Abstract
Organisms capable of rapid physiological colour change have become model taxa in the study of camouflage because they are able to respond dynamically to the changes in their visual environment. Here, we briefly review the ways in which studies of colour changing organisms have contributed to our understanding of camouflage and highlight some unique opportunities they present. First, from a proximate perspective, comparison of visual cues triggering camouflage responses and the visual perception mechanisms involved can provide insight into general visual processing rules. Second, colour changing animals can potentially tailor their camouflage response not only to different backgrounds but also to multiple predators with different visual capabilities. We present new data showing that such facultative crypsis may be widespread in at least one group, the dwarf chameleons. From an ultimate perspective, we argue that colour changing organisms are ideally suited to experimental and comparative studies of evolutionary interactions between the three primary functions of animal colour patterns: camouflage; communication; and thermoregulation.
Keywords: crypsis, signalling, physiological colour change, trade-off, thermoregulation
1. Introduction
Colour change is surprisingly widespread in the animal kingdom, with the ability for rapid change occurring in a broad range of invertebrate and vertebrate ectotherms including crustaceans (Thurman 1988), insects (Hinton & Jarman 1972; Filshie et al. 1975), cephalopods (Norman 2000; Hanlon 2007), amphibians (King et al. 1994; Garcia & Sih 2003), reptiles (Cooper & Greenberg 1992) and fishes (Kodric-Brown 1998). There are two principally different types of colour change, which have different consequences for adaptive camouflage: (i) morphological colour change, which occurs due to changes in the number and quality of pigment-containing cells (chromatophores) in the dermis and usually takes place over a time scale of days or months, and (ii) physiological colour change, which occurs due to movement (dispersion or concentration) of pigment granules within chromatophores and is much more rapid, taking milliseconds to hours (Thurman 1988). Physiological colour change is generally under neuromuscular (cephalopods; Messenger 2001) or neuroendocrine control (most other taxa; Nery & Castrucci 1997), allowing rapid responses to changes in the animal's visual environment. Consequently, organisms capable of physiological colour change show some of the most remarkable and dynamic camouflage strategies in the animal kingdom and have become model taxa in the study of camouflage.
Here, we review the ways in which studies of organisms capable of rapid physiological colour change (which we refer throughout as colour changing animals for simplicity) have contributed to our understanding of camouflage, from both proximate and ultimate perspectives. All terminology relating to types of camouflage follows definitions in Stevens & Merilaita (2009a,b). In terms of proximate mechanisms, we briefly discuss the range of camouflage strategies employed by colour changing animals and how this has contributed to our understanding of visual perception. Next, we propose that colour changing organisms potentially exhibit facultative crypsis, whereby the animal tailors its camouflage response to different predators as well as different backgrounds. This is particularly important, given that most animals exist within a multi-predator environment. We provide new data on the prevalence and evolution of facultative crypsis among 21 lineages of dwarf chameleons (Bradypodion spp.). From an ultimate perspective, we briefly review the interactions between camouflage, signalling and thermoregulation, and their roles in the evolution of colour change. We argue that although studies of colour changing organisms pose some non-trivial challenges, they also present opportunities to gain a better understanding of evolutionary trade-offs between camouflage and other primary functions of animal colour patterns. Finally, we discuss how the conflicting demands of camouflage, signalling and thermoregulation relate to the limits and costs of colour change as well as the evolution of this adaptive strategy.
2. Camouflage strategies and visual perception
Colour changing animals are potentially capable of employing multiple camouflage strategies, which makes them ideal for studying how diverse environmental factors, such as visual background, predator species composition and abundance and the presence of conspecifics, influence camouflage. For example, the three principal physiological colour patterns displayed by juvenile bullethead parrotfish Chlorurus sordidus (stripes, a distinct ‘eyespot’ at the base of the tail fin and a ‘uniformly dark’ pattern) are associated not only with the structural complexity of the background but also with different body sizes and social contexts (Crook 1997). These three patterns are likely to exploit different camouflage or anti-predator mechanisms including disruptive camouflage or motion dazzle (stripes), intimidation of predators or deflecting attention towards the tail (eyespot pattern) and background matching (uniformly dark pattern; Stevens 2007; Stevens et al. 2008). More conclusive evidence for the use of multiple camouflage strategies derives from cephalopods, which have been studied extensively in the laboratory. For instance, cuttlefish employ both mimicry or masquerade and remarkable background matching in terms of colour, pattern and texture (Hanlon 1996, 2007). They also employ a body pattern known as ‘disruptive’ due to the presence of high-contrast light and dark patches with well-defined edges, some of which are found at the body's margin (Hanlon & Messenger 1988; Hanlon 2007). This body pattern is often elicited by backgrounds that contain discrete objects (e.g. pebbles) with size and contrast similar to the cuttlefish's disruptive pattern elements (Chiao & Hanlon 2001; Langridge 2006; Barbosa et al. 2007, 2008; Kelman et al. 2007, 2008; Mathger et al. 2007, 2008), leading to the suggestion that the camouflage mechanism involved is actually crypsis via background matching or ‘general background resemblance’ (Kelman et al. 2007). Whether disruptive body patterns prevent detection or recognition by disrupting object-background segmentation (disruptive camouflage) or by background matching or a combination of the two is the subject of ongoing debate and investigation (Hanlon 2007; Kelman et al. 2007, 2008), highlighting the subtleties and interrelations among camouflage strategies.
