Abstract
Background
Obsessive compulsive disorder (OCD) is characterized by intrusive thoughts, images, or impulses and/or repetitive stereotypical behavior. OCD patients exhibit reduced prepulse inhibition (PPI), and symptom exacerbation following challenge with 5-HT1B receptor agonists. Recently, gain-of-function alleles of the serotonin transporter (5-HTT) have been associated with OCD. We tested the hypothesis that reducing 5-HTT function chronically, either genetically or via serotonin reuptake inhibitor (SRI) treatment, attenuates PPI deficits and perseverative hyperlocomotion induced by 5-HT1B agonists in mice.
Methods
Mice received subchronic or chronic pretreatment with the SRI fluoxetine, and acute treatment with RU24969 (5-HT1A/1B agonist) or 8-OH-DPAT (5-HT1A agonist), and were assessed for PPI, locomotor activity, and spatial patterns of locomotion. The same measures were evaluated in 5-HTT wild-type (WT), heterozygous (HT), and knockout (KO) mice following RU24969 treatment. The effects of WAY100635 (5-HTA antagonist) or GR127935 (5-HT1B/D antagonist) pretreatment on RU24969-induced effects were evaluated. Finally, 5-HT1B binding and functional coupling were assessed in 5-HTT-WT, -HT, and -KO mice, and normal fluoxetine-treated mice.
Results
Chronic, but not subchronic, fluoxetine prevented RU24969-induced PPI deficits and perseverative hyperlocomotion. These RU24969-induced effects were mediated via 5-HT1B, and not 5-HT1A, receptors. 5-HTT-KO mice showed no effects of RU24969, and 5-HTT-HT mice exhibited intermediate phenotypes. 5-HT1B binding and functional coupling were reduced in the globus pallidus and substantia nigra of 5-HTT-KO mice.
Conclusions
Our results demonstrate that chronic, but not subchronic, fluoxetine treatment and 5-HTT knockout robustly attenuate 5-HT1B agonist-induced PPI deficits and perseverative hyperlocomotion. These results may have implications for the etiology and treatment of OCD.
Keywords: 5-HTT, antidepressant, OCD, PPI, 5-HT1B receptor
Introduction
Obsessive compulsive disorder (OCD) is characterized by the inability to inhibit intrusive and distressing thoughts, images, or impulses and/or repetitive behaviors. Alterations in the serotonergic system have been implicated in OCD, although the precise mechanisms underlying these abnormalities have not been identified. Human pharmacological and genetic (1,2) studies have implicated the 5-HT1B receptor (previously termed 5-HT1Dβ) in the pathophysiology of OCD. For example, challenge with 5-HT1B agonists has been shown to exacerbate symptoms in OCD patients in many (3–6), but not all studies (7,8). Recent evidence has also implicated gain-of-function alleles of the serotonin transporter (5-HTT) conferring increased 5-HTT expression (9,10) or constitutive activity of 5-HTT (11,12) in OCD. Furthermore, SRIs provide the only effective pharmacological monotherapy for OCD (13).
OCD has been associated with reduced prepulse inhibition (PPI) of the startle response (14,15). PPI refers to the reduction in magnitude of the startle response that occurs when an abrupt startling stimulus is preceded 30–500 msec by a barely detectable prepulse (16). PPI provides an operational measure of sensorimotor gating, a central inhibitory process theorized to filter out excessive sensory, motor, and cognitive information. Several neuropsychiatric disorders are characterized by deficient PPI, including schizophrenia (17) and OCD (14,15). Numerous animal studies have sought to elucidate the neural substrates underlying the PPI deficits in schizophrenia by identifying the mechanisms by which psychotomimetic drugs disrupt PPI (17,18), and antipsychotics prevent this effect (19,20). However, the PPI paradigm also provides a model for studying the neural substrates of PPI deficits that characterize other disorders, such as OCD.
In rodents, acute treatment with 5-HT1B agonists reduces PPI (21–23) and induces a long-lasting and perseverative form of hyperlocomotion in which animals circle the perimeter of the open field along a fixed path (24–26). The population of 5-HT1B receptors responsible for these behavioral effects of 5-HT1B agonists is currently unknown. Some studies have suggested that postsynaptic receptors mediate 5-HT1B agonist-induced hyperlocomotion, since this effect is not attenuated by degeneration of serotonin neurons by 5,7-dihydroxytryptamine (5,7-DHT)(27). However, 5,7-DHT-induced lesions are often incomplete, complicating the interpretation of results.
The present studies tested the hypothesis that chronically reducing 5-HTT function, either with chronic SRI treatment or genetic knockout of 5-HTT, would diminish the PPI deficits, hyperlocomotion, and perseverative spatial locomotion patterns induced by the 5-HT1B receptor agonist RU24969. Because only long-term SRI treatment reduces OCD symptoms, we assessed the ability of both subchronic and chronic SRI pretreatment to block RU24969-induced effects. Since RU24969 exhibits some affinity for 5-HT1A receptors (Ki=2.5nM) as well as 5-HT1B receptors (Ki=0.38nM)(28), we also assessed the effects of the 5-HT1A agonist 8-OH-DPAT on all measures, and the ability of the 5-HT1A antagonist WAY100635 and the 5-HT1B/D antagonist GR127935 to block RU24969-induced effects. 5-HTT-WT, -HT, and -KO mice were tested similarly. Finally, 5-HT1B receptor binding and functional coupling were also quantified.
Methods
Animals
BALB/cJ mice (Jackson Laboratories, Bar Harbor, ME) 8–12 weeks of age and weighing 20–30g were used for fluoxetine studies. 5-HTT-WT, -HT and -KO mice on a mixed BALB/cJ x 129SvEv F2 background (~75% BALB/cJ) were derived from heterozygous crossings (29). Female BALB/cJ mice were used for all experiments because this strain has been shown to behaviorally respond to chronic SRI treatment (30), and males of this strain exhibit excessive home-cage fighting. Mice were housed in groups of four to five with same-type mice and maintained on a 12L:12D schedule (lights on at 0600). Food and water were provided ad libitum. Behavioral testing occurred during the light phase between 0600 and 1600. Animal testing was conducted in accord with the NIH laboratory animal care guidelines and with IACUC approval.
Chemicals
Fluoxetine (0 or 15mg/kg/day) was delivered ad libitum in the drinking water (0 or 120mg/L) in opaque bottles, and changed weekly. RU24969 and 8-OH-DPAT were dissolved in 0.9% saline as salt doses, and injected intraperitoneally (i.p.) and subcutaneously (s.c.), respectively. Drug doses were selected based on the results of previous dose response studies (21,30). We used RU24969 to stimulate rodent 5-HT1B receptors instead of human 5-HT1B (h5-HT1B) agonists shown to exacerbate OCD, because human and rodent 5-HT1B receptors differ profoundly in drug binding (31,32); for example, RU24969 exhibits >1000-fold higher affinity for rodent 5-HT1B receptors than sumpatriptan (Ki=0.38 vs. 465nM)(28,31). WAY100635 and GR127935 were dissolved in distilled water and injected s.c. Injections were administered at a volume of 5ml/kg bodyweight with 1 cc syringes and 27g needles. For details regarding chemicals see Supplement 1.
