Abstract
Event-related potentials (ERPs) were used to assess arousal (low, high), valence (negative, positive), and stimulus repetition effects for normal and distorted images from the International Affective Pictures System (IAPS). Distorted stimuli were constructed by dividing each image into 108 one cm squares and rearranging the segments randomly to produce a “scrambled” picture. Event-related potentials (ERPs) were elicited by presenting the normal and scrambled images as target stimuli, with a repeated visual pattern used as the standard stimulus. Participants (N=32) were instructed to press a button to the targets and ignore the standard. Stimulus repetition effects were assessed by presenting each stimulus twice in the normal and scrambled condition. High arousal stimuli yielded larger late positive components for both the normal and scrambled pictures. No overall valence effects were obtained, but arousal and valence influenced component amplitudes for middle-latency ERPs from the scrambled stimuli. For the normal pictures, stimulus repetition was associated with increased component amplitudes for all potentials and decreased RTs of all affective categories. For the scrambled pictures, no repetition changes were obtained. The findings suggest that stimulus arousal level contributes more than valence to affective ERP measures for normal as well as perceptually distorted pictures. Stimulus repetition engages memory for previous normal picture items but is not influenced by affective category[jo1]. Theoretical implications are discussed.
Keywords: International Affective Picture System, IAPS, event-related potentials (ERPs), P300, arousal, valence, repetition
1.0 Introduction
The perceptual system utilizes information gained through previous exposure to affective events thereby facilitating subsequent processing of similar stimuli. As event-related potentials (ERPs) can provide fine-grain temporal resolution of the underlying mechanisms, this neuroimaging method has become highly useful for assessing affective neural processing (Bradley et al., 1992; Dolcos and Cabeza, 2002; Ito et al., 1998; Lang et al., 1993, 1999). Although affective ERPs can be generated automatically by motivationally relevant picture motifs (appetitive, fearful, sexual), emotional stimuli also modulate ERPs even if the image content is not readily perceived (Codispoti et al., 2006; Schupp et al., 2006). The present study employs a stimulus repetition paradigm to characterize these issues by comparing affective pictures with “scrambled” image controls.
1.1. ERPs from affective picture stimuli
The International Affective Picture System (IAPS) often is used to provide visual images for assessing emotional outcomes (Lang et al., 1999). These pictures are rated for valence (unpleasant to pleasant) and arousal (calm to exciting) level, and these dimensions have been related to ERP modulations. The temporal course of valence and arousal effects differs, however, as valence influences are observed for early (100-250 ms) whereas arousal influences occur for later (250-850 ms) components (Codispoti et al., 2007; Olofsson et al., 2007). Similar outcomes have been obtained from a wide range of tasks, and as affectively potent stimuli modulate ERPs even when presented subconsciously these effects appear to occur automatically (Bernat et al., 2001; Cuthbert et al., 2000; Delplanque et al., 2006; Roschmann and Wittling, 1992).
Affective ERP effects have been obtained from passive viewing, affect discrimination procedures, and for images presented as distracting or target stimuli in an oddball task (Delplanque et al., 2004, 2005; Keil et al., 2002; Mini et al., 1996; Schupp et al., 2000). In general, negative affective valence produces stronger ERP modulation than positive affective valence pictures (Cacioppo et al., 1999; Crawford and Cacioppo, 2002; Öhman and Mineka, 2001). This “negativity-bias” may reflect rapid amygdala processing of aversive information (LeDoux, 1995; Morris et al., 1998; Ohman and Soares, 1998). Moreover, these early affective ERPs appear related to affective disposition such as trait anxiety and trait fearfulness (Dien, 1999), whereas late affective ERPs are sensitive to individual variation from emotional reappraisal and evaluation strategies (Hajcac et al., 2006, Moser et al., 2006).
In addition, when a P300 component from an affective stimulus is elicited, amplitudes are larger over the parietal cortex and increase with increases in arousal level. Hence, emotional arousal may amplify activity in cortical structures that are normally engaged for target processing (Polich, 2007; Sabatinelli et al., 2007). When arousal level across stimuli is well controlled valence can modulate P300 from frontal sites (Cano et al., 2007; Conroy and Polich, 2007; Delplanque et al., 2005, 2006), although it is unclear whether these effects consistently obtain when both factors are manipulated within the same session (Cuthbert et al., 2000). Despite evidence for ERP modulations from picture valence and arousal (Delplanque et al., 2004, 2006; Olofsson and Polich, 2007; Schupp et al., 2006), these fundamental affective factors are not typically varied systematically. For example, positive/negative valences with generally high arousal level often are compared with neutral valence stimuli that are low-arousing in a fashion that confounds their ERP assessment by differential stimulus probability.
1.2. Stimulus repetition effects
When a stimulus is presented repeatedly, processing changes can be expected because of variation in stimulus novelty, priming mechanisms, and habituation. Recognition memory ERP studies using neutral and affective stimuli have found increased amplitudes for late positive components when a stimulus reoccurs within the stimulus series (Bentin et al., 1992; Friedman, 1990; Olofsson and Polich, 2007; Rugg, 1990; Segalowitz et al., 1997; Smith and Halgren, 1989). Several affective studies have presented stimuli multiple times but collapsed over presentations to obtain their ERP averages, so that repetition effects cannot be assessed directly (Aftanas et al., 2001; Brazdil et al., 2003; Carretie et al., 2004a, 2004b; Ito et al., 1998; Keil et al., 2001; Schupp et al., 2000, 2004).