Irrespective of the camouflage mechanism employed, experimental studies of camouflage in colour changing organisms allow researchers to investigate the visual perception mechanisms of the animal, as well as the predator and prey species to which it must appear camouflaged (Kelman et al. 2007, 2008). By manipulating the visual background and examining the animal's colour response, we have learned a great deal about the cues triggering particular colour patterns and visual processes involved in object recognition (reviewed in Kelman et al. 2008). Specifically, such studies have shown how visual features such as the size, contrast, configuration, texture and edges of background objects influence the type of camouflage pattern adopted (e.g. Chiao et al. 2005; Barbosa et al. 2007, 2008; Kelman et al. 2007; Zylinski et al. 2009 and references therein). Importantly, this extensive body of research has provided protocols for objectively quantifying the full range of colour patterns in cuttlefish (e.g. Hanlon & Messenger 1988; Kelman et al. 2007; Barbosa et al. 2008), which arguably possess one of the largest pattern repertoires of any colour changing animal. The literature on visual perception mechanisms involved in camouflage will not be reviewed here, since it is addressed in detail elsewhere (e.g. Kelman et al. 2008). However, few colour changing organisms have been exposed to systematic experimental manipulation of backgrounds to elicit different camouflage responses or to study visual perception apart from cuttlefish and flatfish (e.g. flounders, sole, turbot, plaice, halibut), which have only been shown to attempt general background resemblance (Saidel 1978; Ramachandran et al. 1996; Healey 1999; Kelman et al. 2006). Whether the visual cues and visual perception mechanisms are similar across different taxonomic groups is of particular interest because it can provide insight into ‘universal visual processing rules’. For instance, Kelman et al. (2008) argued that object recognition in cuttlefish is similar to that in humans and is also likely to resemble that of their predators (see also Zylinski et al. 2009). There is therefore great scope for comparative studies of visual perception mechanisms among different colour changing taxa to elucidate the nature of general visual processing rules.
3. Facultative crypsis
Most animals are exposed to multiple predators, which may differ greatly in their sensory systems, means of prey detection and level of threat. Colour changing organisms have the potential to rapidly change not only their behaviour but also their colour patterns, to different predators. The mimic octopus Thaumoctopus mimicus, for example, can mimic an impressive repertoire of venomous animals, potentially adopting a different guise in response to different types of predator (Norman et al. 2001), although this has yet to be confirmed empirically. Similarly, the cuttlefish, Sepia officinalis, only exhibits a high-contrast eyespot signal, known as the diematic display, towards visual but not chemosensory predators (Langridge et al. 2007). In chameleons, there is some evidence for predator-specific responses involving background matching rather than mimicry or warning signals. Smith's dwarf chameleon Bradypodion taeniabronchum exhibits closer background colour matching in response to a bird than snake predator (Stuart-Fox et al. 2008). Based on models of avian and snake colour perception, the chameleons nevertheless appear more camouflaged in terms of colour contrast to a snake because snakes have poorer colour discrimination. This raises the intriguing possibility that chameleons facultatively adjust their camouflage in relation to differences in predator visual systems.
Whether this ability for facultative crypsis is widespread is not currently known. We therefore tested for predator-specific colour change in 20 additional species or populations of dwarf chameleon Bradypodion spp. The 21 populations, which include B. taeniabronchum, comprise all 15 currently described species and 6 morphologically distinct, genetically divergent lineages as described in Stuart-Fox et al. (2007) and Stuart-Fox & Moussalli (2008). The species are distributed across the more mesic southern and eastern parts of South Africa, but occur in a wide variety of habitat types including both montane and lowland forests, grasslands and heaths (Branch 1998). Methods for the 20 additional populations are identical to those detailed for B. taeniabronchum in Stuart-Fox et al. (2008). Briefly, in field trials conducted within the chameleons' natural habitats, we presented chameleons with a model of each of two widespread predators of dwarf chameleons: a stuffed fiscal shrike Lanius collaris and a resin model (made from a cast of a fresh specimen) of the diurnal, visually hunting snake Dispholidus typus. The ranges of both predators broadly overlap those of all the 21 populations/species of dwarf chameleons (Sinclair et al. 1993; Branch 1998). We measured colour responses of 11–20 individuals per population (from a single locality, mode=16; tables S1 and S2 in the electronic supplementary material) for three body regions (top, middle or bottom flank). We also measured reflectance of the background (the natural perch on which experiments were conducted as chameleons match the branch that they perch on) and irradiance (habitat light), using a spectroradiometer. We then estimated the detectability (visual contrast against the background) of chameleon colour responses relative to the visual systems of these two predators using a model of animal colour perception (Vorobyev & Osorio 1998; Siddiqi et al. 2004). This model has been applied to a range of vertebrates (e.g. Siddiqi et al. 2004; Hemmi et al. 2006; Stuart-Fox et al. 2008 and references therein). It assumes that visual discrimination is limited by photoreceptor noise and can be used to estimate the discriminability of two colours in units of discrimination thresholds or ‘just noticeable differences’. We tested for differences between detectability of chameleons to the two predators using repeated-measures ANOVA (Proc Mixed, SAS v. 9.1).
We found that 11 of the 21 populations showed colour responses to the two predators that differed significantly in chromatic (colour) contrast against the background (table S1 in the electronic supplementary material), while 13 of the 21 populations exhibited responses that differed significantly in achromatic (luminance) contrast (table S2 in the electronic supplementary material). In all populations for which there were significant differences in colour responses, chameleons showed closer background matching in response to birds than snakes. This was evident to both the bird and snake visual systems (tables S1 and S2 in the electronic supplementary material). Although they showed closer colour matching in response to birds, chameleons nevertheless appear more camouflaged (i.e. less chromatically contrasting against the background) to snakes. The most probable reason for this consistent pattern is that chameleons may need to show closer background matching in response to birds than to snakes to achieve a similar level of camouflage because birds have better colour discrimination: diurnal snakes are trichromats, having three different types of visual pigment (Sillman et al. 1997), while birds are tetrachromats (Hart & Hunt 2007). Similarly, chameleons may become notably lighter in response to snakes (more achromatically contrasting) due to potential differences in the viewing angle and visual ecology of the two predators. For instance, snakes may primarily approach their prey from below against a background of high illumination (sun/sky), whereas the opposite is likely to be the case for birds. The fact that chameleons showed poorer background matching in response to snakes than birds could reflect this difference in achromatic response in relation to background illumination intensity, which is not incorporated in discriminability estimates. Alternatively, if colour change is physiologically costly, chameleons may alter their investment towards camouflage in relation to perceived threat, which will, in part, be a function of predator visual capabilities (Stuart-Fox et al. 2006, 2008). This would explain why chameleons do not show ‘maximum camouflage’ at all times. Neurophysiological costs have similarly been invoked to explain the high frequency of only moderately cryptic or conspicuous colour patterns shown by octopuses (which are capable of being highly cryptic) when predators appear to be absent (Hanlon et al. 1999).