Behavioral Experiments
Experiment 1
Mice were pretreated subchronically (7 days average) with 0 or 15mg/kg/day fluoxetine. Mice were then assessed for behavior following acute drug treatments (saline, 10mg/kg RU24969, and 1mg/kg 8-OH-DPAT) on three separate test days in a counterbalanced fashion. On each day, mice received acute drug injection and were immediately assessed for locomotion in the open field for 20 min, and then placed directly into startle chambers to assess PPI. One day of rest separated each test day. Thus, the duration of pretreatment was 5, 7, and 9 days on the three test days.
Experiment 2
All aspects were identical to Experiment 1, except that mice were pretreated chronically (28 days average) with 0 or 15mg/kg/day fluoxetine.
Experiment 3
5-HTT-WT, -HT, and -KO mice were assessed for behavior following acute drug injection as described Experiment 1, except that mice did not receive fluoxetine pretreatment.
Experiment 4
Mice were pretreated with acute injections of vehicle, 1mg/kg WAY100635, or 5mg/kg GR127935. Thirty minutes later, mice received acute drug treatment (saline or 10mg/kg RU24969) and were immediately assessed for behavior. Mice were tested on two separate days and received the same pretreatment, but received the two treatments in a counterbalanced fashion.
The apparati and parameters used to measure PPI and locomotion are described in Supplement 1.
5-HT1B binding and functional coupling
[3H]5-CT
5-HT1B receptor binding was assessed by evaluating [3H]5-CT binding in the presence of 8-OH-DPAT to block 5-HT1A receptors, and SB-269970 to block 5-HT7 receptors. Nonspecific binding was determined in the presence of the 5-HT1B/1D antagonist GR127935.
[35S]GTP-γ-S
5-HT1B receptor functional coupling was evaluated by assessing [35S]GTP-γ-S binding in the presence or absence of the 5-HT1B agonist CP93129. Nonspecific binding was determined in the presence of GR127935.
Optical densities of images were converted to nCi/mg tissue using a calibration curve generated from coexposed microscale standards. For details regarding binding studies see Supplement 1.
Statistical analysis
Analyses for all measures are described in Supplement 1.
Open field
Total distance traveled, total time in the center, and the spatial scaling exponent “spatial d” were evaluated. Spatial d quantifies geometric patterns of locomotor activity, and has been described in detail elsewhere (33). Briefly, spatial d measures the degree to which consecutive movements are straight (d≈1), meandering (d≈1.5), or involve many directional changes (d≈2). For example, highly perseverative patterns of locomotion characterized by straight paths along the perimeter of the open field are reflected by low spatial d values.
Startle
Prepulse inhibition and startle reactivity were analyzed.
Binding studies
Both [3H]5-CT and [35S]GTP-γ-S binding were evaluated.
Results
Chronic, but not subchronic, fluoxetine treatment attenuates RU24969-induced PPI deficits
RU24969 robustly decreased PPI across subchronic fluoxetine pretreatment groups (F(1,28)= 52.70; p<.0001)(Figure 1a). 8-OH-DPAT increased PPI across subchronic fluoxetine pretreatment groups (F(1,28)=6.30; p<.05). No main effect of pretreatment (F(1,23)=0.31; p=0.58) was found, indicating that subchronic fluoxetine pretreatment had no effect on PPI. One fluoxetine-pretreated outlier was removed from analysis.
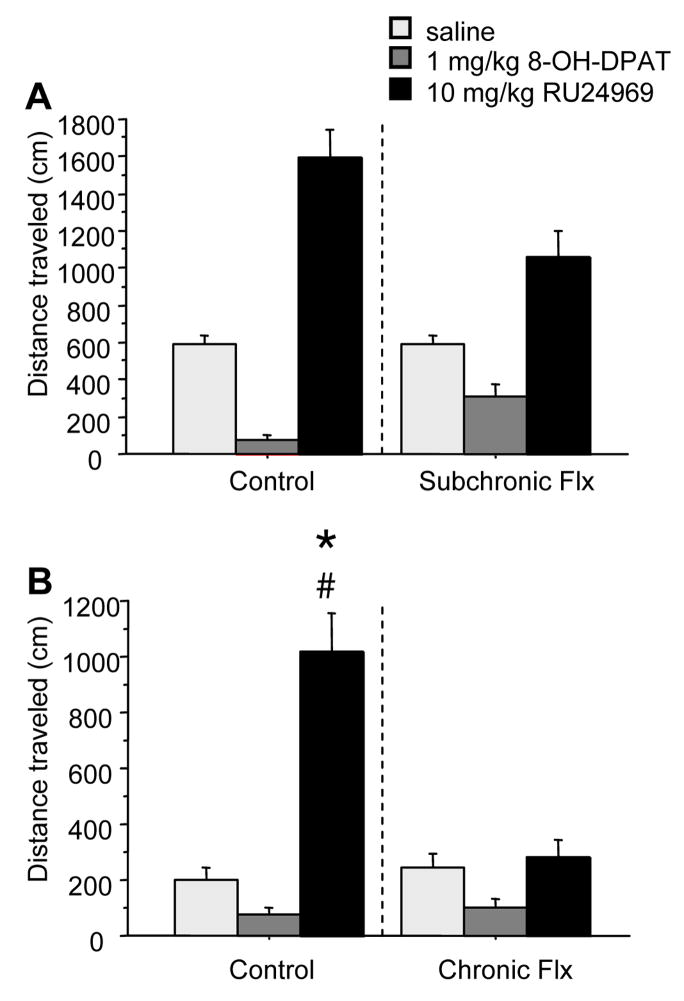
Figure 1.
(A) Percent prepulse inhibition is shown for BALB/cJ mice pretreated subchronically (one week) with 0 (n=15) or 15 (n=15) mg/kg/day fluoxetine. (B) PPI is shown for BALB/cJ mice pretreated chronically (four weeks) with 0 (n=15) or 15 (n=14) mg/kg/day fluoxetine. All mice received saline, RU24969, and 8-OH-DPAT treatment. Chronic, but not subchronic, 15mg/kg/day fluoxetine attenuated the PPI deficits induced by 10mg/kg RU24969. 8-OH-DPAT increased PPI across all pretreatments. Values are means ± SEM. Insets show data with prepulse intensity collapsed. An asterisk (*) indicates a significant difference from saline-treated animals within the same pretreatment group. A pound sign (#) indicates a significant difference from control-pretreated animals within the same treatment group.
Chronic fluoxetine pretreatment diminished the ability of RU24969 to disrupt PPI (Figure 1b) as revealed by a treatment x pretreatment interaction (F(1,27)=9.40; p<.01). RU24969 reduced PPI in both control-pretreated (F(1,14)=28.44; p<.0001) and fluoxetine-pretreated (F(1,13)=9.76; p<.01) mice. However, following RU24969 treatment, control-pretreated mice showed lower PPI levels than chronic fluoxetine-pretreated mice (F(1,27)=15.77; p<.001). Chronic fluoxetine pretreatment had no effect on PPI in control-treated mice (F(1,27)=1.00; p=0.33). A trend for a main effect for 8-OH-DPAT to increase PPI was found (F(1,27)=3.14; p=0.08).
No effects of pretreatment or treatment on startle reactivity were found which could have confounded the interpretation of PPI results in any experiment. Experimental results for startle reactivity are presented in Supplement 1.
