Abstract
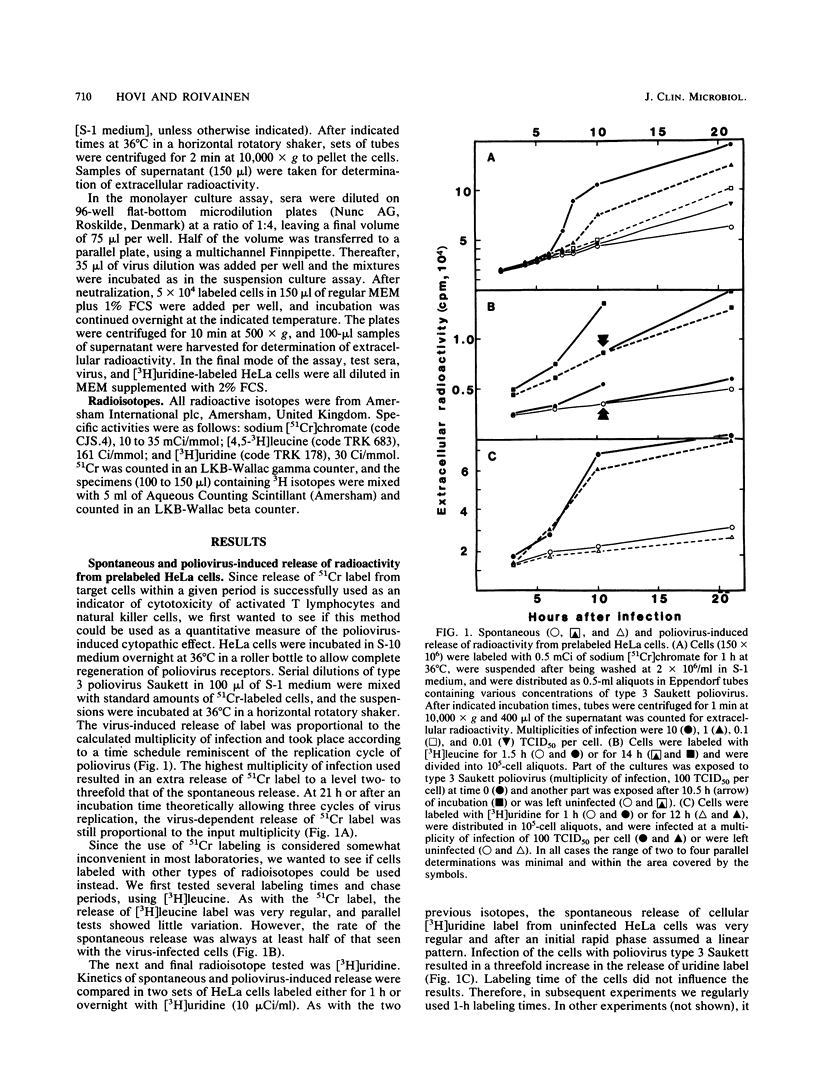
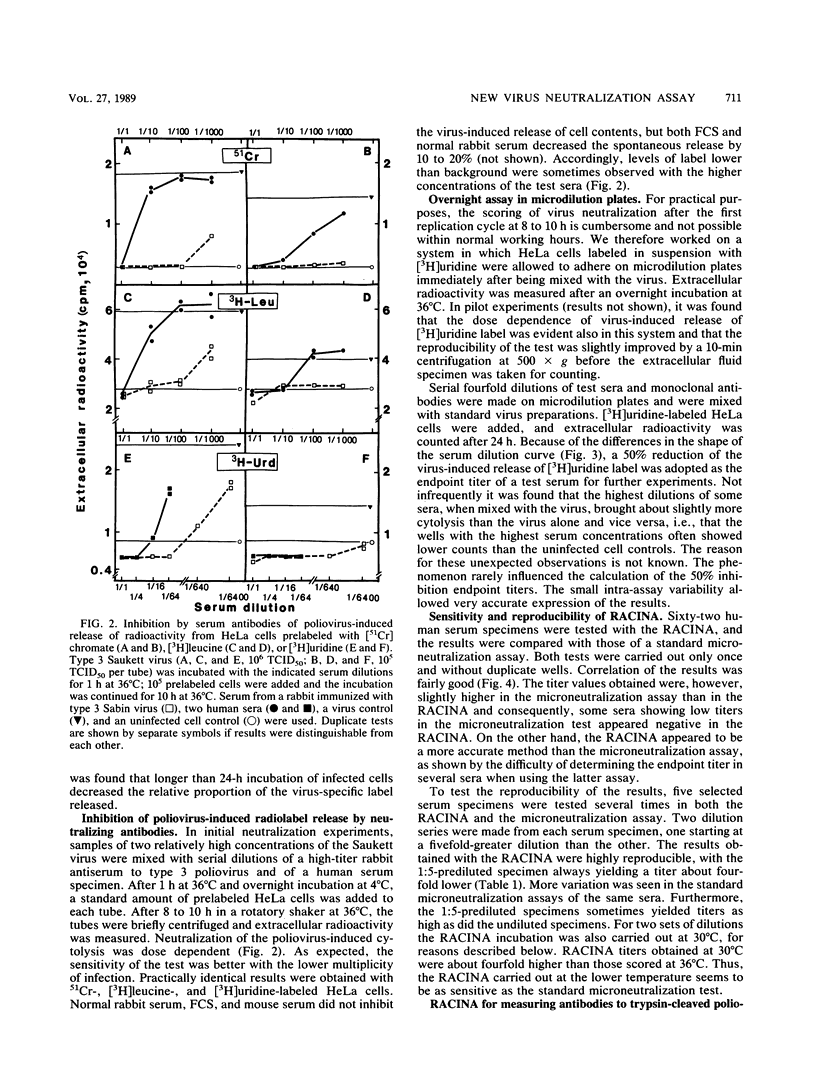
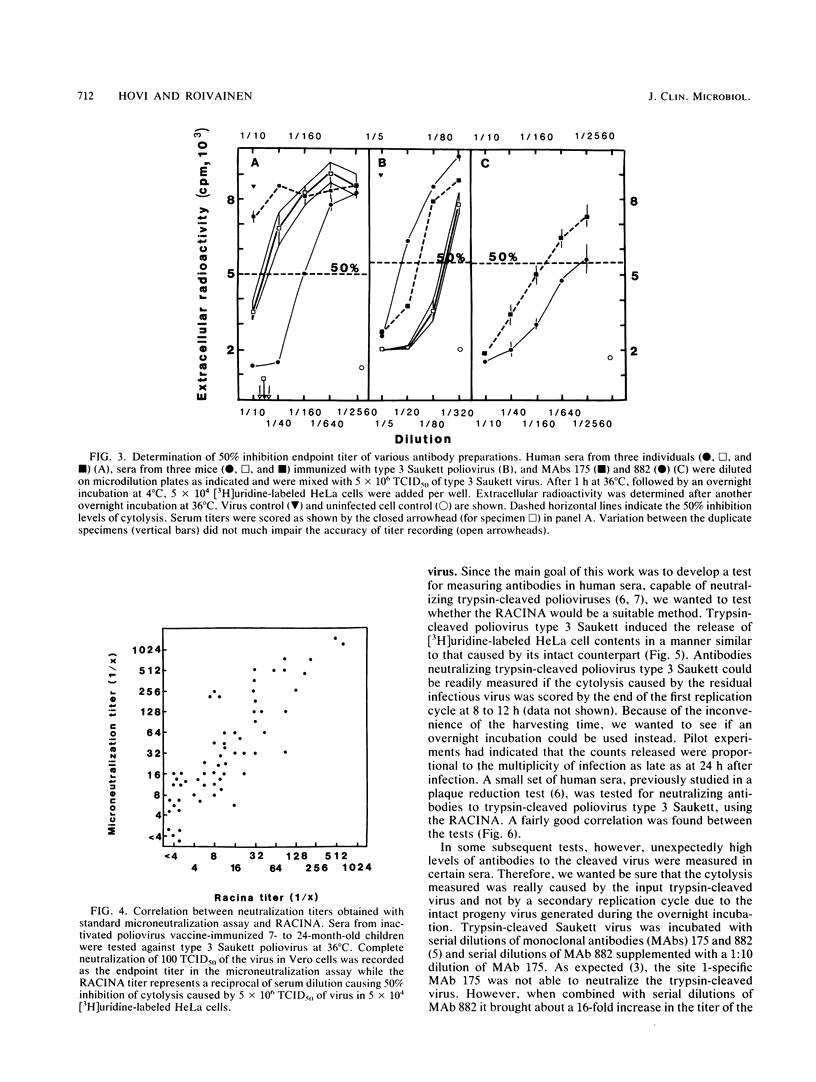
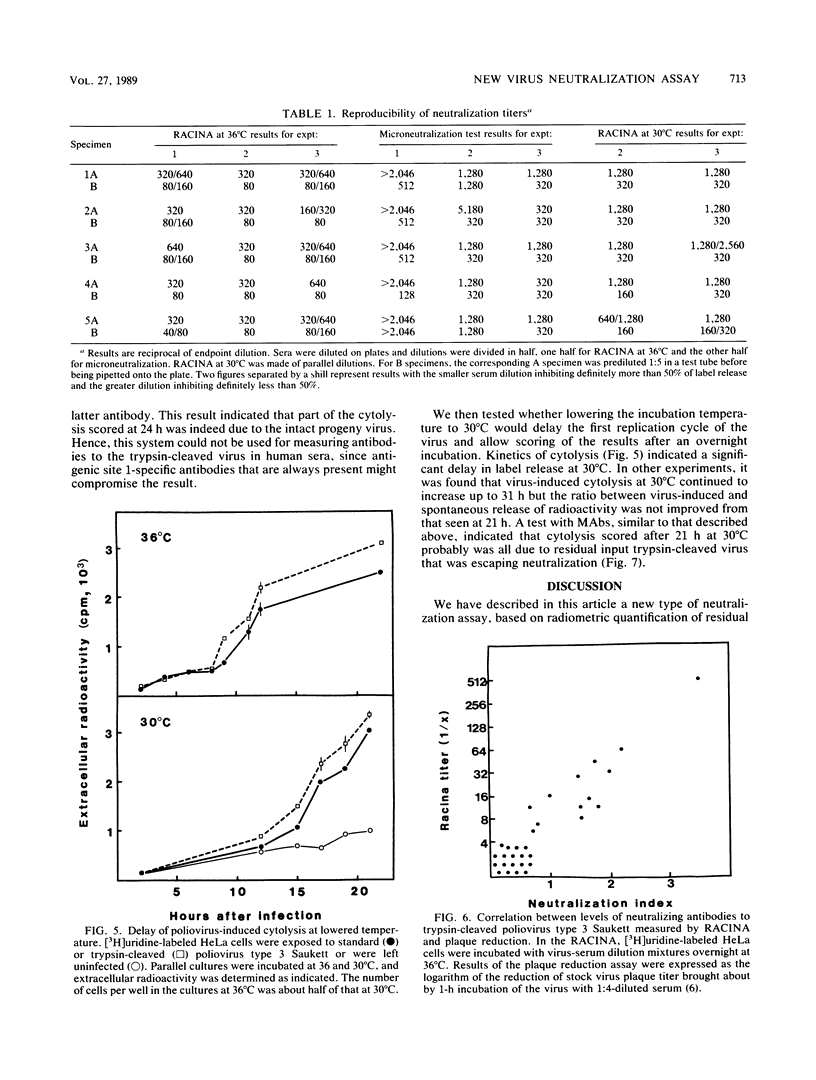
We have developed a new rapid test, the radiometric cytolysis inhibition assay (RACINA), for the determination of neutralizing poliovirus antibodies. HeLa cells prelabeled with 51Cr, [3H]leucine, or, preferentially, with [3H]uridine are used as sensitive quantitative indicators of residual infectious virus. Both suspensions and monolayer cultures of the indicator cells can be used. Neutralization of a fraction of a high-titer virus preparation can be scored after the first replication cycle at 8 to 10 h. By lowering the incubation temperature to 30 degrees C, the completion of the cytolysis due to the first replication cycle of poliovirus was delayed beyond 21 h. This makes it possible to use the RACINA, unlike the standard microneutralization assay, for measuring antibodies to trypsin-cleaved polioviruses. The RACINA was found to be as sensitive as and more reproducible than the standard microneutralization assay in the measurement of neutralizing poliovirus antibodies. The RACINA is a rapid and reliable test for neutralizing antibodies and in principle it may be applicable for quantitation of neutralizing antibodies to other cytolytic agents as well.
