Abstract
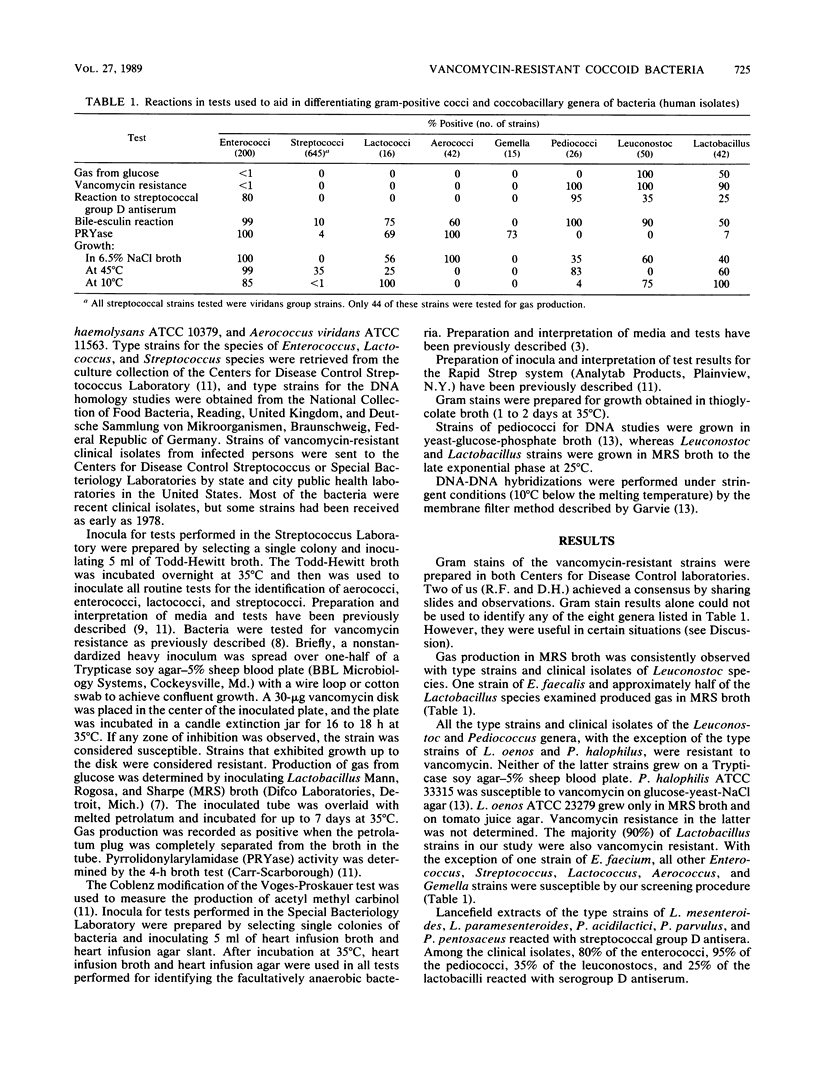
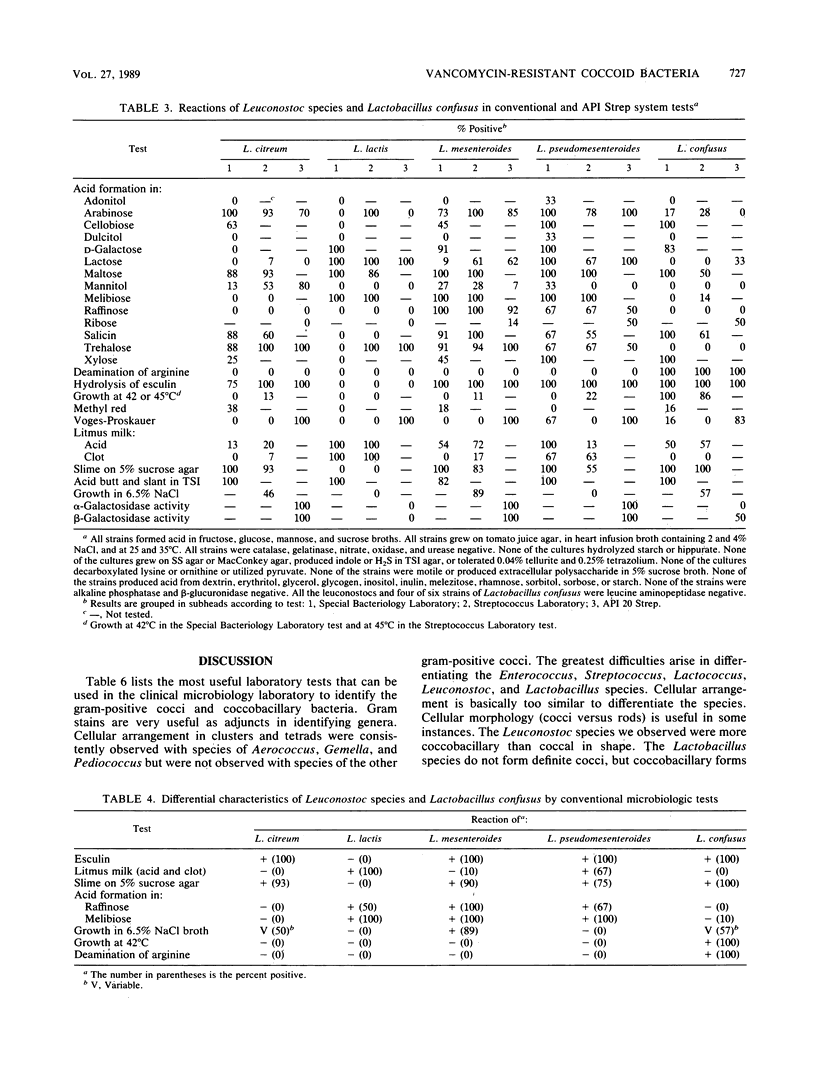
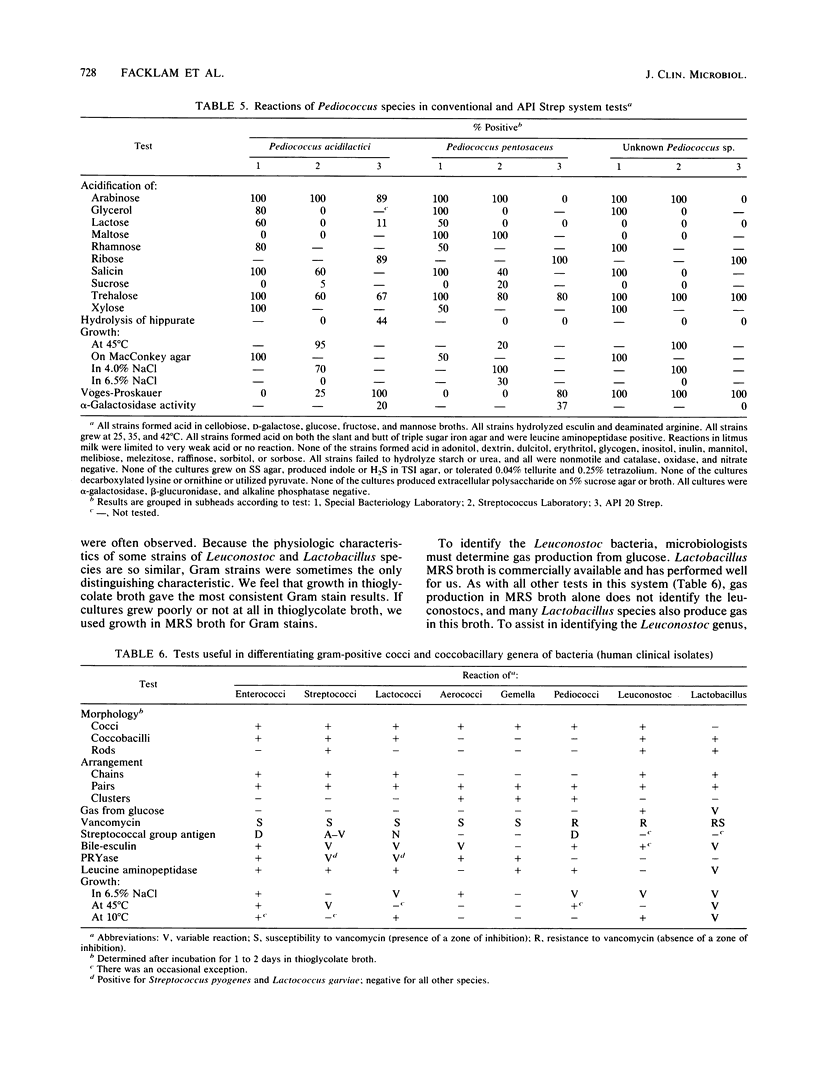
A total of 84 of 150 vancomycin-resistant (defined as no inhibition of bacterial growth around a 30-micrograms vancomycin disk placed on 5% sheep blood-Trypticase soy agar [BBL Microbiology Systems, Cockeysville, Md.]) bacteria were definitively identified by determining the phenotypic criteria. The identity of representatives was also confirmed by DNA-DNA hybridizations. The following strains were identified: 1 Enterococcus faecium, 18 Leuconostoc mesenteroides, 15 Leuconostoc citreum, 9 Leuconostoc pseudomesenteroides, 2 Leuconostoc lactis, 20 Pediococcus acidilactici, 5 Pediococcus pentosaceus, and 14 Lactobacillus confusus. The remaining vancomycin-resistant strains were identified as probable Leuconostoc (6 strains), probable Pediococcus (1 strain), and probable lactobacilli (28 strains). A total of 32 strains of gram-positive coccobacillary bacteria remained unidentified. Tests used for the phenotypic identification of strains to the genus level included a Gram stain of bacteria grown in thioglycolate broth, gas production in Lactobacillus Mann, Rogosa, and Sharpe broth, hydrolysis of pyrrolidonyl-beta-naphthylamide, bile-esculin reaction, demonstration of streptococcal group D antigen, and growth at 10 and 45 degrees C and in 6.5% NaCl broth. Strains were identified to the species level by hydrolysis of esculin, reactions in litmus milk, slime production on 5% sucrose agar, acidification of maltose, melibiose, and raffinose broths, deamination of arginine, and growth at 42 degrees C and in 6.5% NaCl broth.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buu-Hoï A., Branger C., Acar J. F. Vancomycin-resistant streptococci or Leuconostoc sp. Antimicrob Agents Chemother. 1985 Sep;28(3):458–460. doi: 10.1128/aac.28.3.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman G., Ball L. C. Identification of streptococci in a medical laboratory. J Appl Bacteriol. 1984 Aug;57(1):1–14. doi: 10.1111/j.1365-2672.1984.tb02351.x. [DOI] [PubMed] [Google Scholar]
