Abstract
Molecular umbrellas are “amphomorphic” compounds that can produce a hydrophobic or a hydrophilic exterior when exposed to a hydrophobic or hydrophilic microenvironment, respectively. Such molecules are composed of two or more facial amphiphiles that are connected to a central scaffold. Molecular umbrellas that have been synthesized to date, using bile acids as umbrella “walls”, polyamines such as spermidine and spermine as scaffold material, and L-lysine as “branches”, have been found capable of transporting certain hydrophilic peptides, nucleotides, and oligonucleotides across liposomal membranes by passive diffusion. They have also have been shown to increase in the water solubility and hydrolytic stability of a hydrophobic drug, and to exhibit significant antiviral activity. The ability of a fluorescently-labeled molecular umbrella to readily enter live HeLa cells suggests that such conjugates could find use as drug carriers.
INTRODUCTION
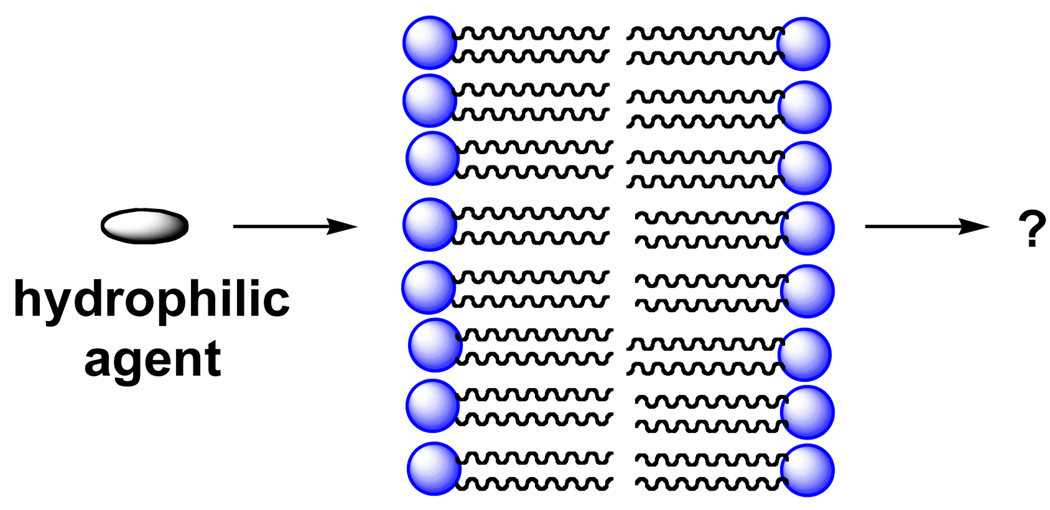
Membranes play a central role in the structure and function of cells by serving as barriers that permit the transport of only those ions and molecules that are necessary for maintaining the living state (1). Due to their strongly hydrophobic interior, delivering hydrophilic drugs across these naturally-occuring lipid bilayers into the cytoplasm of cells has proven to be difficult (2–20). Finding ways to promote the transport of hydrophilic agents across lipid bilayers, in general, by passive diffusion represents a major challenge (Figure 1).
Figure 1.
Styllized illustration of a hydrophilic agent trying to cross a phospholipid bilayer.
A common approach to this problem has been to synthesize drug analogs that are smaller in size and more lipophilic (i.e., lipid-soluble), based (1,4,5,7). Thus, according to the classic solution-diffusion model of bilayer transport, the permeability coefficient (P) of a permeant is directly proportional to its water-membrane partition coefficient (K) and its diffusion coefficient (D), but inversely proportional to the thickness (x) of the bilayer (1). Since P=(K × D)/x, an increase in lipophilicity is expected to increase P by increasing K. Also, a decrease in the size of a permeant is expected to increase P by increasing D, due to a reduction in the resistance toward diffusion.
In this review, we describe a fundamentally different approach to this transport problem that is based on the concept of “molecular umbrellas”. We also show how these unique amphiphilic molecules have potential as drug modifiers, and as drugs, themselves.
MOLECULAR UMBRELLA CONCEPT
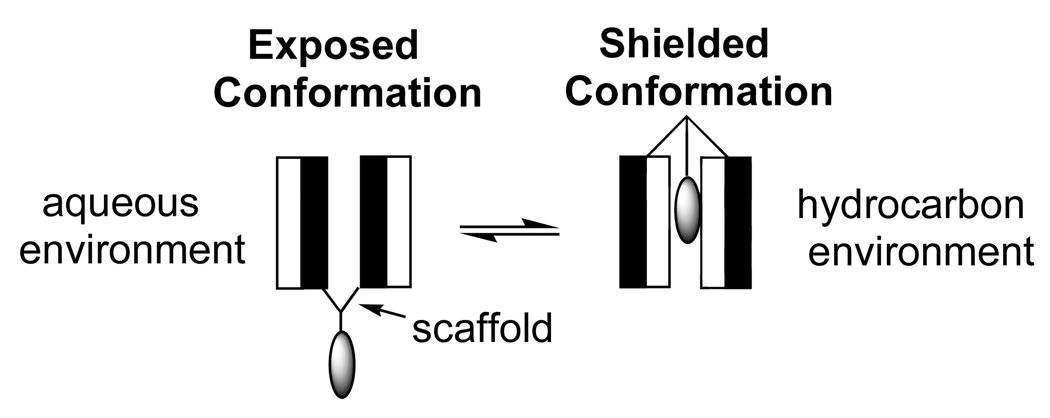
To overcome the incompatibility that exists between a hydrophilic agent and the hydrophobic interior of a lipid bilayer, we conceived of a class of molecules that was intended to function like “molecular umbrellas” (21). Specifically, such molecules were expected to cover the attached agent and shield it from the hydrophobic environment as it crosses the membrane. Our approach to the synthesis of molecular umbrellas has been to couple two or more facial amphiphiles (molecules having a hydrophobic and a hydrophilic face) to a central scaffold. For convenience, we refer to each such amphiphilic unit as a “wall”. When immersed in a hydrocarbon environment, a molecular umbrella is expected to adopt a shielded conformation in which the facially amphiphilic units not only mask their own hydrophilicity, but also that of the attached hydrophilic agent. When immersed in an aqueous environment, they are then expected to form an exposed conformation that allows each hydrophilic face and the attached agent to make direct contact with water. We have introduced the term, “amphomorphic” to describe the ability of such molecules to produce a hydrophobic or hydrophilic exterior on demand.
A stylized illustration of a molecular umbrella in an exposed and in a shielded conformation is shown in Figure 2. Here, the shaded and unshaded rectangles represent the hydrophobic and hydrophilic face of each amphiphilic unit, respectively, and the lightly shaded oval represents a covalently attached, hydrophilic agent.
Figure 2.
Stylized illustration of a di-walled molecular umbrella, bearing a hydrophilic agent, which is in an exposed and a shielded conformation in aqueous and hydrocarbon environments, respectively.
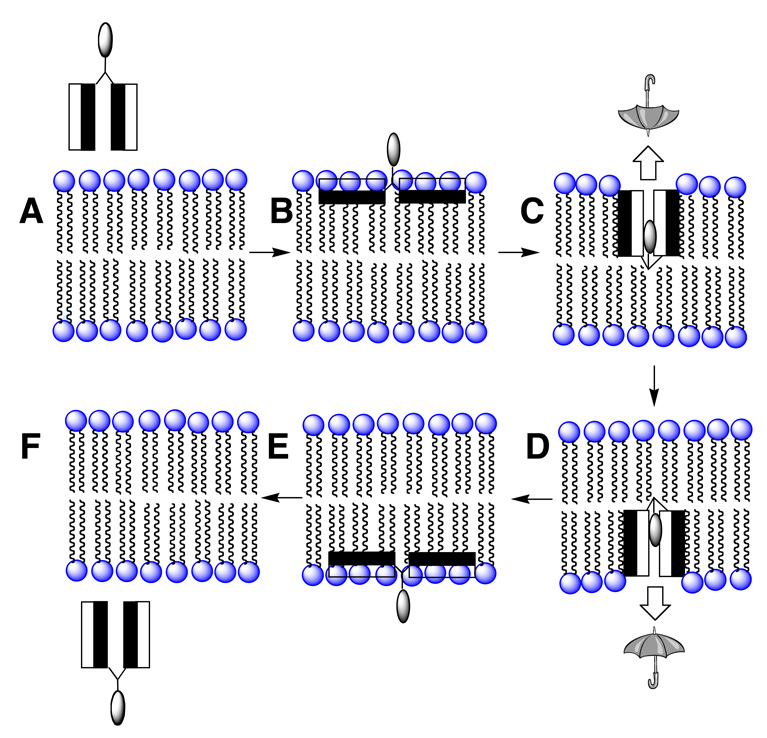
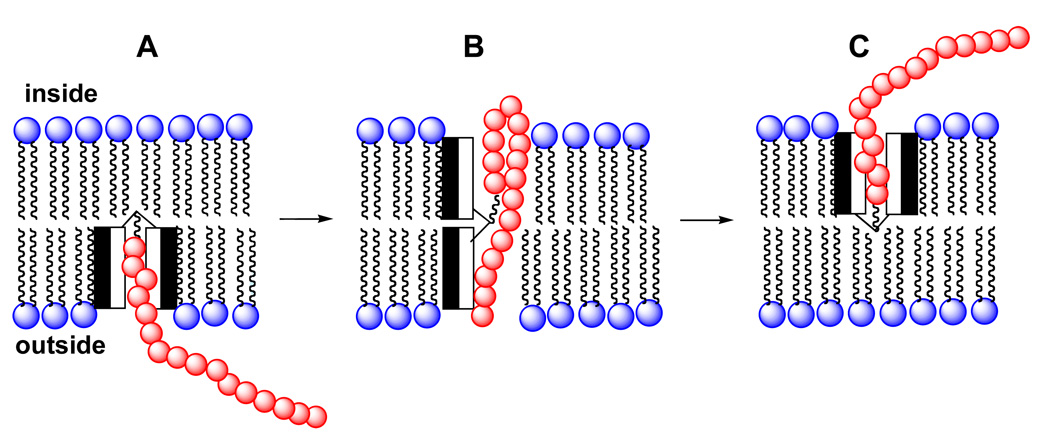
How could a molecular umbrella cross a lipid bilayer? Our working hypothesis is illustrated in Figure 3. In brief, the molecular umbrella first approaches the lipid bilayer in a fully exposed conformation (structure A). Hydrophobic interactions with the membrane interior then leads to an adsorbed state in which the hydrophilic faces are in contact with the polar head group region and the hydrophobic faces are in intimate contact with the hydrocarbon region of the lipid bilayer (structure B). Subsequent absorption into the interior of the membrane, being driven by hydrophobic forces, then affords structure C. Translocation to the adjoining leaflet, 180° rotation then produces D. Finally, reversal of the steps leading to B and A, affording E and F, respectively, then releases the conjugate from the other side of the membrane. In essence, the molecular umbrella functions by masking the hydrophilicity of the hydrophilic agent as it crosses the hydrocarbon interior of the membrane.
