Abstract
Individual cuttlefish, octopus and squid have the versatile capability to use body patterns for background matching and disruptive coloration. We define—qualitatively and quantitatively—the chief characteristics of the three major body pattern types used for camouflage by cephalopods: uniform and mottle patterns for background matching, and disruptive patterns that primarily enhance disruptiveness but aid background matching as well. There is great variation within each of the three body pattern types, but by defining their chief characteristics we lay the groundwork to test camouflage concepts by correlating background statistics with those of the body pattern. We describe at least three ways in which background matching can be achieved in cephalopods. Disruptive patterns in cuttlefish possess all four of the basic components of ‘disruptiveness’, supporting Cott's hypotheses, and we provide field examples of disruptive coloration in which the body pattern contrast exceeds that of the immediate surrounds. Based upon laboratory testing as well as thousands of images of camouflaged cephalopods in the field (a sample is provided on a web archive), we note that size, contrast and edges of background objects are key visual cues that guide cephalopod camouflage patterning. Mottle and disruptive patterns are frequently mixed, suggesting that background matching and disruptive mechanisms are often used in the same pattern.
Keywords: body pattern, vision, colour pattern, contrast, defence
1. Introduction
Camouflage versatility is probably no better developed anywhere in the animal kingdom than in the cephalopods (octopus, squid and cuttlefish), which are marine molluscs possessing soft bodies, diverse behaviour, elaborate skin patterning capabilities and a sophisticated visual system that controls body patterning for communication and camouflage (cf. Packard 1972; Hanlon & Messenger 1996; Messenger 2001). Detailed ethograms of body patterning have been developed for more than 20 species of cephalopods (Hanlon & Messenger 1996), and one surprising finding is that the seemingly vast numbers of camouflage patterns can be synthesized into three pattern types: uniform; mottle; and disruptive (Hanlon & Messenger 1988, 1996; Hanlon 2007). Cephalopods can effectively camouflage themselves on almost any natural habitat they encounter, and they can implement the change in milliseconds (Hill & Solandt 1935; Hanlon 2007). This unique ability, combined with (i) the enormous visual diversity of their habitats (e.g. coral reefs, kelp forests, sand plains, rock reefs, seagrass beds, etc.) and (ii) the vast array and capabilities of their visual predators (e.g. marine mammals, diving birds, teleost and elasmobranch fishes, etc.; Clarke 1996), provide an integrated biological system in which many aspects of camouflage can be described and tested experimentally, and it is hoped that this group may inform us of general principles of how visual camouflage works (Hanlon 2007).
A good deal of fieldwork on cephalopod camouflage has been accomplished (e.g. Forsythe & Hanlon 1988; Hanlon & Messenger 1988, 1996; Mather & Mather 1994; Hanlon et al. 1999, 2007) and very recently a significant literature on laboratory experimentation with cuttlefish has begun to sort out the visual stimuli that evoke different camouflage patterns (Marshall & Messenger 1996; Chiao & Hanlon 2001a,b; Barbosa et al. 2004, 2007, 2008a,b; Chiao et al. 2005, 2007; Mäthger et al. 2006, 2007; Shohet et al. 2006, 2007; Kelman et al. 2007, 2008).
Camouflage is a key evolutionary development in visual predator–prey interactions. Like other researchers who study animal camouflage, we have been positively influenced by the masterful accounts of Poulton (1890), Thayer (1896, 1909) and Cott (1940). A key question—yet unanswered—is whether (and how) disruptive coloration is a distinctive mechanism of camouflage, and how it can be distinguished from background matching. Part of the answer lies in the definitions of disruptiveness, a subject that was formally presented by Cott (1940) but not narrowly defined in his superb comparative treatise (with due credit here to some of his predecessors, including Bates, Poulton and Thayer). In this paper, we (i) provide testable definitions of background matching and disruptiveness in cephalopods, (ii) describe those major pattern types quantitatively, (iii) summarize laboratory psychophysical experiments suggesting that cephalopod camouflage responses are controlled by a modest set of visual cues, (iv) provide field and laboratory evidence that some disruptive patterns have higher contrast than adjacent backgrounds, and (v) show that cephalopods often mix their mottle and disruptive patterns, and speculate on the function of such hybrid responses.
