Abstract
Purpose
Radiation Therapy Oncology Group 85-31 was a randomized trial of androgen suppression for life for patients with locally advanced prostate cancer. However, not all patients continued on the protocol-mandated long-term hormonal therapy despite no evidence of recurrence. We correlated duration of adjuvant hormonal therapy and outcomes among patients who prematurely discontinued hormonal therapy.
Patients and Methods
The protocol mandated pelvic radiotherapy followed by goserelin given indefinitely or until disease progression. There were 189 analyzable patients. Patients were divided in groups based on the tertile of hormonal therapy duration (HTD) as follows: ≤ 1 year, more than 1 year and ≤ 5 years, and more than 5 years. Overall survival (OS), disease-free survival (DFS), cause-specific mortality, local failure (LF), and distant metastasis (DM) were studied. Kaplan-Meier estimation and Cox proportional hazards regression model were used for OS and DFS, and Fine and Gray's regression model was used for the other outcomes.
Results
The median follow-up for surviving patients is 9.6 years. The median duration of adjuvant hormonal therapy was 2.2 years. The HTD more than 5 years group is significantly associated with an improved survival and DFS and fewer DMs than other HTD groups. After adjustment for age, radical prostatectomy, nodal status, Gleason score, and stage variables, the HTD more than 5 years group remains significantly associated with better OS and DFS than other HTD groups.
Conclusion
In this hypothesis-generating analysis, prolonged HTD of more than 5 years seems significantly associated with improvements in most outcomes. Given these data, decreasing HTD to ≤ 5 years may have a detrimental effect on patients with locally advanced prostate cancer. Only a randomized trial will conclusively clarify this issue.
INTRODUCTION
For patients with locally advanced localized prostate cancer, randomized trials have shown an improvement in survival rates when adjuvant hormonal therapy is added to pelvic irradiation.1–3 However, the optimal treatment duration for the adjuvant hormonal component has not been well established.
Radiation Therapy Oncology Group (RTOG) 85-314 was a randomized trial comparing radiotherapy (RT) alone versus RT plus adjuvant androgen suppression for life in locally advanced prognosis carcinoma of the prostate. Long-term results of this trial show a significantly improved absolute survival, lower local failure, and lower incidence of distant disease and disease-specific mortality favoring the combined treatment arm.3 However, not all patients remained on the protocol-mandated long-term hormonal therapy, despite no evidence of recurrent disease.
This article reports on a secondary analysis performed on the RTOG 8531 study correlating duration of adjuvant hormonal therapy and outcomes among patients who prematurely discontinued long-term hormonal therapy.
PATIENTS AND METHODS
Patients Evaluation and Treatment
Details of RTOG 8531 have been published previously.3,4 Briefly, eligible patients had a Karnofsky performance status greater than 60 and histopathologic diagnosis of adenocarcinoma of the prostate with clinical stage T3 or documented involvement of the pelvic lymph nodes. Patients who have undergone a prostatectomy were eligible if pathologically staged as pT3. Pretreatment evaluation included a complete medical history, physical examination with performance status evaluation, chest x-ray, bone scan, complete blood cell counts, acid phosphatase, and serum testosterone. Lymph node assessment was mandatory and could be performed either by lymphangiogram, computed tomography scan, or lymphadenectomy. Prostate-specific antigen (PSA) was not mandatory at the start of the trial because it was not widely available. All institutional, state, and federal regulatory guidelines had to be followed and, before random assignment, all patients gave written informed consent.
Patients were randomly assigned to receive RT and adjuvant goserelin (3.6 mg) monthly (arm 1) or RT alone followed by goserelin at time of relapse (arm 2). For patients randomly assigned to arm 1, goserelin was to be started at the last week of RT and to be given for life or until evidence of disease progression. A dose of 44 to 46 Gy (doses up to 50 Gy were acceptable) was delivered to the pelvic contents, and a prostatic target volume boost of 20 to 25 Gy brought the total dose delivered to the volume to 65 to 70 Gy. In postoperatively treated patients, the prostatic bed received 60 to 65 Gy. Patients were stratified by histologic differentiation, nodal status, acid phosphatase level, and prior radical prostatectomy.
