Abstract
The independence of association between elevated jugular venous pressure (JVP) and outcomes in heart failure (HF) has not been well studied. The objective of this propensity-matched study was to determine if an elevated JVP had intrinsic associations with outcomes in chronic systolic and diastolic HF. Of the 7788 Digitalis Investigation Group trial participants 1020 (13%) had elevated JVP at baseline. Propensity scores for elevated JVP were estimated for all patients based on 32 baseline characteristics and were used to match 827 pairs of patients with normal and elevated JVP. Hazard ratios (HR) and 95% confidence intervals (CI) were estimated to compare outcomes associated with elevated versus normal JVP during 34 months of median follow-up. Before matching, all-cause mortality occurred in 31% and 47% (unadjusted HR, 1.70; 95% CI, 1.54–1.88; P<0.0001) and all-cause hospitalization occurred in 60% and 71% (unadjusted HR, 1.35; 95% CI, 1.25–1.47; P<0.0001) of normal and elevated JVP patients respectively. After matching, all-cause mortality occurred in 48% and 45% (matched HR, 0.95; 95% CI, 0.80–1.12; P=0.521) and all-cause hospitalization occurred in 70% and 70% (matched HR, 0.97; 95% CI, 0.87–1.09; P=0.613) of normal and elevated JVP patients respectively. Elevated JVP had no intrinsic associations with cardiovascular mortality (matched HR, 0.93; 95% CI, 0.77-1.12; P=0.440) or HF hospitalization (matched HR, 0.94; 95% CI, 0.78-1.14; P=0.532). In conclusion, an elevated JVP is a marker of higher burden of sickness and poor outcomes. However, elevated JVP had not intrinsic association with mortality or hospitalization in chronic HF.
Keywords: Heart failure, Jugular venous pressure, Mortality, Morbidity, Outcomes
The assessment of fluid volume status is of crucial importance in patients with chronic heart failure (HF) and estimation of jugular venous pressure (JVP) is one of the most reliable means of assessing fluid volume.1 However, little is known about the association of elevated JVP and outcomes in chronic HF. In one study, elevated JVP was independently associated with adverse outcomes in chronic systolic HF.2 However, this association has not been validated in other similar populations. The objective of this study was to determine whether baseline elevated JVP was associated with poor HF outcomes in a propensity-matched population of ambulatory chronic systolic and diastolic HF in which patients with normal and elevated JVP would be well-balanced in all measured baseline covariates.
Methods
We used a public-use copy of the Digitalis Investigation Group (DIG) dataset obtained from the National Heart Lung and Blood Institute. The rationale, design, and results of the DIG trial have been previously reported.3-6 Briefly, 7788 ambulatory patients with chronic HF in normal sinus rhythm were randomly assigned to receive digoxin or placebo. These patients were recruited from 302 clinical centers in the US (186) and Canada (116) between 1991 and 1993 and followed for a mean length of 37 months. Most patients were receiving diuretics and angiotensin-converting enzyme inhibitors, and 6800 (87%) had left ventricular ejection fraction <45%. Elevated JVP was present in 1020 (13%) patients at the time of randomization or within the previous 30 days. Elevated JVP was estimated by study investigators by physical examination and was described as jugular venous distension. In this manuscript we will use the term elevated JVP and data on elevated JVP were available from all 7788 patients. The primary outcomes for the current analysis were mortality and hospitalizations due to all causes; other outcomes studied included mortality and hospitalizations due to cardiovascular causes, and HF. Data on vital status were 99% complete.7
Because of significant imbalances in baseline covariates between patients with and without elevated JVP (Table 1), we used propensity score matching to assemble a cohort of patients who would be well-balanced in all measured baseline covariates.3-6 We estimated propensity scores for elevated JVP for each of the 7788 patients using a non-parsimonious, multivariate logistic regression model, adjusting for all available baseline covariates presented in Figure 1. Propensity score models are sample-specific adjusters and are not intended to be used for out-of-sample prediction or estimation of coefficients. Therefore, instead of fitness and discrimination, a propensity model’s effectiveness is better assessed by its ability to reduce bias after matching. Using a greedy matching protocol, we matched 827 pairs of patients with and without elevated JVP who had similar propensity scores.8 The details of the matching protocol have been described elsewhere.9-12 We then objectively estimated post-match bias reduction using absolute standardized differences (<10% being inconsequential bias and 0% indicating no residual bias) and presented them as a Love plot.12-15
Table 1.
