Abstract
The cuttlefish, Sepia officinalis, provides a fascinating opportunity to investigate the mechanisms of camouflage as it rapidly changes its body patterns in response to the visual environment. We investigated how edge information determines camouflage responses through the use of spatially high-pass filtered ‘objects’ and of isolated edges. We then investigated how the body pattern responds to objects defined by texture (second-order information) compared with those defined by luminance. We found that (i) edge information alone is sufficient to elicit the body pattern known as Disruptive, which is the camouflage response given when a whole object is present, and furthermore, isolated edges cause the same response; and (ii) cuttlefish can distinguish and respond to objects of the same mean luminance as the background. These observations emphasize the importance of discrete objects (bounded by edges) in the cuttlefish's choice of camouflage, and more generally imply that figure–ground segregation by cuttlefish is similar to that in vertebrates, as might be predicted by their need to produce effective camouflage against vertebrate predators.
Keywords: camouflage, vision, Sepia officinalis, cuttlefish, texture, edge detection
Processes in the psychological plane cause us to overlook the fact that in the physical plane all optical effects whatsoever are fundamentally due to differences of colour and brightness, and of light and shade.(Cott 1940, p. 3)
1. Introduction
(a) Vision and visual camouflage
Accounts of camouflage reflect basic concepts about the relationship between sensory perception and the physical world. The twist is that whereas the discussion of this question normally refers to human perception we must now focus on non-human species. Cott's (1940) book on Adaptive Coloration in Animals remains the most valuable work on camouflage. Cott was familiar with the idea that to achieve verisimilitude an artist has to paint the physical patterns of light and shade created by three-dimensional surfaces. Naive artists overlook these optical effects in favour of ‘higher level’ objects. Only with skill and training is it possible to recover the ‘innocence of the eye’ that is needed to render naturalistic scenes on canvas (Cott 1940; Gombrich 1960). This reasoning led Cott to explicitly reject psychological interpretations of camouflage in favour of what he saw as ‘simple’ optical effects. Cott was however interested in the psychology of attention, as with the suggestion that high-contrast internal features distract the viewer.
Since the 1950s, work in biological and computational vision has drawn attention to the importance of local spatio-temporal filtering and feature detection in low-level visual processing (Mather 2006). That is to say, operations that are performed in parallel across the image by neurons with small receptive fields in structures such as the retina and primary visual cortex of mammals, or the insect optic lobe. The size and complexity of these neural centres, as well as the difficulties of solving equivalent problems in computational vision, imply that substantial resources are required to identify local image features, and then to segregate an image into discrete regions or objects (Troscianko et al. 2009). An appreciation of the costs and complexity of low-level vision draws attention to the importance of psychological mechanisms in object detection. In contrast to Cott, workers such as Julesz (1971) found camouflage interesting precisely because it provides insight into visual mechanisms. Julesz (1971) presented his celebrated demonstrations of how depth and relative motion could be used for figure–ground segregation in random dot patterns as examples of ‘camouflage breaking’. These demonstrations stimulated much work on visual algorithms. There is now evidence for multiple mechanisms in low-level vision, which appear to operate ‘in parallel’, for example, in edge and motion detection, texture coding and local spatial frequency analysis (Mather 2006).
Texture classification is an aspect of visual camouflage that nicely illustrates the importance of visual mechanisms. Image data (including visual textures) can be characterized in terms of the statistics of the intensity at each point or pixel. The first-order statistic is the mean intensity and the second-order statistic specifies the relationship between intensities of pairs of pixels as a function of their separations (see experiment 2). Julesz (1981) showed that visual textures (e.g. isodipole patterns) that are identical in their first- and second-order statistics (spatial frequency power spectrum) are nonetheless visually distinct (Malik & Perona 1990; Victor et al. 2005). The implication is that the eye classifies textures by higher-order statistical properties (e.g. relationships between triplets of pixels), which probably correspond to local features such as edges or corners. These higher-order properties cannot be identified simply from the output of a linear filter (or, equivalently, from the spatial frequency power spectrum). Julesz's (1981) texton theory attempts to define the set of features that humans use to classify visual textures, especially in figure–ground segregation, but despite considerable interest (e.g. from the virtual reality and computer gaming industries) the classification and synthesis of visual textures still cannot be automated (Portilla & Simoncelli 2000). Put simply, this means that for humans there is no simple way to predict whether one visual texture (e.g. on a body) will match another (e.g. a background). A more general conclusion is that accounts of cryptic matching carry significant assumptions, about the mechanisms of edge detections and texture classification, that are more or less untested in non-human species.
Following low-level feature detection, visual systems integrate information from multiple sources to interpret the complex and often ambiguous signals in natural images; this is the problem of higher-level vision, or visual cognition. Once again, work in computational vision has been influential, especially in identifying the ‘problems’ that need to be tackled. Marr (1982) proposed that vision is a multistage process. The first stage locates local features such as edges in the retinal image (Marr & Hildreth 1980). These two-dimensional feature maps are integrated to give a representation of objects in the three-dimensional world. The proposal that vision requires an internal representation, which Marr called the two-and-a-half dimensional sketch, has been criticized owing to its cognitive character. Animate vision (Ballard 1991) and ecological theories of vision (Gibson 1979) suggest that animals' actions and the properties of natural images constrain and simplify visual processing so that deriving an internal representation from local feature maps is computationally wasteful and/or unnecessary. More generally, the relevance of cognitive models to non-human species is controversial. If one holds that humans have internal representations but that animals do not then it may follow that there are fundamental differences between Marr-like human and ‘Gibsonian’ animal vision; for instance, there is doubt that non-human species enjoy our own rich visual perception (or representation) of the external world (Horridge 1991; Stoerig 1998; Troje et al. 1999).