Statistical Methods
Hormonal therapy duration (HTD) is defined from the start of protocol-specified hormone injection date to the earlier of the final hormone injection date. Patients were divided into three groups based on the tertile of HTD as follows: ≤ 1 year, more than 1 year and ≤ 5 years, and more than 5 years. The following outcomes were studied: overall survival (OS), disease-free survival (DFS), cause-specific mortality (CSM), local failure (LF), and distant metastasis (DM). The same definitions of event for outcomes used in the primary article5 were used for this analysis. The failure events for LF were defined as the reappearance of palpable tumor after initial clearance, progression of palpable tumor at any time, persistence of palpable tumor beyond 24 months after study entry, and the biopsy-proven presence of carcinoma of the prostate 2 years after study entry. Persistence of palpable prostate tumor beyond 2 years was recorded as local recurrence as of day 1. The failure event for DM was defined as the clinical or radiographic evidence of disease beyond the pelvis. DFS was defined as survival in the absence of locoregional failure or DM. DFS was also computed using PSA as an end point (either 1.5 ng/mL or 4 ng/mL as the threshold). The failure event for CSM was defined as death from prostate cancer or protocol treatment. Patients who died with disease and for whom the cause of death was unknown were also considered to have failure at this end point. The failure event for OS was defined as death from any cause. Time to event was measured from the randomization date to date of failure event. The Kaplan-Meier method6 was used to estimate the OS and DFS. The log-rank test7,8 was used to test the survival difference between the hormone duration groups. The cumulative incidence method9 was used to estimate the LF rate, DM rate, and CSM rate. Gray's test10 was used to test the difference between the hormone groups. Cox proportional hazards regression11 was used for OS and DFS to estimate the hazard ratios (HRs) with/without adjustment for other covariates using χ2 test. Fine and Gray's proportional hazards regression12 was used for LF, DM, and CSM to estimate the HRs with/without adjustment for other covariates. The following covariates were adjusted in the models: age (< 70 [reference level; RL] or ≥ 70 years), radical prostatectomy (yes [RL] or no), nodal status (negative [RL] or positive), centrally reviewed Gleason score (2 to 6 [RL] or 7 to 10), stage (A/B [RL] or C). Unadjusted and adjusted HRs were calculated for all covariates using the proportional hazards models with associated 95% CIs and P values. All two-sided testing was done at a significance level of .05. Bonferroni correction13 was used in pairwise comparisons to keep the overall significance level of .05.
RESULTS