Figure 3.
A hypothetical mechanism of bilayer transport for a di-walled molecular umbrella.
UMBRELLA FRAMEWORKS
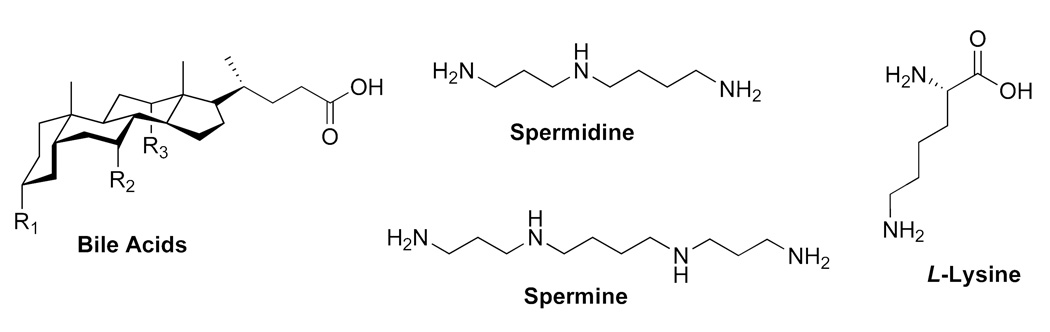
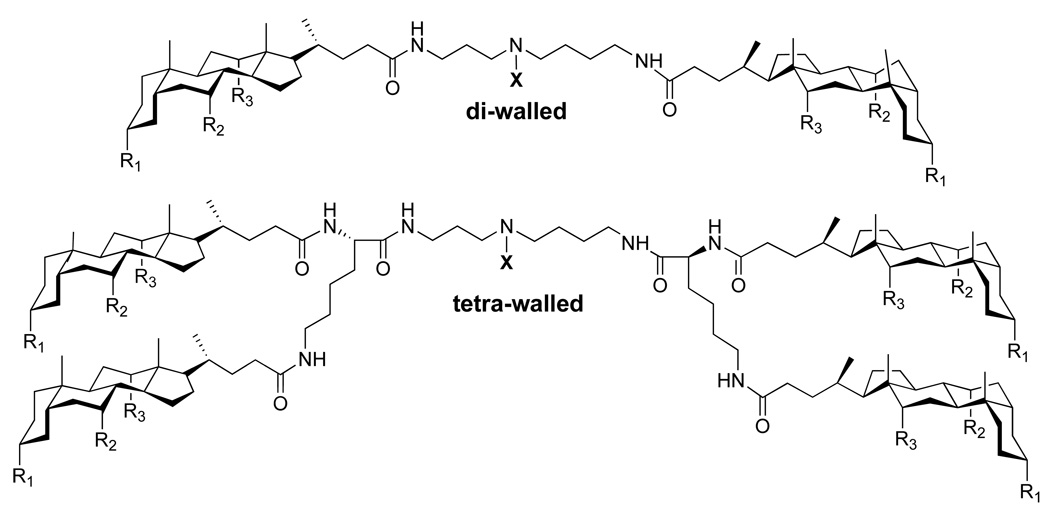
Our work in this area has focused on the use of naturally-occurring starting material for the synthesis of molecular umbrellas, based on the belief that they would be biodegradable and non-toxic. Thus, we have made extensive use of facially amphiphilic bile acids as umbrella “walls”, polyamines such as spermidine and spermine as scaffold material, and L-lysine as “branches” (Figure 4). Representative di-walled and tetra-walled frameworks that we have employed are shown in Figure 5. Here, “X” represents the “handle” of the umbrella where a desired agent is attached. To date, the “R” groups that have been used include: OCH3, OH, OCONH2, and OSO3Na. For all of the studies that are described in the following sections, the molar ratio of molecular umbrella/lipid that has been used is typically <1/100. In all cases where molecular umbrella conjugates have been reacted with glutathione, the conversions have been found to be quantitative
Figure 4.
Representative building blocks for the synthesis of molecular umbrellas. Typical “R” groups that have been used to date include: OCH3, OH, OCONH2, and OSO3Na.
Figure 5.
Common frameworks used in the synthesis of molecular umbrellas. Here, “X” represents the “handle” of the umbrella that can bind to a desired agent is attached. Typical “R” groups that have been used to date include: OCH3, OH, OCONH2, and OSO3Na.
UMBRELLA TRANSPORT OF PEPTIDES, NUCLEOTIDES AND OLIGONUCLEOTIDES ACROSS LIPOSOMAL MEMBRANES
Peptide Transport
Glutathione (Glu-Cys-Gly, GSH) is a simple tripeptide that exists in millimolar concentrations in the cytoplasm of mammalian cells. It is strongly hydrophilic and does not readily cross lipid bilayers by passive diffusion. Because of these properties, and also because its thiol moiety can be used for covalent attachment to a molecular umbrella, we have used GSH for feasibility studies.
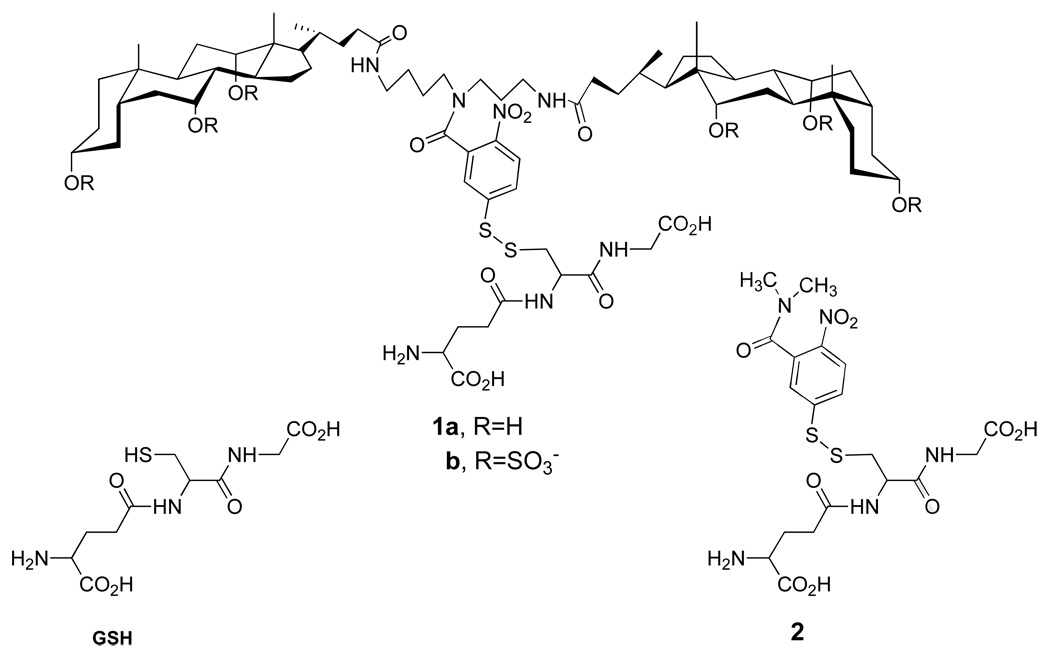
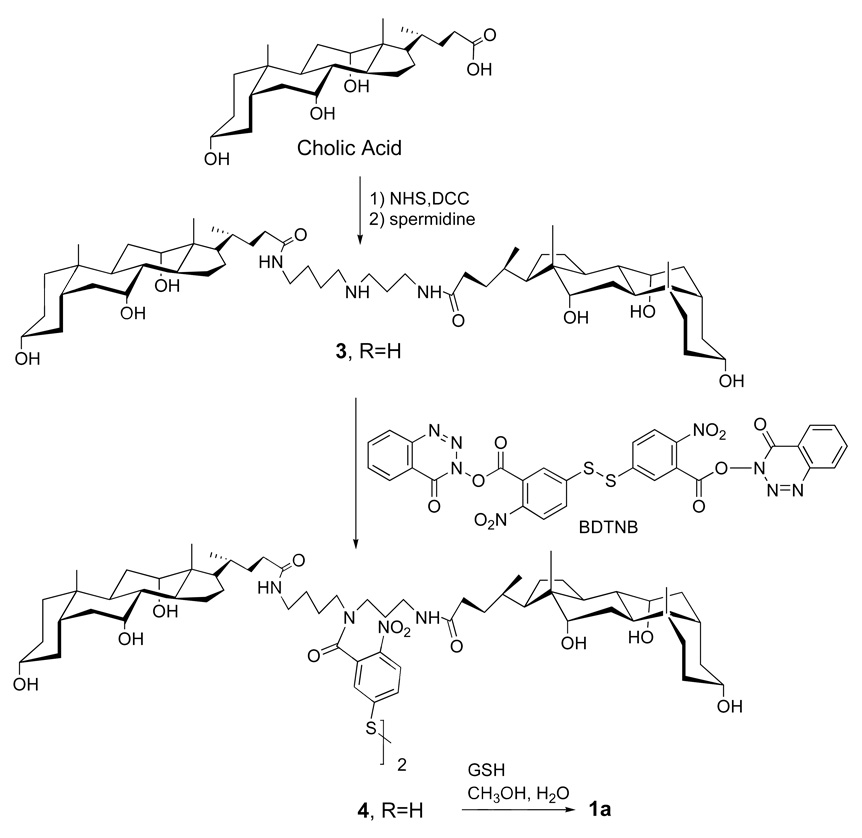
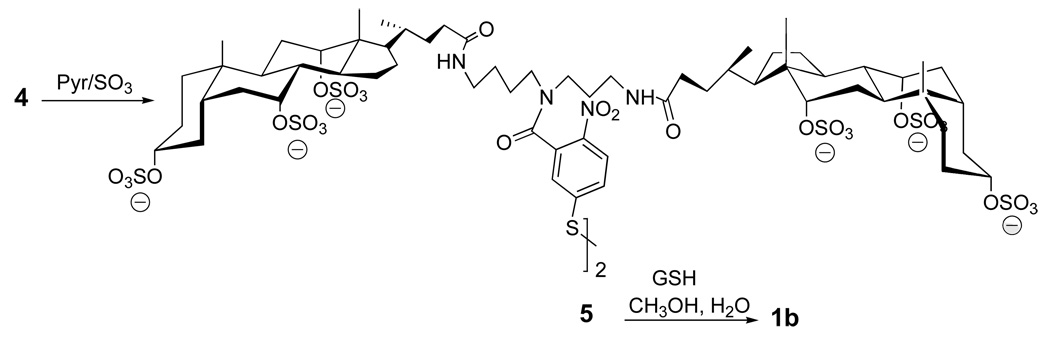
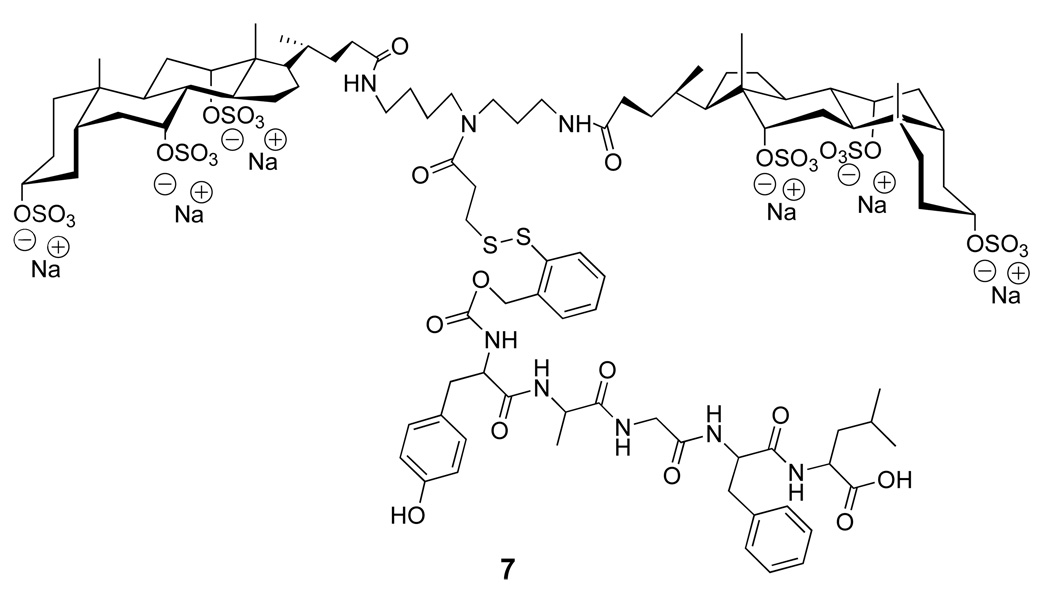
