylation of connexins—by tyrosine protein kinases (especially Src) and by serine protein kinases—is an intensely studied regulatory mechanism. In terms of the effects of v-Src on Cx43, the majority of evidence indicates that v-Src interacts directly with Cx43 through its Src homology 3 (SH3) and SH2 domains (12-14) and then phosphorylates Cx43 on Tyr265 and Tyr247, thereby triggering gap junction channel closure (gating), as measured by decreases in dye transfer or junctional conductance associated with diminished channel open probability (12, 15-19). These data are reminiscent of the Src-dependent phosphorylation of the KCNQ and Shaker family Kv1.3 K+ channels, which also occur at dual tyrosine sites; for the KCNQ channel, this phosphorylation is accompanied by a reduction in the channel open probability (20, 21). On the basis of these data, it has been proposed that the initial binding of Cx43 to v-Src occurs through the interaction of the P274-P284 proline-rich region of Cx43 with the SH3 domain of v-Src (6, 18). This interaction leads to the v-Src-dependent phosphorylation of Cx43 at the Tyr265 site, which provides a binding site for Src's SH2 domain that may stabilize the v-Src-Cx43 interaction and promote the efficient phosphorylation of Cx43 at Tyr247. This second phosphorylation on Tyr247 may be the key molecular event that triggers the closure of the Cx43 channel. A key unanswered question remains: How exactly does phosphorylation of these tyrosine sites in Cx43 result in gap junction channel closure?
c-Src has also been reported to diminish Cx43 function, but demonstration of the phosphorylation of Cx43 on tyrosine has not been straightforward. Thus, although agonist (lysophosphatidic acid, thrombin, and neuropeptide) stimulation of heterotrimeric guanine-nucleotide binding protein (G protein)-coupled receptors activates c-Src and disrupts intercellular communication, the expected phosphorylation of Cx43 on tyrosine could not be demonstrated (22). In contrast, paired cardiomyocytes, isolated from hamsters in late-stage congestive heart failure, contained activated c-Src and Cx43 phosphorylated on tyrosine that was associated with diminished gap junctional conductance (23). Similar to v-Src, activated c-Src interacts with Cx43, an interaction that depends on the SH2 domain and on the phosphorylation of Tyr265 on Cx43 (24, 25). Activation of c-Src is associated with its increased interaction with Cx43 and a reciprocal decrease in the interaction of Cx43 with the ZO-1 cytoskeletal protein (25). The recently described interaction of connexin proteins with other non-connexin cellular proteins, such as ZO-1, has great potential to expand our understanding of connexin regulation, function, and perhaps signal-transducing activity (7, 8).
Regulation of Gap Junction Permeability by Acidification
Intracellular acidification acts as a general regulator of intercellular gap junctional communication in most tissues and experimental cell systems, and it may also exert a protective effect in ischemia-compromised tissues. In the heart and brain, intracellular acidification induced by ischemia triggers the uncoupling of Cx43 gap junctions (known as “pH gating”), which is believed to play a crucial role in attenuating damage to tissues surrounding the ischemic focus (26-28). This effect is not inconsequential, as the ischemia resulting from myocardial infarction, for example, may precipitate cardiac arrhythmias; however, ischemia-induced uncoupling of cardiomyocytes may help to counteract this potentially lethal outcome (27).
The “ball and chain” or “particle-receptor” model, originally invoked as a mechanism for blocking current through sodium and potassium ion channels (29, 30), was proposed as the mechanism underlying the intracellular acidification-induced closure of gap junctions (31). This model posits that one region of connexin forms the “ball” or “particle,” which interacts directly with a second “receptor” region to close gap junctions (31). This model was based on the observation that truncation of Cx43 at amino acid 257, which removes most of the cytoplasmic C-terminal region, blocked acidification-induced channel closure (32). And, remarkably, introduction of a separate C-terminal tail of Cx43 (Cx43CT) into cells expressing truncated gap junction channels restored the ability of the truncated channels to close in response to acidification (33). These data support the concept that the “ball” or “particle” was represented by all or part of the Cx43CT (31, 33). The interacting “receptor” in this early work was undefined, but it is thought to consist of a portion of connexin that is localized to the plasma membrane and may create, or is located near, the gap junction pore (31). The “particle-receptor” model was also proposed to underlie the closure of gap junctions associated with the phosphorylation of Cx43 stimulated by the activated insulin receptor or the v-Src kinase (34, 35).
