Abstract
Diverse functions have been assigned to the visual appearance of webs, spiders and web decorations, including prey attraction, predator deterrence and camouflage. Here, we review the pertinent literature, focusing on potential camouflage and mimicry. Webs are often difficult to detect in a heterogeneous visual environment. Static and dynamic web distortions are used to escape visual detection by prey, although particular silk may also attract prey. Recent work using physiological models of vision taking into account visual environments rarely supports the hypothesis of spider camouflage by decorations, but most often the prey attraction and predator confusion hypotheses. Similarly, visual modelling shows that spider coloration is effective in attracting prey but not in conveying camouflage. Camouflage through colour change might be used by particular crab spiders to hide from predator or prey on flowers of different coloration. However, results obtained on a non-cryptic crab spider suggest that an alternative function of pigmentation may be to avoid UV photodamage through the transparent cuticle. Numerous species are clearly efficient locomotory mimics of ants, particularly in the eyes of their predators. We close our paper by highlighting gaps in our knowledge.
Keywords: camouflage, mimicry, visual cues, pigment, spider, predator
1. Introduction
This paper aims at a broad exploration of the literature pertinent to the subject as defined in the title. Several functions have been assigned to spider web decorations, the most extensively studied being visually related, such as camouflage from predators and/or prey, prey attraction and signalling to animals that are likely to damage the web (Herberstein et al. 2000; Bruce 2006). The function of these structures is highly controversial, as well as other visual aspects of spider ecology, as is the appearance of spiders themselves. Moreover, few spider species have the ability to change their body coloration, a peculiarity that has been suggested to improve camouflage or to constitute a form of aggressive mimicry (Oxford & Gillespie 1998). Are such visual appearances used to lure prey, deter predators or hide from predators or prey?
In this study, we carry out a critical review of the abundant literature on spider and web appearance, predominantly focusing on the potentiality of camouflage and mimicry. For this reason, we will not explore non-visual aspects such as spider olfactory and tactile mimicry or several other hypothetic functions of web decorations. When possible, we will highlight studies considering the visual sensitivities of prey and predators, and the transmission properties of visual signals through the environment. In addition to reviewing possible cases of camouflage, we will report on the nature of pigments used for colour change and evoke physiological and ecological hypotheses for colour change. We will also discuss one neglected hypothesis, the protection against UV photodamage, by making a comparison of the pigmentation of two crab spider species, one being cryptic and the other non-cryptic.
2. Web design, colour and visual environment
Spiders specializing on small prey which are characterized by highly evolved visual systems and flight behaviour face the problem of avoiding detection, and studies of insect vision and flight show that it is surprising that webs capture any prey at all (Craig 1986). However, the sophisticated design of webs enhances prey capture by making the web difficult to detect. Low-frequency oscillations of webs with low fibre density designed to resist only low impact, such as those of Theridiosoma globosum, are specialized to capture small slow-flying prey by fluctuating with the low airflow the web surface in and out of an approaching prey's range of visual resolution (Craig 1986). By contrast, high impact webs such as those of Mangora pia are built with denser and more visible silk and do not oscillate because changes in light intensity across the web surface would cause the web to appear as a visual flag (Craig 1986). As an alternative to dynamic distortion, some spiders in the genera Theridiosoma and Epeirotypus use static distortion by pulling the web centre approximately 3–5 cm with a fibre attached to surrounding vegetation. They build a cone web that escapes the range of visual resolution of potential prey, because when prey are flying at the base of the cone web they are not able to see the web centre or area of the highest fibre density (Craig 1986). The centre thread is released and the web is projected towards a prey when it comes within the reach of the distorted web.
Web visibility is also greatly affected by the light environment. Background pattern has little influence on web visibility in dim-light environments, whereas small background patterns close to the web disrupt the web outline in bright-light environments (Craig 1990). The changing pattern of shade and sunflecks on the web also make the orb difficult to detect (Craig & Freeman 1991). In laboratory experiments, Drosophila melanogaster has difficulty in seeing webs suspended close to backgrounds of high spatial frequency in bright light, and are unable to see and avoid webs characterized by low reflectivity (Craig 1990).
Particular silks affect attraction of prey. Webs of Araneidae and Tetragnathidae, which include viscid droplets of glycoprotein, have a sparkling appearance that functions to attract prey to the web area although at short range they make webs more visible (Craig & Freeman 1991). Viscid silk increases the probability of prey interception of both diurnal and nocturnal species, although this is only true in the brightest habitats for nocturnal species (Craig & Freeman 1991). However, using more sticky viscid silk also makes webs more visible to prey. Consequently, nocturnal spiders or those living in dim habitats are able to enhance web stickiness by using highly visible viscid silk, whereas species foraging in bright habitats are constrained to build less visible and consequently less sticky orbs that are less efficient at retaining large prey (Craig 1988). Nephila clavipes, the golden orb weaver, is unique among spiders studied to date for its ability to adjust web reflectance to local light and to produce pigments that enhance web visibility by increasing light reflected by their silk (Craig et al. 1996). It produces yellow silk that exploits the visual and behavioural systems of insects in the different light environments where it forages. In environments with high light intensity or in forest gaps, N. clavipes produces yellow silk that attracts bees. By contrast, they do not produce pigments in dim sites where silk colours are difficult to see, probably to achieve energetic savings. Similarly, Argiope aetherea and Argiope keyserlingi build more and longer decorations under dim light than bright light, probably to increase the attractive signal for approaching prey or to advertise the web to oncoming birds (Elgar et al. 1996; Herberstein & Fleisch 2003).
3. Web decorations
Web decorations are conspicuous silk structures spun in webs by females of some species of orb-web spiders. While the most studied decorations are entirely made of silk, some spider species combine silk with organic items such as egg sacs and debris. Because empirical studies showed that decorations made of different materials functioned quite differently, we will consider them separately.
(a) Silk decorations
Silk decorations were originally called stabilimenta because they were thought to help the web to stabilize. Several other functions have been advanced, including camouflage, prey attraction, increase in apparent female size, signalling to species likely to damage webs, thermoregulation, stress, regulation of excess silk and male attraction (Herberstein et al. 2000; Starks 2002; Bruce 2006). The general absence of decorations in nocturnal spiders supports a visually mediated function. One common trend is that, when the prey attraction function is supported, the anti-predatory function is not (Herberstein 2000; Bruce et al. 2001; Craig et al. 2001; Cheng & Tso 2007) or the reverse (Blackledge 1998a,b; Blackledge & Wenzel 1999; Eberhard 2006; Jaffé et al. 2006). The only studies simultaneously validating both functions are very speculative and provide no direct evidence for support of both hypotheses (Herberstein & Fleisch 2003; Rao et al. in press). Using silk decorations may constitute a conditional strategy that performs multiple functions both within and across populations (and species) depending on (i) spider developmental stages, (ii) their energetic state or (iii) environmental factors as the relative proportions of predator types, the population-specific prey differences in decoration susceptibility, the presence of bird species likely to damage webs or differences in temperature or ambient light (e.g. Watanabe 1999; Craig et al. 2001; Seah & Li 2002; Starks 2002; Li et al. 2003; Bruce 2006).