Two specific molecular umbrella-glutathione conjugates that were synthesized (i.e., 1a and 1b) are shown in Figure 6 (22, 23). In each case, a 5-thiol(2-nitrobenzoyl) group serves as the "handle". Also shown in Figure 6 is a non-umbrella analog, 2, which has been used as a control. The methods of synthesis for preparing 1a and 1b are outlined in Figure 7 and Figure 8, respectively.
Figure 6.
Structures of glutathione (GSH), di-walled molecular umbrellas that have been covalently attached to glutathione (1a and 1b), and a control conjugate, 2.
Figure 7.
Synthetic scheme for the preparation of 1a.
Figure 8.
Synthetic scheme for the preparation of 1b.
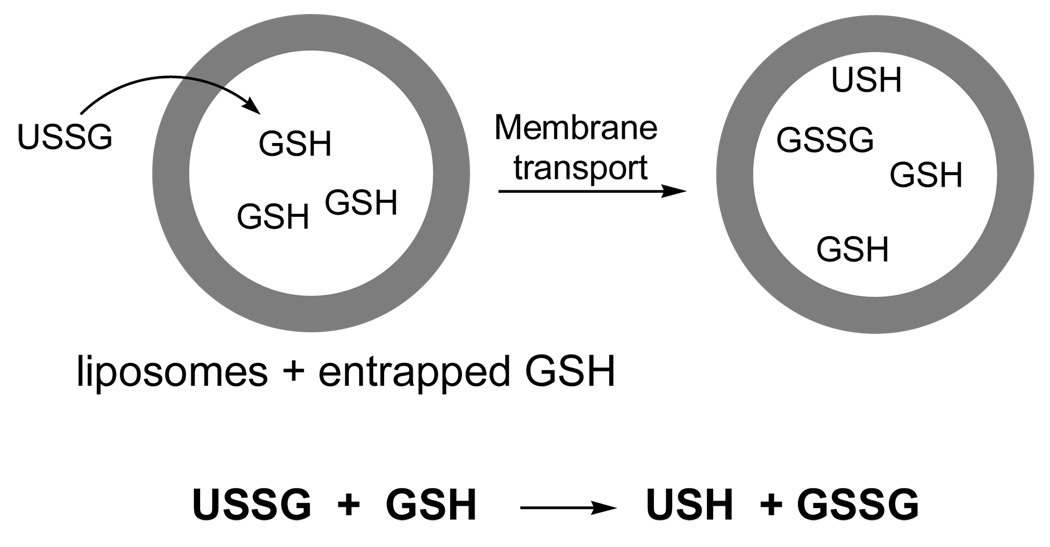
The ability of 1a and 1b to cross phospholipid bilayers by passive diffusion was demonstrated by adding a solution of each conjugate to liposomes made from 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC, 200 nm, extrusion), which contained GSH within their aqueous interior. Thus, the appearance of the 5-thiol(2-nitrobenzoyl) "handle" (i.e., the formation of USH via thiolate-disulfide interchange), provided evidence for liposomal entry (Figure 9). That this exchange reaction occurred within the interior of the liposomes was confirmed by quantifying the amount of cystine (GSSG) that had been produced and entrapped in the liposomes. In sharp contrast to 1a and 1b, addition of 2 to similar liposomal dispersions did not produce any detectable USH under similar experimental conditions.
Figure 9.
Stylized illlustration of a molecular umbrella-glutathione conjugate (USSG) crossing into a liposome loaded with glutathione (GSH), followed by thiolate-disulfide interchange to form oxidized glutathione (GSSG) and USH.
Similar entry by 1a has been found to occur with liposomes having thicker membranes made from 1,2-di(13-cis-docosenoyl)-sn-glycero-3-phosphocholine (C22:1) (24). However, in contrast to POPC membranes, where the formation of USH exhibited a first-order dependency on the molecular umbrella and the entrapped GSH, only a first-order dependency of the molecular umbrella was found when the thicker membranes we used; that is, the rate of formation of USH was independent of the entrapped GSH. These results indicated that the rate-determining step for umbrella entry into POPC liposomes was the thiolate-disulfide interchange reaction, but membrane permeation for the (C22:1) liposomes. At the same time, they indicated the involvement of transport-active monomers for the latter system.