Baseline patient characteristics, by elevated jugular venous pressure (JVP) status, before and after propensity matching
| Variable | Before matching | After matching | ||||
|---|---|---|---|---|---|---|
| Normal JVP (n = 6768) | Elevated JVP (n = 1020) | P Value | Normal JVP (n = 827) | Elevated JVP (n = 827) | P Value | |
| Age (year) | 64 ± 11 | 65 ± 11 | 0.001 | 65 ± 12 | 65 ± 11 | 0.335 |
| Female | 1660 (25%) | 266 (26%) | 0.284 | 229 (28%) | 219 (27%) | 0.629 |
| Non-white | 915 (14%) | 213 (21%) | <0.0001 | 147 (18%) | 150 (18%) | 0.900 |
| Body mass index (kg/m2) | 27 ± 5 | 27 ± 5 | 0.258 | 28 ± 6 | 27 ± 5 | 0.195 |
| Duration of HF (months) | 30 ± 36 | 31 ± 39 | 0.130 | 32 ± 39 | 33 ± 40 | 0.761 |
| Primary cause of HF | ||||||
| Ischemic | 4724 (70%) | 636 (62%) | <0.0001 | 511 (62%) | 525 (64%) | 0.723 |
| Hypertensive | 679 (10%) | 126 (12%) | 119 (14%) | 101 (12%) | ||
| Idiopathic | 953 (14%) | 158 (16%) | 135 (16%) | 126 (15%) | ||
| Others | 412 (6%) | 100 (10%) | 62 (8%) | 75 (9%) | ||
| Prior myocardial infarction | 4344 (64%) | 564 (55%) | <0.0001 | 458 (55%) | 472 (57%) | 0.523 |
| Current angina pectoris | 1846 (27%) | 269 (26%) | 0.546 | 239 (29%) | 230 (28%) | 0.659 |
| Hypertension | 3142 (46%) | 532 (52%) | 0.001 | 434 (53%) | 419 (51%) | 0.486 |
| Diabetes mellitus | 1870 (28%) | 348 (34%) | <0.0001 | 268 (32%) | 270 (33%) | 0.958 |
| Medications | ||||||
| Pre-trial digoxin use | 2887 (43%) | 478 (47%) | 0.011 | 387 (47%) | 390 (47%) | 0.921 |
| Trial use of digoxin | 3391 (50%) | 498 (49%) | 0.446 | 408 (49%) | 407 (49%) | 1.000 |
| ACE inhibitors | 6314 (93%) | 960 (94%) | 0.322 | 769 (93%) | 779 (94%) | 0.363 |
| Diuretics | 5155 (76%) | 921 (90%) | <0.0001 | 729 (88%) | 732 (89%) | 0.873 |
| PS diuretics | 509 (8%) | 87 (9%) | 0.259 | 80 (10%) | 70 (9%) | 0.447 |
| Potassium supplement | 1854 (27%) | 345 (34%) | <0.0001 | 281 (34%) | 281 (34%) | 1.000 |
| Symptoms and signs of HF | ||||||
| Dyspnea at rest | 1179 (17%) | 526 (52%) | <0.0001 | 343 (42%) | 349 (42%) | 0.785 |
| Dyspnea on exertion | 4943 (73%) | 919 (90%) | <0.0001 | 739 (89%) | 727 (88%) | 0.372 |
| Third heart sound | 1320 (20%) | 526 (52%) | <0.0001 | 373 (45%) | 369 (45%) | 0.871 |
| Pulmonary rales | 766 (11%) | 535 (53%) | <0.0001 | 355 (43%) | 348 (42%) | 0.689 |
| Lower extremity edema | 1107 (16%) | 526 (52%) | <0.0001 | 374 (45%) | 350 (42%) | 0.203 |
| NYHA class, % | ||||||
| Class I | 1045 (15%) | 58 (6%) | <0.0001 | 53 (6%) | 55 (7%) | 0.889 |