Evidence for camouflage has been found when decorations conceal the spider from predators or change its apparent shape, although earlier studies did not perform field or laboratory experiment and were more descriptive and speculative (Ewer 1972; Eberhard 1973; Edmunds 1986; Li et al. 2003). Blackledge & Wenzel (2000) argued that decorations are cryptic to insects because their reflectance spectra are flat, but they do not provide any data to test this assumption. On the contrary, Craig & Bernard (1990) showed in a closely related Argiope species that both decorations and spiders reflect UV wavelengths that act as a visual signal to attract prey. Li et al. (2004) also showed that the discoid decoration spun by juvenile Argiope versicolor is a prey attractant under white light containing UV. Spiders that decorate their webs at higher frequency not only grow faster, but also take higher predation risks (Li 2005). Numerous recent studies have indeed shown that silk decorations induce significant cost to spiders by attracting specialized spider-eating predators (e.g. Bruce et al. 2001; Seah & Li 2001; Cheng & Tso 2007). Evidence for prey deception has been suggested when decorations attract pollinating insects by reflecting UV light in patterns similar to UV markers on flowers. Similarly, UV patches created by web decorations may resemble gaps in vegetation that elicit flight behaviour in many insects (Craig & Bernard 1990). However, until recently, the visually mediated functions of web decorations could not be properly tested with regard to the visual sensitivities of prey or predators, as well as the spectral characteristics of the visual background and ambient light.
Bruce et al. (2005) were the first to evaluate the visibility of silk decorations to both prey and predators by considering visual systems, background colour and the ambient light spectrum. Both achromatic and chromatic contrasts were calculated to estimate conspicuousness of the spiders against green vegetation background or against their decorations, at long and short distances, respectively. It was found that decorations were highly conspicuous to both predators and prey at long and short distances. However, the discoid decoration of Argiope mascordi could provide some camouflage for spiders seen by hymenoptera, either prey or predator.
A second study has used visual system modelling to evaluate the conspicuousness of silk decorations to potential prey and predators (Rao et al. in press). In the orb-web spider Argiope radon, it was found that spider abdomens generate pronounced chromatic and achromatic contrasts on web decorations when seen by hymenoptera, and even stronger contrasts when seen by birds. Although, the authors used values of detection threshold (the minimal distance in the colour space allowing separating a target from the background) computed for chromatic contrast (0.01 for honeybees by Dyer & Chittka 2004; 0.06 for birds by Théry & Casas 2002) to estimate the discrimination of achromatic contrast, spiders clearly appear conspicuous to both prey and predators. Because in both visual systems decorations are more conspicuous than spiders, the function of decorations could be to confuse the attack of avian or insect predators. Recently, Blamires et al. (2008) have shown that spiders attract insects with decorations by exploiting visual sensory biases of prey sensitivities in the blue and UV light. However, it is still unknown whether UV, blue light or both are the most important cue.
(b) Detritus decorations
Detritus decorations are generally viewed to function as camouflage for the spider (Eberhard 2003; Chou et al. 2005; Gonzaga & Vasconcellos-Neto 2005). Detritus decorations added to the webs of two Cyclosa species could reduce the intensity of predation, possibly by disrupting the spider's outline (Gonzaga & Vasconcellos-Neto 2005). Egg sac and silk decorations were also suspected to be used for camouflage by Allocyclosa bifurca spiders at the hub, although no rigorous behavioural test was conducted to support this interpretation (Eberhard 2003). However, the odour of decaying plant material incorporated above the orb web may also be used to attract insect prey (Bjorkman-Chiswell et al. 2004).
Physiological models of vision were used to calculate chromatic and achromatic contrasts of Cyclosa spiders and their prey carcass decorations as they are viewed by their hymenopteran predator (Chou et al. 2005). However, the authors compared both chromatic and achromatic contrasts with a discrimination threshold value of 0.05 computed by Théry & Casas (2002) for hymenopteran insects, a value that was estimated for chromatic contrast discrimination, but is not known for achromatic contrast (Théry & Casas 2002; Bruce et al. 2005; Théry et al. 2005). Filming prey interception and predation events showed that carcass decorations do not attract insects and even generate a foraging cost, but that predators redirect their attacks towards decorations, which allows spiders to escape predation (Chou et al. 2005). The function of Cyclosa confusa decorations is neither related to camouflage from predator or to prey attraction, but is apparently to confuse the attacking predator.
4. Spider coloration: generalities
Spider coloration has been reviewed in Oxford & Gillespie (1998) and their excellent overview is still up to date a decade later. Coloration serves multiple purposes, from crypsis and aposematism to sexual selection, and its underlying physiological processes are as numerous. Since then, the biochemical foundation of coloration in spiders has seen little progress compared with the sensory physiology of colour perception. The genetical and evolutionary work on the colour polymorphism is reviewed in Oxford & Gillespie (2001) for the happy-face spider (Theridion grallator) and in Oxford (2005) for the candy-stripe spider (Enoplognatha ovata). The evolutionary forces for the persistence of colour polymorphism in spiders remain generally elusive. By contrast, two areas have attracted most of the attention, the colour changing properties of crab spiders and the striped and bright body coloration in web spiders. The studies conducted on those two aspects are similar in spirit to the work on the web decorations, often produced by the same species. In a recent study, Bush et al. (2008) have carried out ingenious experiments on the wasp spider Argiope bruennichi by masking the spiders behind a leaf or painting their otherwise brightly coloured body, as did Tso et al. (2006) and Chuang et al. (2007, 2008). The marked decrease in prey capture in all cases is strong proof of the attractive nature of the brightly coloured body, and is consistent with the work of Chuang et al. (2007, 2008) and Tso et al. (2007) on brightly coloured, of nocturnal spiders. With these recent studies using physiological models of colour vision, we seem to come to an end of an enduring discussion regarding attraction and crypsis of the bright coloration in web spiders (Craig & Ebert 1994; Hauber 2002; Tso et al. 2002, 2004, 2006; Hoese et al. 2006; Vaclav & Prokop 2006). The next heading deals with the coloration of crab spiders in more detail, as its relationship to camouflage is clear-cut.