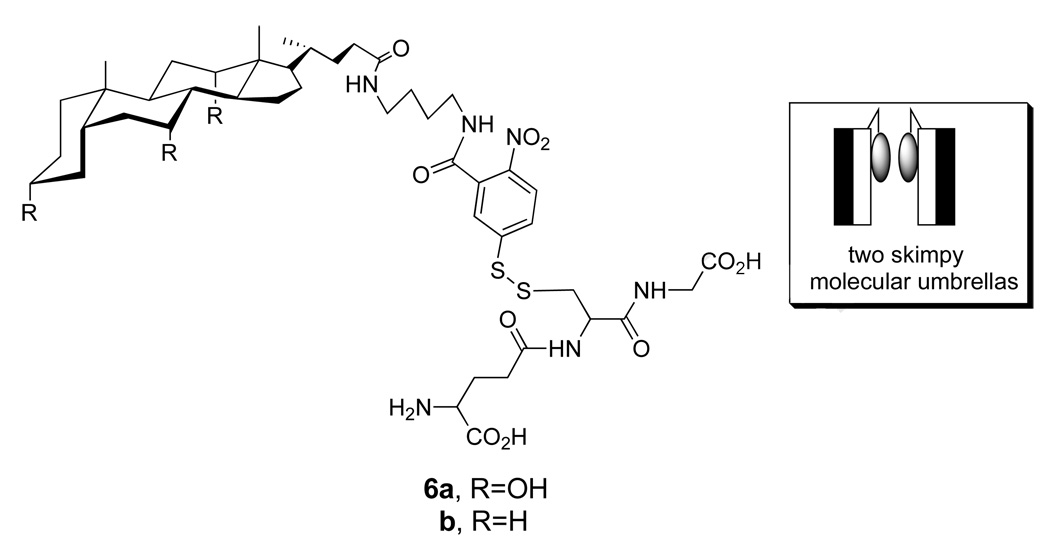
Related experiments that have been carried out using an analog of 1a, which contained only a single facial amphiphile (i.e., 6a), showed that transport across the thicker liposomes was still possible (Chart 4) (24). In this case, however, the rate of appearance of USH showed a second-order dependency on the concentration of this “skimpy” molecular umbrella and independent of the concentration of entrapped GSH. Control experiments established that GSH did not leak out of the liposomes during the thiolate-disulfide exchange reaction, and that GSSG was formed within the aqueous interior. Taken together, these results indicated that membrane permeation was rate-limiting for the skimpy molecular umbrella and that dimers are the transport-active species, most likely operating in a “face-to-face” orientation (Figure 10).
Figure 10.
Structure of a single-walled (“skimpy”) molecular umbrella bearing glutathione (6a), a non-umbrella analog (6b) and a stylized illustration showing two skimpy molecuar umbrellas in a face-to-face orientation.
Similar experiments that were carried out with 6b, which is devoid of facial hydrophilicity, showed no evidence of reaction (24). Control experiments that were carried out in which 6b was first bound to both the inner and outer leaflet of the liposomes (double-sided addition), followed by treatment with external GSH showed that 55% of the conjugate underwent rapid reaction. The fact that ca. half of 6b was unreactive toward externally-added GSH provided strong evidence that this conjugate crosses lipid bilayers very slowly, if at all. These results were significant because they ruled out the possibility that the ability of 6a to cross lipid bilayers was simply due to an increase in the overall hydrophobicity of the peptide after attachment to the molecular umbrella. Thus, it provided strong evidence for an novel mechanism of transport, requiring the presence of facial amphiphilicity.
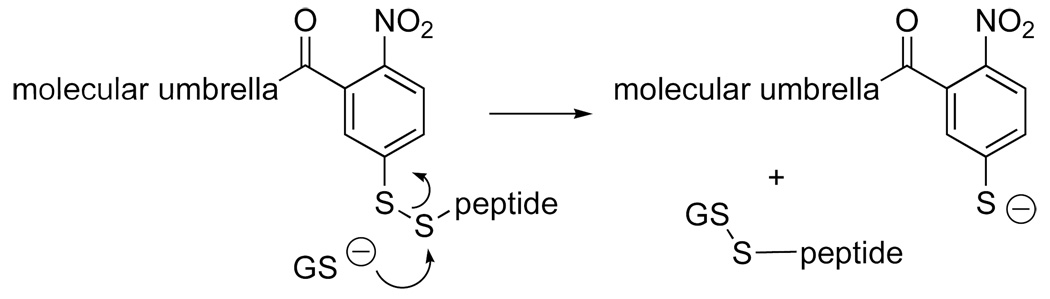
For all of the molecular umbrellas described thus far, a 2-nitrobenzoyl-5-dithio moiety was used as a detachable handle. Since the cytoplasm of cells is rich in glutathione, such a handle could be considered in the design of prodrugs. Two limitations of the use of this handle, however, are: (i) the presence of a cysteine residue is necessary for coupling the peptide to the umbrella, and the (ii) the peptide is released in a non-native state; that is, it leaves as a glutathione conjugate (Figure 11).
Figure 11.
Cleavage of a molecular umbrella-glautathione conjugate by a glutathione molecule.
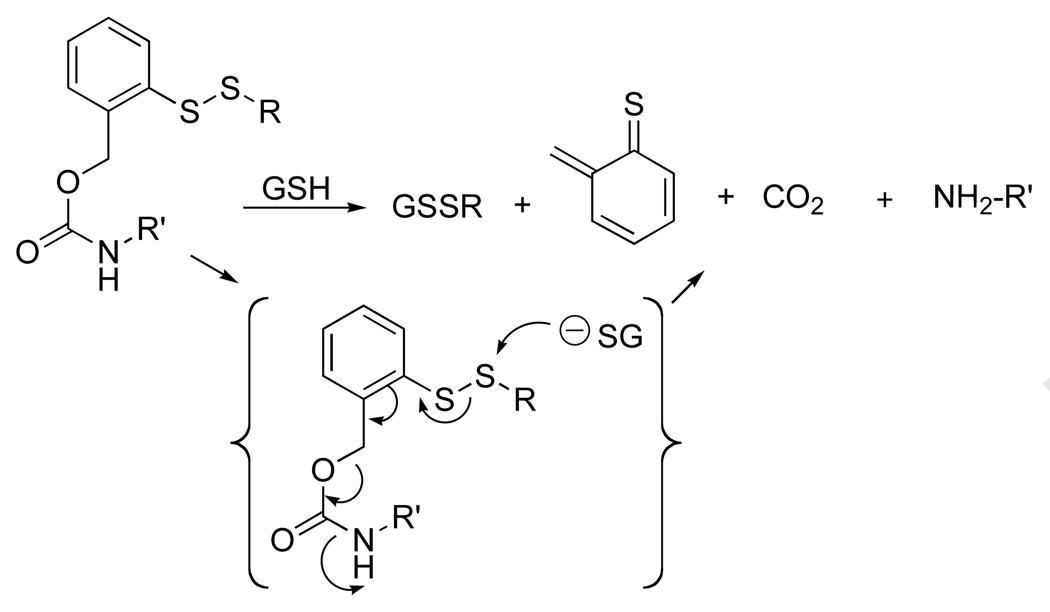
As an alternative to the 2-nitrobenzoyl-5-dithio handle, we explored a prodrug strategy that was based on a thiol-induced reductive fragmentation of a o-dithiobenzyl carbamate (Figure 12) (25, 26). For feasibility studies, a molecular umbrella conjugate of an opioid peptide was synthesized; that is, 7; DADLE, H-Tyr-D-Ala-Gly-Phe-D-Leu-OH (Figure 13) (27).
Figure 12.
Thiol-induced reductive fragmentation of a o-dithiobenzyl carbamate liberating an organic amine.
Figure 13.
Structure of a diwalled molecular umbrella bearing DADLE with a fully detachable handle.
That 7 was capable of releasing the native DADLE was confirmed by treating a solution of the conjugate with excess GSH. As expected, the solution became effervescent and the appearance of the o-thiobenzoquinone methide product was detected by UV (264 nm). At the end of the reaction, thin layer chromatography confirmed the complete disappearance of 7 and the appearance of the free form of DADLE, which could be isolated in 82% yield. Using liposomal targets loaded with excess GSH, it was then demonstrated that 7 could cross lipid bilayers and release DADLE to their aqueous compartment.
Nucleotide Transport
In an effort to expand the scope of molecular umbrellas as membrane transporters, we became keenly interested in antisense oligonucleotides. Currently, antisense oligonucleotides are believed to have considerable promise as therapeutic agents for the treatment of a wide range of diseases; e.g., non-small cell lung cancer, Crohn’s disease, malignant melanoma, chronic lymphocytic leukemia and multiple myeolma. To date, most, if not all, approaches that have been used to deliver antisense oligonucleotides into cells have taken advantage of endocytotic pathways. Release from resulting endosomal-lysosomal compartments by some unknown and inefficient mechanism then delivers a portion of the oligonucleotide to the cytoplasm where it can reach intended targets. In principle, the delivery of antisense oligonucleotides, directly into the cytoplasm via passive transport, could lead to greater overall efficacy.
Before exploring oligonucleotide transport, we sought to demonstrate the feasibility of transporting cleavable forms of adenosine 5'-monophosphate (AMP) and adenosine 5'-triphosphate (ATP) across phospholipid bilayers. Although several studies have been aimed at creating prodrugs of nucleoside 5'monophosphates, reports of membrane transporters of nucleoside 5'-triphosphates are rare.
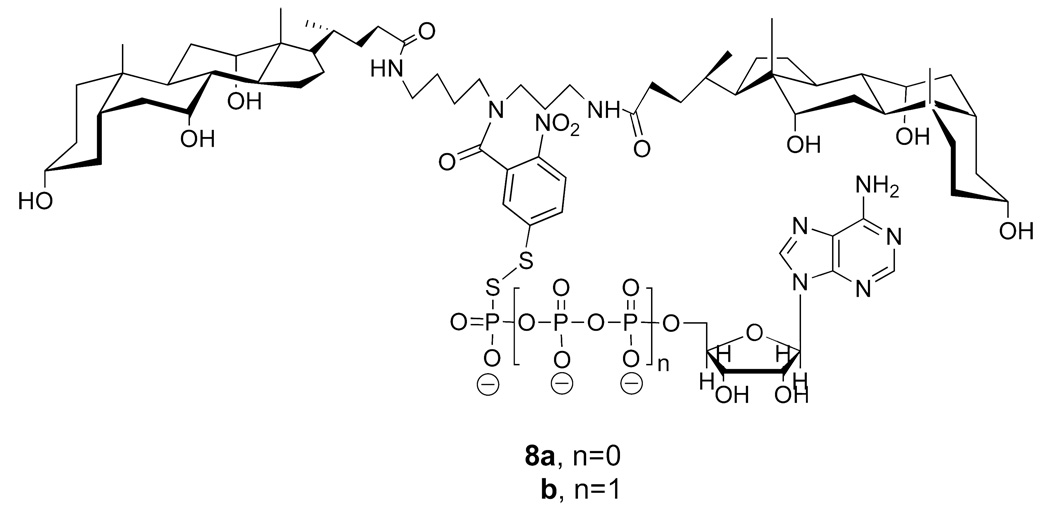
Two conjugates that we prepared, using 5-thiol(2-nitrobenzoyl) as an umbrella handle, and thiol forms of AMP and ATP, were 8a and 8b (Figure 14) (28). Synthetic methods that were used to prepare these conjugates were similar to those used for the molecular umbrella-glutathione conjugates.