Full text
PDF
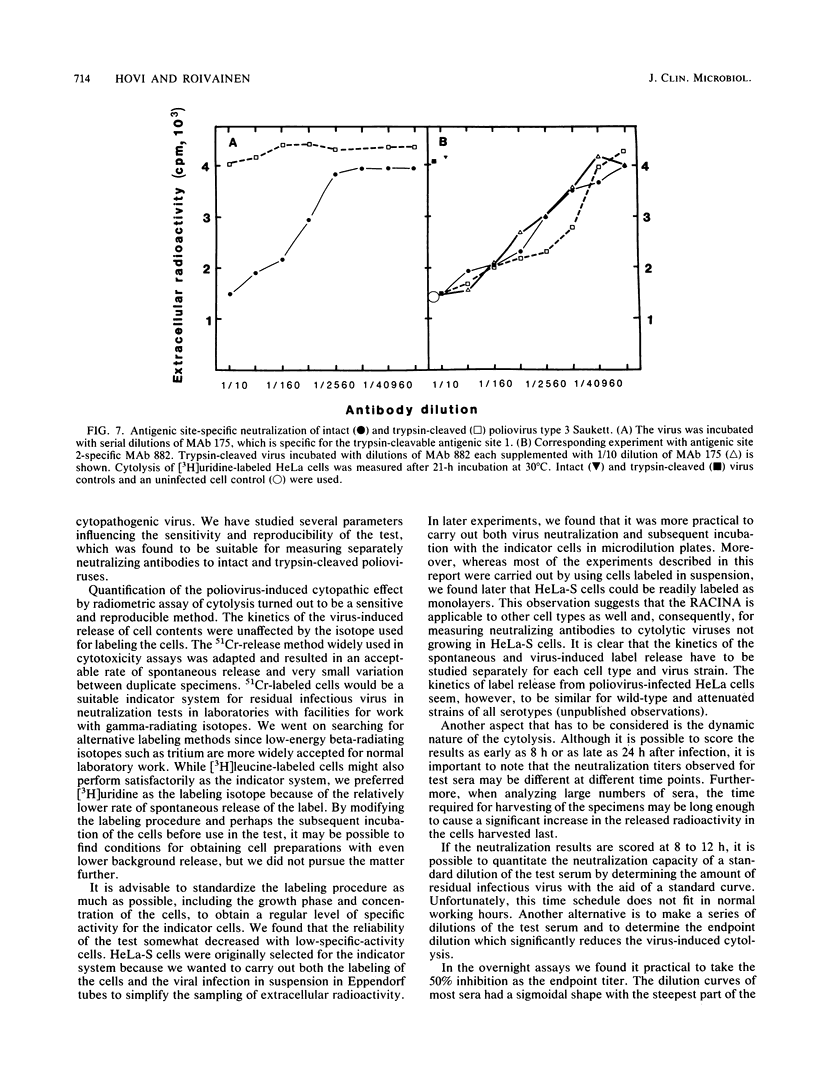

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht P., van Steenis G., van Wezel A. L., Salk J. Standardization of poliovirus neutralizing antibody tests. Rev Infect Dis. 1984 May-Jun;6 (Suppl 2):S540–S544. doi: 10.1093/clinids/6.supplement_2.s540. [DOI] [PubMed] [Google Scholar]
- Fricks C. E., Icenogle J. P., Hogle J. M. Trypsin sensitivity of the Sabin strain of type 1 poliovirus: cleavage sites in virions and related particles. J Virol. 1985 Jun;54(3):856–859. doi: 10.1128/jvi.54.3.856-859.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Icenogle J. P., Minor P. D., Ferguson M., Hogle J. M. Modulation of humoral response to a 12-amino-acid site on the poliovirus virion. J Virol. 1986 Oct;60(1):297–301. doi: 10.1128/jvi.60.1.297-301.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor P. D., Ferguson M., Phillips A., Magrath D. I., Huovilainen A., Hovi T. Conservation in vivo of protease cleavage sites in antigenic sites of poliovirus. J Gen Virol. 1987 Jul;68(Pt 7):1857–1865. doi: 10.1099/0022-1317-68-7-1857. [DOI] [PubMed] [Google Scholar]
- Roivainen M., Hovi T. Cleavage of VP1 and modification of antigenic site 1 of type 2 polioviruses by intestinal trypsin. J Virol. 1988 Sep;62(9):3536–3539. doi: 10.1128/jvi.62.9.3536-3539.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roivainen M., Hovi T. Intestinal trypsin can significantly modify antigenic properties of polioviruses: implications for the use of inactivated poliovirus vaccine. J Virol. 1987 Dec;61(12):3749–3753. doi: 10.1128/jvi.61.12.3749-3753.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roivainen M., Thodén C. J., Stenvik M., Pöyry T., Hovi T. Virus excretion and strain specific antibody responses after oral poliovaccine in previously immunised children. J Med Virol. 1987 Nov;23(3):249–256. doi: 10.1002/jmv.1890230307. [DOI] [PubMed] [Google Scholar]