Patients were accrued from February 1987 to April 1992. Among the 477 eligible patients in arm 1 of RTOG 85-31, 446 received protocol-specified hormonal therapy (31 did not start or refused hormone therapy after random assignment). Three hundred twenty-two patients (72%) discontinued their hormone therapy among 446 patients, and 124 patients are still continuing their hormone therapy. Among those 322 patients, 133 patients (41%) discontinued their hormone therapy due to death, disease progression, or initiation of another hormone therapy and were excluded from the analysis. Therefore, a total of 189 patients (59%) who were randomly assigned to arm 1 were analyzable and comprised the study cohort. A summary of their pretreatment baseline characteristics is listed in Table 1. The median follow-up time for surviving patients was 11.3 years (range, 0.9 to 14.9 years). The median HTD was 2.2 years (range, 0.003 [day 1] to 13.5 years). The tertiles of HTD are 1 year (33% percentile) and 5 years (67% percentile). The HTDs were considered as a continuous variable, and a categoric variable (HTD ≤ 1 year v 1 < HTD ≤ 5 years v HTD > 5 years). Pretreatment characteristics by HTD groups were well balanced, and the follow-up time for surviving patients is similar among the three HTD groups (Table 1).
Table 1.
Pretreatment Characteristics by HTD Groups (N = 189)
| Characteristic | HTD ≤ 1 Years (n = 67) |
HTD > 1 and ≤ 5 Years (n = 61) |
HTD > 5 Years (n = 61) |
Total (N = 189) |
P* | ||||
|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | ||
| Age, years | |||||||||
| Median | 70 | 71 | 70 | 70 | |||||
| Range | 50-84 | 57-81 | 55-80 | 50-84 | |||||
| < 70 | 32 | 48 | 25 | 41 | 27 | 44 | 84 | 44 | .74 |
| ≥ 70 | 35 | 52 | 36 | 59 | 34 | 56 | 105 | 56 | |
| Histologic differentiation | |||||||||
| Well | 19 | 29 | 17 | 28 | 19 | 31 | 55 | 29 | .82 |
| Moderately | 37 | 55 | 33 | 54 | 28 | 46 | 98 | 52 | |
| Poorly | 11 | 16 | 11 | 18 | 14 | 23 | 36 | 19 | |
| Nodal status | |||||||||
| None | 52 | 78 | 45 | 74 | 44 | 72 | 141 | 75 | .76 |
| Present | 15 | 22 | 16 | 26 | 17 | 28 | 48 | 25 | |
| Acid phosphatase | |||||||||
| Not elevated | 41 | 61 | 44 | 72 | 39 | 64 | 124 | 66 | .41 |
| Elevated | 26 | 39 | 17 | 28 | 22 | 36 | 65 | 34 | |
| Radical prostatectomy | |||||||||
| No | 60 | 90 | 50 | 82 | 50 | 82 | 160 | 85 | .38 |
| Yes | 7 | 10 | 11 | 18 | 11 | 18 | 29 | 15 | |
| Stage | |||||||||
| A | 1 | 1 | 2 | 3 | 2 | 3 | 5 | 3 | .65† |
| B | 15 | 22 | 15 | 25 | 17 | 28 | 47 | 25 | |
| C | 51 | 76 | 44 | 72 | 42 | 69 | 137 | 72 | |
| Gleason score | |||||||||
| 2-6 | 23 | 37 | 20 | 34 | 20 | 34 | 63 | 35 | .77 |
| 7 | 27 | 44 | 21 | 36 | 25 | 42 | 73 | 41 | |
| 8-10 | 12 | 19 | 17 | 29 | 14 | 24 | 43 | 24 | |
| Missing | 5 | 3 | 2 | 10 | |||||
| Follow-up time, years | |||||||||
| All patients | |||||||||
| Median | 7.8 | 6.9 | 11.0 | 9.6 | |||||
| Range | 0.7-14.6 | 1.7-14.4 | 5.4-14.9 | 0.7-14.9 | |||||
| Surviving patients | |||||||||
| No. of patients | 25 | 21 | 35 | 81 | |||||
| Median | 11.1 | 11.0 | 11.9 | 11.3 | |||||
| Range | 0.9-14.6 | 4.2-14.4 | 8.1-14.9 | 0.9-14.9 | |||||
| HTD, years | |||||||||
| Median | 0.4 | 2.5 | 9.3 | 2.2 | |||||
| Range | 0.003-0.99 | 1.00-4.9 | 5.1-13.5 | 0.003-13.5 | |||||
Abbreviation: HTD, hormonal therapy duration.
From χ2 test statistics; the P value is computed by excluding missing patients.
Comparing A/B versus C.
Outcomes
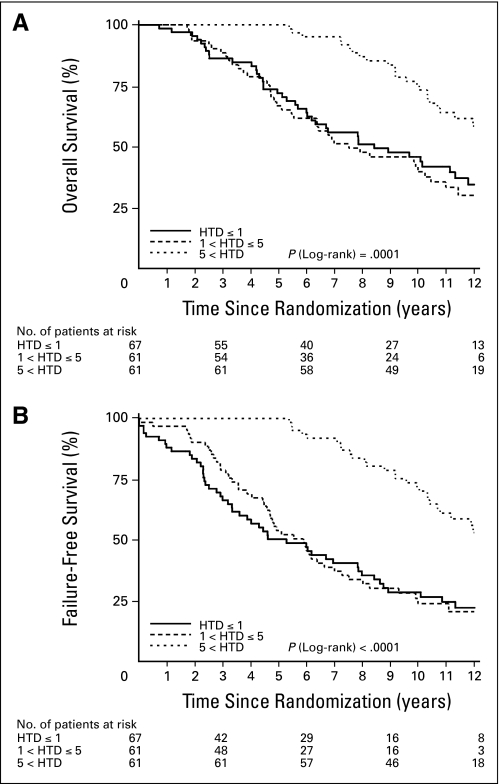
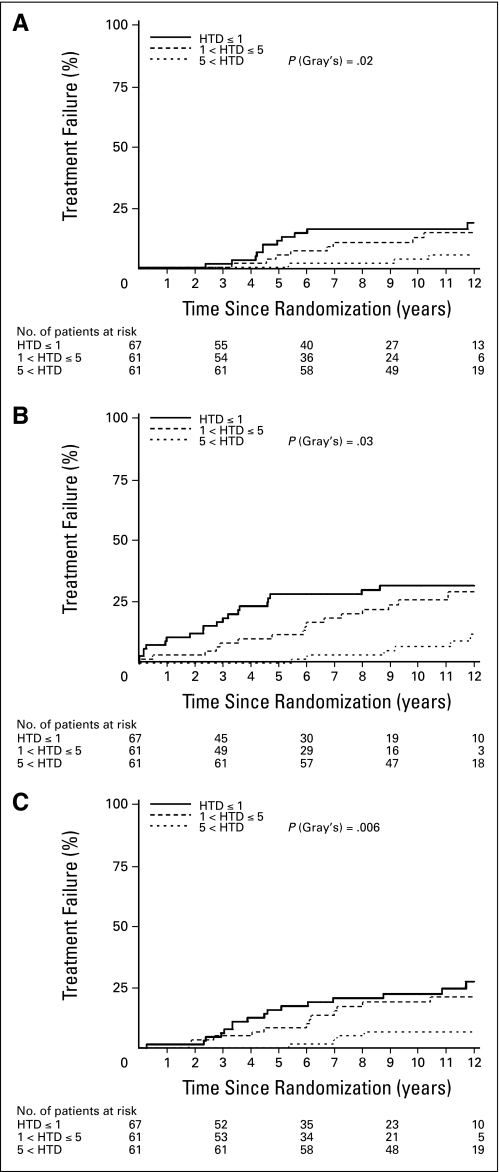