Figure 14.
Structures of di-walled molecular umbrellas bearing a thiolated form of AMP and ATP.
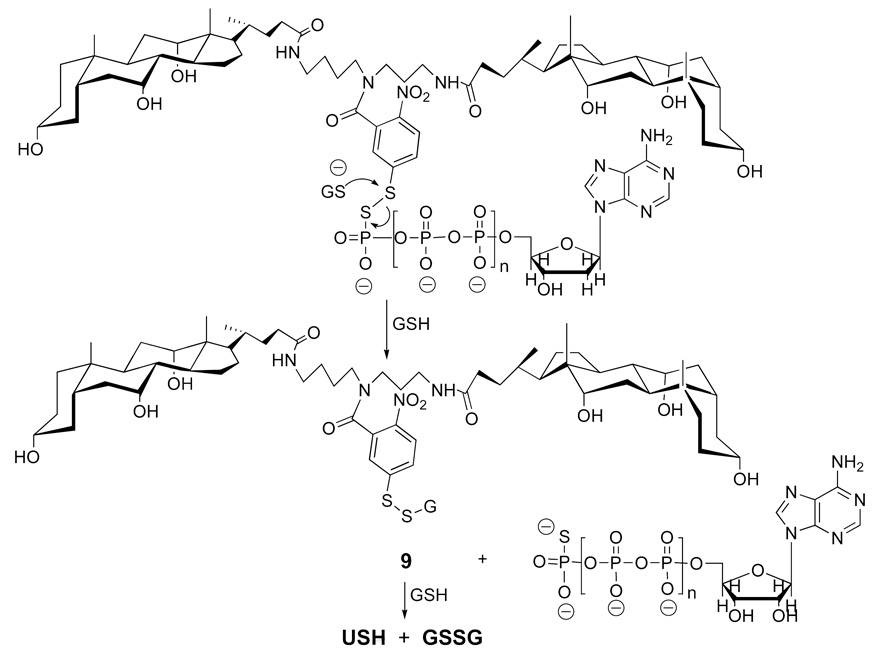
In principle, attack by glutathione on the disulfide moiety could occur by two different pathways. In one pathway, attack on the sulfur atom of the 5-thiol(2-nitrobenzoyl) moiety by glutathione would result in the direct release of the free nucleotide (Figure 15). Subsequent attack by a second glutathione molecule would then liberate USH plus the oxidized form of glutathione (GSSG). An alternative pathway (not shown) is one in which glutathione initially attacks the sulfur atom of the nucleotide, thereby liberating USH, directly. Further attack by GSH on the glutathione-nucleotide conjugate would then afford the free nucleotides plus GSSG. By monitoring the reaction of 8a and 8b with GSH by UV, we were able to obtain strong evidence for a two-stage release that occurs by the first pathway, as shown in Figure 15. Subsequent transport experiments that were carried out with 8a and 8b and liposomes loaded with GSH resulted in the release of these nucleotides within the aqueous compartment of the liposomes.
Figure 15.
One possible pathway for the cleavage of a di-walled molecular umbrella by glutathione to liberate a nucleotide.
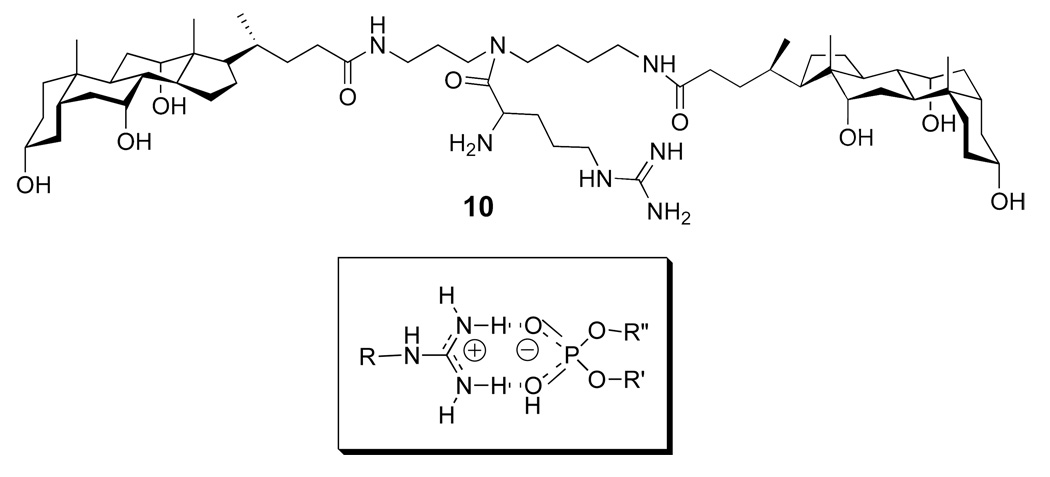
In related studies, molecular umbrella 10 was found capable of transporting ATP across liposomal membranes in a catalytic manner (Figure 16) (29). In this case, a guanidinium moiety was chosen as an umbrella handle because of its ability to bind phosphate groups via hydrogen bonding and electrostatic association. Thus, incubation of liposomes containing co-entrapped ATP and GSH with 10 resulted in the selective release of ATP. The fact that GSH was retained by these liposomes showed that the integrity of the membrane was retained during this transport process.
Figure 16.
Structure of a di-walled molecular umbrella bearing a guanidinium handle, and a probable structure for the guanidinium moiety binding to a phosphate group.
Oligonucleotide Transport
The ability of molecular umbrellas to transport nucleotides across lipid bilayers encouraged us to consider similar transport of an oligonucleotide. An obvious concern, however, was the size of an oligonucleotide relative to that of the molecular umbrella. Specifically, di- and tetra-walled molecular umbrellas would not be capable of shielding an entire oligonucleotide. In Figure 17, we show a mechanism by which umbrella-transport of an oligonucleotide across a lipid bilayer might be possible. Here, the conjugate first penetrates the membrane to produce a shielded conformation (A). Subsequent opening of the umbrella across the bilayer, and simultaneous folding of the oligonucleotide into a partial "U", then leaves most of the membrane-internalized phosphate groups pointing towards the hydrophilic faces of the umbrella (B), allowing for translocation and umbrella closure to form C.
Figure 17.
Stylized illustration of a di-walled molecular umbrella-oligonucleotide conjugate crossing a phospholipid bilayer.
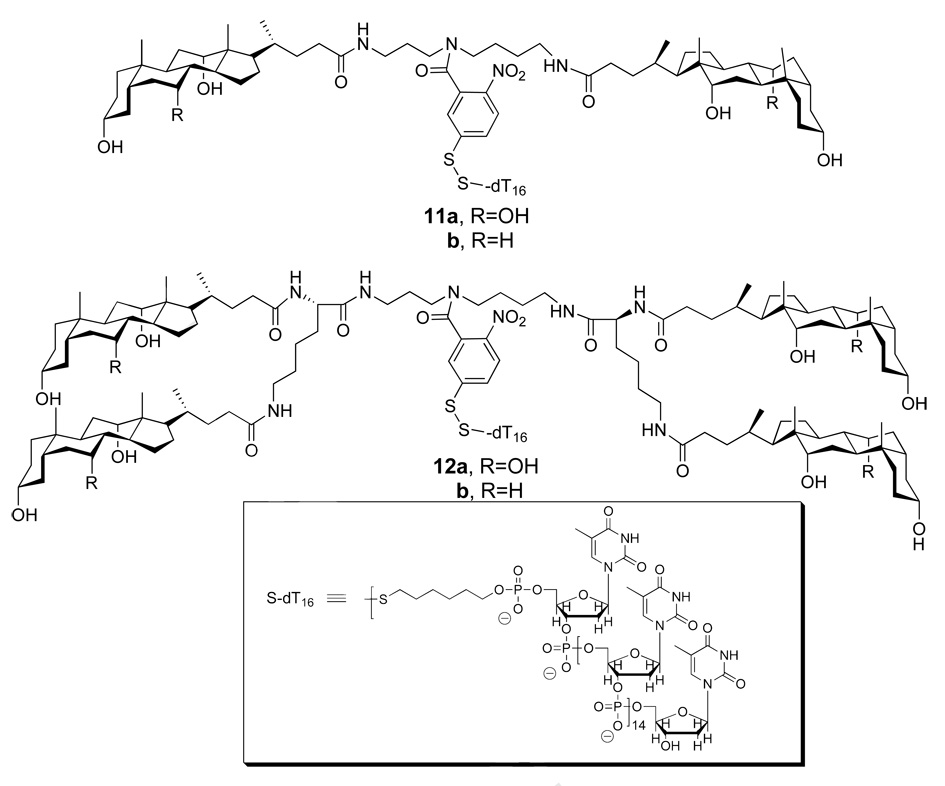
To test the feasibility of umbrella-mediated oligonucleotide transport, conjugates 11a, 11b, 12a and 12b were synthesized using a 16-mer oligonucleotide (S-dT16) as a prototype (Figure 18) (30, 31). Similar to our studies with umbrella-glutathione conjugates, liposomes were loaded with GSH and the release of USH monitored by UV. In all cases, oligonucleotide delivery to the interior of the liposomes was observed. Control experiments confirmed the absence of release of entrapped GSH throughout the course of these influx experiments. Comparison of these molecular umbrella-oligonucleotide conjugates revealed greater transport rates with the tetra-walled umbrellas as compared with their di-walled analogs. The deoxoycholic acid-based, di-walled molecular umbrella conjugate (11b) was found to be ca. 50% more active than the cholic acid-based analog (11a), but the reverse was found to be the case for the tetra-walled molecular umbrella conjugates.