Chronic, but not subchronic, fluoxetine treatment prevents RU24969-induced hyperactivity and perseverative locomotor paths
RU24969 induced hyperlocomotion across subchronic pretreatment conditions (F(1,28)=19.01; p<.001)(Figure 2a). Although a trend for an interaction of treatment x pretreatment was found, (F(1,28)=3.45; p=0.07), RU24969 increased locomotion within both pretreatment groups, and locomotion did not differ between pretreatment groups within treatment groups. Furthermore, RU24969 induced straighter paths of locomotion (lower spatial d) across pretreatment groups (F(1,28)=7.03; p<.01)(Table 1). 8-OH-DPAT reduced locomotion across subchronic pretreatment conditions (F(1,28)=76.09; p<.0001), and induced more circumscribed paths (higher spatial d) in control- but not fluoxetine-pretreated mice as revealed by a pretreatment x treatment interaction (F(1,28)=7.76; p<.01) and Newman Keuls post-hoc tests.
Figure 2.
(A) Total locomotor activity is shown for BALB/cJ mice pretreated subchronically (one week) with 0 (n=15) or 15 (n=15) mg/kg/day fluoxetine. (B) Total locomotor activity is shown for BALB/cJ mice pretreated chronically (four weeks) with 0 (n=15) or 15 (n=15) mg/kg/day fluoxetine. All mice received saline, RU24969, and 8-OH-DPAT treatment. Chronic, but not subchronic, 15mg/kg/day fluoxetine blocked the locomotor hyperactivity induced by 10mg/kg RU24969. 8-OH-DPAT reduced locomotion across all pretreatments. Values are means ± SEM. An asterisk (*) indicates a significant difference from saline-treated animals within the same pretreatment group. A pound sign (#) indicates a significant difference from control-pretreated animals within the same treatment group.
Table 1.
Spatial d values are shown for all experiments. In the subchronic fluoxetine study, RU24969 significantly reduced spatial d (straighter paths) across pretreatment groups. Values are means ± SEM. An asterisk (*) indicates a significant difference from saline-treated animals within the same pretreatment or genotype. A pound sign (#) indicates a significant difference from either control pretreatment or 5-HTT-WT mice within the same drug treatment group.
| Experiment | Pretreatment | Treatment | Spatial d |
|---|---|---|---|
| Subchronic fluoxetine | control | saline | 1.45 ± .06 |
| RU24969 | 1.40 ± .05 | ||
| 8-OH-DPAT | 1.78 ± .05* | ||
| fluoxetine | saline | 1.50 ± .06 | |
| RU24969 | 1.38 ± .05 | ||
| 8-OH-DPAT | 1.52 ± .07# | ||
| Chronic fluoxetine | control | saline | 1.71 ± .06 |
| RU24969 | 1.55 ± .06* | ||
| 8-OH-DPAT | 1.73 ± .07 | ||
| fluoxetine | saline | 1.74 ± .06 | |
| RU24969 | 1.71 ± .07# | ||
| 8-OH-DPAT | 1.81 ± .07 | ||
| Serotonin transporter | WT | saline | 1.50 ± .08 |
| RU24969 | 1.23 ± .07* | ||
| 8-OH-DPAT | 1.57 ± .10 | ||
| HT | saline | 1.63 ± .09 | |
| RU24969 | 1.41 ± .10 | ||
| 8-OH-DPAT | 1.65 ± .09 | ||
| KO | saline | 1.75 ± .08 | |
| RU24969 | 1.76 ± .10# | ||
| 8-OH-DPAT | 1.92 ± .06# | ||
| Antagonist study | control | saline | 1.59 ± .07 |
| RU24969 | 1.61 ± .09 | ||
| WAY100635 | saline | 1.51 ± .05 | |
| RU24969 | 1.32 ± .06*# | ||
| GR127935 | saline | 1.61 ± .06 | |
| RU24969 | 1.66 ± .07 |
RU24969 induced hyperlocomotion in control, but not chronic fluoxetine-pretreated mice (Figure 2b), as revealed by a treatment x pretreatment interaction (F(1,28)=9.58; p<.01). Specifically, control-pretreated mice receiving RU24969 exhibited more locomotor activity than chronic fluoxetine-pretreated mice receiving RU24969, and control-pretreated mice receiving saline. In parallel, RU24969 induced straighter locomotor paths in control-pretreated, but not fluoxetine-pretreated mice (Figure 3), as revealed by a main effect of treatment (F(1,28)=5.65; p<.05), and a trend for a treatment x pretreatment interaction (F(1,28)=3.02; p=0.09). Planned comparisons indicated that RU24969 reduced spatial d in control-pretreated mice (F(1,14)=7.84; p<.01), but not in chronic fluoxetine-pretreated mice (F(1,14)=0.22; p=0.64). 8-OH-DPAT reduced locomotion in mice overall (F(1,28)=11.57; p<.01), and had no effect on spatial d (Table 1).
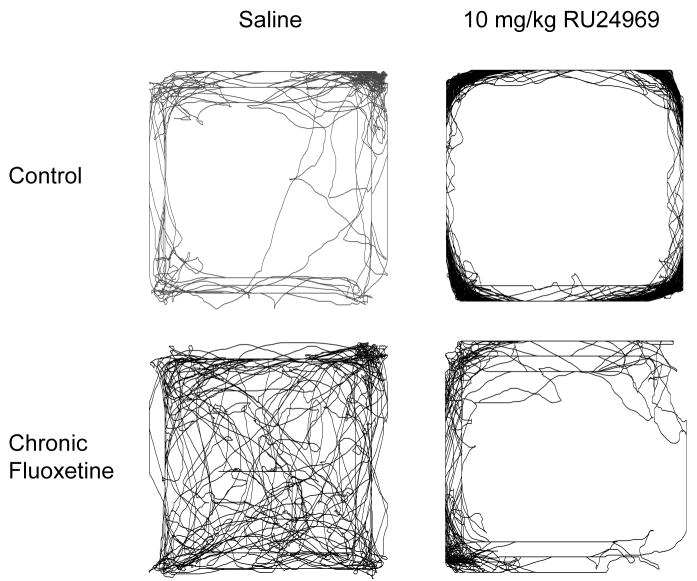
Figure 3.
Locomotor paths taken by BALB/cJ mice receiving chronic fluoxetine (0 or 15mg/kg/day) pretreatment and RU24969 (0 or 10mg/kg) treatment are shown. Paths are representative for spatial d values, but not locomotor activity levels or amount of activity in the center vs. periphery. A control-pretreated mouse receiving RU24969 shows a highly perseverative pattern of locomotion characterized by straight paths along the perimeter of the open field (d=1.2). The remaining three mice show more meandering paths (d=1.4), and varying levels of locomotor activity.
5-HTT-KO mice do not exhibit RU24969-induced PPI deficits, hyperactivity, or perseverative locomotor paths
No significant interactions including genotype and treatment were identified for PPI. To test our a priori hypothesis that reducing 5-HTT function would diminish the effects of RU24969, we performed planned comparisons assessing pretreatment, treatment, and prepulse intensity within each genotype. For 5-HTT-WT mice, a treatment x prepulse intensity interaction (F(2,20)=4.82; p<.05) and Newman Keuls post-hoc tests showed that RU24969 reduced PPI at the 3dB prepulse intensity (Figure 4a). No effects of RU24969 on PPI were found in 5-HTT-HT or -KO mice. 8-OH-DPAT increased PPI at the 6 and 12dB prepulse intensities in 5-HTT-WT and -HT mice, but had no effect in -KO mice, as indicated by a treatment x pretreatment x prepulse intensity interaction (F(4,62)=3.80; p<.01) and post-hoc tests. One 5-HTT-WT and one -HT outlier was removed from PPI analyses.