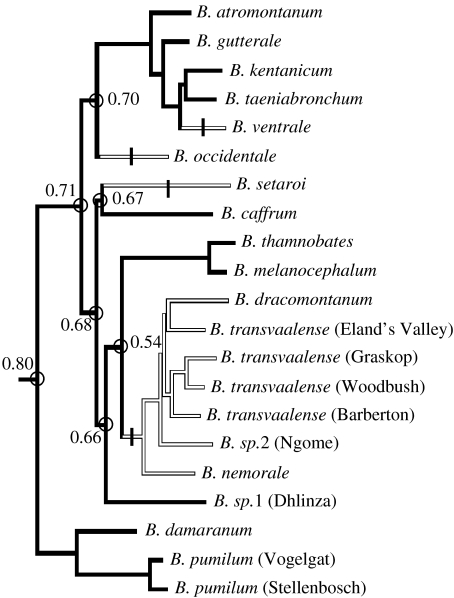
Our results suggest that, as Smith's dwarf chameleon, a number of other dwarf chameleon species appear to show facultative crypsis, although the extent of pattern matching has yet to be qualified. To examine the phylogenetic distribution of the ability for facultative crypsis, we mapped the presence or absence of facultative crypsis onto a molecular phylogeny of the 21 populations of dwarf chameleons (figure 1; see Stuart-Fox et al. (2007) for details of phylogenetic reconstruction). Ancestral state reconstructions were done using both parsimony and maximum likelihood (Markov K1 model), implemented in Mesquite v. 2.5 (Maddison & Maddison 2008). The most parsimonious ancestral state for dwarf chameleons is an ability for facultative crypsis, which appears to have been lost on four independent occasions (figure 1). Maximum-likelihood ancestral state reconstructions were equivocal for several nodes. However, all equivocal nodes had a notably higher proportional likelihood of exhibiting facultative crypsis (figure 1), consistent with the parsimony results. The apparent loss of the ability for facultative crypsis in some species may reflect geographical variation in the relative abundance and species composition of avian and snake predators. There is no obvious relationship between facultative crypsis and habitat since species that show and do not show facultative crypsis occur in all three main habitat types: grassland; forest; and heath. However, detailed data on the ecology, abundance and species composition of bird and snake predators are needed to test whether facultative crypsis is a function of predator diversity, density or behaviour. Overall, our results suggest that at least 11 dwarf chameleon species show facultative crypsis, although additional experimental tests are required to verify that predators perceive the chameleon colour differences and respond to them differently.
Figure 1.
Phylogenetic relationships of the 21 lineages of dwarf chameleons based on mitochondrial 16S and ND2 sequences (see Stuart-Fox et al. 2007). Divergent lineages that are yet to be described are denoted as B. sp. followed by the locality. Terminal taxa showing chromatic facultative crypsis are shown as black branches, while white branches represent those that do not show facultative crypsis. Parsimony ancestral state reconstruction indicates that the ancestral character was an ability for facultative crypsis, and that this ability has been lost four times independently (shown by short vertical bars transecting relevant branches). Nodes with equivocal maximum-likelihood ancestral states are circled with proportional likelihood of exhibiting facultative crypsis shown.
4. Interactions between camouflage, communication and thermoregulation
Conspicuous colour patterns represent the opposite end of the continuum from camouflage and are used by many animals to attract mates and deter rivals (Andersson 1994) or deter predators by signalling distastefulness (aposematism; Cott 1940). Apparently conspicuous coloration is generally assumed to be costly because it increases predation risk. However, recent studies suggest that conspicuous coloration may not necessarily carry a direct predation cost for several reasons. First, conspicuous coloration can simultaneously appear cryptic due to disruptive camouflage or distance effects, whereby colour patterns composed of conspicuous and highly contrasting colours merge to appear uniform and cryptic at the longer viewing distances typical for predators (e.g. Marshall 2000; Tullberg et al. 2005; Bohlin et al. 2008). This may be particularly common in animals with striped colour patterns, such as the blue and yellow stripes of reef fish, which merge to appear similar to the green of their coral backgrounds at greater distances (Marshall 2000). Second, conspicuous coloration may appear conspicuous to conspecifics while remaining concealed from predators due to differences in their visual capabilities. For instance, the colour signalling badges of European songbirds are more conspicuous to other songbirds, which have an ultraviolet-tuned visual system, than to their raptor predators, which have a violet-tuned visual system (Hastad et al. 2005). Third, conspicuous coloration can simultaneously function as warning coloration if it signals toxicity (e.g. Darst et al. 2006). Lastly, the cost of conspicuous coloration may be indirect if individuals compensate behaviourally for conspicuous coloration by, for example, retreating more readily or remaining closer to shelter, which can, in turn, reduce mating or foraging opportunities (Forsman & Appelqvist 1998). Consequently, the trade-off between selection for conspicuous colours and crypsis is not necessarily a simple one. Moreover, colour patterns may carry additional thermoregulatory costs or benefits, particularly in terrestrial ectotherms. Understanding interactions between different functions of animal colour patterns therefore remains an important challenge for evolutionary biologists.
Most experimental studies of the trade-off between camouflage and conspicuousness have manipulated animal colour patterns. This approach is fraught with problems such as differences in the spectral properties of natural and artificial colours used for colour manipulations and, when models are used, differences in the appearance or behaviour of real animals and models (Stuart-Fox et al. 2003). Colour changing organisms provide an opportunity to better understand such trade-offs because colour patterns are expected to vary directly in relation to costs and benefits, which can be experimentally manipulated. For example, Hemmi et al. (2006) studied colour patterns of the fiddler crab Uca vomeris, which is capable of rapid physiological colour change. They first showed that the crabs' mottled coloration appears cryptic against the background while the blue and white display colours are conspicuous to both crabs and their predators. Populations with higher levels of avian predation have mottled, cryptically coloured crabs, suggesting that blue and white display colours carry a predation cost. They were able to verify this experimentally by increasing perceived predation cost of conspicuous coloration (via the use of a model predator). Colourful crabs changed their coloration to appear more cryptic within days (Hemmi et al. 2006), providing convincing experimental evidence for a direct trade-off between signalling and predation risk. Although there is some evidence in colour changing species for colour-dependent anti-predator (e.g. Garcia & Sih 2003) and social behaviours (e.g. Hoglund et al. 2002), surprisingly few studies have examined the trade-off between camouflage and signalling by manipulating predation risk, background colour or social environment.
