Abstract
Of the many visual characteristics of animals, countershading (darker pigmentation on those surfaces exposed to the most lighting) is one of the most common, and paradoxically one of the least well understood. Countershading has been hypothesized to reduce the detectability of prey to visually hunting predators, and while the function of a countershaded colour pattern was proposed over 100 years ago, the field has progressed slowly; convincing evidence for the protective effects of countershading has only recently emerged. Several mechanisms have been invoked for the concealing function of countershading and are discussed in this review, but the actual mechanisms by which countershading functions to reduce attacks by predators lack firm empirical testing. While there is some subjective evidence that countershaded animals match the background on which they rest, no quantitative measure of background matching has been published for countershaded animals; I now present the first such results. Most studies also fail to consider plausible alternative explanations for the colour pattern, such as protection from UV or abrasion, and thermoregulation. This paper examines the evidence to support each of these possible explanations for countershading and discusses the need for future empirical work.
Keywords: countershading, Thayer, crypsis, ultraviolet, abrasion, thermoregulation
1. Introduction
(a) A history of the idea
Prey animals have evolved a variety of visual characteristics in order to avoid detection and attack by predators (see Ruxton et al. 2004a for a review). The diversity and function of these characteristics has attracted the interest of scientists and philosophers for centuries. Aristotle, for example, wrote about the octopus's ability to change its colour so as to resemble adjacent stones (Evans 1965). Colour patterns that protect prey from predation represent some of the earliest and most important examples of the Wallace/Darwinian interpretation of the natural world (Stevens 2007).
In a paper entitled ‘The law which underlies protective colouration’, Abbott H. Thayer (1896) noted that: ‘Animals are painted by nature, darkest on those parts which tend to be most lighted by the sky's light, and vice versa’. Thayer named this ‘obliterative shading’, although the term commonly used now is countershading. Countershading is an extremely common pattern of coloration in numerous terrestrial and aquatic groups (table 1).
Table 1.
Documented examples of countershading in aquatic and terrestrial animals, with the proposed function where available; the absence indicates the occurrence of countershading without stating a function. (SSC, self shadow concealment.)
| species | proposed function | authors |
|---|---|---|
| aquatic animals | ||
| Sepia officinalis, Loligo vulgaris, Octopus vulgaris | SSC | Ferguson & Messenger (1991) |
| Sepia officinalis | body less obvious against the background when the illumination comes from above | Ferguson et al. (1994) |
| Newfoundland and Labrador cod | camouflage | Gosse et al. (2001) |
| fish louse Anilocra physodes L. (Crustacea: Isopoda) | optical flattening | Korner (1982) |
| whale shark | eliminates the optical appearance of relief | Wilson & Martin (2004) |
| shallow water pony fish, (Leiognathus equulus) | eliminating silhouette | Hastings (1971) |
| Abraliopsis sp. | eliminating silhouette | Young & Roper (1976) |
| African upside down catfishes (Mochokidae) | Chapman et al. (1994) | |
| upside down catfish, (Synodontis nigriventris) | not discussed | Nagaishi et al. (1989) |
| common water bug Notonecta glauca | not discussed | Korner (1982) |
| midwater shrimp (Sergestes similis) | crypsis to silhouette-scanning predators | Lindsay et al. (1999) |
| horse mackerel (Trachurus trachurus) | intensity of the reflected light equals the intensity of the background for most angles of view | Cott (1940) and Denton & Nicol (1965) |
| European common squid (Alloteuthis subulata) | reduce detectability | Mathger (2003) |
| mammals | ||
| grey squirrel (Sciurus carolinensis) | some reduction in the dorsoventral gradient, suggesting SSC | Kiltie (1989)) |
| naked mole rats (Heterocephalus glaber) | camouflage | Braude et al. (2001) |
| disc-winged bat Thyroptera | not discussed | Gregorin et al. (2006) |
| mice | background matching | Caro (2005) and Lai et al. (2008) |
| oldfield mouse (Peromyscus polionotus) | not discussed | Kaufman (1974) |
| lagomorphs | minimizes shadow | Stoner et al. (2003a) and Caro (2005) |
| some lemurs, lorises, galagos, tarsiers, New world monkeys, Old world monkeys and apes | SSC and background matching | Bradley & Mundy (2008) |
| insects | ||
| larvae of the Smerinthus ocellata, Mimas tiliae, Sphinx ligustri, Endromis versicolora, Apatura ilia, Papilio podalirius, Macroglossum stellatarum, Cerura vinula, Gonepteryx rhamni, Apatura iris | SSC | de Ruiter (1956) and Tinbergen (1957) |
| larvae of Hyalophora cecropia, Antheraea polyphemus, Actias Luna, Callosamia promethea and Smerinthus ocellata | SSC | Thayer (1909, pp. 186–187) |
| reptiles | ||
| rough green snake, (Opheodrys aestivus) | ‘countershading effect’ | Goldsmith (1984) |
| various lizard species including Phrynosoma coronatum, Gerrhonotus multicarinatus | background matching | Norris & Lowe (1964) |
| green sea turtle Chelonia mydas (L.) | background matching | Bustard (1970) |
| birds | ||
| tropical rainforest birds | silhouette reduction | Gomez & Thery (2007) |
| fishing eating Procellariiformes | not discussed | Bretagnolle (1993) |
| American oystercatchers (Haematopus palliatus) | SSC | Lauro & Nol (1995) |
| penguin | background matching & thermoregulation | Chester (2001) |
| waders | SSC | Ferns (2003) |
| amphibians | ||
| Rana muscosa | background matching | Norris & Lowe (1964) |
‘Countershading’ is often used to refer both to the phenotype (in which prey have darker pigmentation on those surfaces exposed to most lighting) and to the mechanisms by which countershading may protect prey from predation. In his book ‘Concealing colouration in the animal kingdom’, Thayer (1909) outlined his argument for the protective mechanism of countershading: when illuminated from above, animals can cast shadows on their undersides, so that they appear lighter on their upper than their lower surfaces (a ‘self-shadow’ effect Kiltie 1988). If different parts of the organism are differently illuminated, the presence of shading may be used as a visual cue by predators to the existence of a solid three-dimensional object of potential value, or degrade otherwise perfect matching of colour to a uniform background. Hence shadowing on the body may be used as a ‘giveaway cue’ to foraging predators (Hailman 1977). A gradation in shading, Thayer hypothesized, would act to obliterate shadowing, making three-dimensional bodies appear optically flat, and therefore harder to detect as distinct objects.