Recently, resonant mirror spectroscopy and nuclear magnetic resonance (NMR) studies of connexin peptides in solution revealed the pH-dependent induction of α-helical order in the Asp119 to Lys144 (D119-K144) region of the rat Cx43 intracellular loop, located between transmembrane domains 2 and 3, and the interaction of this region with the Cx43CT “particle,” which suggests that acidification-induced α helices enable this region to function as the “receptor” domain (36). In contrast, NMR analyses of the structure of the Cx43CT peptide revealed that, unlike the Cx43 D119-K144 putative “receptor” domain, it exists in solution principally as an elongated random coil with two regions of α-helical structure, which is unaffected by acidification (36, 37). Intracellular acidification of astrocytes had other effects: It stimulated the binding of c-Src to Cx43 but diminished the binding of ZO-1 to Cx43 (38). These data are similar to the previously described effects of activated c-Src on c-Src and ZO-1 binding to Cx43 (25). In this context, it should be noted that intracellular acidification activates c-Src kinase activity (39). Resonant mirror spectroscopy and NMR studies provided a possible explanation for these data, showing that the interaction between the SH3 domain of c-Src and Cx43CT was pH-dependent, whereas the interaction of the PDZ2 domain of ZO-1 with Cx43CT was not (38). Moreover, binding of c-Src to Cx43CT appeared to inhibit the ZO-1-Cx43CT interaction (37, 38). Whether or not the increased association of c-Src with Cx43 stimulated the tyrosine phosphorylation of Cx43 remained unanswered.
A Junction of Two Signaling Pathways
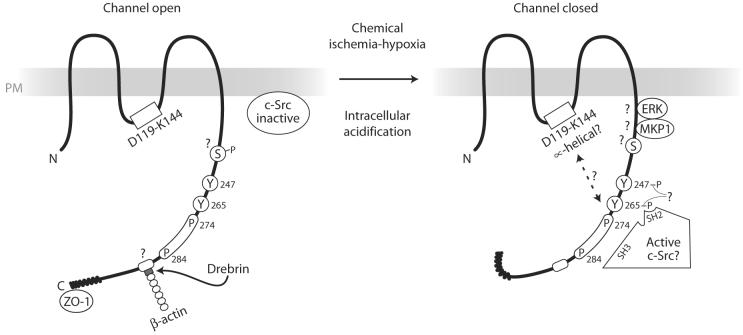
As the Li et al. paper (9) now illustrates, intracellular acidosis stimulates a complex set of downstream molecular events that can affect Cx43 function (Fig. 1). In primary mouse cortical astrocytes, chemical acidosis or ischemia—induced by nigericin or by sodium iodoacetate and sodium cyanide treatments, respectively—resulted in the dephosphorylation of Cx43 on serine/threonine residues and an increased interaction with c-Src. These changes were accompanied by decreased gap junction channel activity, measured by the transfer of fluorescent dye in cells loaded with dye by scraping them. Surprisingly, although acidosis ischemia clearly induced Cx43 dephosphorylation on serine/threonine sites, phosphorylation at unidentified tyrosine sites was stimulated, possibly as a consequence of increased interaction with c-Src. Acidosis ischemia also provoked a shift in the localization of c-Src from the Triton X-100-soluble membrane fraction to the insoluble fraction containing gap junctions, which suggests that the shift in Src's membrane location may permit its interaction with different substrates. It will be important to know whether the associated c-Src is activated and whether the serine-dephosphorylated Cx43 is phosphorylated at the Tyr265 and/or Tyr247 sites targeted by v-Src. These data raise the intriguing prospect that acidification-induced channel gating, until now thought to occur primarily by the interaction between the Cx43CT “particle” and a putative loop “receptor,” may include the interaction of the “particle” with activated (?) c-Src and its phosphorylation on tyrosine. It will be instructive to learn how these distinct mechanisms work together to mediate the closure of gap junctions. Finally, Li et al. (9) discovered that the acidosis-ischemia treatments inhibited the interaction of Cx43 with β-actin (as observed previously for ZO-1) but stimulated the association of Cx43 with the serine kinase ERK1/2 (extracellular signal-regulated kinase 1 and 2) and the ERK phosphatase MKP-1. The apparent loss of interaction of Cx43 with β-actin may result from alterations in the interaction of Cx43 with the drebrin protein, which may link gap junctions to the actin cytoskeleton (40). And, although it is not yet clear whether the interaction of Cx43 with MKP-1 is direct, MKP-1 is a novel interacting protein phosphatase that may regulate Cx43 activity.
Fig. 1.