Of course, the effectiveness and refinement of camouflage suggests that other species do indeed share our strategies of figure–ground detection. Following this line of reasoning, ultimately one might hope to interpret camouflage in terms of visual mechanisms, instead of optical principles (Cott 1940), although as Troscianko and co-workers point out elsewhere in this issue (Troscianko et al. 2009) a complete account is an ambitious objective, because there may be as many camouflage strategies as there are mechanisms of figure detection. It is nonetheless tempting to make inferences from camouflage about texture perception (Kiltie & Laine 1992), edge detection (Osorio & Srinivasan 1991) and so forth. An obvious way to investigate camouflage is to ask how particular types of pattern engage with—and defeat—visual mechanisms, for example, by surveying the relationship between coloration patterns of different species and their habitats. Alternatively, one can study animals such as cephalopod molluscs that can control their appearance, and ask what pattern is selected in a given context. In particular, cuttlefish (Sepia officinalis and Sepia pharaonis) have recently provided a unique and powerful system for investigating camouflage design, and hence the vision, of these remarkable animals.
(b) Visual camouflage in S. officinalis
Cuttlefish, similar to other coleoid cephalopods (squid and octopus), change their body patterns with great facility via intradermal chromatophores, which are under direct neural control and visually driven (Hanlon & Messenger 1996). Although also used in inter- and intraspecific signalling (Adamo et al. 2000; Langridge 2006; Langridge et al. 2007), the flexibility and range of body pattern responses expressed by the cuttlefish is best demonstrated in camouflage. To select a pattern that minimizes the likelihood of detection, S. officinalis must be sensitive to image parameters that are relevant to its predators or prey (Kelman et al. 2007). Behavioural assays can explore how features of the background control the body pattern. Captive cuttlefish readily settle on an artificial background to produce a stable and recordable behavioural output (a body pattern) that is determined by the animal's visual perception.
Much work on cuttlefish camouflage focuses on what visual features in the background promote specific body patterns. Hanlon & Messenger (1988) identified four main body patterns used by juvenile S. officinalis to achieve camouflage, which they named Uniform, Stipple, Mottle and Disruptive. (As Stevens & Merilaita (2009) say elsewhere in this issue, there is a discussion about the definition of disruptive camouflage, and in particular whether disruptive and cryptic camouflage are mutually exclusive principles. We use the terms ‘Disruptive body patterns’ and ‘Disruptive components’ because they are established and well understood in the literature on cuttlefish camouflage. We have capitalized this term, and the other body pattern categories, to indicate that we are referring to a type of pattern, as distinct from a functional class of camouflage.) Each pattern is made from a combination of more than 40 chromatic, textural, postural and locomotor ‘components’, which are flexible in their expression (Hanlon & Messenger 1988; Crook et al. 2002; Langridge 2006). The Disruptive body pattern is made of a number of Disruptive components (Hanlon & Messenger 1988), such as the white square, white head bar and white mantle bar (figure 1), that have well-defined edges as expected for ‘maximum disruptive contrast’ camouflage (Cott 1940; Hanlon & Messenger 1988). However, at least at intermediate levels of expression, individual components, and indeed the entire Disruptive pattern, are likely to be cryptic through background matching (Kelman et al. 2007, 2008). Disruptive camouflage is used by many taxa, yet the perceptual basis for its effectiveness was, until recently, little understood (Cuthill et al. 2005; Merilaita & Lind 2005; Stevens & Cuthill 2006; Stevens et al. 2006). Headway has now been made in understanding the visual mechanisms that are engaged by disruptive camouflage (Stevens 2007). We now turn to look at the factors that cause S. officinalis to select the Disruptive body pattern.
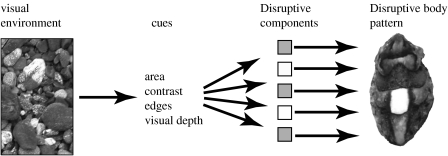
Figure 1.
Summary of the low-level visual cues so far known to be used in the expression of the Disruptive body pattern in S. officinalis. A given visual environment provides low-level cues. If these cues include edgy objects of an area approximately the area of the animal's white square then Disruptive components will be expressed. Visual depth and background contrast increase the expression of some Disruptive components (Kelman et al. 2008). Synchronized expression of Disruptive components leads to the so-called Disruptive body pattern.
Several low-level visual cues are known to drive the expression of the Disruptive body pattern in S. officinalis (figure 1; Kelman et al. 2008). These include: area (objects such as pebbles of an area 70–120% of the animal's ‘white square’ component must be in the visual environment; Barbosa et al. 2007); contrast (these objects should be lighter than the background; Chiao et al. 2005; Barbosa et al. 2008); and edginess (these objects must have defined edges—see below). Visual depth (both real and pictorial) increases the expression of some Disruptive components (Kelman et al. 2008). Cuttlefish probably have specialized (i.e. nonlinear) edge detectors, because they can discriminate conventional chequerboard (i.e. two-dimensional square wave) patterns from patterns with the same spatial frequency power spectrum but with a randomized phase of the spatial frequency components within the pattern (Morrone & Burr 1988; Kelman et al. 2007). The cuttlefish tends to express a Disruptive pattern on the conventional chequerboards and a Mottle on the phase-randomized patterns. Mäthger et al. (2007) used natural substrates to reduce the edginess of pebbles by filling in interstitial spaces around them with sand to similar effect; the expression of Disruptive components weakened as the edginess of the pebbles was reduced.
Edges are relatively cheap to compute yet can be information rich (Morrone & Burr 1988), providing strong visual cues in object recognition. Given that objects with defined edges appear to be key in eliciting a Disruptive response in S. officinalis, how much is edge information used by the animal to select its camouflage pattern? To answer this question, we compare responses of juvenile S. officinalis to a range of stimuli including edgy stimuli without area and to isolated edges (figure 2a). Objects that differ from the background in their mean luminance (first-order information) can be detected directly from the outputs of neurons that behave as linear filters, for instance, by locating points of phase congruence (McGraw et al. 1999). However, objects that do not differ from the background in terms of average luminance will not be detected by such mechanisms, and require additional processing (e.g. signal rectification; Chubb et al. 2001). Such processes are said to be ‘second order’ (Cavanagh & Mather 1989; Landy & Graham 2004). We therefore go on to explore the response of the cuttlefish to objects that are defined by variation in contrast or texture (second-order information) through the use of stimuli, where objects of a size known to give a Disruptive response are made up of much smaller objects, with an overall average luminance that is identical to the background (figure 3).