Even fewer studies have examined interactions between camouflage or signalling and the third important function of colour patterns: thermoregulation. In many colour changing taxa, including fishes, reptiles, amphibians and crustaceans, temperature influences melanocyte-stimulating hormone, which affects melanin dispersion (Fernandez & Bagnara 1991; Castrucci et al. 1997; Visconti et al. 1999; Hoglund et al. 2002). The resulting darkening or lightening, usually of either dorsal surfaces or the entire body, aids heat absorption and reflection, respectively, but may also increase conspicuousness (Norris 1967) by increasing colour contrast or reducing pattern matching. For example, in a laboratory setting, Pacific tree frogs Hyla regilla contrasted more against brown backgrounds at temperatures of 10°C than 25°C (Stegen et al. 2004). In a pioneering study of the interaction between colour change and thermoregulation in 25 species of desert reptiles, Norris (1967) showed that the precision of background colour matching in the visible spectrum was dependent on temperature and the thermal ecology of the species. Thermophilic species became markedly paler than their backgrounds (‘superlight’) at very high temperatures (more than 40°C), but at these temperatures, potential predators are inactive, so costs of increased conspicuousness may be negligible (Norris 1967). Conversely, at cool temperatures, lizards were substantially darker than their backgrounds but compensated behaviourally for increased conspicuousness by maintaining a close distance to shelter (Norris 1967).
As these examples illustrate, there is likely to be a complex interaction between demands of camouflage, thermoregulation and signalling. Many colour changing animals may only display conspicuous colours once they have attained a particular body temperature because only at higher body temperatures can they behaviourally compensate for increased conspicuousness (e.g. with faster escape response). The trade-off between thermoregulation and signalling is supported by evidence for sex-specific differences in colour change at different temperatures (Silbiger & Munguia 2008). An intriguing case occurs in one of few insects known to exhibit physiological colour change, the alpine grasshopper Kosciuscola tristis (Key & Day 1954a,b; Filshie et al. 1975). Both sexes are black or dark coloured at low temperatures (less than 10°C), but within minutes of being exposed to higher temperatures, males turn bright blue, whereas cryptically coloured females show much less dramatic colour change (Key & Day 1954b). The need to thermoregulate is likely to constrain male signalling in this temperate, high-elevation species as males only turn blue on warm days (Key & Day 1954b). This is also true of other colour changing terrestrial ectotherms such as many lizards (Norris 1967; Cooper & Greenberg 1992).
5. Limits and costs of colour change
Colour change provides a ‘solution’ to the conflicting demands of camouflage, signalling and thermoregulation; however, there are limits to colour change, which will affect an animal's ability to express an optimal phenotype in a given situation. For instance, colour changing animals may have evolved the ability to imperfectly match many backgrounds at the expense of superior camouflage against a single type of background. The degree of camouflage may be limited not only by the colour and pattern repertoire but also by the speed of colour change relative to the movement of the animal. The relationship between camouflage and movement requires much more research attention. Strategies adopted by colour changing organisms to compensate for increased conspicuousness due to movement can be informative in this regard. For example, colour changing animals may adopt particular patterns more frequently during movement (e.g. disruptive patterns or stripes), when background matching may be impossible or ineffective. Alternatively, they may use motion camouflage; for example, the very slow jerky walk of chameleons resembles movement of the vegetation, which the animal also resembles in colour and pattern (Nečas 2001). At the interspecific level, the ability to match different backgrounds may be better developed in some species than others, depending on potentially conflicting local selective pressures. This is illustrated by measures of background colour matching in Bradypodion spp. (table S1 in the electronic supplementary material), which show interspecific variation in mean chromatic contrast against the background ranging from less than one JND (i.e. indistinguishable from the background) in some species to more than three JNDs in others. The limits to colour change could be studied by comparing predicted phenotypic optima (potentially derived from mathematical models such as optimality models) with empirical data, an approach that has been successfully applied to understanding the evolution of phenotypic plasticity (Pigliucci 2005).
Logically distinct from limits to colour change are the potential associated physiological and fitness costs. Just as there can be fitness costs of phenotypic plasticity (e.g. Relyea 2002; Merila et al. 2004; but see Steiner & Van Buskirk 2008), there may be non-trivial costs of colour change. As Hanlon et al. (1999) remarked in a study of octopus camouflage ‘…it must be neurophysiologically expensive to operate those hundreds of thousands of chromatophores in synchrony with visual input, and to do so continually…’ and the same is true for animals in which colour change is under neuroendocrine rather than neuromuscular control. Whether there are significant costs of colour change remains to be demonstrated but could be tested by, for example, comparing physiological performance or fitness of individuals exposed to uniform backgrounds (minimal colour change) with those repeatedly exposed to diverse backgrounds (frequent colour change).
6. The evolution of colour change
Although biologists have devoted a great deal of effort to explaining the adaptive function of animal colour patterns, processes driving the evolution of colour change remain almost unexplored. In most groups in which colour change is prevalent, the ability to change colour varies markedly. This begs the question of why some species have evolved a greater colour changing capacity than others. The two most important processes driving the evolution of colour change are likely to be natural selection for camouflage and natural or sexual selection for signalling functions, although in terrestrial taxa, it may also be driven by thermoregulatory requirements. The processes of natural selection for camouflage, signalling and thermoregulation generate different testable predictions. If the capacity for colour change is primarily driven by the need to appear camouflaged against a variety of backgrounds, then the species with the greatest colour changing capacity should (i) show a greater range of body patterns since camouflage against diverse backgrounds requires precise pattern choice, (ii) occupy habitats with greater pressure from visual predators (e.g. shallow, clear waters or habitats with higher predator abundance), (iii) co-occur with predators with a greater range of visual sensitivities, and (iv) occupy habitats with greater variance in background colour relative to the animal's movement patterns. Alternatively, if selection for social or sexual signalling drives the evolution of colour change, then the species showing the greatest colour change are predicted to have (i) more elaborate, ritualized social signalling, (ii) more intense sexual selection (e.g. highly skewed reproductive success, more costly pigment-based colour signals), and (iii) signals that are more conspicuous to conspecific receivers. Finally, if thermoregulatory requirements have driven the evolution of colour change, then the species with the greatest capacity for colour change should occupy more thermally extreme or variable environments.