Figure 18.
Structures of di-walled (11a, 11b) and tetra-walled (12a, 12b) molecular umbrellas bearing an oligonucleotide.
MOLECULAR UMBRELLAS AS DRUG MODIFIERS
While molecular umbrellas were originally designed to promote drug transport across hydrophobic barriers, they can also improve the water-solubility and hydrolytic stability of certain hydrophobic drugs. A molecular umbrella conjugate of the anticancer agent, chlorambucil (CMB), illustrates both of these features.
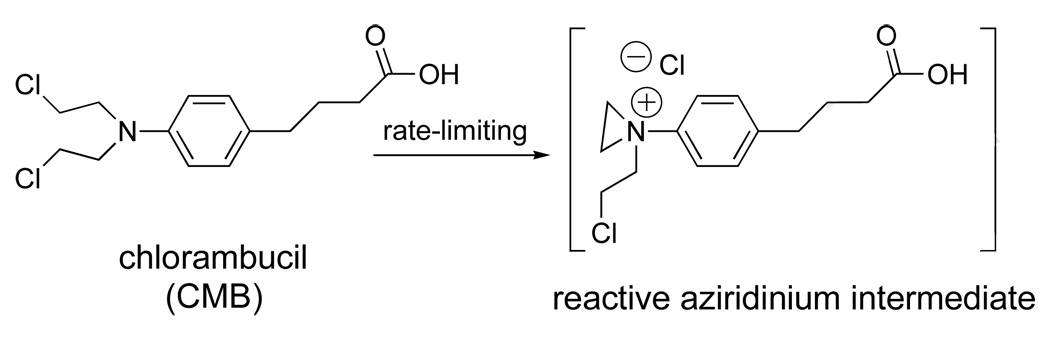
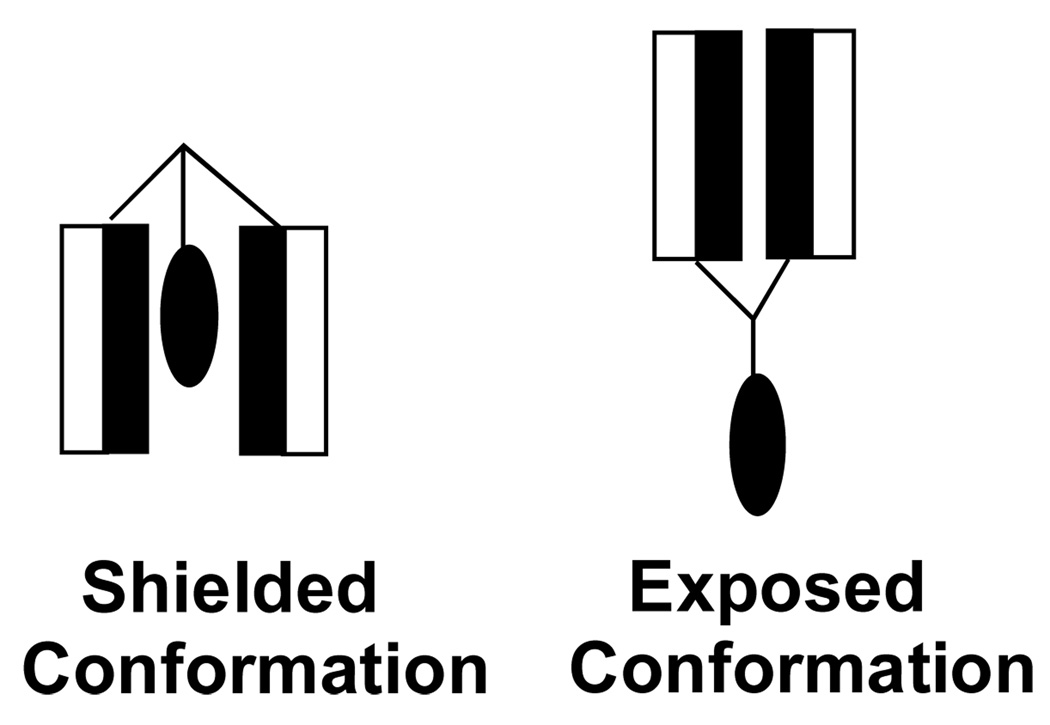
Chlorambucil is an alkylating agent that undergoes a rate-limiting intramolecular displacement to form an aziridinium ion (Figure 19). Subsequent reaction with nucleophiles (e.g., DNA) or water then produces alkylated and hydrolyzed products, respectively. Because charge is developed in the transition state leading to the aziridinium ion, its rate of formation should be faster in a polar environment. Conversely, slower rates are expected in hydrophobic environments. Thus, if CBM were attached to a molecular umbrella, and if the umbrella were to adopt a shielded conformation in water—where the CMB is covered by the hydrophobic faces of the umbrella– this could lead to enhanced stability. An illustration of such shielding is shown in Figure 20, where CMB is represented by a darkened oval, and the shaded and unshaded rectangles represent the hydrophobic and hydrophilic face of each amphiphilic unit, respectively. In addition, by exposing each hydrophilic face of the amphiphilic units to the aqueous phase, a significant increase in water solubility would also be expected.
Figure 19.
Chlorambucil undergoing a rate-limiting intramolecular displacement reaction.
Figure 20.
Stylized illustration of a di-walled molecular umbrella, bearing a hydrophobic agent, which is shown in a shielded and an exposed conformation.
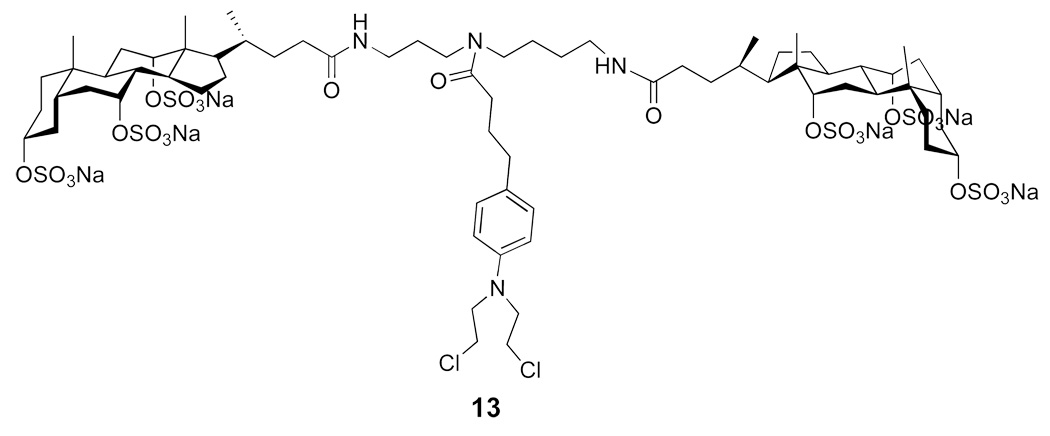
Experimentally, Chlorambucil was found to have a solubility in water that was less than 500 µg/mL. In sharp contrast, 13 showed a water solubility that was 1.20 g/mL (Figure 21) (32). Thus, attachment of CMB resulted in an increase in water-solubility of more than three orders of magnitude! In addition, whereas CMB showed a half-life in phosphate buffer (pH 7.4) of 16 min, the molecular umbrella conjugate 13 had a half-life of ca. 40 min. Thus, covalent attachment of CMB to this di-walled molecular umbrella resulted in a dramatic increase in water solubility and a modest increase in hydrolytic stability. In preliminary studies, we compared the activity of Chlorambucil with 13 in inhibiting the growth of human lymphoblastic leukemia (CCRF-CEM) cells, as well as human colon carcinoma (HCT-116) cells. For the leukemia cells, the activity of the molecular umbrella conjugate was substantially lower relative to CMB; that is, the concentrations needed to inhibit 50% growth were 173 and 2.15 µM for 13 and CMB, respectively. For the colon cancer cells, the difference was less pronounced. In this case, the concentrations needed to inhibit 50% growth were 53.7 and 11.4 µM, respectively.
Figure 21.
Structure of a di-walled molecular umbrella that has been conjugated to Chlorambucil.
MOLEULAR UMBRELLAS AS ANTIVIRAL AGENTS
Anionic polymers such as dextran sulfate and cellulose sulfate are known to inhibit cellular binding of HIV and HSV by competing for viral envelope glycoproteins. The possibility that persulfated molecular umbrellas might behave in a similar way, together with their potential for crossing hydrophobic barriers (e.g., the blood-brain barrier) suggests that such compounds could function as novel therapeutic agents as well as microbicides.