2. Background matching in cephalopods: uniform and mottle pattern descriptions
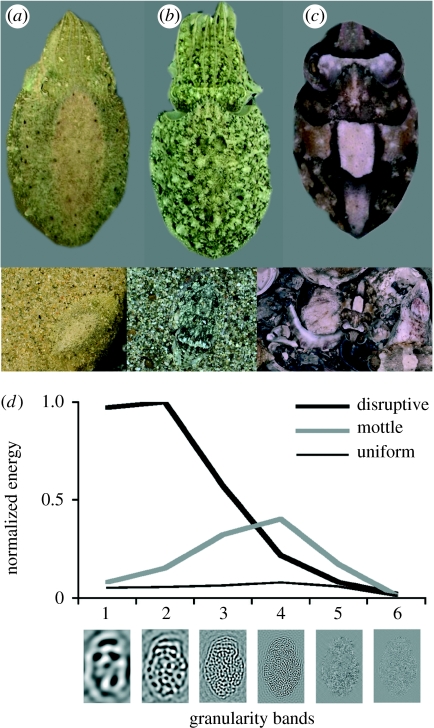
A chief characteristic of uniform body patterns is little or no contrast, i.e. there are no light/dark demarcations that produce spots, lines, stripes or other configurations within the body pattern (figure 1a). Uniform patterns can vary in colour and brightness yet both attributes are held constant, or uniform, within any single uniform body pattern. Stipple patterns are considered a subset of uniform; they usually have small clumps of expanded dark chromatophores that create a uniform distribution of small roundish spots. Stipples represent an early transition phase from uniform to mottle patterns (figure 2b). The individual skin components in uniform and stipple patterns generally match the size of surrounding background objects (e.g. sand, mud, small pebbles) to achieve background matching on a spatial scale (Hanlon & Messenger 1988). Uniform backgrounds are most often observed on open uniform sand, uniformly coloured rocks, in shadows and by squids in the water column.
Figure 1.
(a–c) Representative uniform, mottle and disruptive patterns, respectively, in the cuttlefish Sepia officinalis. (d) The granularity analysis; see text §4 for details. The three spectra shown are typical of uniform, mottle and disruptive patterns (modified from Barbosa et al. 2008b).
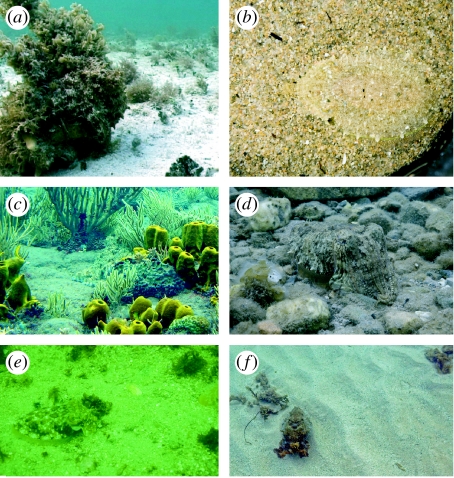
Figure 2.
Three forms of background matching in cephalopods. Specific background match: (a) Octopus vulgaris in mottle showing ‘high-fidelity’ match to calcareous algae at Grand Cayman, BWI, 3 m depth. (b) Sepia officinalis in the laboratory showing high-fidelity match to a coarse sand of moderate contrast. General background match: (c) O. vulgaris in mottle showing a generalist (but not exact) match to a complex background of soft corals, sponges and sand at Saba Island, W. Indies, 2 m depth. Octopus is exactly in the middle of the image. (d) S. officinalis showing mottle with weak striping amidst silt covered rocks and sand near Izmir, Turkey. Deceptive resemblance or masquerade: (e) S. officinalis showing large- and small-scale mottles that match patches of algae on a sand plane near Vigo, Spain at 20 m depth under murky water conditions. (f) Sepia apama showing mottle to resemble or masquerade as clumps of algae on a sand plain at Whyalla, S. Australia, 5 m depth.
Mottle body patterns are characterized by small-to-moderate-scale light and dark patches (or mottles) distributed somewhat evenly and repeatedly across the body surface. There is low-to-moderate contrast between the light and dark patches of the pattern. The light or dark patches can vary in shape and size, yet each corresponds to some adjacent background objects to achieve general matching. Many visual backgrounds consist of small-to-moderate objects of moderate contrast, thus mottle camouflage is extremely common in cephalopods as well as many animals (Cott 1940; Hanlon & Messenger 1996). Figures 1 and 2 and the electronic supplementary material, figure 1, illustrate various examples of mottle patterns in octopus, cuttlefish and squid.