Two of these predictions have been tested in a phylogenetic comparative study of colour change in dwarf chameleons (Stuart-Fox & Moussalli 2008). Among the 21 species of dwarf chameleons (Bradypodion spp.), those with the greatest capacity for colour change had social signals that were more conspicuous to the chameleon visual system but did not occupy habitats with greater variance in background colour (Stuart-Fox & Moussalli 2008). Although colour change clearly serves a camouflage function in chameleons, results of this study suggest that the remarkable ability for chromatic change in dwarf chameleons is likely to have evolved to facilitate social signalling rather than background matching. Whether this is true of other colour changing taxa is currently unknown. In many fish families, rapid colour change is typically expressed more by males than females and functions in both courtship and contests (Kodric-Brown 1998). Physiological colour change occurs in at least 24 families of fishes, the majority of which show permanent or seasonal sexual dichromatism (Kodric-Brown 1998). It is therefore possible that the evolution of colour change in many fishes is driven primarily by selection for sexual signalling, although there are likely to be exceptions (e.g. flatfish). By contrast, in amphibians colour change is relatively slow, largely limited to changes in luminance and appears to function most often in background adaptation (crypsis) and thermoregulation (e.g. King et al. 1994; Garcia & Sih 2003; Stegen et al. 2004), suggesting that selection for signalling is unlikely to be the primary driver of colour changing ability. In other colour changing taxa such as cephalopods, reptiles and crustaceans, however, most species use colour change for both crypsis and signalling, making processes driving the evolution of colour change difficult to infer without detailed experimental and comparative studies. In such groups, where colour change clearly has more than one adaptive function, the capacity for colour change may have evolved as a strategy to accommodate conflicting selective pressures (camouflage, signalling and thermoregulation). Alternatively, colour change may have initially evolved to accommodate camouflage or thermoregulatory requirements and subsequently been co-opted for conspicuous transient signalling (Stuart-Fox & Moussalli 2008).
7. Conclusion
Ironically, the best camouflaged animals are often the hardest to study because they are difficult to find in the wild—and this is particularly true of many colour changing animals. The use of colour changing animals poses additional challenges, not least of which is the non-trivial problem of quantifying colour and colour change in organisms that can change their appearance in minutes, seconds and even milliseconds. However, the studies cited in this review are testament that these problems are surmountable. To date, the great majority of these studies have focused on proximate factors, particularly the structure and arrangement of chromatophores, processes regulating movement of pigments and visual cues triggering different camouflage patterns. The relative paucity of studies that place colour change within an ecological or evolutionary context—or indeed of studies that integrate proximate mechanisms and ultimate explanations—was lamented by Waring (1963) in a monograph on colour change, and is just as true more than 50 years later. We have highlighted the potential of colour changing organisms to provide insight into not only camouflage strategies and visual perception mechanisms but also evolutionary interactions between camouflage, signalling and thermoregulation. We have done so in the hope of stimulating further research into what we see as five key areas: (i) comparative studies of camouflage and visual perception mechanisms, (ii) the evolution of camouflage strategies in a multi-predator environment, (iii) the effect of trade-offs between conflicting selective pressures on the evolution of animal colour patterns, (iv) limits to and costs of colour change, and (v) processes driving its evolution.
Acknowledgments
We thank Martin Stevens, Sami Merilaita, Mike Kearney and two anonymous reviewers for their critical comments. We are grateful to Adrian Armstrong, Bill Branch, Michael Cunningham, George Goode, David Hempson, Kate Henderson, Amanda Lane, Elton Larroux, Georgio Lombardi, Jerry Therron, Stephanie Ritter, Colin Tilbury, Krystal Tolley and Martin Whiting for their assistance. Permits: MPB.5104 (Mpumalanga); 005-00001 (Limpopo); 1721/2003 and 4390/2005 (KwaZulu Natal); 234/2003 (Western Cape); and WRO 11/03 WR (Eastern Cape). Funding for this work was provided by the Claude Leon Foundation, National Research Foundation (NRF), UNESCO-L'Oreal and the Australian Research Council to D.S.-F. A.M. was supported by the Claude Leon and Ian Potter Foundations.
Footnotes
One contribution of 15 to a Theme Issue ‘Animal camouflage: current issues and new perspectives’.
Supplementary Material
Results of repeated measures ANOVA testing for differences in chromatic (Table S1) and achromatic (Table S2) responses to bird and snake predators in 21 species of dwarf chameleon, Bradypodion spp..