Olofsson and Polich (2007) found that successive repetition of affective target stimuli within an oddball sequence produced larger positive amplitudes for pleasant, unpleasant, and neutral stimuli from around 150 ms as well as evidence of priming with shorter P300 and response latencies across repetition trials. This repetition effect occurred independently of stimulus arousal level, with larger positivity from around 200 ms for high compared to low arousing stimuli. Further, N2 and P3 amplitude decreased overall across the experimental session, implying that amplitude habituation was independent of stimulus arousal level. Taken together, these findings suggest that arousal, repetition, and habituation appear to be produced independently of one another and overlap in time, although the time-course of affective stimulus repetition-induced memory effects over longer intervals is unknown.
1.3. Stimulus distortion
Current theories of affective ERP modulation emphasize stimuli with high evolutionary significance as automatic triggers of basic motivational systems whenever the stimuli are perceived (e.g., Schupp et al., 2006). Most ERP studies using affective pictures have employed stimulus durations of >500 ms, enabling a clear perception of picture contents. However, affective information also can be extracted during conditions of limited perception, such that ERP effects of arousal are conveyed in a stream of rapidly (120-333 ms) presented pictures (Junghöfer et al., 2001; Schupp et al., 2003, 2004, 2006). This method alters perceptual processing of affective content and alters higher-level semantic analysis of the picture content. This interpretation is supported by recent findings that physical attributes such as featural composition and complexity modulate affective ERPs perhaps by employing attentional mechanisms not specific to emotion (Bradley et al., 2007; Codispoti et al., 2006, 2007; Cano et al., 2007; Delplanque et al., 2007). Thus, if ERP affective modulations are preserved even when image is distorted the emotional information must be mediated by other perceptual processes.
1.4. Present study
The goals of the present study were to characterize affective valence and arousal processing across stimulus repetition of highly distinct IAPS stimuli and compared to visually distorted controls. IAPS images were selected in a way that the stimuli were either rated as comparatively low or high on arousal level, and relatively strong negative and positive valences selected within each arousal category. No neutral stimuli were employed. The chosen stimuli were presented as targets in an oddball paradigm, with normal pictures compared to their “scrambled” counterparts; and each stimulus occurred twice in each condition.
This approach was devised in order to: (1) assess valence and arousal effects under repetition circumstances within a target detection task[jo2], and (2) temporally track affective ERP effects for these variables across the waveform to characterize when and where they occur. As stimulus repetition is a common procedure and the physical stimulus properties contribute to outcomes, these factors were systematically manipulated to characterize the resulting ERP effects relative to previous studies. It was hypothesized that arousal would modulate the later ERP components, whereas valence would modulate the early ERP components. Valence effects should be found primarily over frontal electrodes, whereas arousal effects should be manifest over parietal areas. Stimulus repetition should produce larger later potentials for normal but not for unrecognizable scrambled pictures that also demonstrated an absence of affective outcomes.
2.0 Method
2.1. Participants
A total of 32 right-handed undergraduates (16 female) were employed as participants. All reported being free of neurological/psychiatric disorders, having normal or corrected-to-normal vision, and provided informed, written consent. Participants were compensated with course credit or $10 per hour.
2.2. Stimuli and procedure
The image stimuli for each of the four categories varied in arousal (low to high) and valence (negative to positive) according to the IAPS ratings, which were obtained using a 1-9 point scale by large cohorts of female and male undergraduates (Lang et al., 1995). The stimuli obtained consisted of 16 images of the four categories based on the mean female and male ratings. The mean (SD) arousal and valence ratings, respectively, were as follows: Low arousal-negative valence=4.46 (0.25) and 2.63 (0.30), high arousal-negative valence=6.76 (0.32) and 2.48 (0.30); low arousal-positive valence=4.45 (0.27) and 7.36 (0.29); high arousal-positive valence=6.68 (0.32) and 7.33 (0.29). Arousal and valence ratings were assessed with separate two-factor (2 arousal × 2 valence) analyses of variance. Low arousal pictures were rated lower on arousal than high arousal pictures, F(1,60)=4242.1, p<.00001; negative valence pictures were rated more negative than positive valence pictures, F(1,60)=971.8, p<.00001; no interactions were obtained (p>0.40, both cases).
These pictures were organized into a 9 × 12 cm format and served as target stimuli presented to each subject in an oddball task, with p=0.40 for a picture. A red and white checked standard image of equal size and a pattern designed to equate visual spatial frequency to the target occurred with p=0.60. This stimulus was adapted from previous reports (Delplanque et al., 2004, 2005, 2006). Additional “scrambled” target images were constructed by randomly rearranging 0.75 cm2 areas of each image (192 square blocks), which largely maintains the picture's visual characteristics (color, spatial frequency, intensity) but eliminates its content. The Appendix lists the IAPS number of each stimulus and presents examples of a normal and scrambled stimulus from each category.
The subjects sat in a chair with 75 cm between the front edge of the chair and the computer screen. All stimuli were displayed for a duration of 1000 ms at the center of a computer screen on a light grey background at normal viewing luminance. A 2000 ms inter-stimulus interval was used. Participants were instructed to respond with a mouse-click using their dominant right hand when a target image was presented and to refrain from responding when the standard stimulus was presented. The 64 target pictures (16 for each of the four affective stimulus types) occurred randomly among 96 presentations of the standard image, resulting in a total of 160 trials. The scrambled pictures occurred as targets in a separate condition. Each stimulus set was presented twice using a different random order to yield 32 ERP trials for each subject and stimulus type, with half the subjects receiving the normal pictures first and the other half receiving the scrambled pictures first. Error rate and response time were recorded.