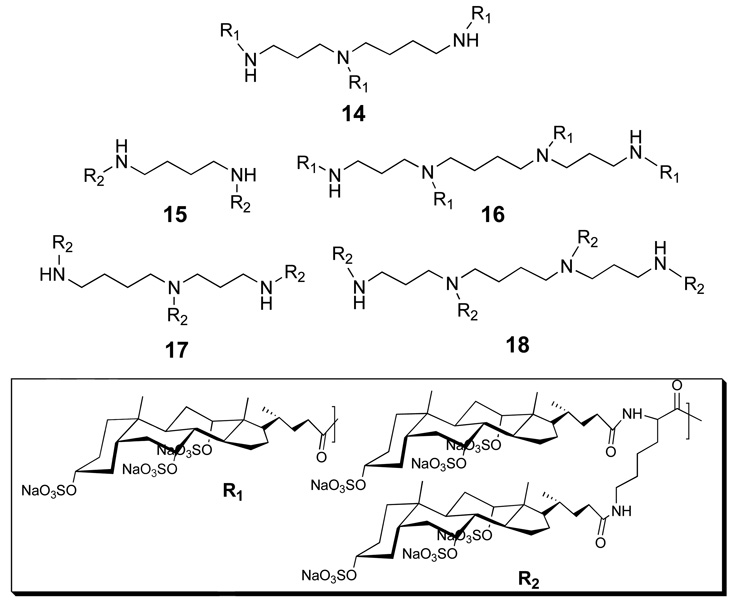
With this thought in mind, five persulfated molecular umbrellas (14, 15, 16, 17 and 18) were synthesized, and their anti-HIV activity measured using U87.CD4.CCR5 cells, replication defective HIV-1 virus and a luciferase assay (Figure 22) (33). At a dosage of 1 µg/mL, 18 showed moderate activity; 15, 16, and 17 were less active and 14 was inactive. At 100 µg/mL, all conjugates showed significant activity and inhibition was >95% with 18. The anti-HSV activity of these conjugates was also compared by conducting plaque reduction assays with HSV-2(G) and human cervical epithelial cells (CaSki). At a concentration of 100 µg/ml, all of the conjugates completely inhibited HSV-2. At a concentration of 10 µg/mL, complete and very significant inhibition was found for 18 and 17, respectively. In contrast, the activities of 14, 15 and 16 were only modest at this concentration.
Figure 22.
Structures of molecular umbrellas bearing three (14), four (15, 16), six (17) and eight (18) persulfated choloyl groups.
Toxicity studies that have been carried out with 18 has shown that it is noncytotoxic towards CaSki cells (33, 34). Thus, human cervical cells were exposed to serial dilutions of 18 (in the absence of virus) overnight (acute) or for 3 h each day for 4 consecutive days (chronic) to examine the effects on cell growth and viability using an MTS assay. No cytotoxicity was observed following chronic or acute exposure even at concentrations as high as 1000 µg/ml.
CELLULAR UPTAKE OF MOLECULAR UMBRELLAS
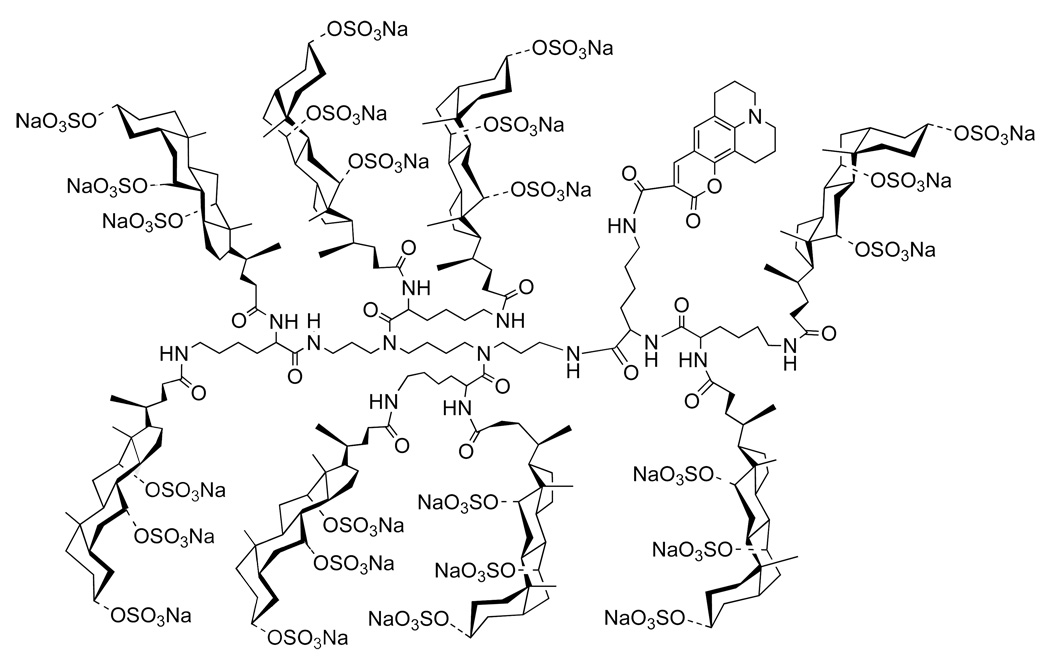
An important question that bears, directly, on the potential of molecular umbrellas as drug carriers is whether they are capable of crossing the plasma membrane of living cells. To address this question, we synthesized a fluorescently-labeled molecular umbrella analog of 18 (i.e., 19) and investigated its interaction with live HeLa cells (Figure 23) (35).
Figure 23.
Structure of a fluorescently-labeled, persulfated molecular umbrella, 19.
In these experiments, confocal microscopy was used to section optically through the cellular volume to establish that the fluorescent label was dispersed within the cell and not bound to its outer surface. Cells were also labeled with Syto-59 to mark their nucleus and their cytoplasm. After a 10-min incubation period with 4 µM of 19, this molecular umbrella could be readily detected inside the cells. Thus, 19 was found to be diffuse in the cytoplasm and punctate in the nucleus. The former is generally considered to be an indication of cellular entry via passive diffusion. In addition, the molecular umbrella within the nucleus was bound in spots that colocalize with nucleoli. In preliminary studies, treatment of HeLa cells with NaN3 and 2-deoxyglucose (to deplete ATP production) only modestly inhibited the cellular uptake of 19. This observation lends further support for passive diffusion playing a significant role in the cellular entry of this molecular umbrella. The fact that 19 is concentrated at the nucleus of these cells is intriguing, but the basis for this localization is not presently understood. It does raise the possibility, however, that molecular umbrellas of this type could be used for the nuclear targeting of drugs.
OTHER RIGID FACIAL AMPHIPHILES USED IN PROMOTING MEMBRANE TRANSPORT
Although this review has focused, sharply, on molecular umbrellas as membrane transporter, it should be noted other types of rigid facial amphiphiles have been devised that promote the transport of ions and small molecules. In a recent report, for example, it was shown that cholic acid could be transformed into cyclotrimeric and cyclotetrameric “toroidal amphiphiles” with inward-directed ammonium substitutents, and that the cyclotrimeric form was capable of transporting chloride anions across vesicle bilayers (36). In an earlier study, four Amphotericin B moelcules were coupled to a calix[4]arene and found to promote the transport of K+ across liposomal membranes (37). Finally, a variety of novel scaffolds (e.g., p-octaphenyl unit) have been used to construct a rich assortment of membrane-spanning pores having facial amphiphilicity (38, 39).
PROSPECTUS
The feasibility of synthesizing molecular umbrellas from bioconjugates, which are capable of transporting hydrophilic agents across lipid bilayers, has been established. Thus, it has been shown that molecular umbrellas derived from certain bile acids, polyamines and amino acids (e.g., cholic acid, spermidine and lysine) can transport hydrophilic peptides, nucleotides, and oligonucleotides across liposomal membranes. It has also been demonstrated that molecular umbrellas can enhance the water solubility and hydrolytic stability of a hydrophobic drug. Certain persulfated molecular umbrellas also show promise as antiviral agents for use as microbicides and as therapeutic agents.
To gain a deeper understanding of how molecular umbrellas cross lipid bilayers, additional studies are needed that provide fundamental insight into the interactions of these novel amphomorphic molecules with lipid bilayers. Further studies that show how transport rates and membrane binding are influenced by systematic changes in the structure and composition of (i) the molecular umbrella, (ii) the attached agent, and (iii) the lipid membrane should also prove valuable in clarifying probable mechanism(s) of membrane transport. The fact that 19 readily enters live HeLa cells, and that passive transport appears to play a significant role in this entry process, implies that fundamental insight gained from model studies should aid the rational design of molecular umbrellas as drug carriers.
ACKNOWLEDGMENT
This work was supported by the National Institues of Health (PHS Grants GM51814).