While Thayer (1896) is generally accepted as the first person to hypothesize this function, Poulton (1888) noted that the appearance of roundness in the chrysalis of the purple emperor butterfly (Apatura iris) was obliterated by the presence of white spots that neutralized the darker tones of its shaded surfaces. Ford (1990) used the theory of countershading to explain the paler undersides of larvae of the purple emperor (A. iris) and brimstone (Gonepteryx rhamni), with Tinbergen (1958), Edmunds (1974) and Sheppard (1975) all discussing the widespread occurrence and accepted role of countershading in prey defence.
Since the last reviews of countershading were published (Kiltie 1988; Ruxton et al. 2004b) more datasets have been added to the literature. In this review therefore, I focus on the evidence and theory supporting Thayer's claim for concealment by reducing ventral shadowing. I discuss the most recent experiments that have added considerably more weight to the idea that countershading does protect prey from predation. I distinguish the various ways that countershading may aid concealment of animals, and add to previous published work by examining avian visual perception of countershaded colour patterns to determine the degree of background matching in live specimens of lepidopteran larvae. I also discuss the objections to the theory that countershading protects prey from detection, and review the alternative explanations for the function of a countershaded colour pattern, which so far have been a neglected route of study. Thus, in this paper, when I use the term countershading, I refer to the appearance of the organism and not to any specific function.
2. Functions of countershading
The function of countershading has often been supported with only indirect evidence from unmanipulated systems, inferring concealment from observed countershaded patterns (see for table 1 for examples). It is therefore possible that countershading could be a vestigial trait with no modern function. However, there is evidence that countershading does reduce shadowing on the bodies of animals. Kiltie (1989) measured the effect of dorsoventral contrast on shadow obliteration in the grey squirrel (Sciurus carolinensis) by photographing under natural conditions the sides or the back of stuffed squirrel skins placed so that the long axis of the mount was orientated either vertically or horizontally, both in the winter and summer (assessing differences in illumination), and in full direct sunlight and partial shade. Kiltie (1989) found that horizontally placed squirrels exhibited some reduction in the dorsoventral gradient, suggesting self-shadow concealment. However, analysis of the vertical photographs found that the same effect did not hold. Whether this reduction in the dorsoventral gradient improved the degree of background matching between the squirrel and the surrounding environment was neither tested nor was the ability of viewers to determine three-dimensional shape. Additionally, Stoner et al. (2003b) conducted a comparative analysis (controlling for shared phylogeny) of colour patterns and found an association between light ventral surfaces, diurnal activity and living in deserts, in both bovids and other ungulates (although the finding was not replicated in a similar study on lagomorphs; Stoner et al. 2003a). Further comparative analyses are required in order to determine whether countershading is a vestige of an ancestral trait. The remainder of this section explores the evidence for the proposed adaptive functions of countershading.
(a) Concealment
Because countershading is generally limited to animals that are thought to be cryptic, the conclusion that countershading renders animals difficult to detect is often accepted without direct evidence (see Ruxton et al. 2004b for review, and de Ruiter 1956; Turner 1961; Edmunds & Dewhirst 1994; Speed et al. 2005 for exceptions).
The most recent studies testing the hypothesis that countershading enhances crypsis have provided further evidence for a concealing function of countershading (Rowland et al. 2007, 2008). Rowland et al. (2007) conducted two experiments where artificial prey resembling lepidopteran larvae were presented either on lawns or on colour matching wooden boards. The first experiment (presentations on lawns) was a replicate of the original experiment of Edmunds & Dewhirst (1994), and showed a large benefit to countershading, and a specific order of preference by the birds (reverse>light>dark>countershaded). In the second experiment, artificial prey were presented on colour matching green boards to create background matching controls. Colour match was achieved by scanning pastry and calculating the predicted photon catches for the double cones of starlings, Sturnus vulgaris. Here prey types were presented in randomized positions to single birds rather than in localized arrangements (e.g. dark in one quarter, light in another, etc. as in Speed et al. 2005) so that any difference found was probably perceptual in origin rather than a by-product of the presentation regime. The study supported the view that countershading enhances crypsis compared with the uniformly pigmented background colour matching dark prey.
Rowland et al. (2008) evaluated the survival benefits of countershading in a series of field experiments where artificial prey resembling lepidopteran larvae were presented on the upper and lower surfaces of beech tree branches, simulating the resting position of many tree-living caterpillars. Rowland et al. (2008) found that when presented on the upper surface of a branch, countershaded prey (with paler coloration on their undersides) gained enhanced protection from predation compared with (i) uniformly coloured prey that manifest natural shading and (ii) prey that showed darker coloration on their undersides (reverse countershaded prey). When prey were presented on the underside of a branch, a reversal of the orientation of countershaded coloration (so that the surface closest to illumination was dark) also enhanced protection from predation. This is consistent with the observation that animals with lighter dorsal coloration are observed to orient upside down (privet hawk moth; Sphinx ligustri; Sheppard 1975). These findings provide definitive evidence that a reduction in pigmentation on the side of an animal furthest from the light source provides a camouflage benefit. However, the actual mechanisms by which countershading functions to reduce attacks by avian predators have yet to be investigated; several mechanisms are invoked for the concealing function of countershading and are discussed below.
(i) Mechanisms by which countershading may aid concealment
Self-shadow concealment which results in improved background matching when viewed from the side
Describing countershading as ‘a fundamental principle of animal colouration’, Cott (1940) reviewed Thayer's (1909) theory of cryptic protection by countershading, reinforcing the view that a gradation in shading would act to eliminate the effects of ventral shadowing. Cott (1940) suggested that if a countershaded animal was seen against a background of similar hue, the animal would ‘fade into a ghostly elusiveness and become invisible from a short distance, its entire contour and surface blending into the background’; the dorsoventral gradation in reflectance exactly balancing the dorsoventral gradation in irradiance, such that radiances of the entire prey animal's body match the radiance of background veiling light when viewed from the side (see figure in Cott 1940, p. 37). Whether countershading results in improved background matching by self-shadow concealment remains untested (although see discussions of Kiltie 1989 above).