There are statistically significant differences in survival/failure rates among the three HTD groups in all outcomes without adjusting for other covariates. The 5- and 11-year overall survival rates for patients with HTD more than 5 years was 100% and 63.9%, as compared with 71.9% and 41.7% and 66.7% and 33.1% for those receiving HTD of ≤ 1 year and between 1 and ≤ 5 years, respectively (Table 2 and Fig 1A; P = .0001). Likewise, DFS rate is improved with HTD more than 5 years, as shown in Table 2 and Figure 1B. There are statistically significant differences in CSM rates favoring the HTD more than 5 years group (Table 2 and Fig 2A, P = .03). The incidence of LF and the development of DM are illustrated in Table 2 and Figures 2B and 2C. Despite unfavorable prognostic factors, the LF rate at 11 years is only 6.7%, and the DM rate at 11 years is also only 6.6% of patients who received hormonal therapy for more than 5 years. The pairwise comparisons show that there are statistically significant differences between the HTD more than 5 years group and the other two HTD groups in OS and DFS at the significance level of .017 (P = .05 and P = .03, respectively). There are statistically significant differences between HTD ≤ 1 year and HTD more than 5 years group in CSM, LF, and DM (data not shown).
Table 2.
Survival/Failure Rates by HTD Group
| Survival/Failure Measure | HTD ≤ 1 Year |
HTD > 1 and ≤ 5 Years |
HTD > 5 Years |
Total |
P* | ||||
|---|---|---|---|---|---|---|---|---|---|
| 5 Years | 11 Years | 5 Years | 11 Years | 5 Years | 11 Years | 5 Years | 11 Years | ||
| Overall survival | .0001 | ||||||||
| % | 71.9 | 41.7 | 66.7 | 33.1 | 100 | 63.9 | 79.5 | 46.3 | |
| 95% CI | 59.1 to 81.3 | 29.1 to 53.8 | 53.3 to 77.1 | 21.0 to 45.7 | 50.1 to 74.8 | 72.9 to 84.6 | 38.7 to 53.5 | ||
| No. of failures | 42 | 40 | 26 | 108 | |||||
| Total No. of patients | 67 | 61 | 61 | 189 | |||||
| Disease-free survival | < .0001 | ||||||||
| % | 50.2 | 24.4 | 53.8 | 23.7 | 100 | 60.9 | 67.7 | 36.3 | |
| 95% CI | 37.4 to 61.7 | 14.4 to 35.9 | 40.5 to 65.3 | 13.5 to 35.4 | 47.2 to 72.1 | 60.5 to 73.9 | 29.2 to 43.4 | ||
| No. of failures | 50 | 47 | 30 | 127 | |||||
| Total No. of patients | 67 | 61 | 61 | 189 | |||||
| Cause-specific mortality | .03 | ||||||||
| % | 11.1 | 15.9 | 5.1 | 14.4 | 0 | 5.2 | 5.4 | 11.8 | |
| 95% CI | 3.3 to 18.9 | 6.8 to 25.0 | 0 to 10.7 | 4.9 to 23.8 | 0 to 10.9 | 2.1 to 8.7 | 7.0 to 16.6 | ||
| No. of failures | 13 | 9 | 3 | 25 | |||||
| Total No. of patients | 67 | 61 | 61 | 189 | |||||
| Local failure | .02 | ||||||||
| % | 27.9 | 31.4 | 11.5 | 25.5 | 0 | 6.7 | 13.4 | 21.3 | |
| 95% CI | 16.8 to 39.0 | 19.8 to 43.0 | 3.4 to 19.6 | 14.1 to 36.9 | 0.3 to 13.1 | 8.5 to 18.3 | 15.3 to 27.2 | ||
| No. of failures | 21 | 16 | 9 | 46 | |||||
| Total No. of patients | 67 | 61 | 61 | 189 | |||||
| Distant metastasis | .006 | ||||||||
| % | 15.7 | 24.4 | 8.3 | 20.9 | 0 | 6.6 | 8.1 | 17.2 | |
| 95% CI | 6.6 to 24.7 | 13.4 to 35.4 | 1.3 to 15.3 | 10.2 to 31.7 | 0.3 to 12.8 | 4.2 to 12.0 | 11.7 to 22.8 | ||
| No. of failures | 17 | 13 | 4 | 34 | |||||
| Total No. of patients | 67 | 61 | 61 | 189 | |||||
Abbreviation: HTD, hormonal therapy duration.
Overall survival and disease-free survival were tested using log-rank test statistics; all others were tested using Gray's test statistics.