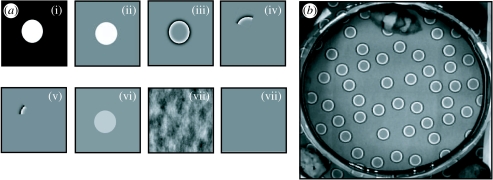
Figure 2.
(a) Visual stimuli used in experiment 1 (edge detection), here shown as a single unit of overall background, not to scale (see (b)). Circles have a diameter of 15 mm throughout. (i) Positive control of high-contrast ‘objects’ of an area approximately 90% of the mean area of the test animal's white square component, which is known to give strong expression of Disruptive components. (ii) Second positive control using the same objects on grey (at same intensity as background (iii)). (iii) High-pass filtered representation of (i), to enhance areas containing high-frequency information (i.e. edges), but attenuate the areas of low frequency, constant grey scale (the black background and the area within the circles), giving ‘edges without objects’. (iv) and (v) are quarter and eighth sections of (iii), with white/light areas of approximately 9 and 4% of the mean white square component, respectively (i.e. an area less than that shown to be necessary for Disruptive components to be expressed). These provide stimuli with isolated edges but no corresponding object. (vi) White circles on grey (ii) with a 60% reduction in contrast (further reduced contrast stimuli at 40 and 20% also tested but not shown). (vii) Phase randomized representation of (ii). Phase components in the frequency domain were randomized and reverse-Fourier transformed to give the resulting image (see Kelman et al. 2007 for further details). (viii) Uniform grey (negative control). (b) Cuttlefish settled in test arena, showing relative size and density of stimulus pattern (brightness and contrast adjusted for viewing purposes).
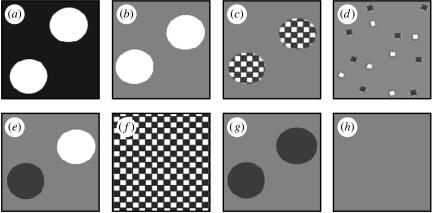
Figure 3.
Main visual stimuli for experiment 2 (second order) shown as units of the whole background (see figure 2b for example of whole stimulus). Where stimuli include circles, then the area, number and configuration remain constant between stimuli. (a) White circles on black background: positive control to ensure circle area produced strong Disruptive response. (b) White circles on grey background: ‘working contrast’ positive control. (c) Three millimetre chequerboard-filled white circles having overall identical power output as the grey background (measured by average pixel value). (d) Three millimetre individual ‘checks’ scattered across same grey in the same numbers as make up the circles in (c), so as to maintain power output across whole stimulus. (e) Equal number of black and white circles to retain same overall power. (f) Uniform 3 mm chequerboard. (g) Black circles on grey: negative control of (b). (h) Uniform grey: negative control. Stimuli (a–f) were also tested at 50 and 25% nominal contrast (not shown).
2. Material and methods
Cuttlefish were reared and maintained as described by Kelman et al. (2007). Subjects in experiment 1 were of 40–50 mm mantle length. Those used in experiment 2 were of 70–80 mm mantle length. We filmed the animals with a digital video camera (Canon XL-1) in an enclosed tank that was designed to prevent disturbance. Lights were arranged to limit shadows, and images were taken via a mirror at a 45° angle above the tank. Individual test subjects were placed in a circular arena of 250 mm diameter, 100 mm depth in seawater.
Test stimuli, printed onto standard A4 paper using a HP 1320 LaserJet printer, were placed under and around the edges of the arena. We describe the test stimuli in figure 2 (experiment 1—edge detection and object recognition) and figure 3 (experiment 2—second-order sensitivity). Images were collected after the animals had settled and the body pattern expressed had remained stable for at least 10 min.
The images of the cuttlefish were cut from the background using Adobe Photoshop v. 6.0 and randomized to ensure that grading was blind to both animal and treatment. Images were graded by eye by a single viewer (S.Z.) with previous grading experience for the expression of 32 body pattern, textural and postural components (Hanlon & Messenger 1988). The level of expression of each component was measured on a four-point scale, 0–3, with 0 representing not expressed and 3 representing strongly expressed.
To aid interpretation and testing of the multivariate datasets from each experiment, the scores for each of the 32 components for each animal were entered in a principal component analysis (PCA), using Matlab v. 7.1 (Jolliffe 1986). MANOVA was used on the PCA scores of individual animals averaged over the sampling occasions to test for significant difference between treatments in terms of the PC scores. Games–Howell tests (as the assumption of equality of the variance was not met; Meyers et al. 2005) were used for post hoc comparisons to determine where differences occurred between stimuli responses.
3. Results
(a) Experiment 1: edge detection and object recognition
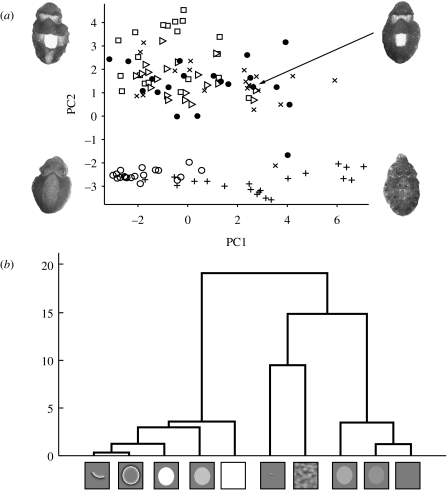
We scored the expression of behavioural components in the 200 images of juvenile S. officinalis resting on patterns with different types of edge information (figure 2). Four PCs were retained using the Kaiser criterion (retaining PCs with a variance greater than 1). These PCs account for over 65 per cent of the variance in the original dataset; PC1 and PC2 account for 30 and 21 per cent, respectively. PCs 1 and 2 correspond well to recognized body patterns (figure 4), which can be characterized as Mottle (or strong Stipple at lower expression) and Disruptive, respectively. The remaining two PCs do not correspond well with a recognizable body pattern, and are probably the result of individual response variation (e.g. the expression of minor body pattern components) as they do not show any meaningful effects of treatment.