| Class II | 3856 (57%) | 388 (38%) | 342 (41%) | 337 (41%) | ||
| Class III | 1787 (26%) | 500 (49%) | 396 (48%) | 389 (47%) | ||
| Class IV | 80 (1%) | 74 (7%) | 36 (4%) | 46 (6%) | ||
| Heart rate (beats /minute) | 78 ± 12 | 83 ± 14 | <0.0001 | 82 ± 13 | 82 ± 14 | 0.417 |
| Blood pressure (mm Hg) | ||||||
| Systolic | 128 ± 20 | 125 ± 21 | 0.001 | 126 ± 21 | 126 ± 21 | 0.651 |
| Diastolic | 75 ± 11 | 75 ± 12 | 0.622 | 75 ± 12 | 75 ± 12 | 0.449 |
| Chest radiograph findings | ||||||
| Pulmonary congestion | 679 (10%) | 430 (42%) | <0.0001 | 258 (31%) | 265 (32%) | 0.709 |
| Cardiothoracic ratio >0.5 | 3924 (58%) | 766 (75%) | <0.0001 | 606 (73%) | 604 (73%) | 0.954 |
| Serum creatinine (mg/dL) | 1.27 ± 0.36 | 1.34 ± 0.41 | <0.0001 | 1.33 ± 0.40 | 1.33 ± 0.41 | 0.793 |
| Serum potassium (mEq/L) | 4.34 ± 0.44 | 4.33 ± 0.45 | 0.366 | 4.34 ± 0.45 | 4.32 ± 0.45 | 0.351 |
| Ejection fraction (%) | 33 ± 13 | 28 ± 12 | <0.0001 | 30 ± 13 | 29 ± 12 | 0.491 |
ACE=angiotensin-converting enzyme; HF=heart failure; NYHA=New York Heart Association; PS=potassium sparing
Figure 1.
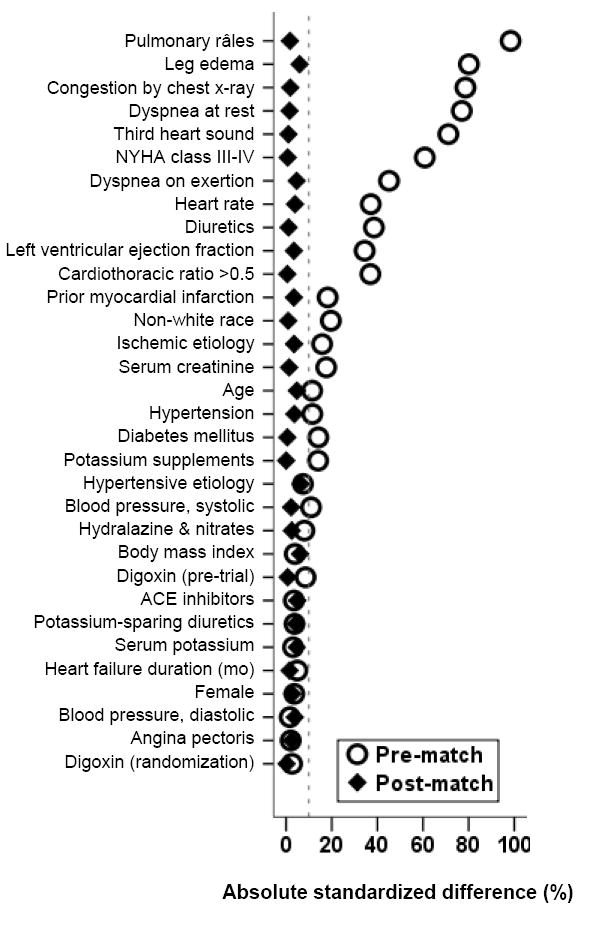
Love plot displaying absolute standardized differences for covariates between chronic heart failure patients with and without elevated jugular venous pressure, before and after propensity score matching (ACE=angiotensin-converting enzyme; NYHA=New York Heart Association)
For descriptive analyses, we used Pearson Chi square and Wilcoxon rank-sum tests for pre-match, and McNemar’s test and paired sample t-test for post-match comparisons, as appropriate. Kaplan-Meier and matched Cox regression analyses were used to determine the association of elevated JVP (relative to normal JVP) with various outcomes. Subgroup analyses and first-order interactions were used to test the heterogeneity of the association between elevated JVP and all-cause mortality. Chronic kidney disease was defined as an estimated glomerular filtration rate <60 ml/min per 1.73 m2 of body surface area. All statistical tests were done using SPSS-15 for Windows.16
Results
Pre-match imbalances in baseline covariates and balances achieved after matching are displayed in Table 1 and Figure 1. Patients with elevated JVP were older, more likely to be nonwhite and generally had higher burden of symptoms and comorbidities all of which were balanced after matching (Table 1). Values of absolute standardized differences for all covariates after matching between patients with normal and elevated JVP were <10% (Figure 1).