- Colman G., Efstratiou A. Vancomycin-resistant leuconostocs, lactobacilli and now pediococci. J Hosp Infect. 1987 Jul;10(1):1–3. doi: 10.1016/0195-6701(87)90025-9. [DOI] [PubMed] [Google Scholar]
- Coovadia Y. M., Solwa Z., van den Ende J. Meningitis caused by vancomycin-resistant Leuconostoc sp. J Clin Microbiol. 1987 Sep;25(9):1784–1785. doi: 10.1128/jcm.25.9.1784-1785.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facklam R., Bosley G. S., Rhoden D., Franklin A. R., Weaver N., Schulman R. Comparative evaluation of the API 20S and AutoMicrobic gram-positive identification systems for non-beta-hemolytic streptococci and aerococci. J Clin Microbiol. 1985 Apr;21(4):535–541. doi: 10.1128/jcm.21.4.535-541.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fertally S. S., Facklam R. Comparison of physiologic tests used to identify non-beta-hemolytic aerococci, enterococci, and streptococci. J Clin Microbiol. 1987 Oct;25(10):1845–1850. doi: 10.1128/jcm.25.10.1845-1850.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg H. D., Vellozzi E. M., Shapiro J., Rubin L. G. Clinical laboratory challenges in the recognition of Leuconostoc spp. J Clin Microbiol. 1988 Mar;26(3):479–483. doi: 10.1128/jcm.26.3.479-483.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A. H., Gilligan P. H., Facklam R. R. Recovery of resistant enterococci during vancomycin prophylaxis. J Clin Microbiol. 1988 Jun;26(6):1216–1218. doi: 10.1128/jcm.26.6.1216-1218.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq R., Derlot E., Duval J., Courvalin P. Plasmid-mediated resistance to vancomycin and teicoplanin in Enterococcus faecium. N Engl J Med. 1988 Jul 21;319(3):157–161. doi: 10.1056/NEJM198807213190307. [DOI] [PubMed] [Google Scholar]