Self-shadow concealment that flattens the form when viewed from the side
Just as painters produce the illusion of three-dimensionality on a flat canvas through shading, Thayer (1896) argued that nature created the opposite effect with countershading—making three-dimensional bodies appear less round and less solid.
A sense of three-dimensional shape arises due to different cues such as contour, shading, perspective and texture (Hoffman 1998). Shading, defined as variation in luminance (Tomonaga 1998), provides an effective source of visual information about the three-dimensional shapes of objects (Ramachandran 1988; Kleffner & Ramachandran 1992; Liu & Todd 2004). Shading is probably phylogenetically one of the most primitive cues to judging shape (Kleffner & Ramachandran 1992). In essence, countershading (dorsoventral gradation in reflectance) is hypothesized to obliterate the perception of the three-dimensional structure of an object when viewed from the side (self-shadow concealment of form or ‘flattening’) by reducing the visual cues of shape. Optical flattening of a caterpillar, for example, could conceal it within a background of flat leaves (background matching of volume and colour); alternatively, flat objects may be harder to detect than three-dimensional ones, which remains unresolved.
In order to accept this as a function of a countershaded pattern, the mechanisms of shape perception in non-human animals need to be identified (shape perception from visual cues has been largely discussed in the human vision literature, see Berbaum et al. 1983; Mingolla & Todd 1986; Ramachandran 1988), and the perceptual or cognitive function that a countershaded colour pattern has evolved to trick in a predator's visual system identified.
There are fewer data from non-human animals on how shading influences shape perception. Tomonaga (1998) tested the perception of shape from shading in two chimpanzees (Pan troglodytes) and five humans (Homo sapiens), using visual search tasks. The results suggested that chimpanzees process shading information in a different way from humans. However, Hess (1950, 1961) tested shading perception in two groups of chicks (Gallus gallus). One group of chicks were reared in an environment where the light always came from below, and a control group where light came from above. Hess (1950, 1961) observed their pecking responses to photographs of grains, some of which had shadows above them on the background, and some had shadows below them. Hess (1950, 1961) found that experimental chicks preferred to peck grains with shadows above them and control chicks preferred to peck grains with shadows below them. Furthermore, Hershberger (1970) found that chicks preferred to peck grains with shadows below them, which suggests that perception of shape from shading by at least some non-human species is in some way comparable with the human ability to perceive depth and projection from shading.
Background matching when viewed from above or below
Thayer's contemporaries discussed the significance of white undersides in pelagic fishes, whales and dolphins, and aquatic birds such as penguins, suggesting that in contrast to providing protection by cancelling the effects of ventral shadowing, a lighter underside would render the animals inconspicuous when the ventral surface is viewed from below against a bright sky, and the dorsum against water or ground—background matching, as opposed to self-shadow concealment by countershading (Wallace 1889, p. 193; Beddard 1895, p. 115; Cott 1940; Craik 1944).
Gotmark (1987) found evidence to support the cryptic function of white undersides in gulls; birds experimentally painted black on their underside were less efficient at catching fishes, possibly because fishes detected them more readily. It is also possible that lighter ventral surfaces may reflect substratum colour and thus promote colour matching in reflected light (Norris & Lowe 1964). The side-blotched lizard (Uta stansburiana) shows both light and dark ventral surfaces that are associated with habitat colour; lighter undersides are found in light-coloured sandy areas, and dark-ventered animals are found on dark rocks or lava flows, which may maintain the degree of background matching.
These accounts bring into focus the need to address each case of countershading separately, and not just to attribute a role of protection without the appropriate tests of survival value against natural predators in the relevant setting and also possible alternative functions.
Assessing the degree of background matching of countershaded lepidopteran larvae to the food plant
Whether the dark and light sections of countershaded animals match the background on which they rest has never been tested for terrestrial animals. While there is some subjective evidence that countershaded animals match the background on which they rest (Grayson & Edmunds 1989; Edmunds & Grayson 1991), no quantitative measure of background matching has been published for countershaded animals. In order to show that countershading reduces detectability by pure background matching when viewed solely from above or below, animals would need to be shown to have a good degree of background matching to the substrate above and below them (as long as predators could approach prey from either direction), and that detectability by predators was reduced by resting on the appropriate background.
Reflectance measurements were taken of 58 larvae of the eyed hawkmoth (Smerinthus ocellata) and 30 larvae of the orange tip butterfly (Anthocharis cardamines) and their associated food plants (white willow (Salix alba) and garlic mustard (Alliaria petiolata) respectively), both larvae are countershaded (the eyed hawkmoth has a reversed pattern associated with a resting position hanging underneath branches). Reflectance was measured using an Ocean Optics USB2000 spectrophotometer, with specimens illuminated at 45° to normal by a DH1000 balanced halogen deuterium light source. Individual larvae were cooled for several minutes prior to measurement to reduce movement. Six measurements were taken from the dorsal, subdorsal (eyed hawkmoth only), lateral/spiracular (from this point termed lateral) and subspiracular/ventral (from this point termed ventral) surfaces of each caterpillar (24 or 18 measurements per larvae). Measurements were always recorded from the first and third thoracic segments, and the 4th, 6th, 8th and 10th abdominal segments, which ensured that spectra were recorded consistently along the length of the larvae, and measurements did not overlap. Four measurements were obtained for each side of the leaf of the food plants.