In the pre-match cohort, all-cause mortality occurred in 31% (rate, 1054/10000 person-years) and 47% (rate, 1789/10000 person-years) of patients with normal and elevated JVP respectively (unadjusted hazard ratio {HR} when elevated JVP is compared with normal JVP, 1.70; 95% confidence interval {CI}, 1.54–1.88; P<0.0001; Table 2). This association lost significance when adjusted for propensity score (propensity-adjusted HR, 1.00; 95% CI, 0.88–1.14; P=0.963). The association of elevated JVP with cardiovascular and HF mortalities are displayed in Table 2. All-cause hospitalization occurred in 60% (rate, 3664/10000 person-years) and 71% (rate, 5186/10000 person-years) of patients with normal and elevated JVP respectively (unadjusted HR, 1.35; 95% CI, 1.25–1.47; P<0.0001; Table 3). This association lost significance when adjusted for propensity score (propensity-adjusted HR, 1.02; 95% CI, 0.93–1.12; P=0.701). The association of elevated JVP with cardiovascular and HF hospitalizations are displayed in Table 3.
Table 2.
Association between elevated jugular venous pressure (JVP) and mortality in chronic heart failure, before and after propensity matching
| Outcomes | Rate, per 10000 person-years (Events / total follow up years) | Absolute rate difference* (per 10000 person-years) | Hazard ratio (95% confidence interval) | P value | |
|---|---|---|---|---|---|
| Pre-match | Normal JVP (n = 6768) | Elevated JVP (n = 1020) | |||
| All-cause | 1054 (2131 / 20219) | 1789 (475 / 2655) | + 735 | 1.70 (1.54–1.88) | <0.0001 |
| Cardiovascular | 829 (1677 / 20219) | 1412 (375 / 2655) | + 583 | 1.70 (1.52–1.90) | <0.0001 |
| Progressive heart failure | 353 (714 / 20219) | 727 (193 / 2655) | + 374 | 2.07 (1.76–2.43) | <0.0001 |
| Post-match | Normal JVP (n = 827) | Elevated JVP (n = 827) | |||
| All-cause | 1866 (398 / 2133) | 1699 (373 / 2196) | - 167 | 0.95.(0.80–1.12) | 0.521 |
| Cardiovascular | 1467 (313 / 2133) | 1321 (290 / 2196) | - 147 | 0.93 (0.77–1.12) | 0.440 |
| Progressive heart failure | 727 (155 / 2133) | 660 (145 / 2196) | - 66 | 0.94 (0.71–1.23) | 0.628 |
Absolute differences in rates of events per 10,000 person-year of follow up were calculated by subtracting the event rates in the normal JVP group from the event rates in the elevated JVP group (before values were rounded).
Table 3.
Association between elevated jugular venous pressure (JVP) and hospitalizations* in chronic heart failure, before and after propensity matching
| Outcomes | Rate, per 10000 person-years (Events / total follow up years) | Absolute rate difference† (per 10000 person-years) | Hazard ratio (95% confidence interval) | P value | |
|---|---|---|---|---|---|
| Pre-match | Normal JVP (n = 6768) | Elevated JVP (n = 1020) | |||
| All-cause | 3664 (4044 / 12019) | 5186 (724 / 1396) | + 1522 | 1.35 (1.25–1.47) | <0.0001 |
| Cardiovascular | 2402 (3416 / 14221) | 3576 (594 / 1661) | + 1174 | 1.42 (1.31–1.55) | <0.0001 |
| Worsening heart failure | 1086 (1888 / 17387) | 1931 (399 / 2066) | + 845 | 1.71 (1.53–1.90) | <0.0001 |
| Post-match | Normal JVP (n = 827) | Elevated JVP (n = 827) | |||
| All-cause | 5056 (583 / 1153) | 4882 (578 / 1184) | - 175 | 0.97 (0.87–1.09) | 0.613 |
| Cardiovascular | 3508 (483 / 1377) | 3338 (470 / 1408) | - 170 | 1.02 (0.87-1.19) | 0.841 |
| Worsening heart failure | 1890 (319 / 1688) | 1813 (314 / 1732) | - 77 | 0.94 (0.78–1.14) | 0.532 |
Data shown include the first hospitalization of each patient for each cause.