Fig 1.
(A) Overall survival rates; (B) disease-free survival rates. HTD, hormone treatment duration.
Fig 2.
(A) Cause-specific mortality rates; (B) local failure rates; (C) distant metastases rates. HTD, hormone treatment duration.
Table 3 shows that patients in the HTD ≤ 1 year group have statistically significantly higher risk of having failure events in all outcomes without adjusting for other covariates. For the HTD more than 1 year and ≤ 5 years group, they also have statistically significantly higher risk of having failure events in OS, DFS, and DM. However, the number of CSM events is too few to make a definite conclusion. After adjusting for age, radical prostatectomy, nodal status, centrally reviewed Gleason score, and stage variables, the HTD more than 5 years group remains statistically significantly associated with having fewer failure events in OS and DFS than other HTD groups at the significance level of .025. The number of events for other outcomes is too small to have a meaningful result in the multiple regression model (Table 3).
Table 3.
Proportional Hazards Model by HTD Group
| Comparison | Unadjusted |
Adjusted* |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P† | HR | 95% CI | P† | |
| Overall survival‡ | ||||||
| HTD ≤ 1 year | 2.37 | 1.45 to 3.86 | .0006 | 2.72 | 1.62 to 4.55 | .0001 |
| HTD > 1 and ≤ 5 years | 2.65 | 1.61 to 4.36 | .0001 | 2.98 | 1.77 to 5.03 | < .0001 |
| HTD > 5 years | RL | RL | ||||
| Disease-free survival‡ | ||||||
| HTD ≤ 1 year | 3.18 | 2.01 to 5.03 | < .0001 | 3.49 | 2.16 to 5.64 | < .0001 |
| HTD > 1 and ≤ 5 years | 3.13 | 1.96 to 4.98 | < .0001 | 3.59 | 2.20 to 5.86 | < .0001 |
| HTD > 5 years | RL | RL | ||||
| Cause-specific mortality§ | ||||||
| HTD ≤ 1 year | 4.81 | 1.39 to 16.60 | .01 | —‖ | — | |
| HTD > 1 and ≤ 5 years | 3.43 | 0.95 to 12.45 | .06 | — | — | |
| HTD > 5 years | RL | RL | ||||
| Local failure§ | ||||||
| HTD ≤ 1 year | 2.81 | 1.33 to 5.96 | .007 | — | — | |
| HTD > 1 and ≤ 5 years | 2.09 | 0.97 to 4.51 | .06 | — | — | |
| HTD > 5 years | RL | RL | ||||
| Distant metastasis§ | ||||||
| HTD ≤ 1 year | 4.86 | 1.66 to 14.28 | .004 | — | — | |
| HTD > 1 and ≤ 5 years | 3.80 | 1.26 to 11.47 | .02 | — | — | |
| HTD > 5 years | RL | RL | ||||
Abbreviations: HTD, hormonal therapy duration; HR, hazard ratio; RL, reference level.
HR is adjusted for age (< 70 [RL] or ≥ 70 years), radical prostatectomy (yes [RL] or no), nodal status (negative [RL] or positive), centrally reviewed Gleason score (2 to 6 [RL] v 7 v 8 to 10), and stage (A/B [RL] or C).
P value from χ2 test.
Cox proportional hazards model was used.
Fine and Gray's model was used.
The number events are too few to have stable estimates.
HTDs were considered as continuous variable and the results remained the same; risk of experiencing failure events, except LF, becomes lower as HTD increases by 1 year (Table 4).
Table 4.
Proportional Hazards Model: Continuous Hormone Therapy Duration
| Comparison | Unadjusted |
Adjusted* |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P† | HR | 95% CI | P† | |
| Overall survival‡ | 0.88 | 0.83 to 0.93 | < .0001 | 0.86 | 0.81 to 0.91 | < .0001 |
| Disease-free survival‡ | 0.86 | 0.82 to 0.90 | < .0001 | 0.85 | 0.80 to 0.89 | < .0001 |
| Cause-specific mortality§ | 0.81 | 0.70 to 0.94 | .004 | —‖ | — | |
| Local failure§ | 0.92 | 0.85 to 1.00 | .052 | — | — | |
| Distant metastasis§ | 0.78 | 0.69 to 0.89 | .0002 | — | — | |
Abbreviation: HR, hazard ratio.
HR is adjusted for age (< 70 [reference level] or ≥ 70 years), radical prostatectomy (yes [reference level] or no), nodal status (negative [reference level] or positive), centrally reviewed Gleason score (2 to 6 [reference level] v 7 v 8 to 10), and stage (A/B [reference level] or C).
P value from χ2 test.
Cox proportional hazards model was used.
Fine and Gray's model was used.
The number events are too few to have stable estimates.