We note several forms of background matching achieved with uniform and mottle patterns in cephalopods. The first is a specific background match to the pattern, contrast, physical surface texture, overall intensity and colour of the immediate background (figure 2a,b). From our extensive field experience and digital library, this sort of ‘high-fidelity’ match to the background occurs infrequently; this makes sense when one considers that cephalopods could not look exactly like each of the 100 plus species of algae and corals on a Caribbean reef, nor exactly match the diversity of rocks and sand. Another form, which is far more common, is general background match in which all the factors above are met except pattern (figure 2c,d). That is, there is a general resemblance but not exact pattern match to the immediate background. Another interesting form is illustrated in figure 2e,f where a cuttlefish does not generally resemble the sand substrate that it is sitting on, but rather it actively chooses to generally match rocks, algae or corals beyond the immediate surrounds. Cott (1940, Part III Disguise) and Hanlon & Messenger (1988) called this ‘deceptive resemblance’, which is quite an appropriate term since the cuttlefish is resembling distant but distinctive objects. The possibility remains, however, that this might be considered masquerade, whose current definition connotes defeat of recognition rather than detection. A future goal might be to determine whether such a camouflaged pattern primarily affects recognition or detection (or both) by the predator.
3. Disruptive camouflage in cephalopods: disruptive pattern descriptions
Disruptive body patterns are characterized in cephalopods by large-scale light and dark components of multiple shapes, orientations, scales and contrasts (figure 1c). Disruptive coloration (see definition by Stevens & Merilaita 2009a,b) has not been proved experimentally in cephalopods but we posit that it occurs and we provide some evidence herein. The common European cuttlefish, Sepia officinalis, has a repertoire of ‘disruptive patterns’ expressed with combinations of 11 skin components (five light and six dark; details in Hanlon & Messenger 1988; see also Holmes 1940; Chiao et al. 2005, 2007; Kelman et al. 2007; Mäthger et al. 2007). Cott (1940) provided some basic features and capabilities of presumed disruptive patterns, yet the specific components of disruptive patterns remain to be described and defined for different taxa.
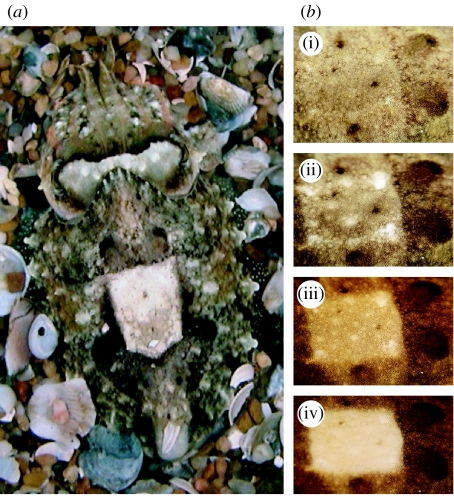
The image in figure 3a suggests that cuttlefish make use of several of the principles of disruption laid out by Cott (1940, pp. 49–65). Differential blending can be seen with the white square standing out emphatically while concurrently the dark patches of the mantle periphery and arms blend in well with the surrounding substrate. Maximum disruptive contrast (figures 3a and 4a,d) is seen in the white square, white head bar and white major lateral papillae that are surrounded by components of lower tonal contrast; note in figure 3b how neural control of the skin grades the appearance and contrast of the white square. Constructive shading and pictorial relief provide enhanced disruptiveness; close examination of the white square in different images (figures 1 and 3–5) shows it to look elevated or depressed depending on how the chromatophores are expressed against the underlying white leucophores; this may help confuse the figure/ground relationship. Coincident disruptive coloration may be achieved when the white head bar also conceals the eye (figure 3a) since the patterning traverses the eye, and the eight appendages and contour of the head are also altered. Coincident coloration may also be occurring when delineation of a skin component coincides with that of the background (figure 5f); Cott (1940, pp. 98–102) listed such occurrences as a form of coincident disruptive coloration similar to ‘background picturing’, which was described in detail by Thayer (e.g. ch. XIII, figs. 62 and 63). This image might also be interpreted as differential blending with the background.
Figure 3.
(a) Cuttlefish showing many attributes of disruptive principles; see text. (b) Four stages (i)–(iv) of increasingly disruptive expression of the white square skin component in the same animal.
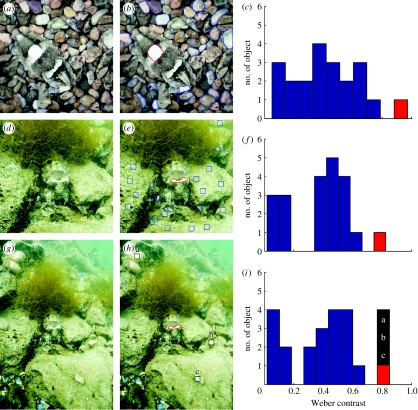
Figure 4.