Figure 4.
(A) Prepulse inhibition and (B) total locomotor activity of serotonin transporter -WT, -HT, and -KO mice treated with control, RU24969, and 8-OH-DPAT. RU24969 reduced PPI only in 5-HTT-WT mice at the 3dB prepulse intensity. 8-OH-DPAT increased PPI in 5-HTT-WT and -HT, but not -KO mice. RU24969 increased locomotion in 5-HTT-WT and -HT, but not -KO mice. Locomotion was reduced overall in 5-HTT-KO mice. Values are means ± SEM. An asterisk (*) indicates a significant difference from saline-treated animals within the same genotype. A pound sign (#) indicates a significant difference from 5-HTT-WT mice within the drug treatment group.
RU24969 induced hyperlocomotion in 5-HTT-WT and -HT, but not -KO mice (Figure 4b). ANOVA found a treatment x genotype interaction (F(2,33)=7.59; p<.01), and post-hoc ANOVAs showed that RU24969 increased locomotion in 5-HTT-WT (F(1,11)=27.08; p<.001), and -HT (F(1,11)=9.41; p<.01), but not -KO mice (F(1,11)=2.65; p=0.13). Locomotor activity levels differed between each genotype following RU24969 treatment, with 5-HTT-WT mice showing the most and -KO mice showing the least locomotion. Lastly, following saline treatment, 5-HTT-KO mice showed lower levels of activity than -WT and -HT mice. 8-OH-DPAT had no effect on locomotor activity, but a main effect of genotype (F(2,33)=4.03; p<.05) and post-hoc tests showed that 5-HTT-KO mice showed lower levels of activity than -WT and -HT mice overall.
RU24969 induced straighter locomotor paths in 5-HTT-WT, but not -HT or -KO mice, as revealed by main effects of genotype (F(2,33)=6.42; p<.01), and drug (F(1,33)=9.44; p<.01), and a trend for a treatment x genotype interaction (F(2,33)=2.74; p=0.07)(Table 1). Planned comparisons indicated that RU24969 induced straighter paths in 5-HTT-WT mice (F(1,11)=10.10; p<.01). Additionally, a trend was found for RU24969 to induce straighter paths in 5-HTT-HT mice (F(1,11)=4.37; p=0.06), but no effect of RU24969 on spatial d was found in -KO mice (F(1,11)=0.02; p=0.90). Furthermore, RU24969-treated 5-HTT-WT mice exhibited straighter paths than RU24969-treated -KO mice. ANOVA assessing the effects of genotype and 8-OH-DPAT on spatial d found only a main effect of genotype (F(2,33)=4.44; p<.05), with 5-HTT-KO mice exhibiting reduced spatial d compared to -WT and -HT mice.
RU24969-induced PPI deficits and perseverative hyperlocomotion are mediated by 5-HT1B receptors
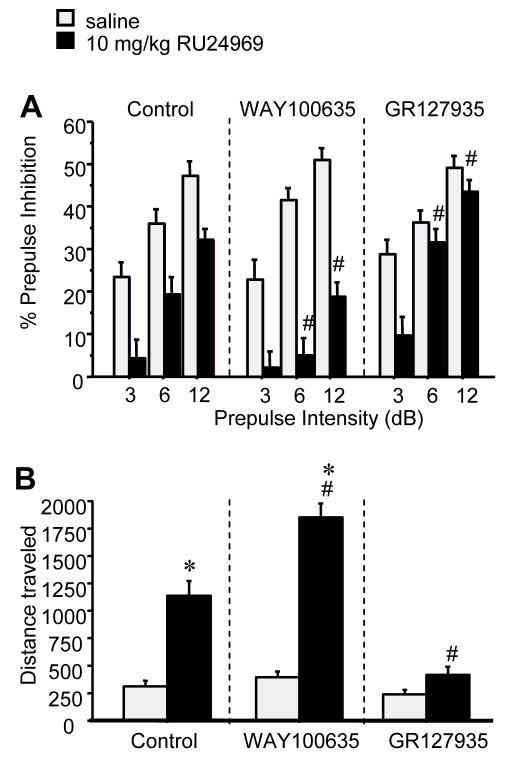
GR127935 pretreatment blocked RU24969-induced PPI deficits, as revealed by an interaction of pretreatment, treatment, and prepulse intensity (F(4,80)=4.97; p<.001)(Figure 5a). RU24969 reduced PPI in saline pretreated mice (F(1,13)=20.11; p<.001) and WAY100635 pretreated mice (F(1,13)=31.60; p<.0001). No significant effect of RU24969 on PPI was found in GR127935 pretreated mice, although a trend was found for RU24969 to reduce PPI at the 3dB prepulse intensity. Pretreatment did not alter PPI in saline-treated mice. However, within RU24969-treated groups, GR127935 increased and WAY100635 decreased PPI relative to control at the 6dB and 12dB prepulse intensities (F(4,80)=3.50; p<.05).
Figure 5.
(A) Prepulse inhibition and (B) total locomotor activity of BALB/cJ mice pretreated with control (n=14), 1mg/kg WAY100635 (n=15), or 5mg/kg GR127935, and treated with 0 or 10mg/kg RU24969 in a counterbalanced fashion. RU24969 treatment reduced PPI compared to control treatment in control- and WAY100635-pretreated, but not GR127935-pretreated mice. 5-HTT-WT mice at the 3dB prepulse intensity. Within RU24969-treatment, WAY100635 pretreatment reduced PPI and GR127935 pretreatment increased PPI at the 6 and 12dB prepulse intensities. RU24969 increased locomotion in control- and WAY100635 pretreated mice, but not GR127935 pretreated mice. Values are means ± SEM. An asterisk (*) indicates a significant difference from saline-treated animals within the same pretreatment group. A pound sign (#) indicates a significant difference from all other pretreatment groups within the same treatment group.
GR127935 pretreatment blocked WAY100,635- and increased RU24969-induced hyperactivity, as revealed by an interaction of pretreatment and treatment (F(2,40)=13.73; p<.0001)(Figure 5b). RU24969 induced hyperactivity in saline pretreated mice (F(1,13)=12.36; p<.01) and WAY100635 pretreated mice (F(1,13)=69.33; p<.0001). No effect of RU24969 was found in GR127935 pretreated mice (F(1,14)=3.24; p=0.09). Pretreatment did not alter locomotion in saline-treated mice; however, within RU24969-treated mice, all pretreatment groups differed from one another.
Activation of 5-HT1B, but not 5-HT1A, receptors by RU24969 induced more perseverative locomotor paths as quantified by spatial d (Table 1). ANOVA revealed an interaction of pretreatment and treatment (F(2,40)=5.57; p<.01), and post hoc tests showed that mice receiving WAY100,635 and RU24969 exhibited lower spatial d values than mice receiving either treatment alone.
5-HT1B agonist-stimulated [35S]GTP-γ-S binding is reduced in 5-HTT-KO mice
CP93129-induced [35S]GTP-γ-S binding in the substantia nigra (F(2,13)=13.76; p<.001) and globus pallidus (F(2,13)=10.55; p<.01) of 5-HTT-KO mice was reduced relative to 5-HTT-WT and -HT mice (Figure 6a, Suppl. Fig. 1). However, chronic fluoxetine had no effect on CP93129-induced [35S]GTP-γ-S binding in either brain region (see Supplement 2).
Figure 6.