Causes of Death
In Appendix Table A1 (online only), causes of death for each HTD group are shown. The rate of deaths from prostate cancer seems higher between the groups receiving fewer than 1 year of hormonal therapy compared with those patients receiving more than 5 years, but this is not statistically different (31% v 15%, P = .11).
DISCUSSION
The use of adjuvant hormonal therapy has been shown in randomized trials to improve outcomes in patients with high-risk prostate cancer1–3 and is now considered standard of care for such patients. Three randomized trials comparing RT plus long-term adjuvant hormonal therapy with RT alone have demonstrated improved OS rates1,3 or biochemical-free survival rate.2 These trials differed in the duration of the hormonal treatment: RTOG 85-313,4 mandated the use of adjuvant analog of luteinizing-hormone releasing hormone (LHRH) agonists for life or until sign of disease progression with the drug starting during the last week of RT; the European Organization for Research and Treatment of Cancer (EORTC) 22863 randomized trial1 initiated LHRH agonist therapy on the first day of RT and continued it for 36 months, and RTOG 92-022 administered LHRH agonist therapy 2 months before RT, during RT, and adjuvantly for another 24 months.
The prolonged use of LHRH agonist therapy results in secondary hypogonadism as a result of downregulation of the gonadotrophin receptors in the pituitary gland14 and, particularly in older patients, may lead to several complications, including osteoporosis,15 decline in bone density and muscle strength,16,17 neurocognitive alterations,18 and cardiac toxicity,19 with a significant impact in patients' quality of life.20,21 Thus investigating whether a shorter course of adjuvant LHRH agonists provides similar outcomes, minimizing toxicity, is a logical step in the management of localized high-risk prostate cancer.
In the current study, we assessed the possible impact hormonal duration could have in outcomes in a group of patients with locally advanced prostate cancer. After adjusting for known prognostic variables, the use of androgen suppression for more than 5 years proved to be significantly better in all end points studied, suggesting that a longer rather than a shorter course of hormonal therapy should be considered for these patients. This secondary analysis, however, has potential limitations and has to be viewed with some caution. First, this is a retrospective analysis from a mixed cohort of patients with prostate cancer, including postprostatectomy and node-positive patients. Second, the reason for the voluntary cessation of the hormonal therapy was not prospectively documented, and the possibility of selection bias has to be entertained. Early termination of hormonal therapy might also be associated with a comorbid condition (ie, obesity, diabetes, and so on) that may be exacerbated by hormonal therapy, leading to a worse survival and/or cancer outcomes. Finally, the trial was initiated before the PSA era, and it is quite possible that some of the patients had more advanced disease than originally thought.
D'Amico et al22 recently evaluated survival outcome from the use of a prolonged compared with a short course of androgen suppression therapy in node-negative, high-risk prostate cancer. These authors carried out a pooled analysis of patients enrolled in three prospective randomized trials1,23,24 and treated either with 36 or 6 months of androgen suppression and pelvic RT. They concluded that the longer use of hormonal therapy was not associated with increased survival.
Like our analysis, the D'Amico et al study22 was not a randomized comparison. Recently, the EORTC reported on a randomized phase III trial25 comparing 6 months versus 36 months of androgen deprivation for patients with locally advanced prostate cancer (EORTC 22961). In this study, 970 eligible patients received pelvic RT and either a short or a longer course of adjuvant LHRH agonist, with the study's end point being a noninferior survival, defined as a mortality HR of 1.35. At a median follow-up of 5.2 years, the 5-year overall survival rate was 85.3% on the longer duration LHRH agonist arm and 80.6% (HR = 1.43) on the short duration arm, with a 5-year biochemical PFS rate of 78.3% and 58.9% for the long and short duration arms, respectively (HR = 2.29). The authors' conclusion was that a noninferior survival outcome could not be confirmed with 6 months of androgen suppression compared with 36 months.
Although not directly comparable to the prostate cancer population, in breast cancer, many randomized trials have evaluated the effect of 1, 2, or more years of adjuvant tamoxifen in localized disease and estrogen receptor–positive patients. These trials have consistently demonstrated a significant benefit in survival with the use of tamoxifen,26 regardless of treatment duration. However, in randomized comparisons between 2 years versus 5 years,27,28 the longer administration of tamoxifen has led to significant improvement in outcomes. Of interest is that further prolongation beyond 5 years was not associated with additional benefit from tamoxifen.29
In summary, in this hypothesis-generating exercise, our results from a secondary analysis of RTOG 85-31 protocol show that prolonged HTD with LHRH agonist for more than 5 years might be associated with improved outcomes in patients with locally advanced localized prostate cancer. Together with the recent results of the EORTC 22961 randomized trial, our data suggest that decreasing the duration of hormonal administration may have a detrimental effect in these patients. A recently completed randomized trial in Quebec, Canada, comparing 18 months versus 36 months of LHRH agonist therapy for high-risk patients (PCS IV Study, A. Nabid, MD, principal investigator) hopefully will help to shed further light on this intriguing question. Further studies are clearly warranted in this area, and only a properly designed randomized trial can conclude that more than 5 years of hormonal therapy is superior to the current standard of 2 to 3 years for men with locally advanced prostate cancer.