The reflectance data were used to assess the degree of background matching of the larval colour according to a model of avian vision. Larval and food-plant reflectance spectra were analysed using a model of avian visual perception, which is based on evidence that discrimination is limited by receptor noise that arises in the photoreceptors. (Vorobyev & Osorio 1998). Based on the cone visual pigment and oil droplet spectra, the model calculates estimated noise in the receptors and opponent colour pathways to get a discrimination value for each colour spectrum (Vorobyev & Osorio 1998). The chromatic contrasts between larvae and food plant were calculated from both spectra (see Vorobyev & Osorio 1998 for equations) and show how much two spectra are separated in receptor space. The units for contrasts are just noticeable differences (JNDs). A JND value of 1 is at the threshold of discrimination: values of JND below 1 indicate that two colours are indistinguishable, and as values of JND increase above 1, objects become easier to discriminate. The model is based on the spectral sensitivities of the blue tit (Cyanistes caeruleus) with relative cone ratios of uvs=0.3704; sws=0.7111; mws=0.9926; and lws=1.0 with a Weber fraction of 0.05, and clear sky irradiance.
Calculations of chromatic contrasts (JNDs) showed that the dorsal surface of the eyed hawkmoth larvae was indistinguishable from the lower willow surface with a JND<1 (table 2), and this match decreased as measurements were taken along the subdorsal, lateral and ventral surfaces, all three surfaces were discriminable from the upper surface with JNDs>1. For the achromatic contrasts (based on the double cones, with a Weber fraction of 0.05), the ventral surface of the larvae was indistinguishable from the upper surface of the willow leaf, which degraded as measurements moved across the body. The dorsal surface had a relatively good match in luminance for the lower surface of the leaf, consistent with the finding that these larvae hang upside down and have a reversed pattern of countershading (light dorsal, dark ventral). For the orange tip, results of the chromatic contrast calculations showed that the larvae had a poor match to the leaves and the pods of the garlic mustard in terms of colour, but the dorsal and ventral surfaces of the larvae provided a relatively good match for luminance. Together, these results indicate that the degree of background matching in these two species of countershaded larvae is stronger in the achromatic signal than the chromatic one. On its own this does not exclusively support the role of pure background matching when viewed solely from above or below because the analyses presented here used the same irradiance spectra for all samples; in addition, the illuminant did not vary from the top to the underside of the larvae, which is one of the crucial elements in countershading. In order to accept background matching as the function of a countershaded colour pattern, the detectability of prey resting on matching and contrasting backgrounds would need to be examined.
Table 2.
Chromatic and achromatic contrasts (JND values) between the mean reflectance of the dorsal, subdorsal (eyed hawkmoth only), lateral and ventral surfaces of countershaded larvae and food-plant backgrounds (upper and lower surface of willow leaves, and leaves/pods of garlic mustard) calculated according to the model of avian vision (Vorobyev & Osorio 1998). (Italics denote a JND less than 1.)
| eyed hawkmoth (Smerinthus ocellata) | orange tip (Anthocharis cardamines) | |||||||
|---|---|---|---|---|---|---|---|---|
| dorsal | subdorsal | lateral | ventral | dorsal | lateral | ventral | ||
| colour (chromatic) | ||||||||
| willow upper | 11.02 | 7.78 | 7.87 | 5.63 | leaf | 14.31 | 20.67 | 13.10 |
| willow lower | 0.746 | 2.43 | 3.51 | 6.69 | pod | 21.18 | 27.67 | 20.69 |
| luminance (achromatic) | ||||||||
| willow upper | 10.15 | 6.36 | 2.57 | 0.94 | leaf | 6.14 | 26.81 | 5.69 |
| willow lower | 1.45 | 3.58 | 5.65 | 8.90 | pod | 2.4445 | 23.11 | 2.00 |
Body outline obliteration when viewed from above
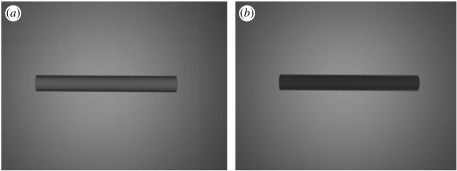
Using a three-dimensional content creation suite (Blender.org 2002) to plot a cylinder with 60 vertices, and a radius of 1, illuminated from above by a Lambertian lamp with illumination intensity of 1, a mechanism not previously discussed in the literature has been identified. When illuminated and viewed from above, a cylinder of uniform colour exhibits unequal reflectance of light across the dorsal surface, with darkening at the edges of the cylinder (figure 1a). Predators have been shown to use edge properties of prey in studies of disruptive coloration (Cuthill et al. 2005). However, with a dorsoventral gradation in colour in a countershaded cylinder, the reflectance at the edge of the body may exactly balance the dorsoventral gradation from which light is reflected, such that the outline of the object is obliterated when it is viewed from above (figure 1b). This may reduce the capacity of predators to detect the edges of a countershaded prey animal when that animal is viewed from above.
Figure 1.
(a,b) When illuminated and viewed from above, a cylinder of uniform colour exhibits unequal reflectance of light across the dorsal surface, with darkening at the edges of the cylinder. Modelled using a three-dimensional content creation suite (Blender.org 2002).
(ii) Objections to the theory of concealment through countershading
Kiltie (1988) noted that self-shadow concealment through countershading in land animals depends heavily on the direction of the light source, which varies with the time of day, season and cloud cover during the day, as well as the position of the viewer. Therefore, the assumption of illumination from directly above is generally not the case in terrestrial habitats, in which case a gradation in dorsoventral pigmentation could make an animal more conspicuous if light was not from directly above. However, in Rowland et al.'s (2008) experiments, prey were left in position for 66 hours, so the suggestion that diurnal variation in the position of the Sun resulting in countershading failing to compensate for the varied shadows cast by solar illumination can be refuted.
Kiltie (1988) also proposed that the dorsal surface of prey species may be the only side typically exposed to predators, and therefore the need for the same level of pigmentation on the ventral surface would be surplus to requirements for protective value. Alternatively, if pigmentation is costly to produce, this may result in reduced amounts of pigment laid down on the ventral surface, particularly when uniformly coloured cryptic animals are at a disadvantage during predation (see Speed et al. 2005 for a fuller discussion of this issue).