5. Spider coloration: pigments responsible for colour change
The colour changing crab spiders of the family Thomisidae, in particular Misumena vatia and Thomisus onustus, have been studied since 1891 (Heckel 1891) with respect to pigmentation. Misumena vatia represents one of the most studied spiders, with a monograph devoted exclusively to its life history (Morse 2007). This spider is unusual as it is able to change reversibly, in a time delay of a few days, from white to yellow and back. Colour change is induced by background colour and colour of prey (Théry 2007 and references therein). The background matching ability of these spiders is at times astonishing, below the discrimination ability of bees for example (see figure 1; Chittka 2001; Théry & Casas 2002; Théry et al. 2005). The sometimes striking match between flower and spider colours found in naturally occurring situations in the field is difficult to attribute to chance alone. Both food and light quality have been found to increase the range of colour change, but the variability in the response level was very high, with many individuals remaining white despite strong yellow stimuli (Théry 2007). This form of crypsis has been interpreted as being potentially both a defensive (hiding from predators) and an aggressive (hiding from prey) one. Bees and other flower-visiting insects are indeed common prey. Predation events by vertebrate predators, however, have never been observed (Morse 2007), whereas predation by mud-dauber and spider wasps has often been observed. The impact of these invertebrate predators on spider populations is nonetheless unknown. Aggressive crypsis might therefore be the only type of crypsis present. Such impressive camouflage begets many questions about its proximate and ultimate mechanisms. In the following, we first report on the nature of pigments. We then move on to the physiological and ecological hypotheses for colour change, and close our discussion with one neglected hypothesis, the protection against UV photodamage, by making a comparison with another, non-cryptic, crab spider.
Figure 1.
Importance of translucent teguments and white reflectance from guanine in background matching by the crab spider M. vatia. The same pale yellow female is represented in the four pictures, taken at an interval of a few minutes. Depending on the exact location of the spider on a plant, (a,b) the different hues between the cephalothorax and legs, and the opisthosoma, may make the animal more difficult to detect, (c) the green coloration of leaves may shine through the translucent legs and (d) the strong yellow hue within the corolla can be reflected by the guanine, leading to a high degree of camouflage. Scale bar, 6 mm.
Older studies assumed that the yellow colour of M. vatia was due to carotenoids (Millot 1926), but ommochromes were later found to be the pigments responsible for this colour (Seligy 1972). Ommochrome pigments are a class of pigments, widespread in insects and other arthropods, which constitute the main chromogenic class in the pathway from tryptophan and range from gold through red, purple and violet, up to brown and black. The reduced form is usually red and the oxidized form usually yellow. The characteristic properties of ommochromes (redox behaviour, absorption of UV and visible light, and low solubility) enable them to act not only as authentic functional pigments (eyes, integument), but also as an electron-accepting or -donating system and as metabolic end products (Needham 1974). Ommochromes, principally xanthommatin, are widely distributed in arthropods as screening pigments in the accessory cells of the eyes and are also present in the retinula cells (Linzen 1974). Ommochrome pigments are poorly known in general and their catabolism is totally unknown, as the biochemical basis for the reversible colour change. One remains simply dismayed at the disappearance of solid biochemical work on a complete class of pigment after the 1970s and 1980s, just before the advent and rise of molecular biology (Linzen 1974; Needham 1974; Fuzeau-Braesch 1985; Kayser 1985). Luckily, the situation is somewhat better in terms of the ultimate reasons for the colour change.
The functions of ommochromes are diverse and several complementary and non-exclusive hypotheses have been suggested for their common occurrence (reviewed in Insausti & Casas 2008). The first hypothesis states that the ommochrome pathway is the main pathway for avoiding excess accumulation of the highly toxic tryptophan. Supporting this hypothesis is the observation that ommochrome formation is strongly correlated with the massive breakdown of proteins at the onset of metamorphosis. This is the oldest and the most popular view for the function of ommochromes. This conclusion has been however, invalidated for M. vatia by Insausti & Casas (2008) on the basis of the red stripes in white spiders. The absence of a change of colour from white to yellow cannot be due to a lack of precursors or enzymes (as found in the white eyes clone of D. melanogaster, Mackenzie et al. 2000), as these spiders have both. Tryptophan might already be neutralized as ommochrome precursor in those granules containing most likely kynurenine.
The second hypothesis states that main raison d'être of ommochromes is signalling, mimicry and crypsis. This is the hypothesis supported by most of the community working on colour changing insects such as stick insects and mantids (Fuzeau-Braesch 1985), including Mantis religiosa, Sphodromantis viridis and Locusta migratoria (Vuillaume 1968), and spiders (Rabaud 1918, 1919; Gabritschevsky 1927; Schmalhofer 2000; Chittka 2001; Théry & Casas 2002; Heiling et al. 2003, 2005a,b; Théry et al. 2005; Théry 2007). In order to test this hypothesis, we need to assess the fitness value of the camouflage and the fitness gain from a change of colour. It can be based on the measurement of some fitness-related trait, such as increased fecundity, survival or simply higher prey capture rate as a function of the degree of flower colour matching. This is the main piece of supporting evidence that is still missing. We also need to assess the likelihood of the ‘nearly perfect’ matching of spiders to their flowers referred to earlier. This in turn, requires the sampling of the colour of all flowers in the neighbourhood of the one chosen by a spider.