Certain skin components of cuttlefish, S. officinalis, have higher contrast than objects in the surrounds. (a) A cuttlefish showing disruptive body pattern on pebble background in the laboratory. (b) White square on the mantle (red) and 20 light objects in the surrounds (blue) were selected for computing their Weber contrasts. (c) The distribution of Weber contrasts for all 21 selected areas in (b). Weber contrast was determined by (MIobject−MIbackground)/MIbackground, where MIobject is the mean intensity of the selected area and MIbackground is the mean intensity of the entire image. Note that the red bar corresponds to the Weber contrast of the white square, and it is the highest one among all other selected areas. (d) A cuttlefish showing distinct disruptive components (white square and white head bar) on a natural habitat in Turkey. (e) White head bar (red) and 20 randomly selected equivalent-sized areas in the surrounds (blue boxes) were chosen for computing their Weber contrasts. (f) The distribution of Weber contrasts for all 21 selected areas in (e). Similarly, the Weber contrast of the white head bar is higher than other areas in the surrounds. (g) The same animal shown in (d), but with a wider view. (h) Besides the 20 areas selected in the surrounds, three more light areas (black boxes) were chosen for computing their Weber contrasts. (i) The distribution of Weber contrasts for all 24 selected areas in (e) and (h), including three areas in the periphery (a–c). In a wider view, there are other areas having similar or even higher Weber contrast than the disruptive component of the animal.
Figure 5.
Background matching or disruptive coloration? (a) Sepia officinalis (in the bottom left of circular arena) showing white square while remainder of body resembles the sand. (b) Sepia pharaonis amidst rocks; its white square is a random sample of other white rocks and its other body components generally resemble other rocks. However, its overall body pattern is disruptive. (c) S. officinalis generally resembling the algae and pectin shell while on a uniform substrate; its body pattern is weakly disruptive as well; photo at 4 m depth near Izmir, Turkey. (d, e) S. officinalis at 20 m near Vigo, Spain, showing a very bright disruptive pattern; the whole animal, with its whiteness and pattern, can be considered to resemble other white objects in the wide field of view. The specific body pattern (e) is highly disruptive and much higher contrast than the immediate surrounds. (f) S. officinalis side view amidst rocks at 2 m near Izmir, Turkey. The transverse mantle bar coincides with the light rock outline in the background.
The disruptive patterns in squids take a slightly different form since these animals are long and thin (see figure 2 in the electronic supplementary material). Squids produce transverse bars that optically break up the longitudinal body. The disruptive patterns in octopuses are expressed mainly by contorting their soft malleable body; usually the arms have transverse bars but the body is uniform or mottle.
4. Quantitative descriptions of uniform, mottle and disruptive patterns in cuttlefish
We recently developed an automated method to statistically characterize each pattern produced by cuttlefish (Barbosa et al. 2008b). The three camouflage pattern types (uniform, mottle and disruptive) differ substantially in spatial scale, i.e. in the sizes of the light and dark components, which we can call ‘granularity’. Thus, we can capture these differences by analysing the pattern in different spatial frequency bands (or granularity bands) accomplished with a fast Fourier transform. Six octave-wide, isotropic filters were chosen for the granularity analysis and are illustrated on the horizontal axis in figure 1d. Note that the light and dark blobs in image 1 (at the left end of the horizontal scale) of figure 1d are comparable in size with the major disruptive components in cuttlefish (figures 1c, 3a, 4 and 5b). By comparison, the black and white blobs in image 3 are much finer, corresponding in spatial scale to the finer-grained components that are typically activated in mottle patterns (figures 1b and 2a,c–f), and images 5 and 6 are very fine-grained and typical of uniform patterns (figures 1a and 2b; see Barbosa et al. (2008b) for detailed description).
