Abstract
Brain-derived neurotrophic factor (BDNF) has been implicated in the mechanism of age-related regional brain volumetric changes. Healthy volunteers with the valine to methionine polymorphism at codon 66 of the BDNF gene (val66met) exhibit decreased volume of a number of brain structures, including hippocampus, temporal and occipital lobar gray matter volumes, and a negative correlation between age and the volume of bilateral dorsolateral prefrontal cortices. We sought to characterize the relationship between age, BDNF and amygdala volumes among healthy volunteers. We measured amygdala volumes in 55 healthy, right-handed volunteers who underwent structural magnetic resonance imaging (MRI) and were also characterized demographically and genotyped with respect to BDNF. Using an ANCOVA model, we found that amygdala volumes were inversely correlated with age in BDNF val66met carriers but not in non-carriers. This is the first report of age-related BDNF val66met polymorphism effects on amygdala volume.
Keywords: Aging, Amygdala, BDNF, MRI
Introduction
Brain-derived neurotrophic factor (BDNF) is the most abundant member of the neurotrophin superfamily and plays a role in differentiation during development (Alcantara et al., 2006; Engelhardt et al., 2007; Sato et al., 2006), neuronal survival in the adult brain (Morse et al., 1993), and regulation of synaptic function (Lessmann et al., 2003). BDNF signaling also has been implicated in learning/memory processes, including episodic memory in humans (Egan et al., 2003). Similarly, in rats, BDNF signaling has a role in the acquisition of conditioned fear (Rattiner et al., 2004) and consolidation of fear extinction (Chhatwal et al., 2006) through binding to TrkB receptors in the amygdala. Murine behavioral expression of conditioned fear has been found to be positively related to amygdala BDNF levels (Yee et al., 2007).
One frequent, nonconservative single nucleotide polymorphism (SNP) has been identified in the human BDNF gene. The SNP (rs6265) at nucleotide 196 (G/A), produces an amino acid substitution of valine to methionine at codon 66 (val66met) in the 5’ prodomain. This polymorphism results in decreased distribution of BDNF val66met to neuronal dendrites. It also is associated with lower activity-dependent secretion in neuronal culture (Chen et al., 2005; Chen et al., 2004; Egan et al., 2003) and in ex-vivo Met-Met homozygous knock-in mice (Chen et al., 2006). BDNF val66met has been reported to have an allele frequency of approximately 19% to 25% in Caucasian samples (Egan et al., 2003; Sen et al., 2003) and 41% in Asian samples (Shimizu et al., 2004). Val66Met allele carriers have previously been found to have smaller volumes of temporal and occipital lobar gray matter (Ho et al., 2006), prefrontal cortex (Pezawas et al., 2004), and hippocampus (Bueller et al., 2006; Frodl et al., 2007; Pezawas et al., 2004), and greater white matter hyperintensity volumes (Taylor et al., 2008), presumably due to decreased BDNF neuroprotection. Among healthy volunteers carrying the val66met polymorphism, there are reports of a negative correlation between age and dorsolateral prefrontal cortical (DLPFC) volume (Nemoto et al., 2006). The val66met polymorphism has been associated with a phenotype of increased anxiety-related behaviors in stressful settings in animal studies (Hashimoto, 2007) and higher levels of trait anxiety/anxious temperament (Jiang et al., 2005; Lang et al., 2005) in humans. Additionally, the val66met polymorphism has been associated with age-related decline of non-verbal reasoning ability. (Harris et al., 2006)
Aging strongly affects brain morphology in humans (Nemoto et al., 2006). It has been reported that BDNF levels in plasma –but not platelet levels- decrease significantly with increasing age (Lommatzsch et al., 2005), and normal aging seems to be associated with a decreased BDNF signaling capacity in the brain (Mattson et al., 2004).Considering the previous findings that BDNF is expressed abundantly in several brain regions and that BDNF has a neuroprotective effect, the val66met polymorphism might have an effect on age-related morphological changes in the brain.
We sought to characterize the relationship between age, BDNF and amygdala volumes among healthy volunteers. We hypothesized a negative correlation between age and amygdala volume only in val66met allele carriers.