5-HT1B functional coupling as assessed by CP93129-induced [35S]GTP-γ-S binding (A), and 5-HT1B receptor expression as assessed by [3H]5-CT binding (B) is shown for 5-HTT-WT, -HT, and -KO mice in the globus pallidus and substantia nigra. Quantification revealed significantly decreased [3H]5-CT binding in 5-HTT-HT and -KO mice relative to 5-HTT-WT mice. [35S]GTP-γ-S binding was reduced in 5-HTT-KO mice relative to 5-HTT-WT and -HT mice (*p<.05). For representative images, see Suppl. Figs. 1 and 2.
5-HT1B receptor binding is reduced in 5-HTT-HT and -KO mice
[3H]5-CT binding was reduced in the substantia nigra of 5-HT-HT (−44%)(F(1,5)=6.82; p<.05) and 5-HTT-KO mice (−44%)(F(1,8)=7.75; p<.05) compared to 5-HTT-WT mice. Furthermore, binding was reduced in the globus pallidus of 5-HTT-KO mice (−48%)(F(1,9)=10.42; p<.01) and 5-HTT-HT mice (−51%)(F(1,6)=4.64; p=0.07; a trend), compared to 5-HTT-WT mice (Figure 6b, Suppl. Fig. 2). However, chronic fluoxetine pretreatment had no effect on [3H]5-CT binding in either brain structure (see Supplement 2).
Discussion
Here we report that both chronic fluoxetine treatment and genetic reduction of 5-HTT expression prevent 5-HT1B agonist-induced PPI deficits, hyperlocomotion, and perseverative locomotor paths in mice. We found that chronic (4 weeks), but not subchronic (1 week), fluoxetine treatment attenuated these RU24969-induced effects, which is consistent with the time-course for SRIs to induce therapeutic effects in OCD. The effects of RU24969 on all three measures were absent in 5-HTT-KO mice, and intermediate in 5-HTT-HT mice. Additionally, we have replicated these behavioral effects in an independent group of animals. Furthermore, we have demonstrated that all three behavioral effects of RU24969 require activation of 5-HT1B, and not 5-HT1A, receptors. Finally, we found that genetically reducing 5-HTT expression, but not chronic fluoxetine treatment, reduced 5-HT1B receptor expression and functional coupling in the substantia nigra and globus pallidus. The present findings may have implications for the etiology and treatment of neuropsychiatric disorders characterized by PPI deficits and perseverative behaviors and treated with SRIs, including OCD.
Our present findings in mice parallel several observations in OCD patients. One, h5-HT1B receptor agonists have been suggested to exacerbate symptoms in OCD patients (3–6). Two, long-term SRI treatment provides effective treatment for OCD (13). Three, recent studies have suggested that lower 5-HTT function might protect against OCD, while increased 5-HTT function may be a risk factor for OCD (9–12). However, autistic patients also exhibit repetitive motor symptoms that respond to chronic SRI treatment (34,35) and are exacerbated by h5-HT1B receptor challenge (36,37). Furthermore, autistic spectrum patients have also been suggested to exhibit PPI deficits (38), Thus, the present mouse model may provide insight into several disorders characterized by these features, such as OCD and autism.
Our results indicate that chronic, but not subchronic, fluoxetine treatment prevents 5-HT1B receptor-induced PPI deficits. RU24969 treatment reduced PPI across all prepulse intensities in pure BALB/cJ mice (Figure 1a), and decreased PPI only at the 3dB prepulse intensity in F2 (75%BALB/cJ, 25%129Sv) 5-HTT-WT mice (Figure 4a). We have previously shown that RU24969 reduces PPI only at low prepulse intensities in 129Sv mice (21,39); thus, this discrepancy was likely due to a strain difference. Chronic fluoxetine treatment might prevent the behavioral effects of RU24969 by increasing extracellular serotonin levels, resulting in desensitization of 5-HT1B receptors. For example, repeated 5-HT1B receptor stimulation attenuates RU24969-induced hyperactivity (40). Neither subchronic nor chronic fluoxetine treatment alone significantly altered PPI (Figure 1), consistent with previous reports in humans (41) and animals (42,43). 8-OH-DPAT treatment increased PPI as previously reported (21,39), regardless of fluoxetine treatment (Figure 1). Thus, any effects of RU24969 mediated by 5-HT1A receptors did not confound the interpretation of the present results.
Chronic, but not subchronic, fluoxetine treatment prevented RU24969-induced hyperlocomotion (Figure 2). Subchronic fluoxetine treatment showed a trend to reduce RU24969-induced hyperactivity. Thus, the attenuation of RU24969-induced hyperactivity by fluoxetine appears to begin in the first week of treatment and develop progressively over four weeks. 8-OH-DPAT reduced locomotion across pretreatment groups in both fluoxetine studies. Although this reduction appears attenuated in subchronic fluoxetine-pretreated mice, this difference was not significant and was not replicated in an independent experiment. In separate studies, we also found that chronic fluoxetine treatment attenuated hyperlocomotion induced by the 5-HT1A/B agonist/5-HT3 antagonist anpirtoline (44)(Suppl. Fig. 4).
Chronic, but not subchronic, fluoxetine treatment prevented the straight, perseverative paths of locomotion induced by RU24969 (Table 1). Spatial d values have been shown to be independent of the amount of locomotor activity (45). Furthermore, RU24969-induced thigmotaxis was not observed because mice spent very little time in the center (<1 min. average), including control-treated mice. Thus, RU24969 reduced spatial d, but did not alter time spent in the center versus periphery due to a floor effect. In the subchronic fluoxetine study, 8-OH-DPAT increased spatial d in mice receiving control pretreatment; however, RU24969 still significantly reduced spatial d in this group. 8-OH-DPAT also induced small nonsignificant increases in spatial d in all other groups.
Our findings that pretreatment with GR127935, but not WAY100635, prevents RU24969-induced PPI deficits and perseverative hyperlocomotion show that activation of 5-HT1B, and not 5-HT1A, receptors is required for these effects (Figure 5). This finding for locomotor activity has been previously reported in C57BL/6 mice (24). Furthermore, RU24969-induced hyperactivity (46) and PPI deficits (39) are absent in 5-HT1B-KO mice. RU24969 also exhibits minor agonist activity at 5-HT2C receptors (Ki=150nM)(Peroutka 1986); however, 5-HT2C activation is unlikely to be involved in RU24969-induced hyperlocomotion, which is unaltered by the 5-HT2C antagonist SB242084 (47) or the 5-HT2A/C antagonist ritanserin (24). Our present results suggest that both chronic fluoxetine and knockout of 5-HTT reduce RU24969-induced PPI deficits and perseverative hyperlocomotion by reducing 5-HT1B receptor function; however, reducing 5-HTT function also functionally desensitizes other receptor populations including presynaptic 5-HT1A receptors (48,49), which do not appear to influence the RU24969-induced behavioral effects studied here. Finally, it is possible that the affinity of 8-OH-DPAT for 5-HT7 receptors (Ki=39nM) contributes to its effects in addition to its affinity for 5-HT1A receptors (Ki=0.65nM)(50).