References
- Andersson M. Princeton University Press; Princeton, NJ: 1994. Sexual selection. [Google Scholar]
- Barbosa A., Mathger L.M., Chubb C., Florio C., Chiao C.C., Hanlon R.T. Disruptive coloration in cuttlefish: a visual perception mechanism that regulates ontogenetic adjustment of skin patterning. J. Exp. Biol. 2007;210:1139–1147. doi: 10.1242/jeb.02741. doi:10.1242/jeb.02741 [DOI] [PubMed] [Google Scholar]
- Barbosa A., Mathger L.M., Buresch K.C., Kelly J., Chubb C., Chiao C.C., Hanlon R.T. Cuttlefish camouflage: the effects of substrate contrast and size in evoking uniform, mottle or disruptive body patterns. Vision Res. 2008;48:1242–1253. doi: 10.1016/j.visres.2008.02.011. doi:10.1016/j.visres.2008.02.011 [DOI] [PubMed] [Google Scholar]
- Bohlin T., Tullberg B.S., Merilaita S. The effect of signal appearance and distance on detection risk in an aposematic butterfly larva (Parnassius apollo) Anim. Behav. 2008;76:577–584. doi:10.1016/j.anbehav.2008.02.012 [Google Scholar]
- Branch W.R. Struik Publishers; Cape Town, Republic of South Africa: 1998. A field guide to snakes and other reptiles of southern Africa. [Google Scholar]
- Castrucci A.M.D., Sherbrooke W.C., Zucker N. Regulation of physiological color change in dorsal skin of male tree lizards, Urosaurus ornatus. Herpetologica. 1997;53:405–410. [Google Scholar]
- Chiao C.C., Hanlon R.T. Cuttlefish camouflage: visual perception of size, contrast and number of white squares on artificial checkerboard substrata initiates disruptive coloration. J. Exp. Biol. 2001;204:2119–2125. doi: 10.1242/jeb.204.12.2119. [DOI] [PubMed] [Google Scholar]
- Chiao C.C., Kelman E.J., Hanlon R.T. Disruptive body patterning of cuttlefish (Sepia officinalis) requires visual information regarding edges and contrast of objects in natural substrate backgrounds. Biol. Bull. 2005;208:7–11. doi: 10.2307/3593095. doi:10.2307/3593095 [DOI] [PubMed] [Google Scholar]
- Cooper, W. E. & Greenberg, N. 1992 Reptilian coloration and behavior. In Biology of the Reptilia, vol. 18 (eds C. Gans & D. Crews), pp. 298–422. Chicago, IL: Chicago University Press.
- Cott H.B. Methuen; London, UK: 1940. Adaptive coloration in animals. [Google Scholar]
- Crook A.C. Colour patterns in a coral reef fish—is background complexity important? J. Exp. Mar. Biol. Ecol. 1997;217:237–252. doi:10.1016/S0022-0981(97)00059-2 [Google Scholar]
- Darst C.R., Cummings M.E., Cannatella D.C. A mechanism for diversity in warning signals: conspicuousness versus toxicity in poison frogs. Proc. Natl Acad. Sci. USA. 2006;103:5852–5857. doi: 10.1073/pnas.0600625103. doi:10.1073/pnas.0600625103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez P.J., Bagnara J.T. Effect of background color and low-temperature on skin color and circulating alpha-MSH in two species of leopard frog. Gen. Comp. Endocrinol. 1991;83:132–141. doi: 10.1016/0016-6480(91)90113-k. doi:10.1016/0016-6480(91)90113-K [DOI] [PubMed] [Google Scholar]
- Filshie B.K., Day M.F., Mercer E.H. Color and color change in the grasshopper, Kosciuscola tristis. J. Insect Physiol. 1975;21:1763–1770. doi:10.1016/0022-1910(75)90238-3 [Google Scholar]
- Forsman A., Appelqvist S. Visual predators imposes correlated selection on prey color pattern and behavior. Behav. Ecol. 1998;9:409–413. doi:10.1093/beheco/9.4.409 [Google Scholar]
- Garcia T.S., Sih A. Color change and color-dependent behavior in response to predation risk in the salamander sister species Ambystoma barbouri and Ambystoma texanum. Oecologia. 2003;137:131–139. doi: 10.1007/s00442-003-1314-4. doi:10.1007/s00442-003-1314-4 [DOI] [PubMed] [Google Scholar]
- Hanlon R.T. Cambridge University Press; Cambridge, UK: 1996. Cephalopod behaviour. [Google Scholar]
- Hanlon R.T. Cephalopod dynamic camouflage. Curr. Biol. 2007;17:R400–R404. doi: 10.1016/j.cub.2007.03.034. doi:10.1016/j.cub.2007.03.034 [DOI] [PubMed] [Google Scholar]
- Hanlon R.T., Messenger J.B. Adaptive coloration in young cuttlefish (Sepia officinalis)—the morphology and development of body patterns and their relation to behavior. Phil. Trans. R. Soc. B. 1988;320:437–487. doi:10.1098/rstb.1988.0087 [Google Scholar]
- Hanlon R.T., Forsythe J.W., Joneschild D.E. Crypsis, conspicuousness, mimicry and polyphenism as antipredator defences of foraging octopuses on Indo-Pacific coral reefs, with a method of quantifying crypsis from video tapes. Biol. J. Linn. Soc. 1999;66:1–22. doi:10.1111/j.1095-8312.1999.tb01914.x [Google Scholar]
- Hart N.S., Hunt D.M. Avian visual pigments: characteristics, spectral tuning, and evolution. Am. Nat. 2007;169:S7–S26. doi: 10.1086/510141. doi:10.1086/510141 [DOI] [PubMed] [Google Scholar]
- Hastad O., Victorsson J., Odeen A. Differences in color vision make passerines less conspicuous in the eyes of their predators. Proc. Natl Acad. Sci. USA. 2005;102:6391–6394. doi: 10.1073/pnas.0409228102. doi:10.1073/pnas.0409228102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healey E.G. The skin pattern of young plaice and its rapid modification in response to graded changes in background tint and pattern. J. Fish Biol. 1999;55:937–971. doi:10.1111/j.1095-8649.1999.tb00732.x [Google Scholar]
- Hemmi J.M., Marshall J., Pix W., Vorobyev M., Zeil J. The variable colours of the fiddler crab Uca vomeris and their relation to background and predation. J. Exp. Biol. 2006;209:4140–4153. doi: 10.1242/jeb.02483. doi:10.1242/jeb.02483 [DOI] [PubMed] [Google Scholar]
- Hinton H.E., Jarman G.M. Physiological colour change in the Hercules beetle. Nature. 1972;238:160–161. doi:10.1038/238160a0 [Google Scholar]
- Hoglund E., Balm P.H.M., Winberg S. Behavioural and neuroendocrine effects of environmental background colour and social interaction in Arctic charr (Salvelinus alpinus) J. Exp. Biol. 2002;205:2535–2543. doi: 10.1242/jeb.205.16.2535. [DOI] [PubMed] [Google Scholar]