Methods
Sample
Healthy volunteers (N=55) were recruited from community referrals and advertisements. They were enrolled in the study after giving written informed consent as approved by the Institutional Review Board. All subjects underwent physical examination, screening for Axis I and II psychiatric illness, and routine laboratory screening. Exclusions were pregnancy, neurologic illness, active medical disease, any Axis I psychiatric diagnosis, current substance use, or first-degree relatives with a mood disorder or schizophrenia.
Polymerase chain reaction (PCR)
The BDNF Val66Met polymorphism (GenBank dbSNP: rs6265) was typed by PCR and using the BsaA I restriction enzyme. Briefly, the oligonucleotide primers, sense MannBF-1F (5’-ATCCCGGTGAAAGAAAGCCCTAAC-3′) and antisense MannBF-1R (5′-CCCCTGCAGCCTTCTTTTGTGTAA-3′), were used to amplify a PCR fragment of 673 bp length. PCR was carried out in a 20 µl volume, containing 100 ng DNA, 40 ng of each primer, 50 mM KCl, 10 mM Tris-HCl (pH 8.3), 2 mM MgCl2, 0.01% gelatin, 200 uM of each dNTP, and 0.8 U of REDTAQ Genomic DNA Polymerase (Sigma, MO, USA). Samples were processed in a Robocycler (Stratagene, CA, USA). Thirty temperature cycles, consisting of 30 sec at 95°C, 30 sec at 60°C, and 40 sec at 72°C, were followed by a final extension step of 72° C for 4 min. The PCR fragments were digested with BsaA I restriction enzyme (NE Biolab, MA, USA), which produces 3 fragments of 275, 321 and 77 bp when guanine is present at nucleotide 1249, and 2 fragments of 321 and 352 bp if cytosine is present at this position. The digested PCR products were separated on a 1.2% agarose gel.
MRI Acquisition
MRI acquisition and image analysis methods have been described previously (Parsey et al., 2006). MRI scans were acquired on a GE 1.5 T Signa Advantage system. A sagittal scout (localizer) was performed to identify the AC-PC plane (1 min). A transaxial T1 weighted sequence with 1.5 mm slice thickness was acquired in a coronal plane orthogonal to the AC-PC plane over the whole brain with the following parameters: 3-dimensional Spoiled Gradient Recalled Acquisition in the Steady State (SPGR); TR 34 msec; TE 5 msec; flip angle of 45 degrees; zero gap; 124 slices; field of view of 22 × 16 cm; with 256 × 192 matrix, reformatted to 256 × 256, yielding a voxel size of 1.5 mm × 0.9 mm × 0.9 mm; time of acquisition 11 min.
Image Analysis
Raw coronal MRI images were cropped to remove non-brain material, utilizing the Exbrain v.2 utility (Lemieux et al., 2003). Where Exbrain was unable to process an MRI, the Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB) Brain Extraction Tool (BET) v1.2 (Smith, 2002) was used along with manual removal of non-brain matter left after utilizing BET. Delineation of the amygdala region of interest (ROI) was performed based on criteria and anatomical landmarks developed by two of the authors (S.R. and V.A.), using an electronic mouse with 3D image analysis software (MEDX, Sensor), on consecutive 1.5-mm-thick coronal slices. Corresponding sagittal and axial views were available to aid in the accuracy of border definition. In anterior slices, the amygdala emerged as a rounded mass of gray matter that increased in size in subsequent slices (Bogerts et al., 1990; Niu et al., 2004). The lateral border consisted of temporal lobe white matter, and the medial border was separated from the entorhinal cortex by the angular bundle (Hammers et al., 2003; Niu et al., 2004). The inferior border was defined by white matter in anterior slices and by the hippocampus and ventricle in more posterior slices. The entorhinal sulcus defined the superior border (Hammers et al., 2003; Pruessner et al., 2000). In the most caudal slices, the amygdala became a thin strip of gray matter located dorsal to the alveus or inferior horn of the lateral ventricle, which serve as boundaries (Convit et al., 1999; Pruessner et al., 2000). The dorsal border was defined with the optic tract and fundus of the inferior portion of the circular sulcus of the insula (Pruessner et al., 2000). The entire rostrocaudal extent of the amygdala was measured for each subject. The areas of all slices were summed, and the sum was multiplied by the slice thickness to obtain volume. Reliability was computed by repeated measurements (two raters traced left and right amygdalae in 9 subjects); the intraclass coefficient (ICC) was 0.87 for left amygdala and 0.92 for right amygdala.