2.3. Recording conditions
Electroencephalographic (EEG) activity was recorded from 21 electrode sites including Fz, Cz, Pz, Fp1/2, F3/4, F7/8, C3/4, T7/8, P3/4, P7/8, O1/2, referenced to linked earlobes, a forehead ground, and impedances of 10KΩ or less, with the reference electrodes balanced. Additional electrodes were placed at the outer canthi as well as above and below the left eye to assess electro-ocular (EOG) activity with a bipolar recording. The bandpass was 0.01-30 Hz (6 dB/octave), and the EEG was digitized at 4.0 ms per point for 1024 ms, with a 100 ms pre-stimulus baseline. Waveforms were averaged off-line, such that trials on which the EEG or EOG exceeded ±100 μV were rejected. Single-trial data also were subjected to an EOG correction procedure to remove any remaining artifact. Rest periods were provided as needed.
3.0 Results
3.1 Behavioral data
Error rate (ER) was defined as the percent of incorrect responses, was less than 1%, and is not considered further. Response time (RT) was defined as the time from stimulus onset to the button press response, and the mean RT was computed over stimulus trials for each subject from each condition across stimulus blocks. RT was assessed with a four-factor (2 arousal × 2 valence × 2 repetition × 2 stimulus type) analysis of variance. Mean RT did not differ among arousal (p>.70) or valence (p>.30) conditions (low arousal-negative valence=509, low arousal-positive valence=502, high arousal-negative valence=504, high arousal-positive valence=502 ms, respectively, p>.15). RT was longer for the first compared to second repetition, F(1,31)=12.7, p<.002, and longer for the normal compared to scrambled stimulus items, F(1,31)=16.2, p<.001. RTs for normal pictures demonstrated a significant 45 ms decrease across repetitions, whereas scrambled pictures resulted in a non-significant change (5 ms decrease), yielding a repetition by stimulus type interaction, F(1,31)=6.7, p<.02. Further, RT from the normal stimuli decreased similarly across arousal and valence levels, but the scrambled stimuli yielded short RT and a stronger decrease for negative stimuli with longer RT[jo3] and less of a decrease for positive stimuli to produce a three-way interaction among arousal by valence by stimulus type, F(1,31)=4.8, p<.05. The origin of this weak behavioral interaction is unknown.
3.2 ERP analyses
After excluding error and artifact trials, the mean number of ERP averages for each stimulus condition across stimulus type for repetition block-1 was 14.7, 14.9, 14.7, 14.9, and for repetition block-2 was 15.1, 14.8, 14.8, 14.8, trials for the low arousal-negative, high-arousal negative, low-arousal positive, and high arousal-positive waveforms, respectively. A four-factor (2 arousal × 2 valence × 2 repetition × 2 stimulus type) analysis of variance performed on the number of trials found no differences for arousal (F<1, p>0.80), valence (F<1, p>0.50), repetition (F<1, p>0.15), or stimulus type (F=2.5, p>0.12). Thus, trial number did not differ among conditions.
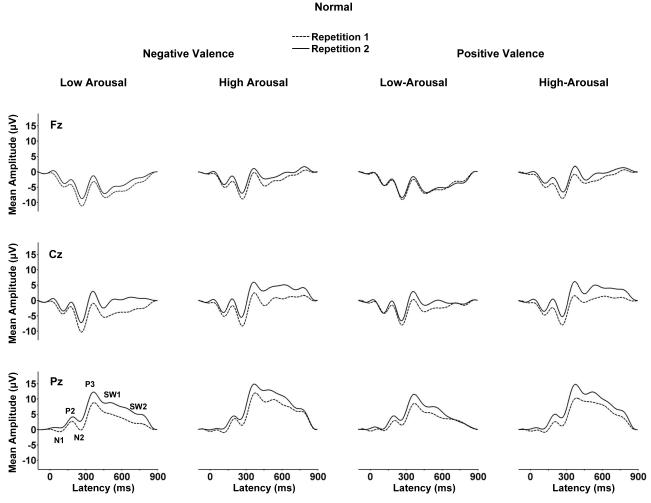
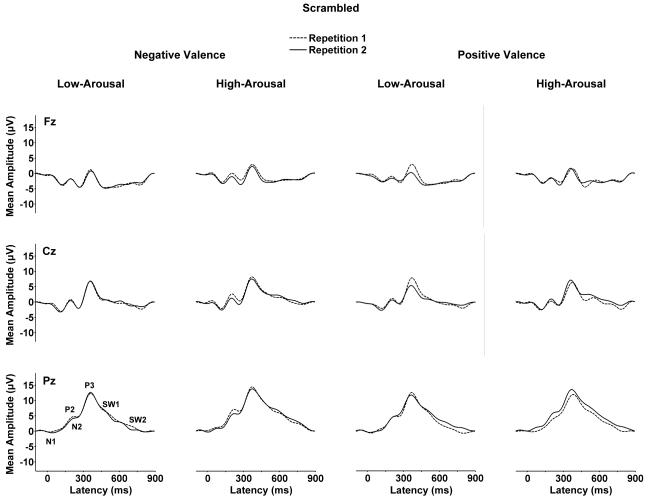
Figure 1 illustrates the grand averages from the normal picture images. Figure 2 illustrates the grand averages from the scrambled images for each arousal and valence category. Averages from each repetition block overlapped for each of the midline electrodes. The ERP components were analyzed by measuring mean waveform amplitude relative to the pre-stimulus baseline within component time windows: N1=120-160 ms, P2=160-220, N2=220-300, P3=300-450 ms, early slow wave=550-700, late slow wave=700-850 ms.