Absolute differences in rates of events per 10,000 person-year of follow up were calculated by subtracting the event rates in the normal JVP group from the event rates in the elevated JVP group (before values were rounded).
In the post-match cohort, all-cause mortality occurred in 48% (rate, 1866/10000 person-years) and 45% (rate, 1699/10000 person-years) of patients with normal and elevated JVP respectively (matched HR, 0.95; 95% CI, 0.80–1.12; P=0.521; Table 2 and Figure 2a). The association of elevated JVP with cardiovascular and HF mortalities are displayed in Table 2. The association between elevated JVP and all-cause mortality was homogeneous across a wide spectrum of subgroups except for the one by gender (Figure 3). All-cause hospitalization occurred in 70% (rate, 5056/10000 person-years) and 70% (rate, 4882/10000 person-years) of patients with normal and elevated JVP respectively (matched HR, 0.97; 95% CI, 0.87–1.09; P=0.613; Table 3 and Figure 2b). The association of elevated JVP with cardiovascular and HF hospitalizations are displayed in Table 3.
Figure 2.
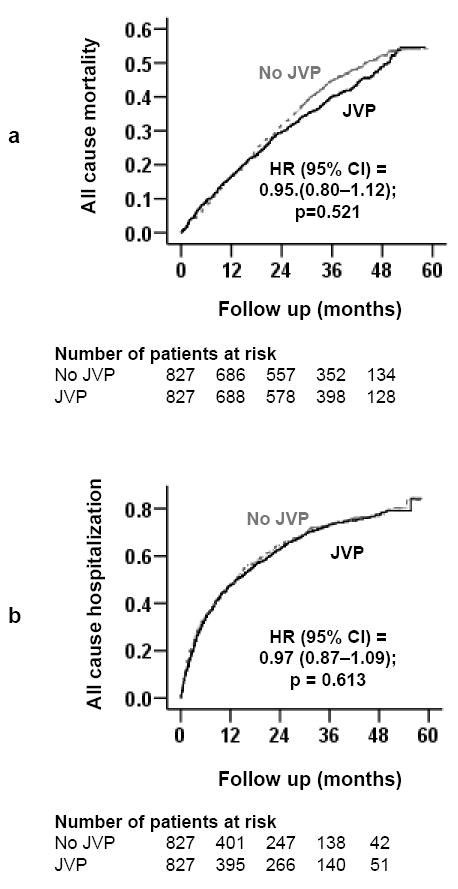
Kaplan-Meier plots for (a) all-cause mortality, and (b) all-cause hospitalization (CI=confidence interval; HR=hazard ratio; JVP=jugular venous pressure)
Figure 3.