Appendix
The Appendix is included in the full-text version of this article, available online at www.jco.org. It is not included in the PDF version (via Adobe® Reader®).
Table A1.
Causes of Death for Cause-Specific Mortality (n = 109)
| Cause of Death | HTD ≤ 1 Year (n = 42) |
HTD > 1 and ≤ 5 Years (n = 40) |
HTD > 5 Years (n = 27) |
Total (N = 109) |
||||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | |
| Prostate cancer | 13 | 31 | 9 | 23 | 4 | 15 | 26 | 24 |
| Second primary | 6 | 14 | 10 | 25 | 2 | 7 | 18 | 17 |
| Other causes | 16 | 38 | 14 | 35 | 18 | 67 | 48 | 44 |
| Unknown | 7 | 17 | 7 | 18 | 3 | 11 | 17 | 16 |
Abbreviation: HTD, hormonal therapy duration.
Footnotes
Supported by National Cancer Institute Grants No. Radiation Therapy Oncology Group U10 CA21661, Community Clinical Oncology Program U10 CA37422, and Stat U10 CA32115.
Presented at the 43rd Annual Meeting of the American Society of Clinical Oncology, June 1-5, 2007, Chicago, IL.
The contents of this article are the sole responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Howard Sandler, Astra Zeneca (C) Stock Ownership: None Honoraria: None Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Luis Souhami, Kyounghwa Bae, Howard Sandler
Administrative support: Kyounghwa Bae
Collection and assembly of data: Kyounghwa Bae
Data analysis and interpretation: Luis Souhami, Kyounghwa Bae, Howard Sandler
Manuscript writing: Luis Souhami, Kyounghwa Bae, Miljenko Pilepich, Howard Sandler
Final approval of manuscript: Luis Souhami, Kyounghwa Bae, Miljenko Pilepich, Howard Sandler
REFERENCES
- 1.Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): A phase III randomised trial. Lancet. 2002;360:103–106. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 2.Hanks GE, Pajak TF, Porter A, et al. Phase III trial of long-term adjuvant androgen deprivation after neoadjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate: The Radiation Therapy Oncology Group Protocol 92-02. J Clin Oncol. 2003;21:3972–3978. doi: 10.1200/JCO.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 3.Pilepich MV, Winter K, Lawton CA, et al. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma: Long-term results of phase III RTOG 85-31. Int J Radiat Oncol Biol Phys. 2005;61:1285–1290. doi: 10.1016/j.ijrobp.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 4.Pilepich MV, Caplan R, Byhardt RW, et al. Phase III trial of androgen suppression using goserelin in unfavorable-prognosis carcinoma of the prostate treated with definitive radiotherapy: Report of Radiation Therapy Oncology Group Protocol 85-31. J Clin Oncol. 1997;15:1013–1021. doi: 10.1200/JCO.1997.15.3.1013. [DOI] [PubMed] [Google Scholar]
- 5.Lawton CA, Winter K, Murray K, et al. Updated results of the phase III Radiation Therapy Oncology Group (RTOG) trial 85-31 evaluating the potential benefit of androgen suppression following standard radiation therapy for unfavorable prognosis carcinoma of the prostate. Int J Radiat Oncol Biol Phys. 2001;49:937–946. doi: 10.1016/s0360-3016(00)01516-9. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan EL MP. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 7.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 8.Kim K, Tsiatis AA. Study duration for clinical trials with survival response and early stopping rule. Biometrics. 1990;46:81–92. [PubMed] [Google Scholar]
- 9.Kalbfleisch JD, Prentice PR. The Statistical Analysis of Failure Time Data. New York, NY: John Wiley & Sons; 1980. [Google Scholar]
- 10.Gray R. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 11.Cox D. Regression models and life tables. J R Stat Soc B. 1972;34:187–229. [Google Scholar]
- 12.Fine J GR. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 13.Bonferroni C. Teoria Statistica delle Classi e Calcolo delle Probabilita:Volume in Onore di Ricardo dalla Volta. Firenza, Italy: Universita di Firenza; 1937. [Google Scholar]