Figure 1d shows typical granularity spectra for uniform, mottle and disruptive body patterns. For readability, we use a scale in which energy is normalized by the maximum value of the granularity spectrum. This energy measure is closely related to the root mean square (r.m.s.) contrast typically used in characterizing the contrast of complex scenes (Bex & Makous 2002); specifically, the square root of the sum of the granularity spectrum values would closely approximate the r.m.s. energy in the image (a small amount of energy in the lowest and highest spatial frequencies is discarded). Note first that the spectrum of the uniform pattern has low energy in all six granularity bands, which corresponds to low contrast in overall appearance. The mottle pattern yields a spectrum with more total energy than the uniform pattern, and the spectral curve has the highest energy in granularity bands 3 and 4. This indicates that the mottle body patterns have moderate contrast with the presence of medium-spatial-scale light/dark components. Finally, the disruptive pattern has a spectrum with more total energy than either the uniform or mottle pattern; moreover, most of this energy is in the two coarsest granularity bands, 1 and 2. This tendency supports the observation that the disruptive patterns have the most contrast in body coloration with large-scale skin components. In summary, the granularity analysis provides a robust method to objectively and quantitatively distinguish the three main body patterns in cuttlefish and shows that both contrast and spatial scale are key attributes for classifying these three camouflage body-pattern types in cephalopods.
In addition to the granularity analysis, we have now developed automated methods for measuring the levels of activation of specific skin components used presumably by S. officinalis for disruptive camouflage (Chiao et al. submitted). From this set of measures, a single score can be derived, which reflects the overall disruptiveness (based upon contrast of the large body-pattern components) of the cuttlefish camouflage pattern. Our recent results show that this objective and automated measure of disruptiveness correlates well with the manual method we used previously (e.g. Chiao et al. 2005). Collectively, these disruptive skin component measures can complement the granularity analysis to quantitatively discriminate among the three main body-pattern types in cuttlefish.
5. Visual background features that evoke uniform, mottle and disruptive patterns in cuttlefish
Distinctive background characteristics drive camouflage behaviour in cuttlefish. Psychophysical experiments have shown that the size of substrate objects can determine which camouflaged body pattern a cuttlefish will show (e.g. Hanlon & Messenger 1988; Chiao & Hanlon 2001a,b; Barbosa et al. 2007, 2008b; Chiao et al. 2007; Mäthger et al. 2007; Shohet et al. 2007). Large light objects on overall dark backgrounds (e.g. chequerboard whose cheques are roughly the size of the animal's white square component) will evoke disruptive coloration (Chiao & Hanlon 2001a; Mäthger et al. 2006; Kelman et al. 2007, 2008; Barbosa et al. 2008b). Similarly, high-contrast chequerboards with a cheque size of approximately 10 per cent of the animal's white square component will elicit mottle coloration (Barbosa et al. 2007, 2008b). Recent experiments showed that there is no strict requirement for discrete objects (rocks or cheques) and that random texture substrates, such as the binary images shown in the electronic supplementary material, figure 3, also generate disruptive coloration as long as the spatial scale is appropriate and contrast is high (Chiao et al. submitted). Uniform body patterns are generally evoked on uniform substrates, such as uniform artificial backgrounds or natural substrates with objects of very small size (Mäthger et al. 2007; Barbosa et al. 2008a), but they can also be elicited on very large-scale chequerboards or objects that contain large patches of uniformity (Chiao & Hanlon 2001b; Barbosa et al. 2007; Mäthger et al. 2008).
Contrast is also a major factor determining body patterning (Chiao & Hanlon 2001a; Mäthger et al. 2006; Kelman et al. 2007, 2008; Barbosa et al. 2008b). A decrease in background contrast causes a decrease in the intensity of the camouflaged pattern or a change in body-pattern type. For example, decreasing the contrast of a chequerboard or textured substrate that evokes disruptiveness at high contrast causes the cuttlefish to show a less-intense disruptive pattern (figure 3c,d in the electronic supplementary material), and at even lower contrast (below approx. 30%), the body pattern turns into uniform/stipple. A similar effect can be observed on small-scale chequerboards and textured substrates that evoke mottle at high contrast: decreasing the contrast causes the animal's body pattern to turn to uniform/stipple (figure 3a,b in the electronic supplementary material).
Average substrate intensity (not ambient light field) also affects cuttlefish body patterns: decreased mean intensity of substrates tends to reduce the expression of disruptive components (Chiao et al. 2007). Even when intensity, contrast and object size are fixed, a change in the global configuration of substrate components can evoke substantially different body patterns (Chiao et al. 2007). Moreover, spatial frequency contents of the substrates affect body patterns; the edges of objects (high-spatial-frequency information) are key visual features that evoke disruptive patterns on both natural substrates (Chiao et al. 2005) and chequerboards (Kelman et al. 2007). Vertical structures, or the presence of three-dimensional objects near the cuttlefish, also influence cuttlefish body patterns (Barbosa et al. 2008a; Kelman et al. 2008).