Figure 1.
Grand averages of the normal picture low-arousal and high-arousal stimuli for the negative valence and positive valence conditions from the repetition 1 and repetition 2 trial blocks (n=32).
Figure 2.
Grand averages of the scrambled picture low-arousal and high-arousal stimuli for the negative valence and positive valence conditions from the repetition 1 and repetition 2 trial blocks (n=32).
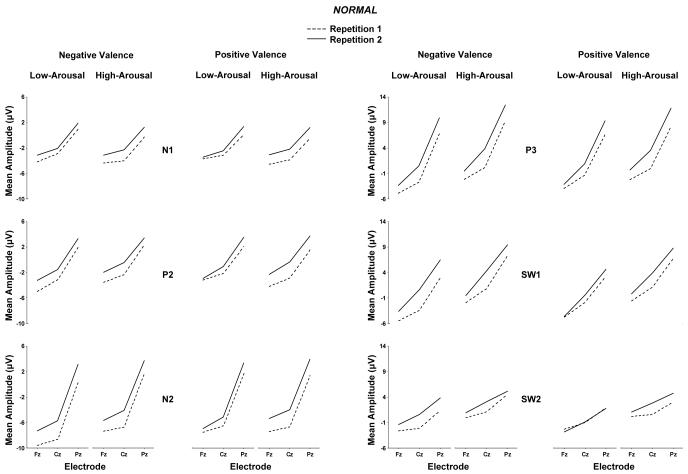
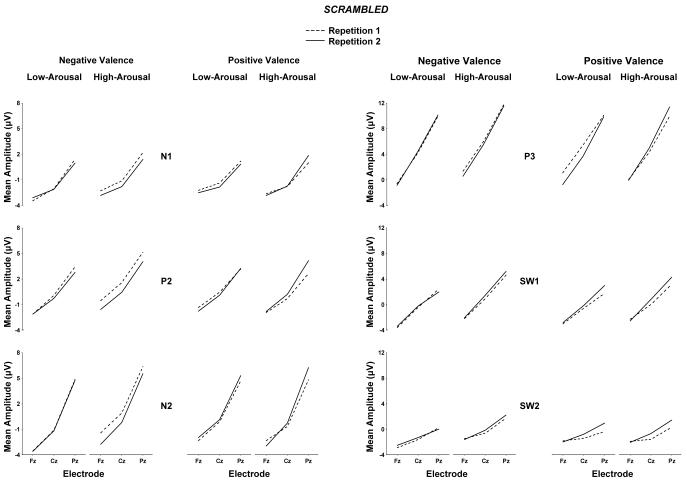
Figure 3 and Figure 4 illustrate the mean component amplitude from the normal and scrambled images, respectively, for each experimental variable at the midline electrode location. Preliminary analyses were conducted to assess the extent to which arousal and valence outcomes were affected by repetition and stimulus type manipulations. No reliable effects for stimulus type order (normal vs. scrambled) were obtained, and this factor will not be considered further. Data were then assessed for the normal and scrambled conditions separately to facilitate interpretation of the findings by applying a four-factor repeated measures analysis of variance (2 arousal × 2 valence × 2 repetition × 3 midline electrodes) on the mean component amplitude from each subject. Greenhouse-Geisser corrections to the df were applied as needed, with the corrected probabilities reported. Additional analyses of hemispheric, frontal, and occipital electrodes were performed, but these did not reveal outcomes that differed appreciably from the overall findings.
Figure 3.
Normal pictures: Mean amplitude for the N1, P2, N2, P3, early slow wave, and later slow wave components from the low-arousal and high-arousal for the negative valence and positive valence stimulus conditions as a function of midline electrode with repetition 1 and repetition 2 trial blocks overlapped.
Figure 4.
Scrambled pictures: Mean amplitude for the N1, P2, N2, P3, early slow wave, and later slow wave components from the low-arousal and high-arousal for the negative valence and positive valence stimulus conditions as a function of midline electrode with repetition 1 and repetition 2 trial blocks overlapped.
Table 1 summarizes the results of the primary analyses of variance performed separately on the data from the normal and scrambled stimulus presentations. The table is organized to permit direct comparison between the two stimulus types with respect to the major independent variables of arousal, valence, and repetition. The electrode main effect reflected increased amplitudes from the frontal to parietal sites for all potentials across normal and scrambled stimulus conditions and will not be considered further.
Table 1.
Summary of analyses of variance (2 arousal × 2 valence × 2 repetition × 3 electrodes) performed separately on the mean amplitude data for each component and stimulus type (normal vs. scrambled) separately.