Recent findings have implicated gain-of-function 5-HTT alleles in OCD, suggesting that lower functioning 5-HTT alleles protect against OCD. Our present findings in 5-HTT-WT, -HT- and -KO mice appear consistent with these human genetic findings. 5-HTT-WT, but not -KO, mice exhibited PPI deficits, hyperlocomotion, and reduced spatial d after receiving RU24969 (Figure 4, Table 1). Furthermore, RU24969 induced intermediate locomotor responses, and straighter locomotor paths that approached significance in 5-HTT-HT mice. Across treatment conditions, 5-HTT-WT and -HT mice showed higher locomotor activity levels and straighter locomotor paths compared to 5-HTT-KO mice, indicating that lower 5-HTT expression levels reduce locomotion and induce more meandering locomotor paths. 8-OH-DPAT did not alter locomotor activity or spatial patterns in any genotype. RU24969 reduced PPI at the 3dB prepulse intensity in 5-HT-WT, but not -HT- or -KO mice. However, we can not rule out that the nonsignificant reductions in PPI observed in 5-HTT-HT and -KO mice at the 3dB prepulse intensity might have obscured PPI disruptive effects of RU24969. 8-OH-DPAT increased PPI in 5-HTT-WT and -HT, but not -KO mice, although this finding can not account for the lack of effect of RU24969 in 5-HTT-KO mice. The differential effects of RU24969 on PPI and locomotor activity in 5-HTT-WT and -KO mice likely reflects lack of 5-HTT, and not flanking alleles, because we obtained similar results in 5-HTTKO mice on a pure 129Sv background (Suppl. Fig. 3; Suppl. Table 1) which lack flanking alleles, and in normal mice following chronic fluoxetine treatment (Figures 1 & 2).
We sought to identify the populations of 5-HT1B receptors that were functionally desensitized by reducing 5-HTT function. Activation of 5-HT1B receptors in the substantia nigra reduces the tonic inhibition exerted by striatal projection neurons onto nigral dopaminergic neurons (51), resulting in increased dopamine release into the striatum (52,53) which disrupts PPI (18), and induces hyperlocomotion (54,55). Likewise, activation of 5-HT1B receptors in the globus pallidus reduces tonic inhibition exerted by GABAergic striatal neurons onto pallidal projection neurons (51), resulting in reduced PPI (18), and increased locomotion (56). Thus, the observed reductions in 5-HT1B receptor binding and/or functional coupling in 5-HTT-HT and -KO mice might underlie their attenuated behavioral responses to RU24969 (Figure 6). Our present findings are also consistent with a previous report examining 5-HT1B binding and functional coupling in 5-HTT-KO mice on a C57BL/6 background (48). However, we found no changes in these measures in chronic fluoxetine-treated mice. Several possibilities could explain this discrepancy. First, reductions in 5-HT1B binding and/or coupling could underlie the reduced behavioral effects of RU24969 in 5-HTT-HT and -KO mice, or may be unrelated. Second, chronic fluoxetine treatment might functionally desensitize a different population of 5-HT1B receptors to achieve the same behavioral effects. For example, desensitization of 5-HT1B receptors on glutamatergic neurons in the dorsal subiculum might reduce RU24969-induced behavioral effects, since activation of these receptors induces dopamine release in the ventral striatum (57). Lastly, reduction in 5-HTT function may reduce RU24969-meditated effects by inducing changes downstream of the 5-HT1B receptor.
In summary, we have established for the first time that chronic SRI treatment and genetic reduction in 5-HTT expression both prevent the PPI deficits, hyperlocomotion, and perseverative locomotor paths induced by 5-HT1B receptor activation. Furthermore, we have shown that chronic, but not subchronic, SRI treatment is required to attenuate these 5-HT1B-mediated behavioral effects. These findings suggest that increased 5-HTT and/or 5-HT1B receptor function, and their functional interaction, contribute to behavioral effects in an animal model that is suggested to be relevant to the etiology of OCD. Accordingly, the 5-HT1B receptor may provide a target for novel therapeutics for OCD. Future work will explore the effects of 5-HT1B receptor ligands on PPI and perseverative hyperlocomotion in mice with gain-of-function mutations in 5-HTT.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grants K01MH071555 and R01MH079424 to S.C.D., R01NS049263 and R21NS052195 to C.W., and DA02925 to M.A.G., as well as NARSAD Awards to S.C.D., M.A., and J.A.G. N.A.S. was supported by T32DA07255-15.
Footnotes
Disclosure/Conflict of Interest
S.C. Dulawa, K.A. Holick, N.A. Shanahan, V. Masten, M. Ansorge, C. Waeber, and J.A. Gingrich reported no biomedical financial interests or potential conflicts of interest. M.A. Geyer holds an equity interest in San Diego Instruments. R. Hen receives compensation as a consultant for BrainCells, Inc., PsychoGenics, Inc., Memory Pharmaceuticals, Roche, Astra Zeneca, and Lundbeck in relation to the generation of novel antidepressants.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sanders AR, Duan J, Gejman PV. DNA variation and psychopharmacology of the human serotonin receptor 1B (HTR1B) gene. Pharmacogenomics. 2002;3:745–762. doi: 10.1517/14622416.3.6.745. [DOI] [PubMed] [Google Scholar]
- 2.Mundo E, Richter MA, Sam F, Macciardi F, Kennedy JL. Is the 5- HT(1Dbeta) receptor gene implicated in the pathogenesis of obsessive-compulsive disorder? Am J Psychiatry. 2000;157:1160–1161. doi: 10.1176/appi.ajp.157.7.1160. [DOI] [PubMed] [Google Scholar]
- 3.Stein DJ, Van Heerden B, Wessels CJ, Van Kradenburg J, Warwick J, Wasserman HJ. Single photon emission computed tomography of the brain with Tc-99m HMPAO during sumatriptan challenge in obsessive-compulsive disorder: investigating the functional role of the serotonin auto-receptor. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:1079–1099. doi: 10.1016/s0278-5846(99)00051-2. [DOI] [PubMed] [Google Scholar]
- 4.Gross-Isseroff R, Cohen R, Sasson Y, Voet H, Zohar J. Serotonergic dissection of obsessive compulsive symptoms: a challenge study with m-chlorophenylpiperazine and sumatriptan. Neuropsychobiology. 2004;50:200–205. doi: 10.1159/000079970. [DOI] [PubMed] [Google Scholar]
- 5.Koran LM, Pallanti S, Quercioli L. Sumatriptan, 5-HT(1D) receptors and obsessive-compulsive disorder. Eur Neuropsychopharmacol. 2001;11:169–172. doi: 10.1016/s0924-977x(01)00082-7. [DOI] [PubMed] [Google Scholar]
- 6.Zohar J, Kennedy JL, Hollander E, Koran LM. Serotonin-1D hypothesis of obsessive-compulsive disorder: an update. J Clin Psychiatry. 2004;65 Suppl 14:18–21. [PubMed] [Google Scholar]