(b) Protection from UV
Burtt (1981) proposed that countershading may function to protect animals from the damaging effects of exposure to UV radiation. For many organisms, exposure to high intensity solar radiation is detrimental (Mitchell et al. 2007; Moan et al. 2008). Animals shield themselves by pigmentation that protects ultraviolet-sensitive tissue or by seeking microhabitats protected from ultraviolet light (Burtt 1981). The whale shark (Rhincodon typus), which has a countershaded pattern (Wilson & Martin 2004), spends a significant proportion of its time in shallow surface waters, and is therefore probably exposed to high levels of ultraviolet radiation. The dark dorsal surface of the whale shark could help shield underlying tissue from the harmful effects of radiation. Lowe & Goodman-Lowe (1996) documented increases in the integumental melanin of juvenile scalloped hammerhead sharks (Sphyrna lewini) in response to experimentally controlled increases in ultraviolet radiation. However, if dorsal pigment darkening is used for UV protection, it is interesting to note that whale sharks possess white regions directly adjacent to the darker melanic regions that would probably be exposed to radiation as well as the shielded regions each time it entered shallow water. The blacktip reef shark (Carcharhinus melanopterus), a resident of shallow reef flats, also exhibits a white band below the melanic region. Such evidence raises doubts that radiation shielding is the primary, or even a major, function of pigmentation patterns in these shallow water sharks.
In order to accept countershading as an adaptation to protect animals from the damaging effects of UV radiation, experimental reduction in ultraviolet flux would need to be shown to decrease mortality, increase the reproductive or growth rate or otherwise enhance fitness. Currently no test of these predictions has been reported.
(c) Thermoregulation
For behaviourally thermoregulating insects, temperature regulation prevents overheating and freezing, optimizes development rate and maximizes muscle performance for locomotion, feeding, mating and competitive interaction (Whitman 1988). Furthermore, survivorship and developmental rates, and ultimate size and weight vary with rearing temperature (Kingsolver 2000). Since the dorsal surface of most animals will be the surface most likely to experience heavy heat loads, Hamilton (1973) hypothesized that the occurrence of countershading may be advantageous for animals, as more concentrated dorsal pigmentation may better moderate radiative heat gain.
While countershading in penguins has been related to concealment through background matching when viewed from above or below, Chester (2001, p. 16) noted that a black dorsal surface and a white ventral surface are used by penguins for thermoregulation, with the animals turning their backs to the Sun when cold, and their white undersides to the light when hot. Naked mole rat body temperature has been shown to vary with ambient temperature (Buffernstein & Yahav 1991), with countershading proposed as a mechanism that might help the mole rats maintain a constant body temperature. However, Braude et al. (2001) found no evidence to suggest a role of thermoregulation for countershading in this species.
Many lizards are known to dorsally darken while basking during early summer mornings (Cowles & Bogert 1944), which has been shown to increase the rate of heat gain (Norris 1967). In this case thermoregulation presumably has influenced coloration, but only in a facultative sense. Conversely, light undersides might reflect light and reduce heat loads (Norris & Lowe 1964). In order to accept countershading as an adaptation to increase radiative heat gain, experimental increases in heat gain by artificially darkening non-countershaded animals would need to be shown to decrease mortality, increase the reproductive or growth rate or otherwise enhance fitness; currently no test of these predictions exist.
(d) Protection from abrasion
The hypothesis that darker dorsal pigmentation is a protection from abrasion leads to the hypothesis that areas exposed to most abrasion should be most darkly pigmented. Dark feathers have been shown to be stronger and resist more abrasion and wear (Ward et al. 2002), and are more likely to be located on areas of the body most vulnerable to abrasion (Burtt 1986). However, acceptance of countershading as an adaptation for abrasion resistance depends on identification of a number of factors: the habitat in which an animal lives and its forces of abrasion, and what parts of the animal are most susceptible to abrasion. Feather damage in desert birds is caused by abrasion from airborne particles, such as blowing sand (Ward et al. 2002), while dark plumage in desert birds is hypothesized to reduce feather damage from airborne particles; dark plumage in marine species (where there are less abrasive forces) is probably favoured for reasons other than abrasion resistance such as rapid drying or crypsis against the sunlit sea or bright sky (Burtt 1981).
Braude et al. (2001) suggested that dominant animals in naked mole rat complexes, which move significantly more within the colony tunnels, should be subjected to more abrasion and therefore have darker dorsal pigmentation; however, the authors found that dominant individuals had less dorsal pigmentation. Although the authors discounted protection from abrasion as a function of countershading, without experimentally manipulating juvenile individuals to have different levels of abrasion through ontogeny the function should not be discounted.
3. Future directions
In Rowland et al.'s (2007) experiments, and the earlier work by Edmunds & Dewhirst (1994) and Speed et al. (2005), countershaded caterpillars were made by fusing small half-cylinders of a darker and a lighter shade of green-coloured pastry dough together along the long axis, to create a two-tone ‘caterpillar’. However, although the experimental results matched predictions, with an advantage of countershaded prey over monotone or pose-inverted countershaded prey, the colour pattern does not match closely that seen in real countershaded prey. In most such prey, the transition from dark dorsal to light ventral surface is not abrupt, but instead graduated. I therefore suggest that testing the advantage of a graduated tonal change (and the slope of that change) would be a useful avenue.
To further evaluate the protective role of countershading, I see that an investigation into the proportion of time spent in various orientations by countershaded animals is required; whether countershaded prey do in fact consistently orient themselves in a manner which counterbalances the effects of illumination remains unresolved. Furthermore, psychophysical evidence for the perception of three-dimensional form by non-human animals is surprisingly scarce and sometimes contradictory. Whether the ‘artistic tricks’ that fool the human visual system also deceive non-human visual systems that differ substantially from the primate visual cortex is unknown.
Finally, no studies examine either the role of body shape on the pattern of countershading, or the effect of ambient light changes and backscattering of light on the optimized level of contrast between the dorsal and ventral surfaces; Heráň (1976) comments on the differences between countershaded patterns and the habitats in which they exist, such as the surface strata of rivers and the sea or open country, in which strong contrasts between the dorsal and ventral coloration is observed. However, no studies exist which examine, while controlling for phylogeny, the level of contrast between the dorsal and ventral surfaces of animals and the habit in which they live (although see Stoner et al. 2003b).