- 14.Engel JB, Schally AV. Drug Insight: Clinical use of agonists and antagonists of luteinizing-hormone-releasing hormone. Nat Clin Pract Endocrinol Metab. 2007;3:157–167. doi: 10.1038/ncpendmet0399. [DOI] [PubMed] [Google Scholar]
- 15.Daniell HW, Dunn SR, Ferguson DW, et al. Progressive osteoporosis during androgen deprivation therapy for prostate cancer. J Urol. 2000;163:181–186. [PubMed] [Google Scholar]
- 16.Higano CS. Side effects of androgen deprivation therapy: Monitoring and minimizing toxicity. Urology. 2003;61:32–38. doi: 10.1016/s0090-4295(02)02397-x. [DOI] [PubMed] [Google Scholar]
- 17.Smith MR, Finkelstein JS, McGovern FJ, et al. Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab. 2002;87:599–603. doi: 10.1210/jcem.87.2.8299. [DOI] [PubMed] [Google Scholar]
- 18.Jenkins VA, Bloomfield DJ, Shilling VM, et al. Does neoadjuvant hormone therapy for early prostate cancer affect cognition? Results from a pilot study. BJU Int. 2005;96:48–53. doi: 10.1111/j.1464-410X.2005.05565.x. [DOI] [PubMed] [Google Scholar]
- 19.Tsai HK, D'Amico AV, Sadetsky N, et al. Androgen deprivation therapy for localized prostate cancer and the risk of cardiovascular mortality. J Natl Cancer Inst. 2007;99:1516–1524. doi: 10.1093/jnci/djm168. [DOI] [PubMed] [Google Scholar]
- 20.Braga-Basaria M, Dobs AS, Muller DC, et al. Metabolic syndrome in men with prostate cancer undergoing long-term androgen-deprivation therapy. J Clin Oncol. 2006;24:3979–3983. doi: 10.1200/JCO.2006.05.9741. [DOI] [PubMed] [Google Scholar]
- 21.Potosky AL, Reeve BB, Clegg LX, et al. Quality of life following localized prostate cancer treated initially with androgen deprivation therapy or no therapy. J Natl Cancer Inst. 2002;94:430–437. doi: 10.1093/jnci/94.6.430. [DOI] [PubMed] [Google Scholar]
- 22.D'Amico AV, Denham JW, Bolla M, et al. Short- vs long-term androgen suppression plus external beam radiation therapy and survival in men of advanced age with node-negative high-risk adenocarcinoma of the prostate. Cancer. 2007;109:2004–2010. doi: 10.1002/cncr.22628. [DOI] [PubMed] [Google Scholar]
- 23.D'Amico AV, Manola J, Loffredo M, et al. 6-month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: A randomized controlled trial. JAMA. 2004;292:821–827. doi: 10.1001/jama.292.7.821. [DOI] [PubMed] [Google Scholar]
- 24.Denham JW, Steigler A, Lamb DS, et al. Short-term androgen deprivation and radiotherapy for locally advanced prostate cancer: Results from the Trans-Tasman Radiation Oncology Group 96.01 randomised controlled trial. Lancet Oncol. 2005;6:841–850. doi: 10.1016/S1470-2045(05)70348-X. [DOI] [PubMed] [Google Scholar]
- 25.Bolla M, van Tiehoven G, de Reijke, et al. Concomitant and adjuvant androgen deprivation (ADT) with external beam irradiation (RT) for locally advanced prostate cancer: 6 months versus 3 years ADT—Results of the randomized EORTC Phase III trial 22961. J Clin Oncol. 2007;25(suppl):238s. abstr 5014. [Google Scholar]
- 26.Tamoxifen for early breast cancer: An overview of the randomised trials—Early Breast Cancer Trialists' Collaborative Group. Lancet. 1998;351:1451–1467. [PubMed] [Google Scholar]
- 27.Randomized trial of two versus five years of adjuvant tamoxifen for postmenopausal early stage breast cancer: Swedish Breast Cancer Cooperative Group. J Natl Cancer Inst. 1996;88:1543–1549. doi: 10.1093/jnci/88.21.1543. [DOI] [PubMed] [Google Scholar]
- 28.Sacco M, Valentini M, Belfiglio M, et al. Randomized trial of 2 versus 5 years of adjuvant tamoxifen for women aged 50 years or older with early breast cancer: Italian Interdisciplinary Group Cancer Evaluation Study of Adjuvant Treatment in Breast Cancer 01. J Clin Oncol. 2003;21:2276–2281. doi: 10.1200/JCO.2003.06.116. [DOI] [PubMed] [Google Scholar]
- 29.Fisher B, Dignam J, Bryant J, et al. Five versus more than five years of tamoxifen for lymph node-negative breast cancer: Updated findings from the National Surgical Adjuvant Breast and Bowel Project B-14 randomized trial. J Natl Cancer Inst. 2001;93:684–690. doi: 10.1093/jnci/93.9.684. [DOI] [PubMed] [Google Scholar]