- 7.Pian KL, Westenberg HG, van Megen HJ, den Boer JA. Sumatriptan (5-HT1D receptor agonist) does not exacerbate symptoms in obsessive compulsive disorder. Psychopharmacology (Berl) 1998;140:365–370. doi: 10.1007/s002130050777. [DOI] [PubMed] [Google Scholar]
- 8.Boshuisen ML, den Boer JA. Zolmitriptan (a 5-HT1B/1D receptor agonist with central action) does not increase symptoms in obsessive compulsive disorder. Psychopharmacology (Berl) 2000;152:74–79. doi: 10.1007/s002130000529. [DOI] [PubMed] [Google Scholar]
- 9.Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, et al. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet. 2006;78:815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wendland JR, Moya PR, Kruse MR, Ren-Patterson RF, Jensen CL, Timpano KR, Murphy DL. A novel, putative gain-of-function haplotype at SLC6A4 associates with obsessive-compulsive disorder. Hum Mol Genet. 2008;17:717–723. doi: 10.1093/hmg/ddm343. [DOI] [PubMed] [Google Scholar]
- 11.Ozaki N, Goldman D, Kaye WH, Plotnicov K, Greenberg BD, Lappalainen J, et al. Serotonin transporter missense mutation associated with a complex neuropsychiatric phenotype. Mol Psychiatry. 2003;8:895, 933–896. doi: 10.1038/sj.mp.4001365. [DOI] [PubMed] [Google Scholar]
- 12.Delorme R, Betancur C, Wagner M, Krebs MO, Gorwood P, Pearl P, et al. Support for the association between the rare functional variant I425V of the serotonin transporter gene and susceptibility to obsessive compulsive disorder. Mol Psychiatry. 2005;10:1059–1061. doi: 10.1038/sj.mp.4001728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hollander E, Pallanti S. Current and experimental therapeutics of OCD. In: Davis K, Charney D, Coyle J, Nemeroff C, editors. Neuropsychopharmacology: The fifth generation of progress. Philadelphia: Lippincott Williams & Wilkins; 2002. pp. 1648–1665. [Google Scholar]
- 14.Swerdlow NR, Benbow CH, Zisook S, Geyer MA, Braff DL. A preliminary assessment of sensorimotor gating in patients with obsessive compulsive disorder. Biol Psychiatry. 1993;33:298–301. doi: 10.1016/0006-3223(93)90300-3. [DOI] [PubMed] [Google Scholar]
- 15.Hoenig K, Hochrein A, Quednow BB, Maier W, Wagner M. Impaired prepulse inhibition of acoustic startle in obsessive-compulsive disorder. Biol Psychiatry. 2005;57:1153–1158. doi: 10.1016/j.biopsych.2005.01.040. [DOI] [PubMed] [Google Scholar]
- 16.Graham FK. Presidential Address, 1974. The more or less startling effects of weak prestimulation. Psychophysiology. 1975;12:238–248. doi: 10.1111/j.1469-8986.1975.tb01284.x. [DOI] [PubMed] [Google Scholar]
- 17.Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl) 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- 18.Swerdlow NR, Caine SB, Braff DL, Geyer MA. The neural substrates of sensorimotor gating of the startle reflex: a review of recent findings and their implications. Journal of Psychopharmacology. 1992;6:176–190. doi: 10.1177/026988119200600210. [DOI] [PubMed] [Google Scholar]
- 19.Bakshi VP, Geyer MA. Multiple limbic regions mediate the disruption of prepulse inhibition produced in rats by the noncompetitive NMDA antagonist dizocilpine. J Neurosci. 1998;18:8394–8401. doi: 10.1523/JNEUROSCI.18-20-08394.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bakshi VP, Geyer MA. Phencyclidine-induced deficits in prepulse inhibition of startle are blocked by prazosin, an alpha-1 noradrenergic antagonist. J Pharmacol Exp Ther. 1997;283:666–674. [PubMed] [Google Scholar]
- 21.Dulawa SC, Geyer MA. Effects of strain and serotonergic agents on prepulse inhibition and habituation in mice. Neuropharmacology. 2000;39:2170–2179. doi: 10.1016/s0028-3908(00)00030-7. [DOI] [PubMed] [Google Scholar]
- 22.Dulawa SC, Hen R, Scearce-Levie K, Geyer MA. 5-HT1B receptor modulation of prepulse inhibition: recent findings in wild-type and 5-HT1B knockout mice. Ann N Y Acad Sci. 1998;861:79–84. doi: 10.1111/j.1749-6632.1998.tb10176.x. [DOI] [PubMed] [Google Scholar]
- 23.Sipes TA, Geyer MA. Multiple serotonin receptor subtypes modulate prepulse inhibition of the startle response in rats. Neuropharmacology. 1994;33:441–448. doi: 10.1016/0028-3908(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 24.Cheetham SC, Heal DJ. Evidence that RU 24969-induced locomotor activity in C57/B1/6 mice is specifically mediated by the 5-HT1B receptor. Br J Pharmacol. 1993;110:1621–1629. doi: 10.1111/j.1476-5381.1993.tb14010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaouloff F, Courvoisier H, Moisan MP, Mormede P. GR 127935 reduces basal locomotor activity and prevents RU 24969-, but not D-amphetamine-induced hyperlocomotion, in the Wistar-Kyoto hyperactive (WKHA) rat. Psychopharmacology (Berl) 1999;141:326–331. doi: 10.1007/s002130050841. [DOI] [PubMed] [Google Scholar]
- 26.Rempel NL, Callaway CW, Geyer MA. Serotonin1B receptor activation mimics behavioral effects of presynaptic serotonin release. Neuropsychopharmacology. 1993;8:201–211. doi: 10.1038/npp.1993.22. [DOI] [PubMed] [Google Scholar]
- 27.Oberlander C, Blaquiere B, Pujol JF. Distinct functions for dopamine and serotonin in locomotor behaviour: evidence using the 5-HT1 agonist RU 24969 in globus pallidus-lesioned rats. Neurosci Lett. 1986;67:113–118. doi: 10.1016/0304-3940(86)90382-4. [DOI] [PubMed] [Google Scholar]
- 28.Peroutka SJ. Pharmacological differentiation and characterization of 5-HT1A, 5-HT1B, and 5-HT1C binding sites in rat frontal cortex. J Neurochem. 1986;47:529–540. doi: 10.1111/j.1471-4159.1986.tb04532.x. [DOI] [PubMed] [Google Scholar]
- 29.Lira A, Zhou M, Castanon N, Ansorge MS, Gordon JA, Francis JH, et al. Altered depression-related behaviors and functional changes in the dorsal raphe nucleus of serotonin transporter-deficient mice. Biol Psychiatry. 2003;54:960–971. doi: 10.1016/s0006-3223(03)00696-6. [DOI] [PubMed] [Google Scholar]
- 30.Dulawa SC, Holick KA, Gundersen B, Hen R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology. 2004;29:1321–1330. doi: 10.1038/sj.npp.1300433. [DOI] [PubMed] [Google Scholar]
- 31.Oksenberg D, Marsters SA, O’Dowd BF, Jin H, Havlik S, Peroutka SJ, Ashkenazi A. A single amino-acid difference confers major pharmacological variation between human and rodent 5-HT1B receptors. Nature. 1992;360:161–163. doi: 10.1038/360161a0. [DOI] [PubMed] [Google Scholar]
- 32.Parker EM, Grisel DA, Iben LG, Shapiro RA. A single amino acid difference accounts for the pharmacological distinctions between the rat and human 5-hydroxytryptamine1B receptors. J Neurochem. 1993;60:380–383. doi: 10.1111/j.1471-4159.1993.tb05865.x. [DOI] [PubMed] [Google Scholar]