4. Conclusion
Several studies now provide evidence that avian predators exert a selection pressure in both natural (Edmunds & Grayson 1991) and artificial (Rowland et al. 2007) systems, which can drive the maintenance of a countershaded colour pattern. This review raises important unanswered questions, and I conclude that further research on countershading is important for the understanding of the evolution of cryptic colour patterns and the psychophysical properties of prey and their associated predators.
Acknowledgments
The author wishes to thank Mike Speed and Graeme Ruxton for their helpful comments on this manuscript. Martin Stevens calculated the avian visual perception of the countershaded larvae, and has also provided useful comments on sections of this paper. Innes Cuthill hypothesized the newly proposed function of countershading, obliteration of body outline. The author also wishes to thank John Endler and Geoff Parker for their constructive criticism of any earlier draft of this manuscript. This work was funded by NERC.
Footnotes
One contribution of 15 to a Theme Issue ‘Animal camouflage: current issues and new perspectives’.
References
- Beddard F.E. Swan Sonnenschein; London, UK: 1895. Animal colouration; an account of the principle facts and theories relating to the colours and markings of animals. [Google Scholar]
- Berbaum K., Bever T., Chung C. Light source position in the perception of object shape. Perception. 1983;12:411–415. doi: 10.1068/p120411. doi:10.1068/p120411 [DOI] [PubMed] [Google Scholar]
- Blender.org 2002 Blender (ed. T. Roosendaal). Amsterdam. See http://www.blender.org/
- Bradley B.J., Mundy N.I. The primate palette: the evolution of primate coloration. Evol. Anthropol. 2008;17:97–111. doi:10.1002/evan.20164 [Google Scholar]
- Braude S., Ciszek D., Berg N.E., Shefferly N. The ontogeny and distribution of countershading in colonies of the naked mole-rat (Heterocephalus glaber) J. Zool. 2001;253:351–357. doi:10.1017/S0952836901000322 [Google Scholar]
- Bretagnolle V. Significance of seabird coloration: the case of procellariiforms. Am. Nat. 1993;142:141–173. doi: 10.1086/285532. doi:10.1086/285532 [DOI] [PubMed] [Google Scholar]
- Buffernstein R., Yahav S. Is the naked mole-rat Heterocephalus glaber an endothermic yet poikilothermic mammal? J. Therm. Biol. 1991;16:227–232. doi:10.1016/0306-4565(91)90030-6 [Google Scholar]
- Burtt E.H. The adaptiveness of animal colors. Bioscience. 1981;31:723–729. doi:10.2307/1308778 [Google Scholar]
- Burtt E.H. An analysis of physical, physiological, and optical aspects of avian coloration with emphasis on wood warblers. Ornithol. Monogr. 1986;38:1–109. [Google Scholar]
- Bustard H.R. The adaptive significance of coloration in hatchling green sea turtles. Herpetologica. 1970;26:224–227. [Google Scholar]
- Caro T. The University of Chicago Press Ltd; London, UK: 2005. Antipredator defenses in birds and mammals. [Google Scholar]
- Chapman L.J., Kaufman L., Chapman C.A. Why swim upside down? A comparative-study of 2 mochokid catfishes. Copeia. 1994:130–135. doi:10.2307/1446679 [Google Scholar]
- Chester J. Celestial Arts; San Francisco, CA: 2001. The nature of penguins. [Google Scholar]
- Cott H.B. Methuen; London, UK: 1940. Adaptive coloration in animals. [Google Scholar]
- Cowles R.B., Bogert C.M. A preliminary study of the thermal requirements of desert reptiles. Bull. Am. Mus. Nat. Hist. 1944;83:265–296. [Google Scholar]
- Craik K.J. White plumage of sea-birds. Nature. 1944;153:288. doi:10.1038/153288a0 [Google Scholar]
- Cuthill I.C., Stevens M., Sheppard J., Maddocks T., Parraga C.A., Troscianko T.S. Disruptive coloration and background pattern matching. Nature. 2005;434:72–74. doi: 10.1038/nature03312. doi:10.1038/nature03312 [DOI] [PubMed] [Google Scholar]
- de Ruiter L. Countershading in caterpillars. An analysis of its adaptive significance. Arch. Neerl. Zool. 1956;11:285–341. [Google Scholar]
- Denton E.J., Nicol J.A.C. Studies on reflexion of light from silvery surfaces of fishes with special reference to bleak, Alburnus alburnus. J. Mar. Biol. Assoc. UK. 1965;45:683–703. [Google Scholar]
- Edmunds M. Longman; Harlow, Essex, UK: 1974. Defence in animals: a survey of anti-predator defences. [Google Scholar]
- Edmunds M., Dewhirst R.A. The survival value of countershading with wild birds as predators. Biol. J. Linn. Soc. 1994;51:447–452. doi:10.1111/j.1095-8312.1994.tb00973.x [Google Scholar]
- Edmunds M., Grayson J. Camouflage and selective predation in caterpillars of the poplar and eyed hawkmoths (Laothoe populi and Smerinthus ocellata) Biol. J. Linn. Soc. 1991;42:467–480. doi:10.1111/j.1095-8312.1991.tb00575.x [Google Scholar]
- Evans M.A. Mimicry and the Darwinian heritage. J. Hist. Ideas. 1965;26:211–220. doi:10.2307/2708228 [Google Scholar]
- Ferguson G.P., Messenger J.B. A countershading reflex in cephalopods. Proc. R. Soc. B. 1991;243:63–67. doi:10.1098/rspb.1991.0011 [Google Scholar]
- Ferguson G.P., Messenger J.B., Budelmann B.U. Gravity and light influence the countershading reflexes of the cuttlefish Sepia officinalis. J. Exp. Biol. 1994;191:247–256. doi: 10.1242/jeb.191.1.247. [DOI] [PubMed] [Google Scholar]