| Normal |
Scrambled |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N1 | P2 | N2 | P3 | SW1 | SW2 | N1 | P2 | N2 | P3 | SW1 | SW2 | |
| Arousal (1,31) | -- | -- | 8.4** | 41.6*** | 75.8*** | 44.2*** | -- | -- | -- | 4.4* | 12.4** | 5.5* |
| Valence (1,31) | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Repetition (1,31) | 17.2*** | 19.9*** | 34.1*** | 39.9*** | 23.4*** | 11.2*** | -- | -- | -- | -- | -- | -- |
| Electrode (2,62) | 51.7*** | 56.0*** | 118.5*** | 189.8*** | 156.6*** | 49.7*** | 42.5*** | 51.9*** | 96.2*** | 182.6*** | 73.9*** | 28.4*** |
| A × V (1,31) | -- | -- | -- | -- | -- | -- | -- | 6.6* | 4.5* | 6.2* | -- | -- |
| A × R (1,31) | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| V × R (1,31) | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| A × E (2,62) | -- | -- | 5.9** | -- | -- | 3.6* | -- | -- | -- | -- | 5.6** | 3.3* |
| V × E (2,62) | -- | -- | -- | 8.9*** | 4.9* | 4.2* | -- | -- | -- | -- | -- | -- |
| R × E (2,62) | -- | -- | -- | 13.5*** | 11.1*** | 7.8** | -- | -- | -- | -- | -- | -- |
| A × V × R (1,31) | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| A × V × E (2,62) | -- | -- | -- | -- | -- | -- | -- | -- | 4.4* | -- | -- | -- |
| A × R × E (2,62) | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| V × R × E (2,62) | -- | 3.5* | -- | -- | -- | -- | -- | 3.8* | -- | -- | -- | 3.5* |
| A × V × R × E (2,62) | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
p<0.05
p<0.01
p<0.001
Arousal
As evident from Figures 3 and 4, high arousal stimuli produced larger late positive potentials compared to low arousal stimuli, for both the normal and scrambled stimulus conditions. These arousal effects were more robust for the normal compared to scrambled images. Arousal and electrode position evinced interactions, such that the largest arousal effects were observed over the parietal electrode for potentials in the normal and scrambled conditions.
Valence
No main effects for valence category were obtained for any potential for either the normal or scrambled stimulus conditions. However, for the scrambled stimulus condition arousal and valence interacted reliably for the P2, N2, and P3 potentials. In general, the component amplitude differences for low compared to high arousal stimuli were somewhat greater for negative compared to positive valence stimuli. The valence and electrode factors yielded interactions for the later potentials only in the normal stimulus condition: Larger differences between valence categories appeared to occur over parietal compared to the frontal and central electrode locations.
Repetition
For the normal image pictures, the first stimulus repetition produced smaller potentials for all components compared to the second stimulus repetition as shown in Figure 3. No main effect of repetition was obtained for the scrambled pictures as shown in Figure 4. For the later components from the normal stimuli, repetition and electrode position interacted, such that repetition amplitudes were larger over the parietal compared to frontal/medial electrodes. The repetition factor also yielded interactions with valence effects for a few potentials from both the normal and scrambled stimuli. However, these interactions were statistically weak and evinced no discernible pattern.
4.0 Discussion
This study employed IAPS images that varied systematically in arousal (low vs. high) and valence (negative vs. positive) as targets in an oddball task to elicit ERPs. Since all stimuli occurred twice, the neuroelectric characteristics of stimulus repetition were obtained for these two affective parameters. Further, stimuli were presented in a separate session as scrambled images to investigate whether stimulus affect can be conveyed from the IAPS without access to global picture motifs. The major findings were that stimulus arousal modulated amplitude of the later components for both normal and scrambled pictures. No overall valence amplitude effects were obtained. Repetition of normal pictures was associated with increased amplitudes for all ERP components and decreased RTs independently of affective stimulus category. For normal stimulus pictures, strong arousal and repetition effects were obtained in the absence of independent valence effects. For the scrambled pictures, arousal effects were found in ERP amplitudes. Repetition of scrambled pictures had no effect on ERPs or RTs.
4.1. Arousal and valence
High-arousal stimuli evinced larger mean amplitudes compared to low-arousal stimuli (Carretie et al., 2004b; Delplanque et al., 2006; Schupp et al., 2000). These effects began approximately with the N2, became appreciably stronger for the P3 and early SW component, and were still robust for the late SW portion of the waveform as has been previously found (Olofsson and Polich, 2007). Arousal level also increased the amplitude of the later potentials for the scrambled pictures, although the effects were statistically weaker than those from the normal pictures. No overall valence effects were obtained for either stimulus type, but arousal level and valence interacted for several components elicited by the scrambled stimuli.
A novel finding in this study is that highly arousing pictures still produce an elevated late ERP positivity for the scrambled stimulus images. The distorted pictures were made from the same IAPS pictures; with each picture segmented into squares and randomly rearranged. These distorted stimuli are unrecognizable and yielded no repetition memory effects for either RT or ERPs components, which were found for the normal pictures. These outcomes are consistent with previous studies that altered stimulus perception by quick serial presentation of affective pictures, as arousing stimuli appear to cause rapid cortical modulations even when picture content cannot be accessed due to perceptual limitations (Junghöfer et al., 2001; Schupp et al., 2003, 2006). These and the present results imply that physical stimulus properties (e.g., color, contrast, texture) can produce arousal ERP effects. Although the exact mechanisms of these influences are unknown, relatively low-level perceptual features of affective pictures appear to contribute to ERP arousal outcomes (Codispoti et al., 2006; Delplanque et al., 2007).
4.2. Stimulus repetition
Stimulus repetition of the normal pictures produced increased component amplitudes from the first to the second trial block, with no repetition main effects found for scrambled images. Recognition memory ERP studies have demonstrated increased amplitudes for late positive components when a stimulus is recognized as having been presented previously in the series (Bentin et al., 1992; Friedman, 1990; Rugg, 1990). Successively repeated emotional stimuli used as targets in an oddball task produced similar amplitude increases from the first to second repetition (Olofsson and Polich, 2007). The current results corroborate previous findings and extend the repetition effect to non-successive and unpredictable stimulus sequences (Aftanas et al., 2001; Carretie et al., 2001a, 2003, 2004a; Ito et al., 1998; Keil et al., 2001; Schupp et al., 2000, 2004). Moreover, the lack of interaction with the arousal or valence factors indicates that the modulation of rapid brain responses to affective images is stable over repetitions, so that ERP modulations from reoccurring affective picture stimuli are unlikely to be confounded by repeated stimulus presentations. That the repetition effects of ERP amplitudes and RTs occurred for the normal but not the scrambled images suggest that only normal images that can be recognized are subject to amplitude increase due to repetition.