Statistical analyses
Statistical analyses were done with SPSS Version 14. An ANCOVA model with amygdala volumes of both hemispheres integrated as “within-subject variable” was used to examine the effects of the presence of the BDNF val66met allele. We added age as a covariate and sex as a cofactor to the model. A separate analysis included age as covariate and race as a cofactor.
Results
Genotype distributions in healthy volunteers (Met-Met N=4 [7%], Met-Val N=18 [33%], Val-Val N=33 [60%]) did not deviate from Hardy-Weinberg expectations (χ2 = 0.48, df=2, p=0.923). There were no differences among subjects between BDNF val66met allele carriers and non-carriers with regard to total cerebral volume (TCV), mean raw amygdala ROI volumes, amygdala volumes after normalization for TCV, sex or age (see Table 1).
Table 1.
Volumetric and demographic characteristics by val66met status.
| Val66met | ||||||
|---|---|---|---|---|---|---|
| Characteristic | Side | + | − | t | df | p |
| Mean total cerebral volume, TCV (L ± SD) | 1.43 ± 0.12 | 1.42 ± 0.20 | 0.07 | 53 | 0.946 | |
| Mean raw amygdala volumes (cm3 ± SD) | left | 1.44 ± 0.21 | 1.37 ± 0.31 | 1.02 | 53 | 0.313 |
| right | 1.50 ± 0.34 | 1.50 ± 0.31 | −0.02 | 53 | 0.987 | |
| Mean amygdala volumes after normalization (% of TCV ± SD) | left | 1.02 ± 0.16 | 1.00 ± 0.19 | 1.09 | 53 | 0.279 |
| right | 1.06 ± 0.24 | 1.06 ± 0.22 | −0.10 | 53 | 0.920 | |
| Mean age (years ± SD [range]) | 32.2 ± 12 [40] | 37.0 ± 16 [49] | −1.22 | 53 | 0.229 | |
| χ2 | df | p | ||||
| Gender (No. [%] males) | 27 [45.5] | 28 [51.5] | 0.194 | 1 | 0.660 | |
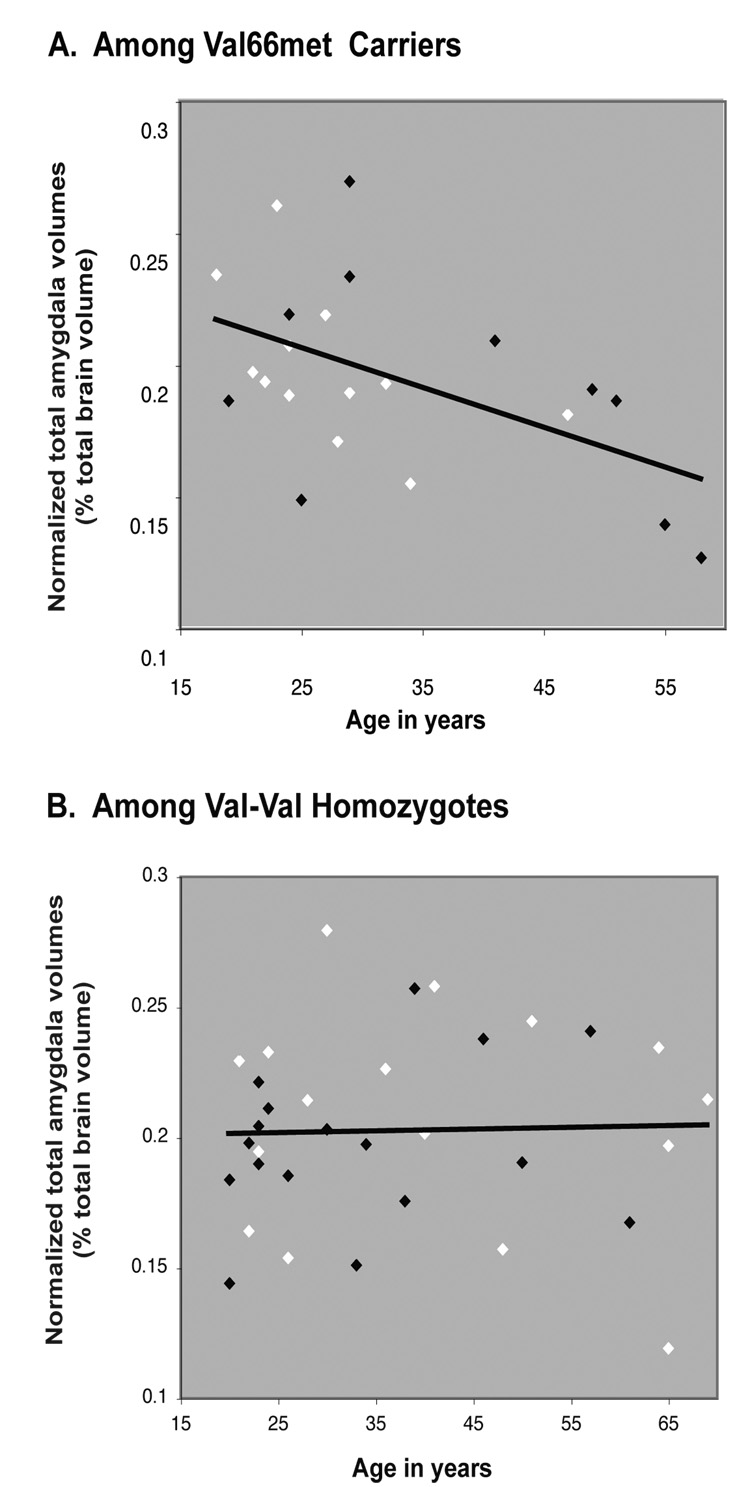