6. Disruptive camouflage patterns with multiple components and higher contrast than the surroundings
Two of Cott's (1940) predictions for disruptive patterns were that (i) more components are expected on the animal's margin than in the background and the patterns will have highly variable and complex components and (ii) there would be high contrast within the animal pattern, comparable with or even higher than the contrast in the surrounding visual field. Regarding point (i), figure 4 in the electronic supplementary material shows representative components of disruptive versus mottle patterns in S. officinalis (Hanlon & Messenger 1988), and the individual light and dark components are more numerous, varied and complex in the disruptive than mottle patterns. Among the five light and six dark skin components that make up disruptive patterns in S. officinalis (op. cit.), two can be expressed particularly brightly: the white square on the mantle and the white (transverse) head bar. As shown in figure 4a–c, the white square has higher Weber contrast than other objects in the immediate surrounds. A field photograph was taken in camera raw mode in 3 m of water near Izmir, Turkey, under natural light conditions. In figure 4d–f, we consider the immediate surrounds of that cuttlefish (i.e. within approx. two body lengths), perhaps comparable with foveal vision of a predator that is nearby; in this animal, the white head bar shows stronger Weber contrast than nearby objects. In figure 4g–i, we consider a wider field of view (a distant predator or sighting by peripheral vision) and in this view the white head bar is comparable by contrast to three randomly scattered rocks in the more distant surrounds. These results conform to a prediction (Thayer 1909; Cott 1940) that disruptive coloration is effective even when some body-pattern components do not match the background, and when maximum disruptive contrast produces some degree of conspicuousness. Such results were reported by Stevens et al. (2006a) who found that disruptive cut-outs of moths that exceeded the background luminance still provided greater protection from birds than equivalent non-disruptive patterns or unpatterned controls.
7. Camouflage patterns that share features of background matching and disruptive coloration
A confusing issue is that disruptive coloration patterns (in many animals) frequently look mottled at a distance, or at least provide background matching in a broader field of view. Thus it is often difficult to sort out disruptiveness from background matching depending on how much of the background is viewed relative to the animal. Cephalopods, with their changeable and fine-tuned body patterns, have the ability to express a continuum of appearances. That is, they combine mottled skin components with disruptive skin components. In practice, a ‘mottle/disruptive pattern’ is perhaps the most common pattern ‘category’ that we observe on heterogeneous backgrounds both in the field and in the laboratory.
Some of the most challenging situations to sort out include the well-camouflaged cuttlefish in figure 5a. It has a body pattern that matches the sand except for the white square, which we have previously considered a disruptive component. Is the animal using disruptive coloration, background matching or both? One explanation could be that the cuttlefish is showing a ‘double case’ of background matching: most of the body closely resembles the sand, while the white square closely resembles white rocks in the substrate. Figure 5b–f (and figure 5 in the electronic supplementary material) shows similar situations in which the animal pattern is disruptive when considered in isolation, but in broad view they show some degree of background matching. Figure 4g is another example; it could be a case of ‘double background matching’ (i.e. white head bar and white square resemble distant white objects and dark parts of the body resemble the immediate surrounds) or disruptive, depending on the distance of viewing. There is no method available (to our knowledge) to distinguish among these possibilities.
A key distinguishing difference between disruptive and mottle patterns in cephalopods is the contrast of the separate light and dark skin components: disruptive patterns have more contrast than mottles (cf. figure 1b–d; also Barbosa et al. 2008b). Cephalopods can, perhaps uniquely, vary the contrast of their pattern while holding all other features steady; thus they use a disruptive pattern to break up their body outline (i.e. with high contrast) and reduce the contrast to make the same pattern achieve background matching by looking mottled from a distance.
8. Discussion
Most systems in biology comprise a continuum of responses, and camouflage is unlikely to be an exception. For over a century, astute biologists have suggested a distinction between the tactics of background matching and disruptive coloration. Excellent recent studies (including others in this volume) have begun to unravel their interrelationships (Merilaita 1998; Cuthill et al. 2005, 2006; Merilaita & Lind 2005; Endler 2006; Schaefer & Stobbe 2006; Stevens & Cuthill 2006; Stevens et al. 2006a,b; Fraser et al. 2007; Stevens 2007; Stobbe & Schaefer 2008). Nonetheless, the concept that each is a separate tactic by which to fool visual predators is still controversial.