- Lütticken R., Kunstmann G. Vancomycin-resistant Streptococcaceae from clinical material. Zentralbl Bakteriol Mikrobiol Hyg A. 1988 Jan;267(3):379–382. doi: 10.1016/s0176-6724(88)80054-3. [DOI] [PubMed] [Google Scholar]
- Orberg P. K., Sandine W. E. Common occurrence of plasmid DNA and vancomycin resistance in Leuconostoc spp. Appl Environ Microbiol. 1984 Dec;48(6):1129–1133. doi: 10.1128/aem.48.6.1129-1133.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucci M. J., Monteschio M. E., Kemker C. L. Intergeneric and intrageneric conjugal transfer of plasmid-encoded antibiotic resistance determinants in Leuconostoc spp. Appl Environ Microbiol. 1988 Feb;54(2):281–287. doi: 10.1128/aem.54.2.281-287.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raccach M. Pediococci and biotechnology. Crit Rev Microbiol. 1987;14(4):291–309. doi: 10.3109/10408418709104442. [DOI] [PubMed] [Google Scholar]
- Rubin L. G., Vellozzi E., Shapiro J., Isenberg H. D. Infection with vancomycin-resistant "streptococci" due to Leuconostoc species. J Infect Dis. 1988 Jan;157(1):216–216. doi: 10.1093/infdis/157.1.216. [DOI] [PubMed] [Google Scholar]
- Ruoff K. L., Kuritzkes D. R., Wolfson J. S., Ferraro M. J. Vancomycin-resistant gram-positive bacteria isolated from human sources. J Clin Microbiol. 1988 Oct;26(10):2064–2068. doi: 10.1128/jcm.26.10.2064-2068.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlaes D. M., Marino J., Jacobs M. R. Infection caused by vancomycin-resistant Streptococcus sanguis II. Antimicrob Agents Chemother. 1984 Apr;25(4):527–528. doi: 10.1128/aac.25.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uttley A. H., Collins C. H., Naidoo J., George R. C. Vancomycin-resistant enterococci. Lancet. 1988 Jan 2;1(8575-6):57–58. doi: 10.1016/s0140-6736(88)91037-9. [DOI] [PubMed] [Google Scholar]