4.3. Theoretical perspective
The straightforward nature of the present task with the same response for each target stimulus in the absence of any emotive judgments implies that the obtained ERP effects were produced automatically (Bernat et al., 2001; Olofsson and Polich, 2007; Schupp et al., 2000). Stimulus arousal influenced late component amplitudes from both normal and scrambled stimuli and implies that fundamental perceptual attributes are possible causes of arousal-related processing modulations, which were statistically weaker for the scrambled stimulus conditions. The variation for component amplitude between arousal and valence levels observed for the scrambled stimuli in the middle latency range also seem to reflect automatic featural processing of affective properties.[jo4] The exact mechanisms by which arousal-related ERP modulations are preserved in distorted images are unknown. However, the results from this and other studies suggest that physical stimulus characteristics (e.g., color, spatial frequency, local/global features) can convey arousing properties (Bradley et al 2007; Cano et al., 2007; Codispoti et al 2006, 2007). A systematic integration of these findings should emerge as additional results accumulate (Olofsson et al. 2007).
Although the origins of arousal/valence interaction effects in the scrambled images remain vague, they may result in part from the use of the extreme negative- and positive-valence ratings coupled with the extreme low- and high-arousal levels used to select the present stimuli. Perhaps normal picture stimuli did not yield this interaction because these images are necessarily perceived as a whole percept rather than the individual components seen in scrambled images. Capturing the image in its entirety could override basic perceptual influences by which valence and arousal interacts (e.g., hue or brightness). Further, cognitive operations are likely to be utilized more efficiently for normal pictures compared to scrambled images, which would enhance memory performance and increase P300/slow wave amplitude (Azizian and Polich, 2007; Karis et al., 1984; Paller et al., 1988). Such ERP amplitude changes have been obtained when affective memory formation is assessed (Dolcos and Cabeza, 2002; Palomba et al., 1997). The present results indicate that emotional stimulus properties also can be conveyed without access to affective content, such that stimulus cues apparently can modulate ERPs when perceptual quality is limited or altered.
Acknowledgements
The first author received an Undergraduate Research Fellowship from University of California, San Diego and is now in the Neuroscience Graduate Program at the University of Southern California. The second author was supported by a fellowship from the American-Scandinavian Foundation and Thord-Gray Memorial Fund. This study was supported by RO1-DA018262 and P50-AA06420. This paper is publication number 18822 from The Scripps Research Institute.
Appendix
Stimulus Category, Numbers of Selected IAPS Pictures, and Sample Normal and Scrambled Picture Stimuli* The images were presented in color.
| Arousal-Valence Category | Selected IAPS Images | Normal | Scrambled |
|---|---|---|---|
| Low- Negative | 2205, 2276, 2375.1, 2455, 2750, 2900.1, 3300, 9000, 9220, 9265, 9280, 9290, 9320, 9330, 9342, 9561 |  |
 |
| High-Negative | 2730, 3030, 3400, 6230, 6250, 6260, 6300, 6313, 6350, 6370, 6510, 6530, 6550, 8485, 9250, 9600 |  |
 |
| Low-Positive | 1463, 1500, 1510, 1590, 1721, 1920, 2080, 2224, 2331, 2395, 2530, 5594, 5820, 7280, 7480, 8461 |  |
 |
| High-Positive | 4607, 4608, 4660, 4670, 4680, 5621, 5629, 8030, 8080, 8180, 8185, 8186, 8370, 8400, 8490, 8501 |  |
 |
The images were presented in color.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aftanas LI, Varlamov AA, Pavlov SV, Makhnev VP, Reva NV. Affective picture processing: Event-related synchronization within individually defined human theta band is modulated by valence dimension. Neuroscience Letters. 2001;303:115–118. doi: 10.1016/s0304-3940(01)01703-7. [DOI] [PubMed] [Google Scholar]
- Azizian A, Polich J. Evidence for attentional gradient in the serial position memory curve from ERPs. Journal of Cognitive Neuroscience. 2007 doi: 10.1162/jocn.2007.19.12.2071. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentin S, Moscovitch M, Heth I. Memory with and without awareness: Performance and electrophysiological evidence of savings. Journal of Experimental Psychology: Learning, Memory, & Cognition. 1992;18:1270–1283. doi: 10.1037//0278-7393.18.6.1270. [DOI] [PubMed] [Google Scholar]
- Bernat E, Bunce S, Shevrin H. Event-related brain potentials differentiate positive and negative mood adjectives during both supraliminal and subliminal visual processing. International Journal of Psychophysiology. 2001;42:11–34. doi: 10.1016/s0167-8760(01)00133-7. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Greenwald MK, Petry MC, Lang PJ. Remembering pictures: Pleasure and arousal in memory. Journal of Experimental Psychology: Learning, Memory, & Cognition. 1992;18:379–390. doi: 10.1037//0278-7393.18.2.379. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Hamby S, Low A, Lang PJ. Brain potentials in perception: picture complexity and emotional arousal. Psychophysiology. 2007;44:364–373. doi: 10.1111/j.1469-8986.2007.00520.x. [DOI] [PubMed] [Google Scholar]