Background matching is generally accepted as a viable tactic of camouflage, yet its multiple mechanisms remain rather poorly defined, quantified or tested in most taxa. Cott (1940) pointed out several ways in which animals use background matching to achieve camouflage. In §2 of this paper, we described several ways in which cephalopods can achieve this: specific background match and general background match that can be manifest in several ways, including general resemblance (or perhaps masquerade) to distant objects in the background. Obviously an animal needs to ‘match’ many of the following features: overall intensity, contrast, colour, spatial scale, texture and pattern. The term match remains ambiguous in the literature, and future efforts should seek to define it quantitatively (see Endler 1984; Mäthger et al. 2008) with modern statistical tools. It seems likely that animals match only the few statistics of the background which happen to be shared across all (or most) predators. The granularity method we introduced in §4 helps quantify spatial scale and texture contrast and can represent one method (among others) to assess the degree to which a camouflage pattern matches the background. However, those matching criteria have to be drawn from specific biological questions. The significance of acknowledging that background matching occurs via several mechanisms (§2) is that it refines the way we measure animal patterns against the surrounding substrate, and which of the six factors above are measured. For example, in figure 2f, we would ask to what degree does the cuttlefish match the distant dark objects (rather than the surrounding sand) to achieve resemblance or masquerade of the algae and rocks? In this case, the match to the algae and rocks may not have to be absolutely exact in terms of spatial scale and overall intensity to achieve sufficient resemblance to fool a predator. Conversely, a cuttlefish sitting on the sand (similar to figure 2b) may need an absolute match of spatial scale to achieve camouflage due to the spatial uniformity of the sandy background.
Disruptive coloration is a more difficult concept to grasp and measure, and therefore is a subject of considerable scepticism. The emergent trend from recent studies (which was hinted at by Cott and others) is that disruptive coloration is indeed a visual tactic of camouflage, but that some components of disruptive patterns appear to enhance background matching when tested with bird or human observers (Cuthill et al. 2005; Schaefer & Stobbe 2006; Stevens et al. 2006a; Fraser et al. 2007; Stobbe & Schaefer 2008). In cephalopods, we have described disruptive coloration but without experimental proof that the patterns we observe are functioning by the disruptive tactic (e.g. Hanlon & Messenger 1988, 1996 and recent publications). Here, we begin to address that issue by pointing out anatomical and optical features of cuttlefish body patterns that fulfil some of the requirements of disruptiveness as outlined by Cott (1940) and many recent investigators. Ten sample images of S. officinalis in the electronic supplementary material, figure 4, illustrate variations in both the marginal edge components as well as those interior to the outline of the cuttlefish in patterns that we have traditionally called mottle or disruptive. The five ‘disruptive’ images have marginal pattern components that touch the outline, as well as large high-contrast markings (what we term ‘white square’ skin component) that can act as distractive markings and false edges throughout the mantle, head and arms (see Stevens & Cuthill 2006); these are typical anatomical features of disruptive patterns used in numerous experiments recently (e.g. Cuthill et al. 2005; Merilaita & Lind 2005; Fraser et al. 2007; Stobbe & Schaefer 2008). In §6, we presented images and relative contrast measurements from cuttlefish camouflage in situ (figures 4 and 5e) indicating a disruptive function. While these features lend support for a disruptive coloration function in cephalopods, proof awaits experimentation.
More interesting is the set of observations by many researchers as well as ours in cephalopods that camouflaged body patterns commonly have features that promote background matching as well as disruptiveness (cf. Thayer 1909; Cott 1940; Hanlon & Messenger 1988; Ruxton et al. 2004; Stevens et al. 2006a; and others). Such ‘hybrid’ patterns in cephalopods have, in our parlance, both mottle and disruptive components (figure 5; electronic supplementary material, figure 5). Disruptive components in a pattern may provide high contrast distractive marks to defeat recognition when a predator or prey is in near viewing distance, while the lower contrast mottle components provide background matching to defeat detection during far viewing or peripheral vision of predators and prey. Cephalopods have many ‘choices’ available to them for how any single body pattern looks (due to neural control of hundreds of thousands of chromatophore organs throughout the skin; Messenger 2001). If we can learn which sensory cues cephalopods extract from the visual field for certain body patterns, it is likely that those cues are also important to the design of the camouflage pattern. That is, the detailed components of a body pattern will have design features that exploit predator perception (cf. review by Stevens 2007).
This raises the question of whether quantitative methods can help distinguish background matching from disruptive coloration. Using computational methods of the sort we have developed (§4), it is possible to obtain a rich statistical characterization of both the animal's response pattern and of the substrate pattern. A crucial factor is that the animal should match those statistics of the background to which its predators are spontaneously sensitive. Thus, a sensible approach might be to first determine which statistics of the background animals do match, because these are the statistics that define the common denominator of their predators' preattentive visual sensitivity.