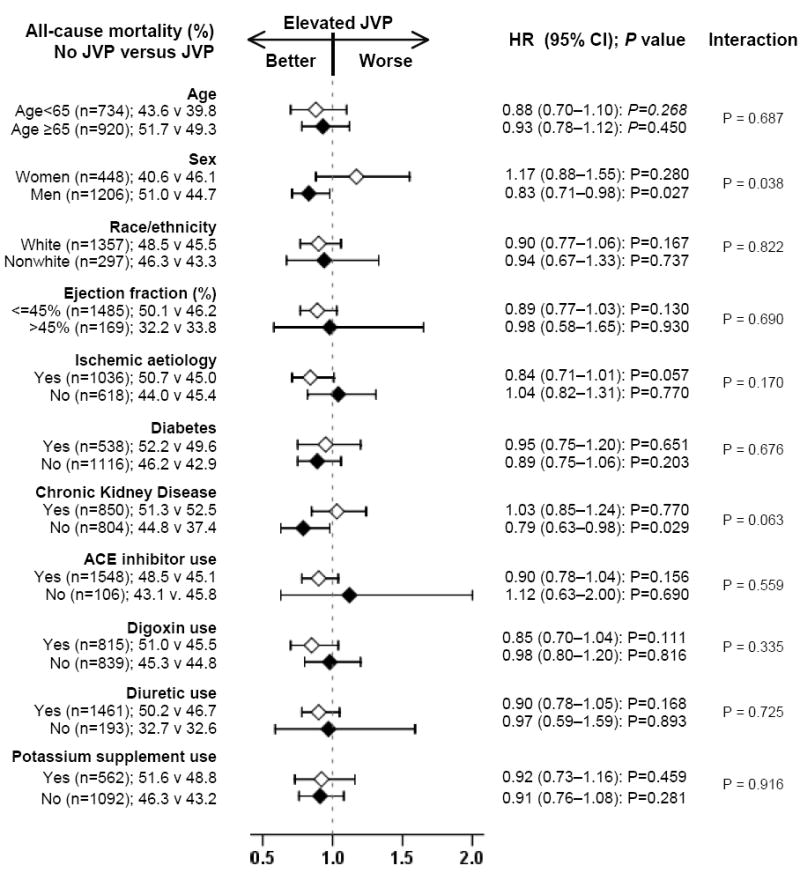
Association of elevated jugular venous pressure (JVP) with all-cause mortality in subgroups of propensity-matched chronic heart failure patients (ACE=angiotensin-converting enzyme; CI=confidence interval; HR=hazard ratio)
Discussion
The findings from the current analysis suggest that elevated JVP was a marker of increased mortality and morbidity in ambulatory patients with chronic HF. However, data from our propensity-matched population in which patients with and without elevated JVP were well balanced in all measured baseline characteristics suggest that elevated JVP had no intrinsic association with outcomes in these patients. These findings are important as elevated JVP is the most reliable sign of fluid overload and can be used to identify HF patients who are at risk for poor outcomes.
Unadjusted associations between elevated JVP and outcomes are likely due to many pre-match imbalances on key prognostic variables between patients with normal and elevated JVP. Patients with elevated JVP were more likely to be older, have diabetes mellitus, renal insufficiency, cardiomegaly, lower mean left ventricular ejection fraction, higher New York Heart Association class symptom, and receive diuretics, all of which are markers of poor prognosis in these patients.10-12, 15, 17-19
This is further confirmed when this association completely disappeared in the propensity-matched cohort and also when adjusted for propensity scores in the pre-matched cohort, which suggest that an elevated JVP is a marker of poor prognosis and does not have any intrinsic prognostic value of its own. This lack of an independent association of elevated JVP with outcomes in chronic HF is mechanistically plausible. The JVP is an indirect clinical measure of right atrial pressure and may reflect left ventricular filling pressure. Although these hemodynamic parameters have been shown to be associated with poor prognosis,20-22 these studies were based on small number of systolic HF patients with short follow up and did not adjust for key prognostically important covariates.
To the best of our knowledge, this is the first study of associations of elevated JVP and outcomes in a propensity-matched population of chronic systolic and diastolic HF. An analysis of the participants in the Studies of Left Ventricular Dysfunction (SOLVD) treatment trial compared the outcomes of 280 chronic systolic HF patients with elevated JVP with those of 2199 patients with normal JVP.2 Although elevated JVP had no independent association with all-cause mortality in that study, it was associated with HF mortality and HF hospitalization. Despite many similarities in baseline characteristics between patients in that analysis and the current analysis, the use of propensity score matching design, the use of a more comprehensive list of variables and the inclusion of both systolic and diastolic HF patients distinguish our study from that study.