- Kelman E.J., Tiptus P., Osorio D. Juvenile plaice (Pleuronectes platessa) produce camouflage by flexibly combining two separate patterns. J. Exp. Biol. 2006;209:3288–3292. doi: 10.1242/jeb.02380. doi:10.1242/jeb.02380 [DOI] [PubMed] [Google Scholar]
- Kelman E.J., Baddeley R.J., Shohet A.J., Osorio D. Perception of visual texture and the expression of disruptive camouflage by the cuttlefish, Sepia officinalis. Proc. R. Soc. B. 2007;274:1369–1375. doi: 10.1098/rspb.2007.0240. doi:10.1098/rspb.2007.0240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelman E.J., Osorio D., Baddeley R.J. A review of cuttlefish camouflage and object recognition and evidence for depth perception. J. Exp. Biol. 2008;211:1757–1763. doi: 10.1242/jeb.015149. doi:10.1242/jeb.015149 [DOI] [PubMed] [Google Scholar]
- Key K.H.L., Day M.F. The physiological mechanism of colour change in the grasshopper Kosciuscola tristis Sjost (Orthoptera, Acrididae) Aust. J. Zool. 1954a;2:340–363. doi:10.1071/ZO9540340 [Google Scholar]
- Key K.H.L., Day M.F. A temperature-controlled physiological colour change in the grasshopper Kosciuscola tristis Sjost (Orthoptera: Acrididae) Aust. J. Zool. 1954b;2:309–339. doi:10.1071/ZO9540309 [Google Scholar]
- King R.B., Hauff S., Phillips J.B. Physiological color change in the green treefrog: responses to background brightness and temperature. Copeia. 1994;1994:422–432. doi:10.2307/1446990 [Google Scholar]
- Kodric-Brown A. Sexual dichromatism and temporary color changes in the reproduction of fishes. Am. Zool. 1998;38:70–81. [Google Scholar]
- Langridge K.V. Symmetrical crypsis and asymmetrical signalling in the cuttlefish Sepia officinalis. Proc. R. Soc. B. 2006;273:959–967. doi: 10.1098/rspb.2005.3395. doi:10.1098/rspb.2005.3395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langridge K.V., Broom M., Osorio D. Selective signalling by cuttlefish to predators. Curr. Biol. 2007;17:R1044–R1045. doi: 10.1016/j.cub.2007.10.028. doi:10.1016/j.cub.2007.10.028 [DOI] [PubMed] [Google Scholar]
- Maddison, W. P. & Maddison, D. R. 2008 Mesquite: a modular system for evolutionary analysis, version 2.5. See http://mesquiteproject.org
- Marshall N.J. Communication and camouflage with the same ‘bright’ colours in reef fishes. Phil. Trans. R. Soc. B. 2000;355:1243–1248. doi: 10.1098/rstb.2000.0676. doi:10.1098/rstb.2000.0676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathger L.M., Chiao C.C., Barbosa A., Buresch K.C., Kaye S., Hanlon R.T. Disruptive coloration elicited on controlled natural substrates in cuttlefish, Sepia officinalis. J. Exp. Biol. 2007;210:2657–2666. doi: 10.1242/jeb.004382. doi:10.1242/jeb.004382 [DOI] [PubMed] [Google Scholar]
- Mathger L.M., Chiao C.C., Barbosa A., Hanlon R.T. Color matching on natural substrates in cuttlefish, Sepia officinalis. J. Comp. Physiol. A. 2008;194:577–585. doi: 10.1007/s00359-008-0332-4. doi:10.1007/s00359-008-0332-4 [DOI] [PubMed] [Google Scholar]
- Merila J., Laurila A., Lindgren B. Variation in the degree and costs of adaptive phenotypic plasticity among Rana temporaria populations. J. Evol. Biol. 2004;17:1132–1140. doi: 10.1111/j.1420-9101.2004.00744.x. doi:10.1111/j.1420-9101.2004.00744.x [DOI] [PubMed] [Google Scholar]
- Messenger J.B. Cephalopod chromatophores: neurobiology and natural history. Biol. Rev. 2001;76:473–528. doi: 10.1017/s1464793101005772. [DOI] [PubMed] [Google Scholar]
- Nečas P. Krieger Publishing; Malabar, FL: 2001. Chameleons: Nature's hidden jewels. [Google Scholar]
- Nery L.E.M., Castrucci A.M.D. Pigment cell signalling for physiological color change. Comp. Biochem. Physiol. A. 1997;118:1135–1144. doi: 10.1016/s0300-9629(97)00045-5. doi:10.1016/S0300-9629(97)00045-5 [DOI] [PubMed] [Google Scholar]
- Norman M.D. Conch Books; Hackenheim, Germany: 2000. Cephalopods: a world guide. [Google Scholar]
- Norman M.D., Finn J., Tregenza T. Dynamic mimicry in an Indo-Malayan octopus. Proc. R. Soc. B. 2001;268:1755–1758. doi: 10.1098/rspb.2001.1708. doi:10.1098/rspb.2001.1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris K.S. Color adaptation in desert reptiles and its thermal relationships. In: Milstead W.W., editor. Lizard ecology—a symposium. University of Missouri Press; Columbia, MO: 1967. pp. 162–229. [Google Scholar]
- Pigliucci M. Evolution of phenotypic plasticity: where are we going now? Trends Ecol. Evol. 2005;20:481–486. doi: 10.1016/j.tree.2005.06.001. doi:10.1016/j.tree.2005.06.001 [DOI] [PubMed] [Google Scholar]
- Ramachandran V.S., Tyler C.W., Gregory R.L., Rogers-Ramachandran D., Duensing S., Pillsbury C., Ramachandran C. Rapid adaptive camouflage in tropical flounders. Nature. 1996;379:815–818. doi: 10.1038/379815a0. doi:10.1038/379815a0 [DOI] [PubMed] [Google Scholar]
- Relyea R.A. Costs of phenotypic plasticity. Am. Nat. 2002;159:272–282. doi: 10.1086/338540. doi:10.1086/338540 [DOI] [PubMed] [Google Scholar]
- Saidel W. Analysis of flatfish camouflage. Am. Zool. 1978;18:579. [Google Scholar]
- Siddiqi A., Cronin T.W., Loew E.R., Vorobyev M., Summers K. Interspecific and intraspecific views of color signals in the strawberry poison frog Dendrobates pumilio. J. Exp. Biol. 2004;207:2471–2485. doi: 10.1242/jeb.01047. doi:10.1242/jeb.01047 [DOI] [PubMed] [Google Scholar]
- Silbiger N., Munguia P. Carapace color change in Uca pugilator as a response to temperature. J. Exp. Mar. Biol. Ecol. 2008;355:41–46. doi:10.1016/j.jembe.2007.11.014 [Google Scholar]
- Sillman A.J., Govardovskii V.I., Rohlich P., Southard J.A., Loew E.R. The photoreceptors and visual pigments of the garter snake (Thamnophis sirtalis): a microspectrophotometric, scanning electron microscopic and immunocytochemical study. J. Comp. Physiol. A. 1997;181:89–101. doi: 10.1007/s003590050096. doi:10.1007/s003590050096 [DOI] [PubMed] [Google Scholar]
- Sinclair, I., Hockey, P. & Tarboton, W. 1993 Illustrated guide to the birds of Southern Africa (illustrated by P. Hayman & N. Arlott). London, UK: New Holland.