Total amygdala volumes were inversely correlated with age in BDNF val66met carriers, but not in non-carriers [BDNF and age interaction (F=4.58; df=1; p=0.037)], (see Figure 1). Mean volumes of left and right amygdala differed among all subjects (left amygdala, 0.98% ± 0.18 vs. right amygdala, 1.06% ± 0.22; t = −2.90, df =54, p = 0.005). However, there was no lateralization of the effects of aging or of the BDNF val66met allele on amygdala volume (data not shown). There was no difference between males and females in mean corrected amygdala volume. Nor was there a difference between the sexes in terms of the age effects on amygdala volume, although statistical power to detect such a difference was low. The sex distribution in the sample is illustrated in Figure 1. Upon racial stratification, in our sample lower rates of Val/Met genotype were seen in African (10%) and Hispanic Americans (20%) than in Caucasian (54%) and Asian Americans (54%). We dichotomized the race variable into two categories of similar Val/Met distribution: African and Hispanic Americans vs. Caucasians and Asian Americans. We tested the contribution of race in terms of its effect on the model (entering race as an additive covariate) and in terms of effects that might differ according to age (race and age interaction). In this adjusted model, there was no main effect of race on amygdala volume (data not shown). Although there was a trend for an interaction of race and age (p=0.09), including it in the model did not modify the significant interaction between BDNF and age in effects on amygdala volume.
Figure 1.
Scatter plot of the correlation between total amygdala volume (expressed as a percentage of total brain volume) and age. White symbols represent females; black symbols, males. Total amygdala volume declined with increasing age in (A) BDNF val66met carriers (r=−0.52, p=0.014) but not in (B) val-val homozygous subjects (r=0.03, p=0.873).