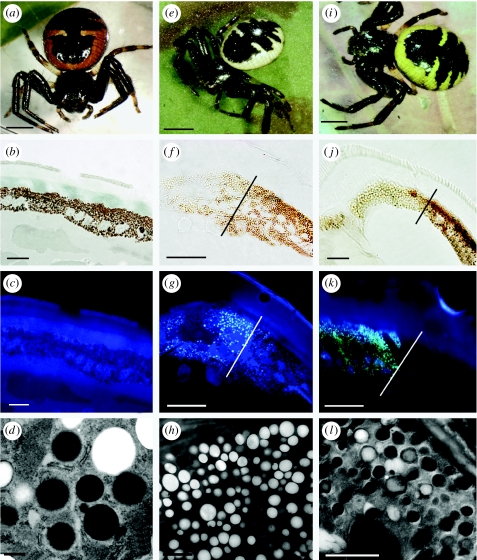
The third hypothesis is based on the observation that the major function of ommochrome in eyes is the protection of photosensitive visual cells against excessive scattered light, and also protection against photodestruction by intense UV light (Langer 1975; Stavenga 1989). Ommochromes participate in the antioxidative system in invertebrate photoreceptors, as melanin in the eyes of vertebrates (Dontsov et al. 1984; Ostrovsky et al. 1987; Sakina et al. 1987; Dontsov 1999). The ommochromes are also effective inhibitors of free radical-induced lipid peroxidation. Lipid peroxidation is also produced by photooxidation and is indicative of photoreceptor damage, expressed in the retina by the deterioration of photoreceptor membranes (Ostrovsky & Fedorovich 1994). The hypothesis that ommochromes in the tegument have a similar function deserves therefore much more attention for the following reasons. First, ommochrome precursors could be sufficient as screening pigments, as in the group of chartreuse mutants of Apis mellifica (Linzen 1974). Indeed, the mutant group accumulates the yellow tinted but still translucent 3-OH-kynurenine in a granular form in the pigment cells of the compound eyes. That pigment precursor therefore assumes a pigment function (Linzen 1974). The intensity of the yellow hue of spiders, due to the mix between 3-OH-kynurenine and ommochromes, might reflect the amount of screening against radiation. Second, M. vatia is both exposed for days to direct solar radiation on the top of flowers and has a transparent cuticle exposing the epidermal cells to direct radiation. This transparency implies a need for protective means in the tissues situated beneath the cuticle and ommochromes might act as such. Here, we show that the comparison with another crab spider, Synaema globosum, supports this conclusion. This species that does not have a camouflage pattern also has a transparent cuticle and comes in three different colour types: white; yellow; and red (figure 2). It is unknown whether this spider does change colour or whether these are different fixed phenotypes. We observed that both the brown-black and the yellow or red-coloured parts of the epidermis contain ommochrome granules, as in M. vatia. The pigmentation of S. globosum is therefore another strong hint that the ommochrome coloration might be related to the transparency of the cuticle in crab spiders. Camouflage profits from such a relationship, but may not be the driving force.
Figure 2.
S. globosum individuals (a–d, e–h and i–l, respectively) of (a) red, (e) white and (i) yellow colours: (a,e,i) habitus, (b,f,j) unstained cross sections of the tegument under light microscopy, (c,g,k) under UV light and (d,h,l) electron micrographs of epithelial cells and pigment granules. The cuticle of both regions, black and coloured (b), is transparent. The absence of fluorescence in the red spider (c) is typical of ommochromes granules (d). In yellow spiders, there is a distinct difference between the black and yellow areas (on the right and left of the dividing mark), both under light microscopy (j) and under UV light (k). The black region contains two types of granules, red and black, whereas the yellow region also contains two types of granules, translucent and light brown (l). Only the yellow portion contains fluorescent granules. In white spiders, the white region (f) contains translucent, fluorescent granules only (g,h). As a result, the white coloration is produced by the guanine layer under the epithelium. Almost the totality of the granules is electron-lucent and homogeneous, indicative of kynurenine (granules type I, Insausti & Casas 2008). There is thus a clear association between body colour and ommochrome metabolites in this non-cryptic crab spider. Scale bars, (a,e,i) 2 mm, (b,c,f,g, j,k) 10 μm, (d) 0.5 μm and (h,l) 2 μm.
Related puzzling aspects of coloration in spiders are widespread fluorescence and UV reflectance. The former aspect has been only very recently assessed (Andrews et al. 2007; Lim et al. 2007). We doubt that the fluorophores observed by these authors are located in the haemolymph, as stated by Andrews et al. (2007), and rather interpret their results and picture as indicative of a pigment located in the epidermis. Several ommochrome precursors based on the tryptophan pathway located in the epidermis are indeed fluorescent (Insausti & Casas 2008) and fluorescence might simply be a side effect of the widespread occurrence of ommochromes in spider colours. On the basis of several behavioural tests and ingenious experiments using both native and European bees, it was conclusively demonstrated that UV reflective body colours of Australian crab spiders attract prey (i.e. bees) to the flowers they are positioned on (Heiling et al. 2003; Heiling & Herberstein 2004; Heiling et al. 2005a,b, 2006; Herberstein et al. in press). While the tropical and subtropical distribution of UV reflectance in crab spiders raises a number of very exciting evolutionary questions about coevolution and trait evolution, the much higher amount of UV radiation received in Australia compared with Europe (Godar 2005) should not be forgotten as an easier potential explanation. UV reflectance might act as protective means in tropical and subtropical regions.
6. Spiders mimic ants
More than 300 species of spiders, belonging to 13 families, mimic ants (Cushing 1997; Nelson & Jackson 2007a). Myrmecomorphic species are defined as spiders mimicking ant morphology and/or behaviour. Morphological adaptations include colour and form modification, which make the spider look as though it has three body segments instead of two, and long slender legs instead of shorter robust legs (review by Cushing 1997). Adaptation of the chelicerae, spinnerets and cuticle coloration allow the spider to mimic the mandibles, sting, compound eyes and antennae of their ant model. Behavioural adaptation includes ant-like erratic movements and the raising of a pair of legs to mimic the movements of ant antennae. Several species of myrmecomorphic spiders evolved transformational mimicry in which successive instars mimic different ant models. Also, several ant-mimicking spiders use polymorphic mimicry in which each morph mimics a different ant morph or species. Some species have each sex mimicking a different ant model. The limited space for this paper precluded us from doing full justice to movement camouflage that needs more studies in general, as it seems the most striking type of camouflage spiders have used in the course of evolution.
A minority of spider myrmecomorphs are aggressive mimics (McIver & Stonedahl 1993; Cushing 1997), and use their morphology and behaviour to attract and prey on ant models. However, in order for the myrmecomorphic spider to be considered as an aggressive mimic by the ant species, the ant model must be a selective agent able to see resemblance of the mimic. This is unlikely for the majority of ant species that have poor eyesight or which do not investigate the spider myrmecomorphs (Cushing 1997). Most myrmecomorphic spiders are considered as Batesian mimics because ant unpalatability offers protection against generalistic arthropod predators. Both direct and indirect evidence support this hypothesis (review in Cushing 1997; see also Nelson et al. 2005, 2006a–c; Nelson & Jackson 2006a,b, 2007a,b). Recent experimental studies in the genus Myrmarachne have shown that salticid spider resemblance to ants holds in the eyes of their predators, other salticid species and mantises (Nelson & Jackson 2006b; Nelson et al. 2006b,c). It has also been demonstrated that an ant-mimicking jumping spider is able to discriminate between ant models, conspecifics and prey by sight alone (Nelson & Jackson 2006b, 2007b). A recent unpublished study using a physiological model of bird vision has shown that although the head and thoracic regions of Myrmarachne gisti are visible to bird predators from a long distance, this myrmecomorphic spider is unlikely to be detected at short distance (D. Li 2008, personal communication). By giving the choice between living M. gisti and model ants under light conditions with and without UV, specialized ant-eating salticids are able to distinguish between ant-mimics and ants based on M. gisti's specific display behaviour but not on coloration. These findings provide evidence that this classic ant mimicry has extended into UV light wavelengths, and that Batesian mimicry of M. gisti is an effective defence against avian predators.