Ischemia-induced intracellular acidosis induces gap junction closure that is associated with the increased interaction of c-Src with Cx43 and phosphorylation of Cx43 on tyrosine. As reported by Li et al. (9), the induction of intracellular acidosis in astrocytes by ischemia or hypoxia produces a complex series of molecular events, including dephosphorylation of unidentified serines on Cx43; induced phosphorylation at undetermined tyrosines (Tyr247 and Tyr265?); increased interaction of Cx43 with c-Src, ERK, and MKP-1; and interruption of the Cx43-β-actin association. The Cx43 binding domains for ERK and MKP-1 have not yet been reported. Disruption of Cx43-β-actin binding may involve alterations of the interaction of Cx43 with drebrin, an actin-binding protein, which was recently reported to interact with Cx43 (40). Src-induced disruption of gap junctional communication relies on the interactions of the SH3 and SH2 domains of Src and the P274-P284 proline-rich region and phosphorylated Tyr265 of Cx43, respectively. Acidification-induced channel closure may involve the interaction of these Cx43 “particle” elements and the “receptor,” in part comprising the D119-K144 region of the Cx43 intracellular loop. Acidification induces α-helical order in the D119-K144 peptide, which may promote junction closure (36). Intracellular acidification also disrupts the interaction of ZO-1 with the C terminus of Cx43, which correlates with the augmented binding of c-Src to Cx43 (38). PM, plasma membrane.
These complex alterations in Cx43 phosphorylation and Cx43-binding partners, induced by intracellular acidification, raise critical mechanistic questions involving the concept wherein connexins participate in the formation of a dynamic protein complex, or “nexus” (7), at the plasma membrane; such a nexus may influence gap junction function and may also initiate signaling events that affect other cell activities (41). How does the appearance of one or more phosphotyrosine sites on Cx43 close gap junctions? Does it help to create an active “particle” that triggers an intramolecular interaction, as predicted by the “particle-receptor” model, or does it precipitate an interaction with an SH2-domain containing protein that somehow prompts channel closure? And how does the acidosis-induced association of a phosphoserine phosphatase with Cx43 contribute to channel closure? These and other fascinating questions must await future investigations.
References
- 1.Goodenough DA, Goliger JA, Paul DL. Connexins, connexons, and intercellular communication. Annu. Rev. Biochem. 1996;65:475–502. doi: 10.1146/annurev.bi.65.070196.002355. [DOI] [PubMed] [Google Scholar]
- 2.Bruzzone R, White TW, Paul DL. Connections with connexins: The molecular basis of direct intercellular signaling. Eur. J. Biochem. 1996;238:1–27. doi: 10.1111/j.1432-1033.1996.0001q.x. [DOI] [PubMed] [Google Scholar]
- 3.Saez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC. Plasma membrane channels formed by connexins: Their regulation and functions. Physiol. Rev. 2003;83:1359–1400. doi: 10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- 4.Gerido DA, White TW. Connexin disorders of the ear, skin, and lens. Biochim. Biophys. Acta. 2004;1662:159–170. doi: 10.1016/j.bbamem.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 5.Lampe PD, Lau AF. The effects of connexin phosphorylation on gap junctional communication. Int. J. Biochem. Cell Biol. 2004;36:1171–1186. doi: 10.1016/S1357-2725(03)00264-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warn-Cramer BJ, Lau AF. Regulation of gap junctions by tyrosine protein kinases. Biochim. Biophys. Acta. 2004;1662:81–95. doi: 10.1016/j.bbamem.2003.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duffy HS, Delmar M, Spray DC. Formation of the gap junction nexus: Binding partners for connexins. J. Physiol. (Paris) 2002;96:243–249. doi: 10.1016/s0928-4257(02)00012-8. [DOI] [PubMed] [Google Scholar]