Discussion
We observed an interaction between BDNF and age, such that total amygdala volumes were inversely correlated with age in BDNF val66met carriers but not in non-carriers. Our results in amygdala are consistent with a report of an increased age-related volume reduction of the DLPFC in healthy BDNF val66met carriers (Nemoto et al., 2006), although unlike the latter study, we did not find sex effects associated with amygdala volume or BDNF val66met allele carrier status. On the other hand, smaller hippocampal volumes in BDNF val66met carriers were found to be age and sex-independent. (Pezawas et al., 2004) Perhaps these inconsistencies reflect different mechanisms by which the BDNF val66met variant can affect brain volumes. It has been suggested that the BDNF val66met allele may result in reduced BDNF synthesis during neurodevelopment, causing decreased neuronal proliferation and survival, small soma size and diminished dendritic growth; alternatively, BDNF val66met may exert its influence through modulating synaptic activity in mature neurons. (Ho et al., 2006)
Reduced amygdala volumes could be a marker for abnormal fear-driven responses and/or anxiety levels, affecting the ability of an organism to assess the dangerousness of stimuli and respond appropriately. Future studies might examine relationships between amygdala volumes, BDNF val66met status, and anxiety in the elderly.
This study has limitations due to sample size, and therefore these results should be considered as preliminary, e.g. 1) the sample included relatively few subjects of older age in the BDNF-Met carrier group, with no subjects older than 60 yrs; and 2) there was not adequate statistical power to detect sex or racial differences in three-way interactions with effect of age and BDNF genotype on amygdala volume.
Conclusions
We observed an inverse correlation between amygdala volumes and age in BDNF val66met carriers but not in non-carriers. Our results support the role of the val66met polymorphism in age-related morphological changes in the brain.
Acknowledgments
This study was supported by NIMH grant MH62185. Dr. Baca-Garcia is a Lilly Suicide Scholar at Columbia University. Dr. Sublette’s work was supported in part through a T32 National Research Service Award Training Grant MH016434.
Abbreviations
- AC-PC
Anterior commisure – Posterior commisure
- ANCOVA
Analysis of Covariance
- BDNF
Brain-Derived Neurotrophic Factor
- BET
Brain Extraction Tool
- DLPFC
Dorsolateral Prefrontal Cortex
- FMRIB
Functional Magnetic Resonance Imaging of the Brain
- ICC
Intraclass Coefficient
- MRI
Magnetic Resonance Imaging
- ROI
Region of Interest
- SNP
Single Nucleotide Polymorphism
- SPGR
Spoiled Gradient Recalled Acquisition in the Steady State
- TCV
Total Cerebral Volume
- TE
Echo time
- TR
Repetition time
- Val66met
Valine to methionine polymorphism at codon 66 of the BDNF gene
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was performed at the New York State Psychiatric Institute and Columbia University.
References
- Alcantara S, Pozas E, Ibanez CF, Soriano E. BDNF-modulated spatial organization of Cajal-Retzius and GABAergic neurons in the marginal zone plays a role in the development of cortical organization. Cereb Cortex. 2006;16:487–499. doi: 10.1093/cercor/bhi128. [DOI] [PubMed] [Google Scholar]
- Bogerts B, Ashtari M, Degreef G, Alvir JM, Bilder RM, Lieberman JA. Reduced temporal limbic structure volumes on magnetic resonance images in first episode schizophrenia. Psychiatry Res. 1990;35:1–13. doi: 10.1016/0925-4927(90)90004-p. [DOI] [PubMed] [Google Scholar]