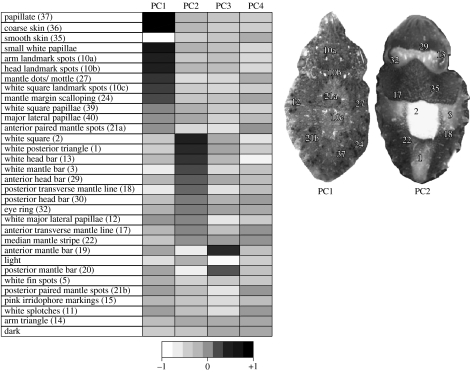
Figure 4.
Contribution of original body component variables to the four PCs retained. Numbers in brackets relate to component numbers as described in Hanlon & Messenger (1988). Examples of cuttlefish show body patterns with components of high weighting for PC1 and PC2, with some body components labelled.
A plot of treatment scores of six of the most relevant stimuli (figure 5a) shows how responses differ in terms of PC1 and 2. As would be expected, responses to high-contrast black and white circles tend to score highly positive on PC2 (corresponding to the high expression of Disruptive components) and low or negatively on PC1 (i.e. the low expression of Mottle body components). Responses to white circles on grey, high-passed whole circles and quarter sections of high-passed circles also score relatively highly on PC2 but show more variation across PC1.
Figure 5.
Results from experiment 1. (a) Plot of individual responses on PCs 1 and 2 for six of the stimuli. Cuttlefish images show the type of body pattern typical to highly positive and negative scores, and intermediate response for both PCs. Here, it can be seen how the PC scores can be used to characterize and cluster responses (squares, white circle on black; triangles, white circle on grey; filled circles, high-passed full circle; crosses, high-passed quarter circle; pluses, phase randomized; open circles, uniform grey). (b) Hierarchical cluster tree showing statistical relationship between stimuli responses, as determined by MANOVA for PCs 1–4, showing two major clades with Disruptive-type responses on the left and Mottle/Uniform responses on the right. Quarter sections of high-passed circles, full high-pass circles and white circles on grey show little statistical distance between them.
Phase randomization of white circles on grey results in negative scores on PC2 and high positive scores on PC1. This effect of phase randomization corroborates the finding that removing phase information from an originally edgy stimulus leads to a Mottle response (Kelman et al. 2007). Responses to the homogeneous grey stimulus (negative control) are characterized by negative scores on both PCs, which corresponds to the Uniform body pattern (Hanlon & Messenger 1988).
Responses to the remaining stimuli can also be characterized in terms of their PC1 and PC2 scores. For example, reducing the contrast of white circles reduces PC1 and PC2 scores, with responses to 40 and 20 per cent of the nominal contrast stimuli tending to be highly negative on both axes (i.e. Uniform or weak Stipple). Interestingly, responses to eighth section of high-passed circles tended to have low PC2 scores, but relatively high PC1 scores. This demonstrates that although these edges do not provide the necessary perceived area to promote a higher PC2 score (i.e. strong Disruptive pattern), they are perceived as different from low-contrast objects. This is in agreement with the observation that small high-contrast, edgy stimuli promote a Mottle response (Barbosa et al. 2007, 2008).
MANOVA of PC1–4 scores for all treatments showed that significant differences existed between the stimuli (Hotelling's T-square, F36,342=16.69, p≪0.005). Crucially, post hoc comparison of responses to full white, high-passed and quarter high-passed circles confirm that there are no significant differences between these treatments (p>0.1 on all PCs). Figure 5b shows a hierarchical cluster tree generated from the group means after the MANOVA by Mahalanobis distance (Martinez & Martinez 2005). This representation of the statistical distances/similarities between the responses to the stimuli illustrates the close relationship between responses to isolated edges and whole objects.
(b) Experiment 2: second-order sensitivity
Experiment 1 shows that edge information alone is a sufficient cue for S. officinalis to express Disruptive components. This experiment goes on to investigate further how cuttlefish identify objects by testing for sensitivity to second-order information, i.e. patterns where figure and ground have the same mean intensity but differ in their visual texture. This was done by comparing responses to conventional light circles on a dark background (figure 3b) with responses to circular patches of 3 mm chequerboard that had the same mean intensity as the background (figure 3c). Two control backgrounds were included in the study, one in which the same number of checks were scattered at random across the background (figure 3d), and another that consisted simply of a uniform 3 mm chequerboard (figure 3f; Barbosa et al. 2008).
Three PCs were retained under the Kaiser criterion, accounting for over 62 per cent of the variance in the original dataset. PC1 and PC2 accounted for 35 and 19 per cent, respectively. As with the previous experiment, PC1 corresponds well to the Mottle body pattern and PC2 to the Disruptive body pattern (figure 4). PC3 does not correspond to a recognizable body pattern.
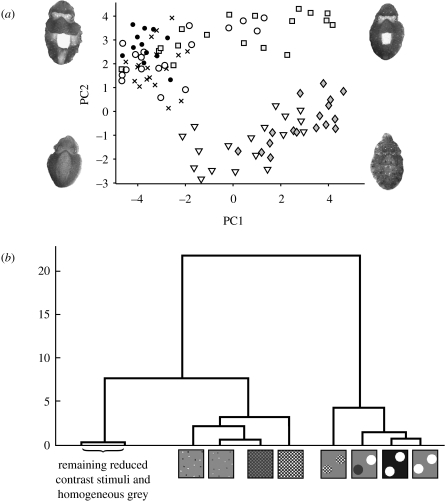
Figure 6a shows a scatter plot of the PC1 and PC2 scores of the responses to the main experimental stimuli. White circles on a black background give, as expected, a strong Disruptive pattern, characterized by a relatively high PC2 score combined with a very negative PC1 score. White circles on a grey background again give high scores on PC2, but show more variation across PC1.