- Ferns P.N. Plumage colour and pattern in waders. Wader Study Group Bull. 2003;100:122–129. [Google Scholar]
- Ford E.B. Bloomsbury Books; London, UK: 1990. Butterflies. [Google Scholar]
- Goldsmith S.K. Aspects of the natural history of the rough green snake, Opheodrys aestivus (Colubridae) Southwest. Nat. 1984;29:445–452. doi:10.2307/3670997 [Google Scholar]
- Gomez D., Thery M. Simultaneous crypsis and conspicuousness in color patterns: comparative analysis of a neotropical rainforest bird community. Am. Nat. 2007;169:S42–S61. doi: 10.1086/510138. doi:10.1086/510138 [DOI] [PubMed] [Google Scholar]
- Gosse, K., Wroblewski, J. & Neis, B. 2001 Closing the loop: commercial fish harvesters' local ecological knowledge and science in a study of coastal cod in Newfoundland and Labrador. See http://www.coastsunderstress.ca/publications.php
- Gotmark F. White underparts in gulls function as hunting camouflage. Anim. Behav. 1987;35:1786–1792. doi:10.1016/S0003-3472(87)80071-4 [Google Scholar]
- Grayson J., Edmunds M. The causes of color and color-change in caterpillars of the poplar and eyed hawkmoths (Laothoe populi and Smerinthus ocellata) Biol. J. Linn. Soc. 1989;37:263–279. [Google Scholar]
- Gregorin R., Goncalves E., Lim B.K., Engstrom M.D. New species of disk-winged bat Thyroptera and range extension for T. discifera. J. Mamm. 2006;87:238–246. doi:10.1644/05-MAMM-A-125R1R1.1 [Google Scholar]
- Hailman J.P. Indiana University Press; London, UK: 1977. Optical signals: animal communication and light. [Google Scholar]
- Hamilton W.J. McGraw-Hill; London, UK: 1973. Life's colour code. [Google Scholar]
- Hastings J.W. Light to hide by: ventral luminescence to camouflage the silhouette. Science. 1971;173:1016–1017. doi: 10.1126/science.173.4001.1016. doi:10.1126/science.173.4001.1016 [DOI] [PubMed] [Google Scholar]
- Hershberger W. Attached-shadow orientation perceived as depth by chickens reared in an environment illuminated from below. J. Comp. Physiol. Psychol. 1970;73:407–411. doi: 10.1037/h0030223. doi:10.1037/h0030223 [DOI] [PubMed] [Google Scholar]
- Heráň I. Hamlyn; London, UK: 1976. Animal colouration. The nature and purpose of colours in vertebrates. [Google Scholar]
- Hess E.H. Development of chick's responses to light and shade cues of depth. J. Comp. Physiol. Psychol. 1950;43:112–122. doi: 10.1037/h0059235. doi:10.1037/h0059235 [DOI] [PubMed] [Google Scholar]
- Hess E.H. Shadows and depth perception. Sci. Am. 1961;204:138–148. doi: 10.1038/scientificamerican0361-138. [DOI] [PubMed] [Google Scholar]
- Hoffman D.D. W. W. Norton & Company Ltd; London, UK: 1998. Visual intelligence: how we create what we see. [Google Scholar]
- Kaufman D.W. Adaptive coloration in Peromyscus polionotus: experimental selection by owls. J. Mamm. 1974;55:271–283. doi:10.2307/1378997 [Google Scholar]
- Kiltie R.A. Countershading—universally deceptive or deceptively universal. Trends Ecol. Evol. 1988;3:21–23. doi: 10.1016/0169-5347(88)90079-1. doi:10.1016/0169-5347(88)90079-1 [DOI] [PubMed] [Google Scholar]
- Kiltie R.A. Testing Thayer's countershading hypothesis—an image-processing approach. Anim. Behav. 1989;38:542–544. doi:10.1016/S0003-3472(89)80048-X [Google Scholar]
- Kingsolver J.G. Feeding, growth, and the thermal environment of cabbage white caterpillars, Pieris rapae L. Physiol. Biochem. Zool. 2000;73:621–628. doi: 10.1086/317758. doi:10.1086/317758 [DOI] [PubMed] [Google Scholar]
- Kleffner D.A., Ramachandran V.S. On the perception of shape from shading. Percept. Psychophys. 1992;52:18–36. doi: 10.3758/bf03206757. [DOI] [PubMed] [Google Scholar]
- Korner H.K. Countershading by physiological color-change in the fish louse Anilocra physodes L. (Crustacea, Isopoda) Oecologia. 1982;55:248–250. doi: 10.1007/BF00384495. doi:10.1007/BF00384495 [DOI] [PubMed] [Google Scholar]
- Lai Y.C., Shiroishi T., Moriwaki K., Motokawa M., Yu H.T. Variation of coat colour in house mice throughout Asia. J. Zool. 2008;274:270–276. doi:10.1111/j.1469-7998.2007.00382.x [Google Scholar]
- Lauro B., Nol E. Patterns of habitat use for pied and sooty oystercatchers nesting at the Furneaux Islands, Australia. Condor. 1995;97:920–934. doi:10.2307/1369531 [Google Scholar]
- Lindsay S.M., Frank T.M., Kent J., Partridge J.C., Latz M.I. Spectral sensitivity of vision and bioluminescence in the midwater shrimp Sergestes similis. Biol. Bull. 1999;197:348–360. doi: 10.2307/1542789. doi:10.2307/1542789 [DOI] [PubMed] [Google Scholar]
- Liu B., Todd J.T. Perceptual biases in the interpretation of 3D shape from shading. Vis. Res. 2004;44:2135–2145. doi: 10.1016/j.visres.2004.03.024. doi:10.1016/j.visres.2004.03.024 [DOI] [PubMed] [Google Scholar]
- Lowe C., Goodman-Lowe G. Suntanning in hammerhead sharks. Nature. 1996;383:677. doi: 10.1038/383677a0. doi:10.1038/383677a0 [DOI] [PubMed] [Google Scholar]
- Mathger L.M. The response of squid and fish to changes in the angular distribution of light. J. Mar. Biol. Assoc. UK. 2003;83:849–856. doi:10.1017/S0025315403007884h [Google Scholar]
- Mingolla E., Todd J.T. Perception of solid shape from shading. Biol. Cybern. 1986;53:137–151. doi: 10.1007/BF00342882. doi:10.1007/BF00342882 [DOI] [PubMed] [Google Scholar]