The strong bivariate associations of elevated JVP with major natural history endpoints in chronic systolic and diastolic HF in our study suggest that an elevated JVP is an excellent marker of poor outcomes in these patients. Further, an elevated JVP is the most reliable sign of fluid overload in HF. However, proper estimation of JVP remains a challenge and an emphasis on the use of the internal jugular vein may likely underestimate elevated JVP in these patients, which was evident from the low prevalence of elevated JVP in our study. A similar low prevalence of elevated JVP has also been reported in HF patients with acute dyspnea in the emergency department or in the hospital.23, 24 This low prevalence of elevated JVP may be due to the fact that the internal jugular vein is behind the sternocleidomastoid muscle in the neck and may not be clearly visible in chronic HF.25 An alternative approach may be to use the external jugular vein, keeping in mind its limitation as a superficial vein.26 Therefore, a distended external jugular vein is unreliable unless the venous pulsation can be seen, the top of which should be used to estimate JVP. The distance between right atrium and sternal angle varies with body position and should be taken into account while estimating JVP.27
Several limitations of our study must be acknowledged. DIG participants were predominantly young men in normal sinus rhythm from the pre-beta-blocker era of HF therapy which may limit generalizability. The low prevalence of elevated JVP at baseline indicate that many patients with elevated JVP may have been misclassified as having normal JVP which may have underestimated the true association. However, the prevalence of elevated JVP in DIG participants was very similar to that of SOLVD participants.2 In conclusion, despite the lack of an intrinsic association between an elevated JVP and outcomes, because of its strong and significant bivariate association, an elevated JVP will remain a useful marker of prognosis in chronic systolic and diastolic HF. The usefulness of JVP may be enhanced by routine assessment of JVP in all patients with HF
Acknowledgments
The Digitalis Investigation Group (DIG) study was conducted and supported by the NHLBI in collaboration with the DIG Investigators. This manuscript was prepared using a limited access dataset obtained by the NHLBI and does not necessarily reflect the opinions or views of the DIG Study or the NHLBI.
Funding/Support: Dr. Ahmed is supported by the National Institutes of Health through grants from the National Heart, Lung, and Blood Institute (R01-HL085561 and P50-HL077100), and a generous gift from Ms. Jean B. Morris of Birmingham, Alabama.
Footnotes
Conflict of Interest Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2005;112:e154–235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 2.Drazner MH, Rame JE, Stevenson LW, Dries DL. Prognostic importance of elevated jugular venous pressure and a third heart sound in patients with heart failure. N Engl J Med. 2001;345:574–581. doi: 10.1056/NEJMoa010641. [DOI] [PubMed] [Google Scholar]
- 3.Rosenbaum PR, Rubin DB. The central role of propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 4.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127:757–763. doi: 10.7326/0003-4819-127-8_part_2-199710151-00064. [DOI] [PubMed] [Google Scholar]
- 5.Rubin DB. Using propensity score to help design observational studies: Application to the tobacco litigation. Health Services and Outcomes Research Methodology. 2001;2:169–188. [Google Scholar]
- 6.Rubin DB. On principles for modeling propensity scores in medical research. Pharmacoepidemiol Drug Saf. 2004;13:855–857. doi: 10.1002/pds.968. [DOI] [PubMed] [Google Scholar]
- 7.Collins JF, Howell CL, Horney RA. Determination of vital status at the end of the DIG trial. Control Clin Trials. 2003;24:726–730. doi: 10.1016/j.cct.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 8.Levesque R. SPSS. SPSS programming and data management 2nd ed. A guide for SPSS and SAS users. SPSS; Chicago (Ill): 2005. Macros. available for download at http://www.spsstools.net/spss_programming.htm. [Google Scholar]