Figure 6.
Results from experiment 2. (a) Scores of responses to six experimental stimuli showing two main clusters of data points, one characterized by positive PC1 and negative PC2 scores (scattered 3 mm squares, diamonds; and 3 mm chequerboard, down triangles), and the other by negative PC1 and positive PC2 scores (white circles on black, filled circles; white circles on grey, open circles; and mixed black/white circles on grey, crosses). The responses to the second-order stimuli (‘objects’ formed from 3 mm checks on grey, squares) are not closely clustered with other groups and often have positive scores on both PC1 and 2. Images of cuttlefish illustrate the response typical to that area of the plot. (b) Hierarchical tree illustrating the statistical relationships of the responses to the stimuli as determined by MANOVA for PCs 1–3. This shows that the second-order stimulus elicits a response more similar to that of whole white circles than to 3 mm checks, which are separated by a large distance. Most reduced contrast stimuli resulted in responses that were closely related to responses to uniform grey, and these have been grouped here for ease of interpretation.
Responses to second-order stimuli (objects made of 3 mm squares, figure 3c) were very similar to those of white circles on grey on PC2, but tended to have higher scores on PC1 (i.e. this stimulus elicited Disruptive components combined with Mottle components). Responses to the same 3 mm squares scattered across the grey background are very distinct from those where the squares are grouped as objects, characterized by much lower PC2 scores, and a linear-type increase of PC1 with PC2. The extended 3 mm chequerboard stimulus gave negative PC2 scores, with many PC1 scores also negative. Reducing the contrast of the stimuli tends to reduce the scores of PC1 and 2 as in experiment 1. At 50 per cent nominal contrast most responses and at 25 per cent nominal contrast all responses were similar to that shown by a homogeneous grey background (see cluster tree, figure 6b).
A MANOVA of all the stimuli shows that there are significant differences in the responses to the stimuli (Hotelling's T-square, F51,260=7.92, p<0.005). Post hoc comparison of groups confirmed that the response to the second-order stimulus (objects formed of 3 mm checks) did not significantly differ on PC1 to responses to either the scattered 3 mm squares or 3 mm chequerboard (p=0.801 and 0.971, respectively), but was significantly different on PC2 (p<0.005 in both cases). Conversely, when compared with the responses to stimuli known to elicit Disruptive responses (i.e. white circles on black and white circles on grey) the second-order stimulus responses were not significantly different on PC2 (p=0.867 and 0.973, respectively), but were significantly different on PC1 (p=0.013 and 0.022, respectively). This suggests certain image parameters, such as texture, drive Mottle components independently of image parameters that elicit Disruptive components (see §4).
Figure 6b shows the hierarchical cluster tree of the statistical relationship between all of the treatments. This tree demonstrates that the responses to the second-order stimulus are statistically closer to the ‘Disruptive stimuli’ (white circles on grey or black) than to the 2 mm chequerboard or scattered stimuli. However, the second-order stimulus is not closely nested with these disruptive stimuli, suggesting that responses to this stimulus were distinctive among the experimental treatments.
4. Discussion
The findings reported here afford several insights into how S. officinalis regulates its camouflage. Firstly, they demonstrate the importance of local edge information in eliciting Disruptive components in the body pattern. When presented with isolated edges taken from high-passed circles, the body pattern response is indistinguishable from that to whole white circles on the same background. Secondly, S. officinalis is sensitive to second-order information, responding to cues beyond mean intensity to determine the presence of background objects. A fine pattern organized as a textured ‘object’ results in a very different body pattern response from the same fine pattern presented as a whole background, even when the object has the same average luminance as the background. The sensitivity of S. officinalis to such textural information was suggested by Chiao et al. (2007) who investigated the effect of configuration and size of white squares on the expression of Disruptive body patterns. They found that the strength of the Disruptive pattern was dependent on the configuration of clusters of small light ‘elements’ when contrast, intensity and area were constant. Typically, real objects are not uniform but have a distinct visual texture, so it is not surprising that the visual system of the cuttlefish can use more complex methods of feature detection. Texture is a property of an image region which, in human vision, can be characterized and used to segregate a visual image into regions at a relatively early stage of processing, to ease the computational load at later stages (Landy & Graham 2004). It seems that cuttlefish use a similar process, which entails a nonlinear transformation of the image, such as rectification (Malik & Perona 1990).
The common cuttlefish occurs in a wide range of habitats in coastal European and sub-African waters to depths of approximately 200 m (Sherrard 2000; Wang et al. 2003). Its use of second-order information and edge cues are likely to be a testament to the complexity of the visual environment it naturally encounters; edge detection and texture segregation in the wild will be a more complex task than in the laboratory. First-order edge detectors work well where objects are defined by step edges indicated by changes in intensity (such as the chequerboard stimuli commonly used in cuttlefish vision experiments). However, such clearly defined objects are unlikely to be commonplace in the heterogeneous shallow benthic environment. Noisy objects and edges might be caused by factors such as variation in scene illumination, relief, partial occlusion by surrounding objects or substrate or biofilm growth. The successful detection of such objects may still be crucial if the animal is to effectively catch prey and escape predator detection: many marine fish and invertebrates show a preference for complex habitats, both in near-shore and offshore regions (Stoner & Titgen 2003). In the latter, features such as shell debris, sand waves, cobble and biogenic objects provide structure (Scharf et al. 2006), suggesting that using complex camouflage in order to hunt and avoid predation may still be the name of the game even in this otherwise visually and structurally homogeneous habitat.