- Mitchell D., Paniker L., Sanchez G., Trono D., Nairn R. The etiology of sunlight-induced melanoma in Xiphophorus hybrid fish. Mol. Carcin. 2007;46:679–684. doi: 10.1002/mc.20341. doi:10.1002/mc.20341 [DOI] [PubMed] [Google Scholar]
- Moan J., Porojnicu A.C., Dahlback A. Ultraviolet radiation and malignant melanoma. Sunlight Vit. D Skin Cancer. 2008;624:104–116. doi: 10.1007/978-0-387-77574-6_9. [DOI] [PubMed] [Google Scholar]
- Nagaishi H., Nishi H., Fujii R., Oshima N. Correlation between bosy colour and behaviour in upside-down catfish, Synodontis nigriventis. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 1989;92:323–326. [Google Scholar]
- Norris K.S. Color adaptation in desert reptiles and its thermal relationships. In: Milstead W.W., editor. Liazard ecology: a symposium. University of Missouri Press; Columbia, MO: 1967. pp. 163–229. [Google Scholar]
- Norris K.S., Lowe C.H. An analysis of background colour-matching in amphibians and reptiles. Ecology. 1964;45:565–580. doi:10.2307/1936109 [Google Scholar]
- Poulton E.B. Notes in 1887 upon lepidopterous larvae, etc., including a complete account of the life-history of the larvae of Sphinx convolvuli and Aglia tau. Trans. Entomol. Soc. Lond. 1888:515–606. [Google Scholar]
- Ramachandran V.S. Perception of shape from shading. Nature. 1988;331:163–166. doi: 10.1038/331163a0. doi:10.1038/331163a0 [DOI] [PubMed] [Google Scholar]
- Rowland H.M., Speed M.P., Ruxton G.D., Edmunds M., Stevens M., Harvey I.F. Countershading enhances cryptic protection: an experiment with wild birds and artificial prey. Anim. Behav. 2007;74:1249–1258. doi:10.1016/j.anbehav.2007.01.030 [Google Scholar]
- Rowland, H. M., Cuthill, I. C., Harvey, I. F., Speed, M. & Ruxton, G. D. 2008 Can't tell the caterpillars from the trees: countershading enhances survival in a woodland. Proc. R. Soc. B.275, 2539–2546. (doi:10.1098/rspb.2008.0812) [DOI] [PMC free article] [PubMed]
- Ruxton G.D., Sherratt T.N., Speed M.P. Oxford University Press; Oxford, UK: 2004a. Avoiding attack—the evolutionary ecology of crypsis, warning signals and mimicry. [Google Scholar]
- Ruxton G.D., Speed M., Kelly D.J. What, if anything, is the adaptive function of countershading. Anim. Behav. 2004b;68:445–451. doi:10.1016/j.anbehav.2003.12.009 [Google Scholar]
- Sheppard P.M. Hutchinson & Co Ltd; London, UK: 1975. Natural selection and heredity. [Google Scholar]
- Speed M.P., Kelly D.J., Davidson A.M., Ruxton G.D. Countershading enhances crypsis with some bird species but not others. Behav. Ecol. 2005;16:327–334. doi:10.1093/beheco/arh166 [Google Scholar]
- Stevens M. Predator perception and the interrelation between different forms of protective coloration. Proc. R. Soc. B. 2007;274:1457–1464. doi: 10.1098/rspb.2007.0220. doi:10.1098/rspb.2007.0220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoner C.J., Bininda-Emonds O.R.P., Caro T. The adaptive significance of coloration in lagomorphs. Biol. J. Linn. Soc. 2003a;79:309–328. doi:10.1046/j.1095-8312.2003.00190.x [Google Scholar]
- Stoner C.J., Caro T.M., Graham C.M. Ecological and behavioral correlates of coloration in artiodactyls: systematic analyses of conventional hypotheses. Behav. Ecol. 2003b;14:823–840. doi:10.1093/beheco/arg072 [Google Scholar]
- Thayer A.H. The law which underlie protective coloration. Auk. 1896;13:124–129. [Google Scholar]
- Thayer G.H. Macmillan; New York, NY: 1909. Concealing-colouration in the animal kingdom: and exposition of the laws of disguise through colour and pattern: being a summary of Abbot H. Thayer's discoveries. [Google Scholar]
- Tinbergen N. Defense by colour. Sci. Am. 1957;197:49. [Google Scholar]
- Tinbergen N. Country Life Limited; London, UK: 1958. Curious naturalists. [Google Scholar]
- Tomonaga M. Perception of shape from shading in chimpanzees (Pan troglodytes) and humans (Homo sapiens) Anim. Cogn. 1998;1:25–35. doi:10.1007/s100710050004 [Google Scholar]
- Turner E.R.A. Survival values of different methods of camouflage as shown in a model population. Proc. Zool. Soc. Lond. 1961;136:273–284. [Google Scholar]
- Vorobyev M., Osorio D. Receptor noise as a determinant of colour thresholds. Proc. R. Soc. B. 1998;265:351–358. doi: 10.1098/rspb.1998.0302. doi:10.1098/rspb.1998.0302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace A.R. Macmillan & Co; London, UK: 1889. Darwinism. An exposition of the theory of natural selection with some of its applications. [Google Scholar]
- Ward J.M., Blount J.D., Ruxton G.D., Houston D.C. The adaptive significance of dark plumage for birds in desert environments. Ardea. 2002;90:311–323. [Google Scholar]
- Whitman D.W. Function and evolution of thermoregulation in the sesert grasshopper Taeniopoda eques. J. Anim. Ecol. 1988;57:369–383. doi:10.2307/4911 [Google Scholar]
- Wilson S.G., Martin A.R. Body markings of the whale shark, vestigial or functional. West Aust. Nat. 2004;124:118–134. [Google Scholar]
- Young R.E., Roper C.F.E. Bioluminescent countershading in midwater animals: evidence from living squid. Science. 1976;191:1046–1048. doi: 10.1126/science.1251214. doi:10.1126/science.1251214 [DOI] [PubMed] [Google Scholar]