7. Future prospects
Spider camouflage and mimicry is attracting attention, mainly from behavioural ecologist quarters. While we enthusiastically welcome this renewed interest, we caution against glossing over physiological mechanisms. As so often with integrative biology, we need both more detailed mechanistic studies within the animal, on the biochemical pathways or the colour perception processes for example, and evolutionary behavioural or ecological work, both in the laboratory and in the field. As an example to the point, it is still unclear whether a crab spider changes colour to match its background or chooses an appropriate flower colour to match its imminent colour change.
Our paper identified major advances and gaps in our understanding and an untapped potential of studying mimicry and camouflage in spiders. Recent studies do take into account the visual systems of prey and predators and light environments. This approach is necessary, and has clearly improved our knowledge on the functions of web, decoration and spider coloration. By contrast, we still lack a comprehensive understanding of colour vision in the very same spiders, an approach that requires painstaking electrophysiological work, furthermore, on all four pairs of eyes. The study of mimicry and camouflage centred on the classical models systems, such as Octopus or Heliconius, is plagued with the recurring difficulty of observing and quantifying the ecological impact and evolutionary forces of predators on the studied traits. Spiders, by being comparatively immobile and constructing trapping devices that often contain a portion of their predatory history, represent an excellent model devoid of the above difficulties. The almost complete lack of theoretical studies of colour mimicry and camouflage using spiders is therefore even more striking.
Acknowledgments
We thank T. Insausti for carrying the work displayed in the two figures, and Martin Stevens, Sami Merilaita and two anonymous reviewers for their comments that improved the manuscript.
Footnotes
One contribution of 15 to a Theme Issue ‘Animal camouflage: current issues and new perspectives’.
References
- Andrews K., Reed S.M., Masta S.E. Spiders fluoresce variably across many taxa. Biol. Lett. 2007;3:265–267. doi: 10.1098/rsbl.2007.0016. doi:10.1098/rsbl.2007.0016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkman-Chiswell B.T., Kulinski M.M., Muscat R.L., Nguyen K.A., Norton B.A., Symonds M.R.E., Westhorpe G.E., Elgar M.A. Web-building spiders attract prey by storing decaying matter. Naturwissenschaften. 2004;91:245–248. doi: 10.1007/s00114-004-0524-x. doi:10.1007/s00114-004-0524-x [DOI] [PubMed] [Google Scholar]
- Blackledge T.A. Stabilimentum variation and foraging success in Argiope aurantia and Argiope trifasciata (Araneae: Araneidae) J. Zool. Lond. 1998a;246:21–27. doi:10.1111/j.1469-7998.1998.tb00128.x [Google Scholar]
- Blackledge T.A. Signal conflict in spider webs driven by predators and prey. Proc. R. Soc. B. 1998b;265:1991–1996. doi:10.1098/rspb.1998.0530 [Google Scholar]
- Blackledge T.A., Wenzel J.W. Do stabilimenta in orb webs attract prey or defend spiders? Behav. Ecol. 1999;10:372–376. doi:10.1093/beheco/10.4.372 [Google Scholar]
- Blackledge T.A., Wenzel J.W. The evolution of cryptic spider silk: a behavioral test. Behav. Ecol. 2000;11:142–145. doi:10.1093/beheco/11.2.142 [Google Scholar]
- Blamires S.J., Hochuli D.F., Thompson M.B. Why cross the web: decoration spectral properties and prey capture in an orb spider (Argiope keyserlingi) web. Biol. J. Linn. Soc. 2008;94:221–229. doi:10.1111/j.1095-8312.2008.00999.x [Google Scholar]
- Bruce M.J. Silk decorations: controversy and consensus. J. Zool. Lond. 2006;269:89–97. doi:10.1111/j.1469-7998.2006.00047.x [Google Scholar]
- Bruce M.J., Herberstein M.E., Elgar M.A. Signalling conflict between prey and predator attraction. J. Evol. Biol. 2001;14:786–794. doi:10.1046/j.1420-9101.2001.00326.x [Google Scholar]
- Bruce M.J., Heiling A.M., Herberstein M.E. Spider signals: are web decorations visible to birds and bees? Biol. Lett. 2005;1:299–302. doi: 10.1098/rsbl.2005.0307. doi:10.1098/rsbl.2005.0307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush A.A., Yu D.W., Herberstein M.E. Function of bright coloration in the wasp spider Argiope bruennichi (Araneae: Araneidae) Proc. R. Soc. B. 2008;275:1337–1342. doi: 10.1098/rspb.2008.0062. doi:10.1098/rspb.2008.0062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng R.-C., Tso I.-M. Signaling by decorating webs: luring prey or deterring predators? Behav. Ecol. 2007;18:1085–1091. doi:10.1093/beheco/arm081 [Google Scholar]
- Chittka L. Camouflage of predatory crab spiders on flowers and the colour perception of bees (Aranida: Thomisidae/Hymenoptera: Apidae) Entomol. Gen. 2001;25:181–187. [Google Scholar]
- Chou I.-C., Wang P.-H., Shen P.-S., Tso I.-M. A test of prey-attracting and predator defence functions of prey carcass decorations built by Cyclosa spiders. Anim. Behav. 2005;69:1055–1061. doi:10.1016/j.anbehav.2004.09.010 [Google Scholar]
- Chuang C.-Y., Yang E.-C., Tso I.-M. Diurnal and nocturnal prey luring of a colorful predator. J. Exp. Biol. 2007;210:3830–3837. doi: 10.1242/jeb.007328. doi:10.1242/jeb.007328 [DOI] [PubMed] [Google Scholar]
- Chuang C.-Y., Yang E.-C., Tso I.-M. Deceptive color signaling in the night: a nocturnal predator attracts prey with visual lures. Behav. Ecol. 2008;19:237–244. doi:10.1093/beheco/arm106 [Google Scholar]