- Brazdil M, Roman R, Daniel P, Rektor I. Intracerebral somatosensory event-related potentials: Effect of response type (button pressing versus mental counting) on P3-like potentials within the human brain. Clinical Neurophysiology. 2003;114:1489–1496. doi: 10.1016/s1388-2457(03)00135-4. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Gardner WL, Berntson GG. The affect system has parallel and integrative processing components: form follows function. Journal of Personality and Social Psychology. 1999;76:839–855. [Google Scholar]
- Cano ME, Class QA, Polich J. Affective valence, stimulus attributes, and P300: Color vs. black/white and normal vs. scrambled images. International Journal of Psychophysiology. 2007 doi: 10.1016/j.ijpsycho.2008.07.016. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy MA, Polich J. Affective valence and P300 when stimulus arousal level is controlled. Cognition & Emotion. 2007;21:891–901. [Google Scholar]
- Carretie L, Hinojosa JA, Martin-Loeches M, Mercado F, Tapia M. Automatic attention to emotional stimuli: Neural correlates. Human Brain Mapping. 2004a;22:290–299. doi: 10.1002/hbm.20037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carretie L, Hinojosa JA, Mercado F. Cerebral patterns of attentional habituation to emotional visual stimuli. Psychophysiology. 2003;40:381–388. doi: 10.1111/1469-8986.00041. [DOI] [PubMed] [Google Scholar]
- Carretie L, Martin-Loeches M, Hinojosa JA, Mercado F. Emotion and attention interaction studied through event-related potentials. Journal of Cognitive Neuroscience. 2001a;13:1109–1128. doi: 10.1162/089892901753294400. [DOI] [PubMed] [Google Scholar]
- Carretie L, Mercado F, Hinojosa JA, Martin-Loeches M, Sotillo M. Valence-related vigilance biases in anxiety studied through event-related potentials. Journal of Affective Disorders. 2004b;78:119–130. doi: 10.1016/s0165-0327(02)00242-2. [DOI] [PubMed] [Google Scholar]
- Carretie L, Mercado F, Tapia M, Hinojosa JA. Emotion, attention, and the 'negativity bias', studied through event-related potentials. International Journal of Psychophysiology. 2001b;41:75–85. doi: 10.1016/s0167-8760(00)00195-1. [DOI] [PubMed] [Google Scholar]
- Codispoti M, Ferrari V, Bradley MM. Repetition and event-related potentials: distinguishing early and late processes in affective picture perception. Journal of Cognitive Neuroscience. 2007;19:577–586. doi: 10.1162/jocn.2007.19.4.577. [DOI] [PubMed] [Google Scholar]
- Codispoti M, Ferrari V, De Cesarei A, Cardinale R. Implicit and explicit categorization of natural scenes. Progress in Brain Research. 2006;156:53–65. doi: 10.1016/S0079-6123(06)56003-0. [DOI] [PubMed] [Google Scholar]
- Crawford LE, Cacioppo JT. Learning where to look for danger: integrating affective and spatial information. Psychological Science. 2002;13:449–453. doi: 10.1111/1467-9280.00479. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ. Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biological Psychology. 2000;52:95–111. doi: 10.1016/s0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- Delplanque S, Lavoie ME, Hot P, Silvert L, Sequeira H. Modulation of cognitive processing by emotional valence studied through event-related potentials in humans. Neuroscience Letters. 2004;356:1–4. doi: 10.1016/j.neulet.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Delplanque S, Silvert L, Hot P, Rigoulot S, Sequeira H. Arousal and valence effects on event-related P3a and P3b during emotional categorization. International Journal of Psychophysiology. 2006;60:315–322. doi: 10.1016/j.ijpsycho.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Delplanque S, Silvert L, Hot P, Sequeira H. Event-related P3a and P3b in response to unpredictable emotional stimuli. Biological Psychology. 2005;68:107–120. doi: 10.1016/j.biopsycho.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Delplanque S, N'diaye K, Scherer K, Granjean D. Spatial frequencies or emotional effects? A systematic measure of spatial frequencies for IAPS pictures by a discrete wavelet analysis. J Neurocience Methods. 2007 doi: 10.1016/j.jneumeth.2007.05.030. in press. [DOI] [PubMed] [Google Scholar]
- Dien J. Differential lateralization of trait anxiety and trait fearfulness: Evoked potential correlates. Personality and Individual Differences. 1999;26:333–356. [Google Scholar]
- Dolcos F, Cabeza R. Event-related potentials of emotional memory: Encoding pleasant, unpleasant, and neutral pictures. Cognitive, Affective and Behavioral Neuroscience. 2002;2:252–263. doi: 10.3758/cabn.2.3.252. [DOI] [PubMed] [Google Scholar]
- Friedman D. Cognitive event-related potential components during continuous recognition memory for pictures. Psychophysiology. 1990;27:136–148. doi: 10.1111/j.1469-8986.1990.tb00365.x. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Nieuwenhuis S. Reappraisal modulates the electrocortical response to unpleasant pictures. Cognitive Affective and Behavioral Neuroscience. 2006 doi: 10.3758/cabn.6.4.291. [DOI] [PubMed] [Google Scholar]
- Ito TA, Cacioppo JT, Lang PJ. Eliciting affect using the international affective picture system: Trajectories through evaluative space. Personality and Social Psychology Bulletin. 1998;24:855–879. [Google Scholar]
- Junghöfer M, Bradley MM, Elbert TR, Lang PJ. Fleeting images: A new look at early emotion discrimination. Psychophysiology. 2001;38:175–178. [PubMed] [Google Scholar]