- Stegen J.C., Gienger C.M., Sun L.X. The control of color change in the Pacific tree frog, Hyla regilla. Can. J. Zool. 2004;82:889–896. doi:10.1139/z04-068 [Google Scholar]
- Steiner U.K., Van Buskirk J. Environmental stress and the costs of whole-organism phenotypic plasticity in tadpoles. J. Evol. Biol. 2008;21:97–103. doi: 10.1111/j.1420-9101.2007.01463.x. doi:10.1111/j.1420-9101.2007.01463.x [DOI] [PubMed] [Google Scholar]
- Stevens M. Predator perception and the interrelation between different forms of protective coloration. Proc. R. Soc. B. 2007;274:1457–1464. doi: 10.1098/rspb.2007.0220. doi:10.1098/rspb.2007.0220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M., Merilaita S. Animal camouflage: current issues and new perspectives. Phil. Trans. R. Soc. B. 2009a;364:423–427. doi: 10.1098/rstb.2008.0217. doi:10.1098/rstb.2008.0217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M., Merilaita S. Defining disruptive coloration and distinguishing its functions. Phil. Trans. R. Soc. B. 2009b;364:481–488. doi: 10.1098/rstb.2008.0216. doi:10.1098/rstb.2008.0216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M., Hardman C.J., Stubbins C.L. Conspicuousness, not eye mimicry, makes “eyespots” effective antipredator signals. Behav. Ecol. 2008;19:525–531. doi:10.1093/beheco/arm162 [Google Scholar]
- Stuart-Fox D., Moussalli A. Selection for social signalling drives the evolution of chameleon colour change. PLoS Biol. 2008;6:e25. doi: 10.1371/journal.pbio.0060025. doi:10.1371/journal.pbio.0060025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart-Fox D., Moussalli A., Marshall J., Owens I.P.F. Conspicuous males suffer higher predation risk: visual modelling and experimental evidence from lizards. Anim. Behav. 2003;66:541–550. doi:10.1006/anbe.2003.2235 [Google Scholar]
- Stuart-Fox D., Whiting M.J., Moussalli A. Camouflage and colour change: antipredator responses to bird and snake predators across multiple populations in a dwarf chameleon. Biol. J. Linn. Soc. 2006;88:437–446. doi:10.1111/j.1095-8312.2006.00631.x [Google Scholar]
- Stuart-Fox D., Moussalli A., Whiting M.J. Natural selection on social signals: signal efficacy and the evolution of chameleon display coloration. Am. Nat. 2007;170:916–930. doi: 10.1086/522835. doi:10.1086/522835 [DOI] [PubMed] [Google Scholar]
- Stuart-Fox D., Moussalli A., Whiting M.J. Predator-specific camouflage in chameleons. Biol. Lett. 2008;4:326–329. doi: 10.1098/rsbl.2008.0173. doi:10.1098/rsbl.2008.0173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman C.L. Rhythmic physiological colour change in Crustacea—a review. Comp. Biochem. Physiol. C. 1988;91:171–185. doi:10.1016/0742-8413(88)90184-3 [Google Scholar]
- Tullberg B.S., Merilaita S., Wiklund C. Aposematism and crypsis combined as a result of distance dependence: functional versatility of the colour pattern in the swallowtail butterfly larva. Proc. R. Soc. B. 2005;272:1315–1321. doi: 10.1098/rspb.2005.3079. doi:10.1098/rspb.2005.3079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visconti M.A., Ramanzini G.C., Camargo C.R., Castrucci A.M.L. Elasmobranch color change: a short review and novel data on hormone regulation. J. Exp. Zool. 1999;284:485–491. doi: 10.1002/(sici)1097-010x(19991001)284:5<485::aid-jez3>3.0.co;2-5. doi:10.1002/(SICI)1097-010X(19991001)284:5<485::AID-JEZ3>3.0.CO;2-5 [DOI] [PubMed] [Google Scholar]
- Vorobyev M., Osorio D. Receptor noise as a determinant of colour thresholds. Proc. R. Soc. B. 1998;265:351–358. doi: 10.1098/rspb.1998.0302. doi:10.1098/rspb.1998.0302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring H. Academic Press; New York, NY: 1963. Colour change mechanisms of cold-blooded vertebrates. [Google Scholar]
- Zylinski S., Osorio D., Shohet A.J. Perception of edges and visual texture in the camouflage of the common cuttlefish Sepia officinalis. Phil. Trans. R. Soc. B. 2009;364:439–448. doi: 10.1098/rstb.2008.0264. doi:10.1098/rstb.2008.0264 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Results of repeated measures ANOVA testing for differences in chromatic (Table S1) and achromatic (Table S2) responses to bird and snake predators in 21 species of dwarf chameleon, Bradypodion spp..