- Bueller JA, Aftab M, Sen S, Gomez-Hassan D, Burmeister M, Zubieta JK. BDNF Val66Met allele is associated with reduced hippocampal volume in healthy subjects. Biol Psychiatry. 2006;59:812–815. doi: 10.1016/j.biopsych.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Ieraci A, Teng H, Dall H, Meng CX, Herrera DG, Nykjaer A, Hempstead BL, Lee FS. Sortilin controls intracellular sorting of brain-derived neurotrophic factor to the regulated secretory pathway. J Neurosci. 2005;25:6156–6166. doi: 10.1523/JNEUROSCI.1017-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, Herrera DG, Toth M, Yang C, McEwen BS, Hempstead BL, Lee FS. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Patel PD, Sant G, Meng CX, Teng KK, Hempstead BL, Lee FS. Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. J Neurosci. 2004;24:4401–4411. doi: 10.1523/JNEUROSCI.0348-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhatwal JP, Stanek-Rattiner L, Davis M, Ressler KJ. Amygdala BDNF signaling is required for consolidation but not encoding of extinction. Nat Neurosci. 2006;9:870–872. doi: 10.1038/nn1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Convit A, McHugh P, Wolf OT, de Leon MJ, Bobinski M, De Santi S, Roche A, Tsui W. MRI volume of the amygdala: a reliable method allowing separation from the hippocampal formation. Psychiatry Res. 1999;90:113–123. doi: 10.1016/s0925-4927(99)00007-4. [DOI] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Engelhardt M, Di Cristo G, Berardi N, Maffei L, Wahle P. Differential effects of NT-4, NGF and BDNF on development of neurochemical architecture and cell size regulation in rat visual cortex during the critical period. Eur J Neurosci. 2007;25:529–540. doi: 10.1111/j.1460-9568.2006.05301.x. [DOI] [PubMed] [Google Scholar]
- Frodl T, Schule C, Schmitt G, Born C, Baghai T, Zill P, Bottlender R, Rupprecht R, Bondy B, Reiser M, Moller HJ, Meisenzahl EM. Association of the brain-derived neurotrophic factor Val66Met polymorphism with reduced hippocampal volumes in major depression. Arch Gen Psychiatry. 2007;64:410–416. doi: 10.1001/archpsyc.64.4.410. [DOI] [PubMed] [Google Scholar]
- Hammers A, Allom R, Koepp MJ, Free SL, Myers R, Lemieux L, Mitchell TN, Brooks DJ, Duncan JS. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Human Brain Mapping. 2003;19:224–247. doi: 10.1002/hbm.10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SE, Fox H, Wright AF, Hayward C, Starr JM, Whalley LJ, Deary IJ. The brain-derived neurotrophic factor Val66Met polymorphism is associated with age-related change in reasoning skills. Mol. Psychiatry. 2006;11:505–513. doi: 10.1038/sj.mp.4001799. [DOI] [PubMed] [Google Scholar]
- Hashimoto K. BDNF variant linked to anxiety-related behaviors. Bioessays. 2007;29:116–119. doi: 10.1002/bies.20534. [DOI] [PubMed] [Google Scholar]
- Ho BC, Milev P, O'Leary DS, Librant A, Andreasen NC, Wassink TH. Cognitive and magnetic resonance imaging brain morphometric correlates of brain-derived neurotrophic factor Val66Met gene polymorphism in patients with schizophrenia and healthy volunteers. Arch Gen Psychiatry. 2006;63:731–740. doi: 10.1001/archpsyc.63.7.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Xu K, Hoberman J, Tian F, Marko AJ, Waheed JF, Harris CR, Marini AM, Enoch MA, Lipsky RH. BDNF variation and mood disorders: a novel functional promoter polymorphism and Val66Met are associated with anxiety but have opposing effects. Neuropsychopharmacology. 2005;30:1353–1361. doi: 10.1038/sj.npp.1300703. [DOI] [PubMed] [Google Scholar]
- Lang UE, Hellweg R, Kalus P, Bajbouj M, Lenzen KP, Sander T, Kunz D, Gallinat J. Association of a functional BDNF polymorphism and anxiety-related personality traits. Psychopharmacology (Berl) 2005;180:95–99. doi: 10.1007/s00213-004-2137-7. [DOI] [PubMed] [Google Scholar]
- Lemieux L, Hammers A, Mackinnon T, Liu RS. Automatic segmentation of the brain and intracranial cerebrospinal fluid in T1-weighted volume MRI scans of the head, and its application to serial cerebral and intracranial volumetry. Magn Reson Med. 2003;49:872–884. doi: 10.1002/mrm.10436. [DOI] [PubMed] [Google Scholar]
- Lessmann V, Gottmann K, Malcangio M. Neurotrophin secretion: current facts and future prospects. Prog Neurobiol. 2003;69:341–374. doi: 10.1016/s0301-0082(03)00019-4. [DOI] [PubMed] [Google Scholar]