- Karis D, Druckman D, Lissak R, Donchin E. A psychophysiological analysis of bargaining. ERPs and facial expressions. Annals of the New York Academy of Sciences. 1984;425:230–235. doi: 10.1111/j.1749-6632.1984.tb23539.x. [DOI] [PubMed] [Google Scholar]
- Keil A, Bradley MM, Hauk O, Rockstroh B, Elbert T, Lang PJ. Large-scale neural correlates of affective picture processing. Psychophysiology. 2002;39:641–649. doi: 10.1017.S0048577202394162. [DOI] [PubMed] [Google Scholar]
- Keil A, Muller MM, Gruber T, Wienbruch C, Stolarova M, Elbert T. Effects of emotional arousal in the cerebral hemispheres: A study of oscillatory brain activity and event-related potentials. Clinical Neurophysiology. 2001;112:2057–2068. doi: 10.1016/s1388-2457(01)00654-x. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Instruction manual and affective ratings. University of Florida; Gainsville: 1999. [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: Affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30:261–273. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Cognitive emotional interactions in the brain. Cognition & Emotion. Special Issue: Development of emotion cognition relations. 1989;3:267–289. [Google Scholar]
- LeDoux JE. Emotion: clues from the brain. Annual Review of Psychology. 1995;46:209–235. doi: 10.1146/annurev.ps.46.020195.001233. [DOI] [PubMed] [Google Scholar]
- Mini A, Palomba D, Angrilli A, Bravi S. Emotional information processing and visual evoked brain potentials. Perceptual and Motor Skills. 1996;83:143–152. doi: 10.2466/pms.1996.83.1.143. [DOI] [PubMed] [Google Scholar]
- Morris JS, Öhman A, Dolan RJ. Conscious and unconscious emotional learning in the human amygdala. Nature. 1998;393:467–470. doi: 10.1038/30976. [DOI] [PubMed] [Google Scholar]
- Moser E, Hajcac G, Bukay E, Simons RF. Intentional modulation of emotional responding to unpleasant pictures: An ERP study. Psychophysiology. 2006;43:292–296. doi: 10.1111/j.1469-8986.2006.00402.x. [DOI] [PubMed] [Google Scholar]
- Öhman A, Mineka S. Fears, phobias, and preparedness: Toward an evolved module of fear and fear learning. Psychological Review. 2001;108:483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- Öhman A, Soares JJ. Emotional conditioning to masked stimuli: Expectancies for aversive outcomes following nonrecognized fear-relevant stimuli. J Exp Psychol:Gen. 1998;127:69–82. doi: 10.1037//0096-3445.127.1.69. [DOI] [PubMed] [Google Scholar]
- Olofsson JK, Nordin S, Sequeira H, Polich J. Affective picture processing: An integrative review of ERP findings. 2007 doi: 10.1016/j.biopsycho.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson JK, Polich J. Affective visual event-related potentials: Arousal, repetition, and time-on-task. Biological Psychology. 2007 doi: 10.1016/j.biopsycho.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paller KA, Mccarthy G, Wood CC. ERPs predictive of subsequent recall and recognition performance. Biological Psychology. 1988;26:269–276. doi: 10.1016/0301-0511(88)90023-3. [DOI] [PubMed] [Google Scholar]
- Palomba D, Angrilli A, Mini A. Visual evoked potentials, heart rate responses and memory to emotional pictorial stimuli. International Journal of Psychophysiology. 1997;27:55–67. doi: 10.1016/s0167-8760(97)00751-4. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clinical Neurophysiology. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roschmann R, Wittling W. Topographic brain mapping of emotion-related hemisphere asymmetries. International Journal of Neuroscience. 1992;63:5–16. doi: 10.3109/00207459208986656. [DOI] [PubMed] [Google Scholar]
- Rugg MD. Event-related brain potentials dissociate repetition effects of high- and low-frequency words. Memory and Cognition. 1990;18:367–379. doi: 10.3758/bf03197126. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D, Lang PJ, Keil A, Bradley MM. Emotional perception: correlation of functional MRI and event-related potentials. Cerebral Cortex. 2007;17:1085–1091. doi: 10.1093/cercor/bhl017. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Cacioppo JT, Ito T, Lang PJ. Affective picture processing: The late positive potential is modulated by motivational relevance. Psychophysiology. 2000;37:257–261. [PubMed] [Google Scholar]
- Schupp HT, Flaisch T, Stockburger J, Junghöfer M. Emotion and attention: Event-related brain potential studies. Progress in Brain Research. 2006;156:31–51. doi: 10.1016/S0079-6123(06)56002-9. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Junghöfer M, Weike AI, Hamm AO. Attention and emotion: An ERP analysis of facilitated emotional stimulus processing. Neuroreport. 2003;14:1107–1110. doi: 10.1097/00001756-200306110-00002. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Junghöfer M, Weike AI, Hamm AO. The selective processing of briefly presented affective pictures: An erp analysis. Psychophysiology. 2004;41:441–449. doi: 10.1111/j.1469-8986.2004.00174.x. [DOI] [PubMed] [Google Scholar]
- Segalowitz SJ, Van Roon P, Dywan J. The ERP late positivity: A graduated response to stimulus repetition. Neuroreport. 1997;8:757–760. doi: 10.1097/00001756-199702100-00035. [DOI] [PubMed] [Google Scholar]
- Smith ME, Halgren E. Dissociation of recognition memory components following temporal lobe lesions. Journal of Experimental Psychology. 1989;15:50–60. doi: 10.1037//0278-7393.15.1.50. [DOI] [PubMed] [Google Scholar]