- 9.Ahmed A, Allman RM, Fonarow GC, Love TE, Zannad F, Dell’italia LJ, White M, Gheorghiade M. Incident heart failure hospitalization and subsequent mortality in chronic heart failure: a propensity-matched study. J Card Fail. 2008;14:211–218. doi: 10.1016/j.cardfail.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmed A, Perry GJ, Fleg JL, Love TE, Goff DC, Jr, Kitzman DW. Outcomes in ambulatory chronic systolic and diastolic heart failure: a propensity score analysis. Am Heart J. 2006;152:956–966. doi: 10.1016/j.ahj.2006.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmed A, Husain A, Love TE, Gambassi G, Dell’Italia LJ, Francis GS, Gheorghiade M, Allman RM, Meleth S, Bourge RC. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J. 2006;27:1431–1439. doi: 10.1093/eurheartj/ehi890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmed A, Rich MW, Sanders PW, Perry GJ, Bakris GL, Zile MR, Love TE, Aban IB, Shlipak MG. Chronic kidney disease associated mortality in diastolic versus systolic heart failure: a propensity matched study. Am J Cardiol. 2007;99:393–398. doi: 10.1016/j.amjcard.2006.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 14.Normand ST, Landrum MB, Guadagnoli E, Ayanian JZ, Ryan TJ, Cleary PD, McNeil BJ. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54:387–398. doi: 10.1016/s0895-4356(00)00321-8. [DOI] [PubMed] [Google Scholar]
- 15.Wahle C, Adamopoulos C, Ekundayo OJ, Mujib M, Aronow WS, Ahmed A. A propensity-matched study of outcomes of chronic heart failure (HF) in younger and older adults. Arch Gerontol Geriatr. 2008 doi: 10.1016/j.archger.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.SPSS for Windows, Rel. 15 [computer program]. Version. Chicago: SPSS Inc.; 2008. [Google Scholar]
- 17.Ahmed A, Aban IB, Vaccarino V, Lloyd-Jones DM, Goff DC, Jr, Zhao J, Love TE, Ritchie C, Ovalle F, Gambassi G, Dell’Italia LJ. A propensity-matched study of the effect of diabetes on the natural history of heart failure: variations by sex and age. Heart. 2007;93:1584–1590. doi: 10.1136/hrt.2006.113522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giamouzis G, Sui X, Love TE, Butler J, Young JB, Ahmed A. A propensity-matched study of the association of cardiothoracic ratio with morbidity and mortality in chronic heart failure. Am J Cardiol. 2008;101:343–347. doi: 10.1016/j.amjcard.2007.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmed A. A propensity matched study of New York Heart Association class and natural history end points in heart failure. Am J Cardiol. 2007;99:549–553. doi: 10.1016/j.amjcard.2006.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keogh AM, Baron DW, Hickie JB. Prognostic guides in patients with idiopathic or ischemic dilated cardiomyopathy assessed for cardiac transplantation. Am J Cardiol. 1990;65:903–908. doi: 10.1016/0002-9149(90)91434-8. [DOI] [PubMed] [Google Scholar]
- 21.Unverferth DV, Magorien RD, Moeschberger ML, Baker PB, Fetters JK, Leier CV. Factors influencing the one-year mortality of dilated cardiomyopathy. Am J Cardiol. 1984;54:147–152. doi: 10.1016/0002-9149(84)90320-5. [DOI] [PubMed] [Google Scholar]
- 22.Morley D, Brozena SC. Assessing risk by hemodynamic profile in patients awaiting cardiac transplantation. Am J Cardiol. 1994;73:379–383. doi: 10.1016/0002-9149(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 23.Mueller C, Scholer A, Laule-Kilian K, Martina B, Schindler C, Buser P, Pfisterer M, Perruchoud AP. Use of B-type natriuretic peptide in the evaluation and management of acute dyspnea. N Engl J Med. 2004;350:647–654. doi: 10.1056/NEJMoa031681. [DOI] [PubMed] [Google Scholar]
- 24.Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, O’Connor CM, Sun JL, Yancy CW, Young JB. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol. 2007;50:768–777. doi: 10.1016/j.jacc.2007.04.064. [DOI] [PubMed] [Google Scholar]
- 25.Ahmed A. DEFEAT heart failure: clinical manifestations, diagnostic assessment, and etiology of geriatric heart failure. Heart Fail Clin. 2007;3:389–402. doi: 10.1016/j.hfc.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Vinayak AG, Levitt J, Gehlbach B, Pohlman AS, Hall JB, Kress JP. Usefulness of the external jugular vein examination in detecting abnormal central venous pressure in critically ill patients. Arch Intern Med. 2006;166:2132–2137. doi: 10.1001/archinte.166.19.2132. [DOI] [PubMed] [Google Scholar]
- 27.Seth R, Magner P, Matzinger F, van Walraven C. How far is the sternal angle from the mid-right atrium? J Gen Intern Med. 2002;17:852–856. doi: 10.1046/j.1525-1497.2002.20101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