Visual cues rarely exist in isolation and variation in first- and second-order attributes may co-occur (Schofield 2000). For example, textural change might be combined with luminance, colour, motion or depth (Landy & Graham 2004). Mounting evidence shows that cuttlefish perceive and use multiple cues to determine what body pattern should be used (figure 1). Here, we see that although the second-order stimulus (objects defined by texture) results in the use of Disruptive components such as the white square and head bar (i.e. PC2 characteristics), this response was combined with Mottle components (PC1 characteristics) not seen in responses to untextured objects. Likewise, Kelman et al. (2008) have shown that a three-dimensional background led to a stronger expression of the Disruptive pattern and the suppression of Mottle components compared with a two-dimensional image of the same background. This highlights the range and flexibility of the cuttlefishes' ability to use visual information to select camouflage, and suggests that valuable information may be lost if such a complex system is oversimplified. Indeed, the true range of body patterns available to the animal may well extend beyond those that are currently acknowledged. The cuttlefish camouflage has most probably evolved in response to predation from teleost fish, which suggests that fish have similar abilities in figure–ground segregation and object recognition to the cephalopods. To respond to a visual environment in an appropriate way and to produce effective camouflage cuttlefish need to be sensitive to the parameters used by their predators.
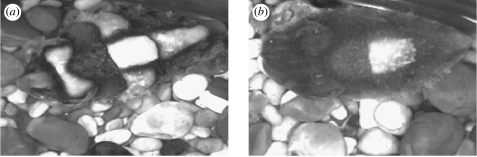
The importance of cues regarding area, contrast and edginess of objects in the use of Disruptive components by S. officinalis point towards some interesting questions about where a functioning disruptive camouflage (sensu Cott 1940) ends and a functioning background matching begins, and what happens intermediately. Placing S. officinalis on a background containing high-contrast, edgy objects of an area approximating that of the animal's white square elicits a strongly expressed, high-contrast Disruptive response. Is this designed to convey disruptive camouflage, or is the animal simply attempting to match the background for object area and contrast within the limits of its abilities? Cuttlefish undoubtedly use Disruptive components to camouflage in the wild (Hanlon & Messenger 1988; Hanlon et al. 2007), and they are seen expressed on naturalistic backgrounds in the laboratory. Often, such components appear to be used in the differential blending sense of disruptive camouflage, where some components appear cryptic with certain background elements whereas others stand out and are strongly contrasting. There is also evidence that cuttlefish may position themselves to obtain coincident disruptive advantages (Hanlon et al. 2007). Strongly expressed Disruptive body patterns often include areas that appear to be supernormal stimuli designed to enhance false borders, where a light component is edged with a high contrasting thin dark line (figure 7a). Such markings have been shown to exploit vertebrate-like edge detectors and aid outline segmentation (Osorio & Srinivasan 1991; Stevens & Cuthill 2006).
Figure 7.
Illustrating examples of how Disruptive components might be used in both background matching and disruptive camouflage. (a) True disruptive camouflage advantage may be conveyed when components are strongly expressed and coordinated, with the use of exaggerated edges to create false boundaries. At lower levels of expression, or the expression of individual Disruptive components, it is more likely to convey a cryptic background matching advantage in the same visual environment. (b) Here, the white square component alone is expressed by a different animal on the same background, with ‘shading’ giving it a relief similar to the pebbles in the environment.
The intermediate expression of Disruptive components, as seen expressed on many of the experimental stimuli used here, fail to meet the conditions thought to be necessary for a disruptive advantage to be conveyed to the animal (Cott 1940; Cuthill et al. 2005; Stevens et al. 2006). For example, Disruptive components often do not extend to the body margins and therefore fail to break up the body outline (figure 7b). This suggests that background matching may be a more likely mechanism to achieve camouflage under these conditions. To fully understand how these body patterns are used, and to assess their effectiveness in real terms, further data are needed in relation to the shallow water visual environments S. officinalis lives in, and the visual capabilities of the predators and prey it encounters there.
Our findings emphasize the similarities between cuttlefish and vertebrate vision. It is demonstrated that S. officinalis uses multiple strategies to perceive and interpret its visual surroundings. We predict that these are comparable to those used by the teleost fish, as we expect image segregation and object recognition strategies to have evolved in tandem with visual predators. The extraordinarily flexible range of body patterns used by the cuttlefish affords us a unique insight into camouflage design and an increased understanding of how camouflage exploits visual mechanisms.
Acknowledgements
Experiments were carried out in accordance with Association for the study of Animal Behaviour and University of Sussex guidelines, and met UK legal requirements.We thank N. Scott-Samuel for advice on experiment 2, T. Troscianko for comments on the manuscript, and the staff at SeaLife Brighton for help with animal care. This work was supported by a CASE award to S.Z. funded by QinetiQ and the BBSRC.
Footnotes
One contribution of 15 to a Theme Issue ‘Animal camouflage: current issues and new perspectives’.