- Craig C.L. Orb-web visibility: the influence of insect flight behaviour and visual physiology on the evolution of web designs within the Araneoidea. Anim. Behav. 1986;34:54–68. doi:10.1016/0003-3472(86)90006-0 [Google Scholar]
- Craig C.L. Insect perception of spider webs in three light environments. Funct. Ecol. 1988;2:277–282. doi:10.2307/2389398 [Google Scholar]
- Craig C.L. Effects of background pattern on insect perception of webs spun by orb-weaving spiders. Anim. Behav. 1990;39:135–144. doi:10.1016/S0003-3472(05)80733-X [Google Scholar]
- Craig C.L., Bernard G.D. Insect attraction to ultraviolet-reflecting spider webs and web decorations. Ecology. 1990;71:616–623. doi:10.2307/1940315 [Google Scholar]
- Craig C.L., Ebert K. Colour and pattern in predator–prey interactions: the bright body colours and patterns of a tropical orb-spinning spider attract flower-seeking prey. Funct. Ecol. 1994;8:616–620. doi:10.2307/2389923 [Google Scholar]
- Craig C.L., Freeman C.R. Effects of predator visibility on prey encounter: a case study on aerial web weaving spiders. Behav. Ecol. Sociobiol. 1991;29:249–254. doi:10.1007/BF00163981 [Google Scholar]
- Craig C.L., Weber R.S., Bernard G.D. Evolution of predator–prey systems: spider foraging plasticity in response to the visual ecology of prey. Am. Nat. 1996;147:205–229. doi:10.1086/285847 [Google Scholar]
- Craig C.L., Wolf S.G., Davis J.L.D., Hauber M.E., Maas J.L. Signal polymorphism in the web-decorating spider Argiope argentata is correlated with reduced survivorship and the presence of stingless bees, its primary prey. Evolution. 2001;55:986–993. doi: 10.1554/0014-3820(2001)055[0986:spitwd]2.0.co;2. doi:10.1554/0014-3820(2001)055[0986:SPITWD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Cushing P.E. Myrmecomorphy and myrmecophily in spiders: a review. Fla. Entomol. 1997;80:165–193. doi:10.2307/3495552 [Google Scholar]
- Dontsov A.E. Comparative study of spectral and antioxidant properties of pigments from the eyes of two Mysis relicta (Crustacea, Mysidacea) populations, with different light damage resistence. J. Comp. Physiol. B. 1999;169:157–164. doi:10.1007/s003600050206 [Google Scholar]
- Dontsov A.E., Lapina V.A., Ostrovsky M.A. Photoregeneration of O2 by ommochromes and their role in the system of antioxidative protection of invertebrate eye cells. Biofizika. 1984;29:878–882. [Google Scholar]
- Dyer A.G., Chittka L. Biological significance of discriminating between similar colours in spectrally variable illumination: bumblebees as a study case. J. Comp. Physiol. A. 2004;190:105–114. doi: 10.1007/s00359-003-0475-2. doi:10.1007/s00359-003-0475-2 [DOI] [PubMed] [Google Scholar]
- Eberhard W.G. Stabilimenta on the webs of Uloborus diversus (Araneae: Uloboridae) and other spiders. J. Zool. Lond. 1973;68:1090–1097. [Google Scholar]
- Eberhard W.G. Substitution of silk stabilimenta for egg sacs by Allocyclosa bifurca (Araneae: Araneidae) suggests that silk stabilimenta function as camouflage devices. Behaviour. 2003;140:847–868. doi:10.1163/156853903770238346 [Google Scholar]
- Eberhard W.G. Stabilimenta of Philoponella vicina (Araneae: Uloboridae) and Gasteracantha cancriformis (Araneae: Araneidae): evidence against a prey attractant function. Biotropica. 2006;39:216–220. doi:10.1111/j.1744-7429.2006.00254.x [Google Scholar]
- Edmunds J. The stabilimenta of Argiope flavipalpis and Argiope trifasciata in West Africa, with a discussion of the function of stabilimenta. In: Eberhard W.G., Lubin Y.D., Robinson B.C., editors. Proc. 9th Int. Cong. Arachnology, Panama 1983. Smithsonian Institute Press; Washington, DC: 1986. pp. 61–72. [Google Scholar]
- Elgar M.A., Allan R.A., Evans T.A. Foraging strategies in orb-spinning spiders: ambient light and silk decorations in Argiope aetherea Walckenaer (Araneae: Araneoidea) Austral Ecol. 1996;21:464–467. doi:10.1111/j.1442-9993.1996.tb00633.x [Google Scholar]
- Ewer R.F. The devices in the web of the African spider Argiope flavipalpis. J. Nat. Hist. 1972;6:159–167. [Google Scholar]
- Fuzeau-Braesch S. Colour changes. In: Kerkut G.A., Gilbert L.I., editors. Comprehensive insect physiology biochemistry and pharmacology. vol. 9. Pergamon Press; Oxford, UK: 1985. pp. 549–589. [Google Scholar]
- Gabritschevsky E. Experiments on color changes and regeneration in the crab-spider, Misumena vatia. J. Exp. Zool. 1927;47:251–267. doi:10.1002/jez.1400470207 [Google Scholar]
- Godar D.E. UV doses worldwide. Photochem. Photobiol. 2005;81:736–749. doi: 10.1562/2004-09-07-ir-308r.1. doi:10.1562/2004-09-07-IR-308R.1 [DOI] [PubMed] [Google Scholar]
- Gonzaga M.O., Vasconcellos-Neto J. Testing the functions of detritus stabilimenta in webs of Cyclosa fililineata and Cyclosa morretes (Araneae: Araneidae): do they attract prey or reduce the risk of predation? Ethology. 2005;111:479–491. doi:10.1111/j.1439-0310.2005.01074.x [Google Scholar]
- Hauber M.E. Conspicuous colouration attracts prey to a stationary predator. Ecol. Entomol. 2002;27:686–691. doi:10.1046/j.1365-2311.2002.00457.x [Google Scholar]
- Heckel E. Sur le mimétisme de Thomisus onustus. Bull. Sci. Fr. Belg. 1891;23:347–354. [Google Scholar]
- Heiling A.M., Herberstein M.E. Predator–prey coevolution: Australian native bees avoid their spider predators. Proc. R. Soc. B. 2004;271(Suppl.):S196–S198. doi: 10.1098/rsbl.2003.0138. doi:10.1098/rsbl.2003.0138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiling A.M., Herberstein M.E., Chittka L. Crab-spiders manipulate flower signals. Nature. 2003;421:334. doi: 10.1038/421334a. doi:10.1038/421334a [DOI] [PubMed] [Google Scholar]