- 8.Giepmans BNG. Gap junctions and connexin-interacting proteins. Cardiovasc. Res. 2004;62:233–245. doi: 10.1016/j.cardiores.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 9.Li W, Hertzberg EL, Spray DC. Regulation of connexin43-protein binding in astrocytes in response to chemical ischemia/hypoxia. J. Biol. Chem. 2005;280:7941–7948. doi: 10.1074/jbc.M410548200. [DOI] [PubMed] [Google Scholar]
- 10.Frame MC. Newest findings on the oldest oncogene; how activated src does it. J. Cell Sci. 2004;117:989–998. doi: 10.1242/jcs.01111. [DOI] [PubMed] [Google Scholar]
- 11.Yeatman TJ. A renaissance for SRC. Nat. Rev. Cancer. 2004;4:470–480. doi: 10.1038/nrc1366. [DOI] [PubMed] [Google Scholar]
- 12.Loo LW, Berestecky JM, Kanemitsu MY, Lau AF. pp60src-mediated phosphorylation of connexin 43, a gap junction protein. J. Biol. Chem. 1995;270:12751–12761. doi: 10.1074/jbc.270.21.12751. [DOI] [PubMed] [Google Scholar]
- 13.Kanemitsu MY, Loo LWM, Simon S, Lau AF, Eckhart W. Tyrosine phosphorylation of connexin 43 by v-Src is mediated by SH2 and SH3 domain interactions. J. Biol. Chem. 1997;272:22824–22831. doi: 10.1074/jbc.272.36.22824. [DOI] [PubMed] [Google Scholar]
- 14.Loo LW, Kanemitsu MY, Lau AF. In vivo association of pp60v-src and the gap-junction protein connexin 43 in v-src-transformed fibroblasts. Mol. Carcinog. 1999;25:187–195. [PubMed] [Google Scholar]
- 15.Swenson KI, Piwnica-Worms H, McNamee H, Paul DL. Tyrosine phosphorylation of the gap junction protein connexin43 is required for pp60src-induced inhibition of communication. Cell Regul. 1990;1:989–1002. doi: 10.1091/mbc.1.13.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crow DS, Beyer EC, Paul DL, Kobe SS, Lau AF. Phosphorylation of connexin43 gap junction protein in uninfected and Rous sarcoma virustransformed mammalian fibroblasts. Mol. Cell. Biol. 1990;10:1754–1763. doi: 10.1128/mcb.10.4.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Filson AJ, Azarnia R, Beyer EC, Loewenstein WR, Brugge JS. Tyrosine phosphorylation of a gap junction protein correlates with inhibition of cell-to-cell communication. Cell Growth Differ. 1990;1:661–668. [PubMed] [Google Scholar]
- 18.Lin R, Warn-Cramer BJ, Kurata WE, Lau AF. v-Src phosphorylation of connexin43 on tyr247 and tyr265 disrupts gap junctional communication. J. Cell Biol. 2001;154:815–827. doi: 10.1083/jcb.200102027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cottrell GT, Lin R, Warn-Cramer BJ, Lau AF, Burt JM. Mechanism of v-Src and mitogen-activated protein kinase-induced reduction of gap junction communication. Am. J. Physiol. Cell Physiol. 2003;284:C511–C520. doi: 10.1152/ajpcell.00214.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Langlais P, Gamper N, Liu F, Shapiro MS. Dual phosphorylations underlie modulation of unitary KCNQ K+ channels by Src tyrosine kinase. J. Biol. Chem. 2004;279:45399–45407. doi: 10.1074/jbc.M408410200. [DOI] [PubMed] [Google Scholar]
- 21.Fadool DA, Holmes TC, Berman K, Dagan D, Levitan IB. Tyrosine phosphorylation modulates current amplitude and kinetics of a neuronal voltage-gated potassium channel. J. Neurophysiol. 1997;78:1563–1573. doi: 10.1152/jn.1997.78.3.1563. [DOI] [PubMed] [Google Scholar]
- 22.Postma FR, Hengeveld T, Alblas J, Giepmans BN, Zondag GC, Jalink K, Moolenaar WH. Acute loss of cell-cell communication caused by G protein-coupled receptors: A critical role for c-Src. J. Cell Biol. 1998;140:1199–1209. doi: 10.1083/jcb.140.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toyofuku T, Yabuki M, Otsu K, Kuzuya T, Tada M, Hori M. Functional role of c-Src in gap junctions of the cardiomyopathic heart. Circ. Res. 1999;85:672–681. doi: 10.1161/01.res.85.8.672. [DOI] [PubMed] [Google Scholar]
- 24.Giepmans BN, Hengeveld T, Postma FR, Moolenaar WH. Interaction of c-Src with gap junction protein connexin-43. Role in the regulation of cell-cell communication. J. Biol. Chem. 2001;276:8544–8549. doi: 10.1074/jbc.M005847200. [DOI] [PubMed] [Google Scholar]
- 25.Toyofuku T, Akamatsu Y, Zhang H, Kuzuya T, Tada M, Hori M. c-Src regulates the interaction between connexin-43 and ZO-1 in cardiac myocytes. J. Biol. Chem. 2001;276:1780–1788. doi: 10.1074/jbc.M005826200. [DOI] [PubMed] [Google Scholar]