LITERATURE CITED
- 1.Stein WD. San Diego, CA: Academic Press; 1986. Transport and Diffusion Across Cell Membranes. [Google Scholar]
- 2.Langel U. Boca Raton, FL: CRC Press; 2002. Cell-Penetrating Peptides: Processes and Applications. [Google Scholar]
- 3.Curiel DT, Douglas JT, editors. John Wiley & Sons, Inc; 2002. Vector Targeting for Therapeutic Gene Delivery. [Google Scholar]
- 4.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Del. Rev. 2001;46:3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 5.Pidgeon C, Ong S, Liu H, Qiu X, Pidgeon M, Dantzig AH, Munroe J, Homback WJ, Kasher JS, Glunz L, Szczerba T. IAM chromatography: an in vitro screen for predicting drug membrane permeability. J. Med. Chem. 1995;38:590–594. doi: 10.1021/jm00004a004. [DOI] [PubMed] [Google Scholar]
- 6.Juliano RL, Alahari S, Yoo H, Kole R, Cho M. Antisense pharmacodynamics: critical issues in the transport & delivery of antisense oligonucleotides. Pharm. Res. 1999;16:494–502. doi: 10.1023/a:1011958726518. [DOI] [PubMed] [Google Scholar]
- 7.Boguslavsky V, Hruby VJ, O’Brien DF, Misicka A, Lipkowski A. Effect of peptide conformation on membrane permeability. J. Peptide Res. 2003;61:287–297. doi: 10.1034/j.1399-3011.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 8.Dias N, Stein CA. Antisense oligonucleotides: basic concepts and mechanisms. Mol. Cancer Ther. 2002;1:347–355. [PubMed] [Google Scholar]
- 9.Rothbard JB, Jessop TC, Lewis RS, Murray BA, Wender PA. Role of membrane potential and hydrogen bonding in the mechanism of translocation of guanidinium-rich peptides into cells. J. Am. Chem. Soc. 2004;126:9506–9507. doi: 10.1021/ja0482536. [DOI] [PubMed] [Google Scholar]
- 10.Wender PA, Rothbard JB, Jessop TC, Kreider EL, Wylie BL. Oligocarbamate molecular transporters: design, synthesis, and biological evaluation of a new class of transporters for drug delivery. J. Am. Chem. Soc. 2002;124:13382–13383. doi: 10.1021/ja0275109. [DOI] [PubMed] [Google Scholar]
- 11.Rothbard JB, Kreider E, VanDeusen CL, Wright L, Wylie BL, Wender PA. Arginine-rich molecular transporters for drug delivery: role of backbone spacing in cellular uptake. J. Med. Chem. 2002;45:3612–3618. doi: 10.1021/jm0105676. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki T, Futaki S, Niwa M, Tanaka S, Ueda K, Sugiura Y. Possible existence of common internalization mechanisms among arginine-rich peptides. J. Biol. Chem. 2002;277:2437–2443. doi: 10.1074/jbc.M110017200. [DOI] [PubMed] [Google Scholar]
- 13.Terrone D, Leung S, Sang W, Roudaia L, Silvius JR. Penetratin and related cell-penetrating cationic peptides can translocate across lipid bilayers in the presence of a transbilayer potential. Biochemistry. 2003;42:13787–13799. doi: 10.1021/bi035293y. [DOI] [PubMed] [Google Scholar]
- 14.Sakai N, Takeuchi T, Futaki S, Matile S. Direct observation of anion-mediated translocation of fluorescent oligoarginine carriers into and across bulk liquid and anionic bilayer membranes. ChemBioChem. 2005;6:114–122. doi: 10.1002/cbic.200400256. [DOI] [PubMed] [Google Scholar]
- 15.Sakai N, Futaki S, Matile S. Anion hopping of (and on) functional oligoarginines: from chloroform to cells. Soft Matter. 2006;2:636–641. doi: 10.1039/b606955j. [DOI] [PubMed] [Google Scholar]
- 16.Takeuchi T, Kosuge M, Tadokoro A, Sugiura Y, Nishi M, Kawata M, Sakai N, Matile S, Futaki S. Direct and rapid cytosolic delivery using cell-penetrating peptides mediated by pyrenebutyrate. ACS Chem. Biol. 2006;1:299–303. doi: 10.1021/cb600127m. [DOI] [PubMed] [Google Scholar]
- 17.Ziegler A, Blatter XL, Seelig A, Seelig J. Protein transduction domains of HIV-1 and SIV TAT interact with charged lipid vesicles: binding mechanism and thermodynamic analysis. Biochemistry. 2003;42:9185–9194. doi: 10.1021/bi0346805. [DOI] [PubMed] [Google Scholar]
- 18.Binder H, Lindblom G. Charge-dependent translocation of the trojan peptide penetratin across lipid membranes. Biophys. J. 2003;85:982–995. doi: 10.1016/S0006-3495(03)74537-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuchs SM, Raines RT. Pathway for polyarginine entry into mammalian cells. Biochemistry. 2003;43:2438–2444. doi: 10.1021/bi035933x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Potocky TB, Menon AK, Gellman SH. Cytoplasmic and nuclear delivery of a TAT-derived peptide and a β-Peptide after endocytic u,ptake into HeLa Cells. J. Biol. Chem. 2003;278:50188–50194. doi: 10.1074/jbc.M308719200. [DOI] [PubMed] [Google Scholar]
- 21.Janout V, Lanier M, Regen SL. Molecular umbrellas. J. Am. Chem. Soc. 1996;118:1573–1574. [Google Scholar]
- 22.Janout V, DiGiorgio C, Regen SL. Molecular umbrella-assisted transport of a hydrophilic peptide across a phospholipid membrane. J. Am. Chem. Soc. 2000;122:2671–2672. [Google Scholar]
- 23.Janout V, Zhang LH, Staina IV, DiGiorgio C, Regen SL. Molecular umbrella-assisted transport of glutathione across a phospholipid membrane. J. Am. Chem. Soc. 2001;123:5401–5406. doi: 10.1021/ja010124r. [DOI] [PubMed] [Google Scholar]
- 24.Janout V, Staina V, Bandyopadhyay P, Regen SL. Evidence for an umbrella mechanism of bilayer transport. J. Am. Chem. Soc. 2001;123:9926–9927. doi: 10.1021/ja016265a. [DOI] [PubMed] [Google Scholar]
- 25.Senter PD, Pearce WE, Greenfield RS. Development of a drug-release strategy based on the reductive fragmentation of benzyl carbamate disulfides. J. Org. Chem. 1990;55:2975–2978. [Google Scholar]
- 26.Zalipsky S, Qazen M, Walker JA, Mullah N, Quinn YP, Huang SK. New detachable poly(ethylene glycol) conjugate: cysteine-cleavable lipopolymers regenerating natural phospholipid, diacyl phosphatidylethanolamine. Bioconjugate Chem. 1999;10:703–707. doi: 10.1021/bc990031n. [DOI] [PubMed] [Google Scholar]
- 27.Jing B, Janout V, Regen SL. Fully-detachable molecular umbrellas as peptide delivery agents. Bioconjugate Chem. 2003;14:1191–1196. doi: 10.1021/bc034074m. [DOI] [PubMed] [Google Scholar]
- 28.Janout V, Jing B, Regen SL. Molecular umbrella-assisted transport of thiolated AMP and ATP across phospholipid bilayers. Bioconjugate Chem. 2002;13:351–356. doi: 10.1021/bc015564m. [DOI] [PubMed] [Google Scholar]
- 29.Janout V, Jing B, Staina IV, Regen SL. Selective transport of ATP across a phospholipid bilayer by a molecular umbrella. J. Am. Chem. Soc. 2003;125:4436–4437. doi: 10.1021/ja0291336. [DOI] [PubMed] [Google Scholar]
- 30.Janout V, Regen SL. A needle and thread approach towards bilayer transport: permeation of a molecular umbrella-oligonucleotide conjugate across a phospholipid membrane. J. Am. Chem. Soc. 2005;127:22–23. doi: 10.1021/ja044257z. [DOI] [PubMed] [Google Scholar]
- 31.Janout V, Jing B, Regen SL. Molecular umbrella-assisted transport of an oligonucleotide across cholesterol-rich phospholipid bilayers. J. Am. Chem. Soc. 2005;127:15862–15870. doi: 10.1021/ja053930x. [DOI] [PubMed] [Google Scholar]
- 32.Vijayaraghavan S, Jing B, Vrablik T, Chou TC, Regen SL. Enhanced hydrolytic stability and water solubility of an aromatic nitrogen mustard by conjugation with molecular umbrellas. Bioconjugate Chem. 2003;14:667–671. doi: 10.1021/bc034007s. [DOI] [PubMed] [Google Scholar]
- 33.Jing B, Janout V, Herold BC, Klotman ME, Heald T, Regen SL. Persulfated molecular umbrellas as anti-HIV and anti-HSV agents. J. Am. Chem. Soc. 2004;126:15930–15931. doi: 10.1021/ja044400o. [DOI] [PubMed] [Google Scholar]
- 34.Madan RP, Mesquita PMM, Cheshenko N, Jing B, Shende V, Guzman E, Healt T, Keller MJ, Regen SL, Shattock RJ, Herold BC. Molecular umbrellas: a novel class of candidate microbicides to prevent human immunodeficiency virus and herpes simplex virus infections. Journal of Virology. 2007;81:7636–7646. doi: 10.1128/JVI.02851-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehiri M, Jing B, Ringhoff D, Janout V, Cassimeris L, Regen SL. Cellular entry and nuclear targeting by a highly anionic molecular umbrella. Bioconjugate Chem. 2008;19:1510–1513. doi: 10.1021/bc8001826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whitmarsh SD, Redmond AP, Sgarlata V, Davis AP. Cationic cyclocholamides; toroidal amphiphiles with potential for anion transport. Chem. Commun. 2008:3669–3671. doi: 10.1039/b805777j. [DOI] [PubMed] [Google Scholar]
- 37.Paquet V, Zumbuehl A, Carreira EM. Biologically active amphotericin B— calix[4]arene conjugates. Bioconjugate Chem. 2006;17:1460–1463. doi: 10.1021/bc060205i. [DOI] [PubMed] [Google Scholar]
- 38.Matile S, Som A, Sorde N. Recent synthetic ion channels and pores. Tetrahedron. 2004;60:6405–6435. [Google Scholar]
- 39.Saki N, Mareda J, Matile S. Artificial β-barrels. Acc. Chem. Res. 2008 doi: 10.1021/ar700229r. Web: 07/01/2008. [DOI] [PubMed] [Google Scholar]