- Heiling A.M., Cheng K., Chittka L., Goeth A., Herberstein M.E. The role of UV in crab spider signals: effects on perception by prey and predators. J. Exp. Biol. 2005a;208:3925–3931. doi: 10.1242/jeb.01861. doi:10.1242/jeb.01861 [DOI] [PubMed] [Google Scholar]
- Heiling A.M., Chittka L., Cheng K., Herberstein M.E. Colouration in crab spiders: substrate choice and prey attraction. J. Exp. Biol. 2005b;208:1785–1792. doi: 10.1242/jeb.01585. doi:10.1242/jeb.01585 [DOI] [PubMed] [Google Scholar]
- Heiling A.M., Cheng K., Herberstein M.E. Picking the right spot: crab spiders position themselves on flowers to maximize prey attraction. Behaviour. 2006;143:957–968. doi:10.1163/156853906778623662 [Google Scholar]
- Herberstein M.E. Foraging behaviour in orb-web spiders (Araneidae): do web decorations increase prey capture success in Argiope keyserlingi Karsch, 1878? Aust. J. Zool. 2000;48:217–223. doi:10.1071/ZO00007 [Google Scholar]
- Herberstein M.E., Fleisch A.F. Effect of abiotic factors on the foraging strategy of the orb-web spider Argiope keyserlingi (Araneae: Araneidae) Austral Ecol. 2003;28:622–628. doi:10.1046/j.1442-9993.2003.t01-1-01319.x [Google Scholar]
- Herberstein M.E., Craig C.L., Coddington J.A., Elgar M.A. The functional significance of silk decorations of orb-web spiders: a critical review of the empirical evidence. Biol. Rev. 2000;75:649–669. doi: 10.1111/j.1469-185x.2000.tb00056.x. [DOI] [PubMed] [Google Scholar]
- Herberstein, M. A., Heiling, A. M. & Cheng, K. In press. Evidence for UV-based sensory exploitation in Australian but not European crab spiders. Evol. Ecol. (doi:10.1007/s10682-008-9260-6)
- Hoese F.J., Law E.A.J., Rao D., Herberstein M.E. Distinctive yellow bands on a sit-and-wait predator: prey attractant or camouflage? Behaviour. 2006;143:763–781. doi:10.1163/156853906777791333 [Google Scholar]
- Insausti T.C., Casas J. The functional morphology of color changing in a spider: development of ommochrome pigment granules. J. Exp. Biol. 2008;211:780–789. doi: 10.1242/jeb.014043. doi:10.1242/jeb.014043 [DOI] [PubMed] [Google Scholar]
- Jaffé R., Eberhard W., De Angelo C., Eusse D., Gutierrez A., Quijas S., Rodrìguez A., Rodrìguez M. Caution, webs in the way! Possible functions of silk stabilimenta in Gasteracantha cranciformis (Araneae, Araneidae) J. Arachnol. 2006;34:448–455. doi:10.1636/S04-28.1 [Google Scholar]
- Kayser H. Pigments. In: Kerkut G.A., Gilbert L.I., editors. Comprehensive insect physiology, biochemistry and pharmacology. Pergamon Press; Oxford, UK: 1985. pp. 367–415. [Google Scholar]
- Langer H. Properties and functions of screening pigments in insects eyes. In: Snyder A.W., Menzel R., editors. Photoreceptor optics. Springer; Berlin, Germany: 1975. pp. 429–455. [Google Scholar]
- Li D. Spiders that decorate their webs at higher frequency intercept more prey and grow faster. Proc. R. Soc. B. 2005;272:1753–1757. doi: 10.1098/rspb.2005.3160. doi:10.1098/rspb.2005.3160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Kok L.M., Seah W.K., Lim M.L.M. Age-dependent stabilimentum-associated predator avoidance behaviours in orb-weaving spiders. Behaviour. 2003;140:1135–1152. doi:10.1163/156853903322589678 [Google Scholar]
- Li D., Lim M.L.M., Seah W.K., Tay S.L. Prey attraction as a possible function of discoid stabilimenta of juvenile orb-spinning spiders. Anim. Behav. 2004;68:629–635. doi:10.1016/j.anbehav.2003.12.018 [Google Scholar]
- Lim M.L.M., Land M.F., Li D. Sex-specific UV and fluorescence signals in jumping spiders. Science. 2007;315:481. doi: 10.1126/science.1134254. doi:10.1126/science.1134254 [DOI] [PubMed] [Google Scholar]
- Linzen B. The tryptophan→ommochrome pathway in insects. In: Treherne J.E., Berridge M.J., Wigglesworth V.B., editors. Advances in insect physiology. vol. 10. Academic Press; London, UK/New York, NY: 1974. pp. 117–246. [Google Scholar]
- Mackenzie S.M., Howells A.J., Cox G.B., Ewart G.D. Sub-cellular localization of the White/Scarlet ABC transporter to pigment granule membranes within the compound eye of Drosophila melanogaster. Genetica. 2000;108:239–252. doi: 10.1023/a:1004115718597. doi:10.1023/A:1004115718597 [DOI] [PubMed] [Google Scholar]
- McIver J.D., Stonedahl G. Myrmecomorphy: morphological and behavioral mimicry of ants. Annu. Rev. Entomol. 1993;38:351–379. [Google Scholar]
- Millot J. Contributions à l'histophysiologie des araneides. Bull. Biol. Fr. Bel. 1926;8(Suppl.):1–283. [Google Scholar]
- Morse D.H. Harvard University Press; Cambridge, MA: 2007. Predator upon a flower: life history and fitness in a crab spider. [Google Scholar]
- Needham A.E. Springer; Berlin, Germany: 1974. The significance of zoochromes. [Google Scholar]
- Nelson X.J., Jackson R.R. Compound mimicry and trading predators by the males of sexually dimorphic Batesian mimics. Proc. R. Soc. B. 2006a;273:367–372. doi: 10.1098/rspb.2005.3340. doi:10.1098/rspb.2005.3340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson X.J., Jackson R.R. Vision-based innate aversion to ants and ant mimics. Behav. Ecol. 2006b;17:676–681. doi:10.1093/beheco/ark017 [Google Scholar]
- Nelson X.J., Jackson R.R. Complex display behaviour during the intraspecific interactions of myrmecomorphic jumping spiders (Araneae, Salticidae) J. Nat. Hist. 2007a;41:1659–1678. doi:10.1080/00222930701450504 [Google Scholar]
- Nelson X.J., Jackson R.R. Vision-based ability of an ant-mimicking jumping spider to discriminate between models, conspecific individuals and prey. Insect. Soc. 2007b;54:1–4. doi:10.1007/s00040-006-0901-x [Google Scholar]