- 26.Kleber AG, Riegger CB, Janse MJ. Electrical uncoupling and increase of extracellular resistance after induction of ischemia in isolated, arterially perfused rabbit papillary muscle. Circ. Res. 1987;61:271–279. doi: 10.1161/01.res.61.2.271. [DOI] [PubMed] [Google Scholar]
- 27.Peters NS, Coromilas J, Severs NJ, Wit AL. Disturbed connexin43 gap junction distribution correlates with the location of reentrant circuits in the epicardial border zone of healing canine infarcts that cause ventricular tachycardia. Circulation. 1997;95:988–996. doi: 10.1161/01.cir.95.4.988. [DOI] [PubMed] [Google Scholar]
- 28.Lin JH, Weigel H, Cotrina ML, Liu S, Bueno E, Hansen AJ, Hansen TW, Goldman S, Nedergaard M. Gap-junction-mediated propagation and amplification of cell injury. Nat. Neurosci. 1998;1:494–500. doi: 10.1038/2210. [DOI] [PubMed] [Google Scholar]
- 29.Armstrong CM, Bezanilla F. Inactivation of the sodium current: II. Gating current experiments. J. Gen. Physiol. 1977;70:567–590. doi: 10.1085/jgp.70.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zagotta WN, Hoshi T, Aldrich RW. Restoration of inactivation in mutants of Shaker potassium channels by a peptide derived from ShB. Science. 1990;250:568–571. doi: 10.1126/science.2122520. [DOI] [PubMed] [Google Scholar]
- 31.Delmar M, Coombs W, Sorgen P, Duffy HS, Taffet SM. Structural bases for the chemical regulation of connexin43 channels. Cardiovasc. Res. 2004;62:268–275. doi: 10.1016/j.cardiores.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 32.Liu S, Taffet S, Stoner L, Delmar M, Vallano ML, Jalife J. A structural basis for the unequal sensitivity of the major cardiac and liver gap junctions to intracellular acidification: The carboxyl tail length. Biophys. J. 1993;64:1422–1433. doi: 10.1016/S0006-3495(93)81508-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morley GE, Taffet SM, Delmar M. Intramolecular interactions mediate pH regulation of Cx43 channels. Biophys. J. 1996;70:1294–1302. doi: 10.1016/S0006-3495(96)79686-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Homma N, Alvarado JL, Coombs W, Stergiopoulos K, Taffet SM, Lau AF, Delmar M. A particle-receptor model for the insulin-induced closure of connexin43 channels. Circ. Res. 1998;83:27–32. doi: 10.1161/01.res.83.1.27. [DOI] [PubMed] [Google Scholar]
- 35.Zhou L, Kasperek EM, Nicholson BJ. Dissection of the molecular basis of pp60v-src induced gating of connexin 43 gap junction channels. J. Cell Biol. 1999;144:1033–1045. doi: 10.1083/jcb.144.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duffy HS, Sorgen PL, Girvin ME, O'Donnell P, Coombs W, Taffet SM, Delmar M, Spray DC. pH-dependent intracellular binding and structure involving Cx43 cytoplasmic domains. J. Biol. Chem. 2002;277:36706–36714. doi: 10.1074/jbc.M207016200. [DOI] [PubMed] [Google Scholar]
- 37.Sorgen PL, Duffy HS, Sahoo P, Coombs W, Delmar M, Spray DC. Structural changes in the carboxyl terminus of the gap junction protein connexin43 indicates signaling between binding domains for c-Src and zonula occludens-1. J. Biol. Chem. 2004;279:54695–54701. doi: 10.1074/jbc.M409552200. [DOI] [PubMed] [Google Scholar]
- 38.Duffy HS, Ashton AW, O'Donnell P, Coombs W, Taffet SM, Delmar M, Spray DC. Regulation of connexin43 protein complexes by intracellular acidification. Circ. Res. 2004;94:215–222. doi: 10.1161/01.RES.0000113924.06926.11. [DOI] [PubMed] [Google Scholar]
- 39.Yamaji Y, Tsuganezawa H, Moe OW, Alpern RJ. Intracellular acidosis activates c-Src. Am. J. Physiol. Cell Physiol. 1997;272:C886–C893. doi: 10.1152/ajpcell.1997.272.3.C886. [DOI] [PubMed] [Google Scholar]
- 40.Butkevich E, Hulsmann S, Wenzel D, Shirao T, Duden R, Majoul I. Drebrin is a novel connexin-43 binding partner that links gap junctions to the submembrane cytoskeleton. Curr. Biol. 2004;14:650–658. doi: 10.1016/j.cub.2004.03.063. [DOI] [PubMed] [Google Scholar]
- 41.Stout C, Goodenough DA, Paul DL. Connexins: Functions without junctions. Curr. Opin. Cell Biol. 2004;16:507–512. doi: 10.1016/j.ceb.2004.07.014. [DOI] [PubMed] [Google Scholar]