References
- Adamo S.A., Brown W.M., King A.J., Mather D.L., Mather J.A., Shoemaker K.L., Wood J.B. Agonistic and reproductive behaviours of the cuttlefish Sepia officinalis in a semi-natural environment. J. Mollusc. Stud. 2000;66:417–419. doi:10.1093/mollus/66.3.417 [Google Scholar]
- Ballard D.H. Animate vision. Artif. Intell. 1991;48:57–86. doi:10.1016/0004-3702(91)90080-4 [Google Scholar]
- Barbosa A., Mathger L.M., Chubb C., Chiao C.-C., Florio C., Hanlon R.T. Disruptive coloration in cuttlefish: a visual perception mechanism that regulates ontogenetic adjustment of skin patterning. J. Exp. Biol. 2007;210:1139–1147. doi: 10.1242/jeb.02741. doi:10.1242/jeb.02741 [DOI] [PubMed] [Google Scholar]
- Barbosa A., Mäthger L.M., Buresch K.C., Kelly J., Chubb C., Chiao C.-C., Hanlon R.T. Cuttlefish camouflage: the effects of substrate contrast and size in evoking uniform, mottle or disruptive body patterns. Vision Res. 2008;48:1242–1253. doi: 10.1016/j.visres.2008.02.011. doi:10.1016/j.visres.2008.02.011 [DOI] [PubMed] [Google Scholar]
- Cavanagh P., Mather G. Motion: the long and short of it. Spatial Vis. 1989;4:103–129. doi: 10.1163/156856889x00077. doi:10.1163/156856889X00077 [DOI] [PubMed] [Google Scholar]
- Chiao C.-C., Kelman E.J., Hanlon R.T. Disruptive body patterning of cuttlefish (Sepia officinalis) requires visual information regarding edges and contrast of objects in natural substrate backgrounds. Biol. Bull. 2005;208:7–11. doi: 10.2307/3593095. doi:10.2307/3593095 [DOI] [PubMed] [Google Scholar]
- Chiao C.-C., Chubb C., Hanlon R.T. Interactive effects of size, contrast, intensity and configuration of background objects in evoking disruptive camouflage in cuttlefish. Vision Res. 2007;47:2223–2235. doi: 10.1016/j.visres.2007.05.001. doi:10.1016/j.visres.2007.05.001 [DOI] [PubMed] [Google Scholar]
- Chubb C., Olzak L., Derrington A. Second-order processes in vision: introduction. J. Opt. Soc. Am. A. 2001;18:2175–2178. doi: 10.1364/josaa.18.002175. doi:10.1364/JOSAA.18.002175 [DOI] [PubMed] [Google Scholar]
- Cott H.B. Methuen; London, UK: 1940. Adaptive colouration in animals. [Google Scholar]
- Crook A.C., Baddeley R., Osorio D. Identifying the structure in cuttlefish visual signals. Phil. Trans. R. Soc. B. 2002;357:1617–1624. doi: 10.1098/rstb.2002.1070. doi:10.1098/rstb.2002.1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthill I.C., Stevens M., Sheppard J., Maddocks T., Parraga C.A., Troscianko T.S. Disruptive coloration and background pattern matching. Nature. 2005;434:72–74. doi: 10.1038/nature03312. doi:10.1038/nature03312 [DOI] [PubMed] [Google Scholar]
- Gibson J.J. Houghton Mifflin; Boston, MA: 1979. Ecological approach to visual perception. [Google Scholar]
- Gombrich E.H. Pantheon; New York, NY: 1960. Art and illusion: a study in the psychology of pictorial representation. [Google Scholar]
- Hanlon R.T., Messenger J.B. Adaptive coloration in young cuttlefish (Sepia officinalis L)—the morphology and development of body patterns and their relation to behaviour. Phil. Trans. R. Soc. B. 1988;320:437–487. doi:10.1098/rstb.1988.0087 [Google Scholar]
- Hanlon R.T., Messenger J.B. Cambridge University Press; Cambridge, MA: 1996. Cephalopod behaviour. [Google Scholar]
- Hanlon R.T., Naud M.-J., Forsythe J.W., Hall K., Watson A.C., McKechnie J. Adaptable night camouflage by cuttlefish. Am. Nat. 2007;169:543–551. doi: 10.1086/512106. doi:10.1086/512106 [DOI] [PubMed] [Google Scholar]
- Horridge G.A. Ratios of template responses as the basis of semivision. Phil. Trans. R. Soc. B. 1991;331:189–197. doi: 10.1098/rstb.1991.0007. doi:10.1098/rstb.1991.0007 [DOI] [PubMed] [Google Scholar]
- Jolliffe I.T. Springer series in statistics. Springer; New York, NY: 1986. Principle component analysis. [Google Scholar]
- Julesz B. University of Chicago Press; Chicago, IL: 1971. Foundations of cyclopean perception. [Google Scholar]
- Julesz B. Textons, the elements of texture perception, and their interactions. Nature. 1981;290:91–97. doi: 10.1038/290091a0. doi:10.1038/290091a0 [DOI] [PubMed] [Google Scholar]
- Kelman E.J., Badderley R.J., Shohet A.J., Osorio D. Perception of visual texture and the expression of disruptive camouflage by the cuttlefish Sepia officinalis. Proc. R. Soc. B. 2007;274:1369–1375. doi: 10.1098/rspb.2007.0240. doi:10.1098/rspb.2007.0240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelman E.J., Osorio D., Baddeley R. Review on sensory neuroethology of cuttlefish camouflage and visual object recognition. J. Exp. Biol. 2008;211:1757–1763. doi: 10.1242/jeb.015149. doi:10.1242/jeb.015149 [DOI] [PubMed] [Google Scholar]
- Kiltie R.A., Laine A.F. Visual textures, machine vision and animal camouflage. Trends. Ecol. Evol. 1992;7:163–167. doi: 10.1016/0169-5347(92)90211-S. doi:10.1016/0169-5347(92)90211-S [DOI] [PubMed] [Google Scholar]
- Landy M.S., Graham N. Visual perception of texture. In: Landy L.M., Graham N., editors. The visual neurosciences. MIT Press; Cambridge, MA: 2004. pp. 1106–1118. [Google Scholar]
- Langridge K.V. Symmetrical crypsis and asymmetrical signalling in the cuttlefish Sepia officinalis. Proc. R. Soc. B. 2006;273:959–967. doi: 10.1098/rspb.2005.3395. doi:10.1098/rspb.2005.3395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langridge K., Broom M., Osorio D. Selective signalling by cuttlefish to predators. Curr. Biol. 2007;17:R1044–R1045. doi: 10.1016/j.cub.2007.10.028. doi:10.1016/j.cub.2007.10.028 [DOI] [PubMed] [Google Scholar]