- Lommatzsch M, Zingler D, Schuhbaeck K, Schloetcke K, Zingler C, Schuff-Werner P, Virchow JC. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol Aging. 2005;26:115–123. doi: 10.1016/j.neurobiolaging.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Maudsley S, Martin B. BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2004;27:589–594. doi: 10.1016/j.tins.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Morse JK, Wiegand SJ, Anderson K, You Y, Cai N, Carnahan J, Miller J, DiStefano PS, Altar CA, Lindsay RM, et al. Brain-derived neurotrophic factor (BDNF) prevents the degeneration of medial septal cholinergic neurons following fimbria transection. J Neurosci. 1993;13:4146–4156. doi: 10.1523/JNEUROSCI.13-10-04146.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto K, Ohnishi T, Mori T, Moriguchi Y, Hashimoto R, Asada T, Kunugi H. The Val66Met polymorphism of the brain-derived neurotrophic factor gene affects age-related brain morphology. Neurosci Lett. 2006;397:25–29. doi: 10.1016/j.neulet.2005.11.067. [DOI] [PubMed] [Google Scholar]
- Niu L, Matsui M, Zhou SY, Hagino H, Takahashi T, Yoneyama E, Kawasaki Y, Suzuki M, Seto H, Ono T, Kurachi M. Volume reduction of the amygdala in patients with schizophrenia: a magnetic resonance imaging study. Psychiatry Res. 2004;132:41–51. doi: 10.1016/j.pscychresns.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Kent JM, Oquendo MA, Richards MC, Pratap M, Cooper TB, Arango V, Mann JJ. Acute Occupancy of Brain Serotonin Transporter by Sertraline as Measured by [(11)C]DASB and Positron Emission Tomography. Biol Psychiatry. 2006;59:821–828. doi: 10.1016/j.biopsych.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Verchinski BA, Mattay VS, Callicott JH, Kolachana BS, Straub RE, Egan MF, Meyer-Lindenberg A, Weinberger DR. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. J Neurosci. 2004;24:10099–10102. doi: 10.1523/JNEUROSCI.2680-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Li LM, Serles W, Pruessner M, Collins DL, Kabani N, Lupien S, Evans AC. Volumetry of hippocampus and amygdala with high-resolution MRI and three-dimensional analysis software: minimizing the discrepancies between laboratories. Cereb Cortex. 2000;10:433–442. doi: 10.1093/cercor/10.4.433. [DOI] [PubMed] [Google Scholar]
- Rattiner LM, Davis M, French CT, Ressler KJ. Brain-derived neurotrophic factor and tyrosine kinase receptor B involvement in amygdala-dependent fear conditioning. J Neurosci. 2004;24:4796–4806. doi: 10.1523/JNEUROSCI.5654-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Suzuki K, Nakanishi S. Expression profile of BDNF-responsive genes during cerebellar granule cell development. Biochem Biophys Res Commun. 2006;341:304–309. doi: 10.1016/j.bbrc.2005.12.184. [DOI] [PubMed] [Google Scholar]
- Sen S, Nesse RM, Stoltenberg SF, Li S, Gleiberman L, Chakravarti A, Weder AB, Burmeister M. A BDNF coding variant is associated with the NEO personality inventory domain neuroticism, a risk factor for depression. Neuropsychopharmacology. 2003;28:397–401. doi: 10.1038/sj.npp.1300053. [DOI] [PubMed] [Google Scholar]
- Shimizu E, Hashimoto K, Iyo M. Ethnic difference of the BDNF 196G/A (val66met) polymorphism frequencies: the possibility to explain ethnic mental traits. Am J Med Genet B Neuropsychiatr Genet. 2004;126:122–123. doi: 10.1002/ajmg.b.20118. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WD, Zuchner S, McQuoid DR, Payne ME, Macfall JR, Steffens DC, Speer MC, Krishnan KR. The Brain-Derived Neurotrophic Factor VAL66MET Polymorphism and Cerebral White Matter Hyperintensities in Late-Life Depression. Am. J. Geriatr. Psychiatry. 2008;16:263–271. doi: 10.1097/JGP.0b013e3181591c30. [DOI] [PubMed] [Google Scholar]
- Yee BK, Zhu SW, Mohammed AH, Feldon J. Levels of neurotrophic factors in the hippocampus and amygdala correlate with anxiety- and fear-related behaviour in C57BL6 mice. J Neural Transm. 2007;114:431–444. doi: 10.1007/s00702-006-0548-9. [DOI] [PubMed] [Google Scholar]