- Nelson X.J., Jackson R.R., Edwards G.B., Barrion A.T. Living with the enemy: jumping spiders that mimic weaver ants. J. Arachnol. 2005;33:813–819. doi:10.1636/S04-12.1 [Google Scholar]
- Nelson X.J., Jackson R.R., Li D. Conditional use of honest signaling by a Batesian mimic. Behav. Ecol. 2006a;17:575–580. doi:10.1093/beheco/arj068 [Google Scholar]
- Nelson X.J., Jackson R.R., Li D., Barrion A.T., Edwards G.B. Innate aversion to ants (Hymenoptera: Formicidae) and ant mimics: experimental findings from mantises (Mantodea) Biol. J. Linn. Soc. 2006b;88:23–32. doi:10.1111/j.1095-8312.2006.00598.x [Google Scholar]
- Nelson X.J., Li D., Jackson R.R. Out of the frying pan and into the fire: a novel trade-off for Batesian mimics. Ethology. 2006c;112:270–277. doi:10.1111/j.1439-0310.2006.01155.x [Google Scholar]
- Ostrovsky M.A., Fedorovich I.B. Retinal as sensitizer of photodamage to retinal proteins of eye retina. Biofisika. 1994;39:13–25. [PubMed] [Google Scholar]
- Ostrovsky M.A., Sakina N.L., Dontsov A.E. An antioxidative role of ocular screening pigments. Vis. Res. 1987;27:893–899. doi: 10.1016/0042-6989(87)90005-8. doi:10.1016/0042-6989(87)90005-8 [DOI] [PubMed] [Google Scholar]
- Oxford G.S. Genetic drift within a protected polymorphism: enigmatic variation in color-polymorph frequencies in the candy-stripe spider, Enoplognatha ovata. Evolution. 2005;59:2170–2184. doi: 10.1554/05-046.1. doi:10.1554/05-046.1 [DOI] [PubMed] [Google Scholar]
- Oxford G.S., Gillespie R.G. Evolution and ecology of spider coloration. Annu. Rev. Entomol. 1998;43:619–643. doi: 10.1146/annurev.ento.43.1.619. doi:10.1146/annurev.ento.43.1.619 [DOI] [PubMed] [Google Scholar]
- Oxford G.S., Gillespie R.G. Portraits of evolution: studies of coloration in Hawaiian spiders. Biosciences. 2001;51:521–528. doi:10.1641/0006-3568(2001)051[0521:POESOC]2.0.CO;2 [Google Scholar]
- Rabaud E. Note sommaire sur l'adaptation chromatique des Thomisides. Bull. Soc. Zool. Fr. 1918;43:195–197. [Google Scholar]
- Rabaud E. Deuxième note sur l'adaptation chromatique des Thomisides. Bull. Soc. Zool. Fr. 1919;44:327–329. [Google Scholar]
- Rao, D., Webster, M., Heiling, A. M., Bruce, M. J., Herberstein, M. E. In press. The aggregating behaviour of Argiope radon, with special reference to web decorations. J. Ethol. (doi:10.1007/s10164-007-0080-x)
- Sakina N.L., Dontsov A.E., Lapina V.A., Ostrovsky M.A. Protective system of eye structures from photoinjury. II. Screening pigments of arthropods—ommochromes—as inhibitors of photooxidative processes. J. Evol. Biochem. Physiol. 1987;23:702–706. [Google Scholar]
- Schmalhofer V.R. Diet-induced and morphological color changes in juvenile crab spiders (Araneae, Thomisidae) J. Arachnol. 2000;28:56–60. doi:10.1636/0161-8202(2000)028[0056:DIAMCC]2.0.CO;2 [Google Scholar]
- Seah W.K., Li D. Stabilimenta attract unwelcome predators to orb-webs. Proc. R. Soc. B. 2001;268:1553–1558. doi: 10.1098/rspb.2001.1709. doi:10.1098/rspb.2001.1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seah W.K., Li D. Stabilimentum variations in Argiope versicolor (Araneae: Araneidae) from Singapore. J. Zool. Lond. 2002;258:531–540. doi:10.1017/S0952836902001693 [Google Scholar]
- Seligy V.L. Ommochrome pigments of spiders. Comp. Biochem. Physiol. A. 1972;42:699–709. doi:10.1016/0300-9629(72)90448-3 [Google Scholar]
- Starks P.T. The adaptive significance of stabilimentum in orb-webs: a hierarchical approach. Ann. Zool. Fenn. 2002;39:307–315. [Google Scholar]
- Stavenga D.G. Pigments in compound eyes. In: Stavenga D.G., Hardie R.C., editors. Facets of vision. Springer; Berlin, Germany: 1989. pp. 152–172. [Google Scholar]
- Théry M. Colours of background reflected light and of the prey's eye affect adaptive coloration in female crab spiders. Anim. Behav. 2007;73:797–804. doi:10.1016/j.anbehav.2006.06.015 [Google Scholar]
- Théry M., Casas J. Predator and prey views of spider camouflage. Nature. 2002;415:133. doi: 10.1038/415133a. doi:10.1038/415133a [DOI] [PubMed] [Google Scholar]
- Théry M., Debut M., Gomez D., Casas J. Specific color sensitivities of prey and predator explain camouflage in different visual systems. Behav. Ecol. 2005;16:25–29. doi:10.1093/beheco/arh130 [Google Scholar]
- Tso I.-M., Tai P.-L., Ku T.-H., Kuo C.-H., Yang E.-C. Colour-associated foraging success and population genetic structure in a sit-and-wait predator Nephila maculata (Araneae: Tetragnathidae) Anim. Behav. 2002;63:175–182. doi:10.1006/anbe.2001.1878 [Google Scholar]
- Tso I.-M., Lin C.-W., Yang E.-C. Colourful orb-weaving spiders, Nephila pilipes, through a bee's eyes. J. Exp. Biol. 2004;207:2631–2637. doi: 10.1242/jeb.01068. doi:10.1242/jeb.01068 [DOI] [PubMed] [Google Scholar]
- Tso I.-M., Liao C.-P., Huang R.-P., Yang E.-C. Function of being colorful in web spiders: attracting prey or camouflaging oneself? Behav. Ecol. 2006;17:606–613. doi:10.1093/beheco/ark010 [Google Scholar]
- Tso I.-M., Huang J.-P., Liao C.-P. Nocturnal hunting of a brightly coloured sit-and-wait predator. Anim. Behav. 2007;74:787–793. doi:10.1016/j.anbehav.2006.09.023 [Google Scholar]
- Vaclav R., Prokop P. Does the appearance of orbweaving spiders attract prey? Ann. Zool. Fenn. 2006;43:65–71. [Google Scholar]
- Vuillaume M. Pigmentations et variations pigmentaires de trois insectes: Mantis religiosa, Sphodromantis viridis, et Locusta migratoria. Bull. Biol. Fr. Belg. 1968;102:147–232. [Google Scholar]
- Watanabe T. The influence of energetic state on the form of stabilimentum built by Octonoba sybotides (Araneae: Uloboridae) Ethology. 1999;105:719–725. doi:10.1046/j.1439-0310.1999.00451.x [Google Scholar]