- Malik J., Perona P. Preattentive texture discrimination with early vision mechanisms. J. Opt. Soc. Am. A. 1990;7:923–932. doi: 10.1364/josaa.7.000923. [DOI] [PubMed] [Google Scholar]
- Marr D. Henry Holt and Co; New York, NY: 1982. Vision: a computational investigation into the human representation and processing of visual information. [Google Scholar]
- Marr D., Hildreth E. Theory of edge detection. Proc. R. Soc. B. 1980;207:187–217. doi: 10.1098/rspb.1980.0020. doi:10.1098/rspb.1980.0020 [DOI] [PubMed] [Google Scholar]
- Martinez W.L., Martinez A.R. Computer science and data analysis series. CRC Press; Boca Raton, FL: 2005. Exploratory data analysis with Matlab. [Google Scholar]
- Mather G. Psychology Press; Hove, UK: 2006. Foundations of perception. [Google Scholar]
- Mäthger L.M., Chiao C.C., Barbosa A., Buresch K.C., Kaye S., Hanlon R.T. Disruptive coloration elicited on controlled natural substrates in cuttlefish, Sepia officinalis. J. Exp. Biol. 2007;210:2657–2666. doi: 10.1242/jeb.004382. doi:10.1242/jeb.004382 [DOI] [PubMed] [Google Scholar]
- McGraw P.V., Levi D.M., Whitaker D. Spatial characteristics of the second-order visual pathway revealed by positional adaptation. Nat. Neurosci. 1999;2:479–484. doi: 10.1038/8150. doi:10.1038/8150 [DOI] [PubMed] [Google Scholar]
- Merilaita S., Lind J. Background-matching and disruptive coloration, and the evolution of cryptic coloration. Proc. R. Soc. B. 2005;272:665–670. doi: 10.1098/rspb.2004.3000. doi:10.1098/rspb.2004.3000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers L.S., Gamst G., Guarino A.J. Sage Publications; London, UK: 2005. Applied multivariate research: design and interpretation. [Google Scholar]
- Morrone M.C., Burr D.C. Feature detection in human vision: a phase-dependent energy model. Proc. R. Soc. B. 1988;235:221–245. doi: 10.1098/rspb.1988.0073. doi:10.1098/rspb.1988.0073 [DOI] [PubMed] [Google Scholar]
- Osorio D., Srinivasan M.V. Camouflage by edge enhancement in animal coloration patterns and its implications for visual mechanisms. Proc. R. Soc. B. 1991;244:81–85. doi: 10.1098/rspb.1991.0054. doi:10.1098/rspb.1991.0054 [DOI] [PubMed] [Google Scholar]
- Portilla J., Simoncelli E.P. A parametric texture model based on joint statistics of complex wavelet coefficients. Int. J. Comput. Vision. 2000;40:49–71. doi:10.1023/A:1026553619983 [Google Scholar]
- Scharf F.S., Manderson J.P., Fabrizio M.C. The effects of seafloor habitat complexity on survival of juvenile fishes: species-specific interactions with structural refuge. J. Exp. Mar. Biol. Ecol. 2006;335:167–176. doi:10.1016/j.jembe.2006.03.018 [Google Scholar]
- Schofield A.J. What does second-order vision see in an image? Perception. 2000;29:1071–1086. doi: 10.1068/p2913. doi:10.1068/p2913 [DOI] [PubMed] [Google Scholar]
- Sherrard K.M. Cuttlebone morphology limits habitat depth in eleven species of Sepia (Cephalopoda: Sepiidae) Biol. Bull. 2000;198:404–414. doi: 10.2307/1542696. doi:10.2307/1542696 [DOI] [PubMed] [Google Scholar]
- Stevens M. Predator perception and the interrelation between different forms of protective coloration. Proc. R. Soc. B. 2007;274:1457–1464. doi: 10.1098/rspb.2007.0220. doi:10.1098/rspb.2007.0220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M., Cuthill I.C. Disruptive coloration, crypsis and edge detection in early visual processing. Proc. R. Soc. B. 2006;273:2141–2147. doi: 10.1098/rspb.2006.3556. doi:10.1098/rspb.2006.3556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M., Merilaita S. Defining disruptive coloration and distinguishing its functions. Phil. Trans. R. Soc. B. 2009;364:481–488. doi: 10.1098/rstb.2008.0216. doi:10.1098/rstb.2008.0216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M., Cuthill I.C., Windsor A.M.M., Walker H.J. Disruptive contrast in animal camouflage. Proc. R. Soc. B. 2006;273:2433–2438. doi: 10.1098/rspb.2006.3614. doi:10.1098/rspb.2006.3614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoerig P. Wavelength information processing versus color perception: evidence from blindsight and color-blind sight. In: Backhaus W.G.K., Kliegl R., Werner J.S., editors. Color vision: perspectives from different disciplines. Walter de Gruyter; Berlin, Germany: 1998. pp. 131–147. [Google Scholar]
- Stoner A.W., Titgen R.H. Biological structures and bottom type influence habitat choices made by Alaska flatfishes. J. Exp. Mar. Biol. Ecol. 2003;292:43–59. doi:10.1016/S0022-0981(03)00144-8 [Google Scholar]
- Troje N.F., Huber L., Loidolt M. Categorical learning in pigeons: the role of texture and shape in complex static stimuli. Vision Res. 1999;39:353–366. doi: 10.1016/s0042-6989(98)00153-9. doi:10.1016/S0042-6989(98)00153-9 [DOI] [PubMed] [Google Scholar]
- Troscianko T., Benton C.P., Lovell P.G., Tolhurst D.J., Pizlo Z. Camouflage and visual perception. Phil. Trans. R. Soc. B. 2009;364:449–461. doi: 10.1098/rstb.2008.0218. doi:10.1098/rstb.2008.0218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor J.D., Conte M.M., Chubb C. Interaction of luminance and higher-order statistics in texture discrimination. Vision Res. 2005;45:311–328. doi: 10.1016/j.visres.2004.08.013. doi:10.1016/j.visres.2004.08.013 [DOI] [PubMed] [Google Scholar]
- Wang J.J., Pierce G.J., Boyle P.R., Denis V., Robin J.P., Bellido J.M. Spatial and temporal patterns of cuttlefish (Sepia officinalis) abundance and environmental influences—a case study using trawl fishery data in French Atlantic coastal, English Channel, and adjacent waters. ICES J. Mar. Sci. 2003;60:1149–1158. doi:10.1016/j.visres.2004.08.013 [Google Scholar]