- 33.Paulus MP, Geyer MA. Quantitative assessment of the microstructure of rat behavior: I, f(d), the extension of the scaling hypothesis. Psychopharmacology (Berl) 1993;113:177–186. doi: 10.1007/BF02245695. [DOI] [PubMed] [Google Scholar]
- 34.Gordon CT, State RC, Nelson JE, Hamburger SD, Rapoport JL. A double-blind comparison of clomipramine, desipramine, and placebo in the treatment of autistic disorder. Arch Gen Psychiatry. 1993;50:441–447. doi: 10.1001/archpsyc.1993.01820180039004. [DOI] [PubMed] [Google Scholar]
- 35.McDougle CJ. Current and emerging therapeutics of autistic disorder and related pervasive developmental disorders. In: Davis K, Charney D, Coyle J, Nemeroff C, editors. Neuropsychopharmacology: The fifth generation of progress. Philadelphia: Lippincott Williams & Wilkins; 2002. pp. 566–577. [Google Scholar]
- 36.Hollander E, Novotny S, Allen A, Aronowitz B, Cartwright C, DeCaria C. The relationship between repetitive behaviors and growth hormone response to sumatriptan challenge in adult autistic disorder. Neuropsychopharmacology. 2000;22:163–167. doi: 10.1016/S0893-133X(99)00121-9. [DOI] [PubMed] [Google Scholar]
- 37.Novotny S, Hollander E, Allen A, Mosovich S, Aronowitz B, Cartwright C, et al. Increased growth hormone response to sumatriptan challenge in adult autistic disorders. Psychiatry Res. 2000;94:173–177. doi: 10.1016/s0165-1781(00)00134-7. [DOI] [PubMed] [Google Scholar]
- 38.McAlonan GM, Daly E, Kumari V, Critchley HD, van Amelsvoort T, Suckling J, et al. Brain anatomy and sensorimotor gating in Asperger’s syndrome. Brain. 2002;125:1594–1606. doi: 10.1093/brain/awf150. [DOI] [PubMed] [Google Scholar]
- 39.Dulawa SC, Hen R, Scearce-Levie K, Geyer MA. Serotonin1B receptor modulation of startle reactivity, habituation, and prepulse inhibition in wild-type and serotonin1B knockout mice. Psychopharmacology (Berl) 1997;132:125–134. doi: 10.1007/s002130050328. [DOI] [PubMed] [Google Scholar]
- 40.Callaway CW, Geyer MA. Tolerance and cross-tolerance to the activating effects of 3,4-methylenedioxymethamphetamine and a 5-hydroxytryptamine1B agonist. J Pharmacol Exp Ther. 1992;263:318–326. [PubMed] [Google Scholar]
- 41.Quednow BB, Kuhn KU, Stelzenmuelle R, Hoenig K, Maier W, Wagner M. Effects of serotonergic and noradrenergic antidepressants on auditory startle response in patients with major depression. Psychopharmacology (Berl) 2004;175:399–406. doi: 10.1007/s00213-004-1842-6. [DOI] [PubMed] [Google Scholar]
- 42.Pouzet B, Andersen MP, Hogg S. Effects of acute treatment with antidepressant drugs on sensorimotor gating deficits in rats. Psychopharmacology (Berl) 2005;178:9–16. doi: 10.1007/s00213-004-1976-6. [DOI] [PubMed] [Google Scholar]
- 43.Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- 44.Gothert M, Hamon M, Barann M, Bonisch H, Gozlan H, Laguzzi R, et al. 5-HT3 receptor antagonism by anpirtoline, a mixed 5-HT1 receptor agonist/5-HT3 receptor antagonist. Br J Pharmacol. 1995;114:269–274. doi: 10.1111/j.1476-5381.1995.tb13222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paulus MP, Dulawa SC, Ralph RJ, Mark AG. Behavioral organization is independent of locomotor activity in 129 and C57 mouse strains. Brain Res. 1999;835:27–36. doi: 10.1016/s0006-8993(99)01137-3. [DOI] [PubMed] [Google Scholar]
- 46.Saudou F, Amara DA, Dierich A, LeMeur M, Ramboz S, Segu L, et al. Enhanced aggressive behavior in mice lacking 5-HT1B receptor. Science. 1994;265:1875– 1878. doi: 10.1126/science.8091214. [DOI] [PubMed] [Google Scholar]
- 47.Fletcher PJ, Sinyard J, Higgins GA. The effects of the 5-HT(2C) receptor antagonist SB242084 on locomotor activity induced by selective, or mixed, indirect serotonergic and dopaminergic agonists. Psychopharmacology (Berl) 2006;187:515–525. doi: 10.1007/s00213-006-0453-9. [DOI] [PubMed] [Google Scholar]
- 48.Fabre V, Beaufour C, Evrard A, Rioux A, Hanoun N, Lesch KP, et al. Altered expression and functions of serotonin 5-HT1A and 5-HT1B receptors in knock-out mice lacking the 5-HT transporter. Eur J Neurosci. 2000;12:2299–2310. doi: 10.1046/j.1460-9568.2000.00126.x. [DOI] [PubMed] [Google Scholar]
- 49.Pejchal T, Foley MA, Kosofsky BE, Waeber C. Chronic fluoxetine treatment selectively uncouples raphe 5-HT(1A) receptors as measured by [(35)S]-GTP gamma S autoradiography. Br J Pharmacol. 2002;135:1115–1122. doi: 10.1038/sj.bjp.0704555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sprouse J, Reynolds L, Li X, Braselton J, Schmidt A. 8-OH-DPAT as a 5-HT7 agonist: phase shifts of the circadian biological clock through increases in cAMP production. Neuropharmacology. 2004;46:52–62. doi: 10.1016/j.neuropharm.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 51.Johnson SW, Mercuri NB, North RA. 5-hydroxytryptamine1B receptors block the GABAB synaptic potential in rat dopamine neurons. J Neurosci. 1992;12:2000–2006. doi: 10.1523/JNEUROSCI.12-05-02000.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guan XM, McBride WJ. Serotonin microinfusion into the ventral tegmental area increases accumbens dopamine release. Brain Res Bull. 1989;23:541–547. doi: 10.1016/0361-9230(89)90198-6. [DOI] [PubMed] [Google Scholar]
- 53.Higgins GA, Jordan CC, Skingle M. Evidence that the unilateral activation of 5-HT1D receptors in the substantia nigra of the guinea-pig elicits contralateral rotation. Br J Pharmacol. 1991;102:305–310. doi: 10.1111/j.1476-5381.1991.tb12170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Delfs JM, Kelley AE. The role of D1 and D2 dopamine receptors in oral stereotypy induced by dopaminergic stimulation of the ventrolateral striatum. Neuroscience. 1990;39:59–67. doi: 10.1016/0306-4522(90)90221-o. [DOI] [PubMed] [Google Scholar]
- 55.Koshikawa N, Aoki S, Tomiyama K, Maruyama Y, Kobayashi M. Sulpiride injection into the dorsal striatum increases methamphetamine-induced gnawing in rats. Eur J Pharmacol. 1987;133:119–125. doi: 10.1016/0014-2999(87)90213-5. [DOI] [PubMed] [Google Scholar]
- 56.Wisniecki A, Correa M, Arizzi MN, Ishiwari K, Salamone JD. Motor effects of GABA(A) antagonism in globus pallidus: studies of locomotion and tremulous jaw movements in rats. Psychopharmacology (Berl) 2003;170:140–149. doi: 10.1007/s00213-003-1521-z. [DOI] [PubMed] [Google Scholar]
- 57.Boulenguez P, Peters SL, Mitchell SN, Chauveau J, Gray JA, Joseph MH. Dopamine release in the nucleus accumbens and latent inhibition in the rat following microinjections of a 5-HT1B agonist into the dorsal subiculum: implications for schizophrenia. J Psychopharmacol. 1998;12:258–267. doi: 10.1177/026988119801200305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.