We anticipate close (yet seldom or never exact) statistical matches between the animal's body pattern and the background when its response aims at specific background matching or masquerade, and divergence when the response is general background matching or disruptive coloration. A future step might be to determine what aspects of the visual background lead to divergences between the pattern deployed by the animal and the statistical properties of the background. Moreover, if a certain background evokes a body pattern that has background matching as well as disruptive features in it (figure 5), then we can (hypothetically) begin to sort out which visual background features cause this intermediate, or hybrid, pattern. Perhaps these approaches can begin to bridge the continuum between the seemingly interrelated tactics of background matching and disruptive coloration.
In our work with cephalopods and fishes (i.e. having access to video and thousands of images of camouflaged animals under natural conditions), it seems that there may not be a compelling reason to separate background matching and disruptive coloration too distinctively. These two mechanisms are, after all, to some extent human conveniences to help understand the complexities, the compromises and the continuum of camouflage. It may be beneficial to move beyond generic terms such as background matching and to acknowledge and define quantitatively those examples of animal patterns that may be designed to achieve specific versus general background matching, and begin to develop and test quantitative methods of comparing animal patterns with visual surrounds after posing detailed biological questions about what is being compared between animal and background.
We posit that it will be useful to define animal patterns (verbally and statistically) by taxon, provide more detailed and measurable criteria by which to measure them against backgrounds and eventually do so ‘in the eyes of the predator’ as every researcher recognizes as essential. Of course a major gap remains, i.e. knowledge of the visual capabilities of the predators (cf. Lythgoe 1979; Marshall et al. 2003; Stevens 2007). This gap is likely to retard full understanding of camouflage for a long time.
Acknowledgments
Laboratory experiments conformed to standard guidelines for animal care.
We thank the Sholley Foundation, National Geographic Society and the Office of Naval Research for funding portions of this work. We gratefully acknowledge assistance from many interns and MBL staff for helping culture cuttlefish in the laboratory, and for dive partners for R.T.H. for the field shots. We are indebted to numerous colleagues over the years for stimulating discussions on camouflage; notable among them are John Messenger, Justin Marshall, Hanumant Singh, John Endler, Phil McFadden and Sönke Johnsen. Two anonymous reviewers as well as Martin Stevens and Sami Merilaita provided very constructive improvements for the manuscript. We thank the editors for organizing this volume and the opportunity to contribute to it.
Footnotes
One contribution of 15 to a Theme Issue ‘Animal camouflage: current issues and new perspectives’.
Supplementary Material
References
- Barbosa A., Florio C.F., Chiao C.-C., Hanlon R.T. Visual background features that elicit mottled body patterns in cuttlefish, Sepia officinalis. Biol. Bull. 2004;207:154. doi: 10.1086/BBLv207n2p154. [DOI] [PubMed] [Google Scholar]
- Barbosa A., Mäthger L.M., Chubb C., Florio C., Chiao C.-C., Hanlon R.T. Disruptive coloration in cuttlefish: a visual perception mechanism that regulates ontogenetic adjustment of skin patterning. J. Exp. Biol. 2007;210:1139–1147. doi: 10.1242/jeb.02741. doi:10.1242/jeb.02741 [DOI] [PubMed] [Google Scholar]
- Barbosa A., Litman L., Hanlon R.T. Changeable cuttlefish camouflage is influenced by horizontal and vertical aspects of the visual background. J. Comp. Physiol. A. 2008a;194:405–413. doi: 10.1007/s00359-007-0311-1. doi:10.1007/s00359-007-0311-1 [DOI] [PubMed] [Google Scholar]
- Barbosa A., Mäthger L., Buresch K., Kelly J., Chubb C., Chiao C.-C., Hanlon R.T. Cuttlefish camouflage: the effects of substrate contrast and size in evoking uniform, mottle or disruptive body patterns. Vision Res. 2008b;48:1242–1253. doi: 10.1016/j.visres.2008.02.011. doi:10.1016/j.visres.2008.02.011 [DOI] [PubMed] [Google Scholar]
- Bex P.J., Makous W. Spatial frequency, phase, and the contrast of natural images. J. Opt. Soc. Am. A. 2002;19:1096–1106. doi: 10.1364/josaa.19.001096. doi:10.1364/JOSAA.19.001096 [DOI] [PubMed] [Google Scholar]
- Chiao C.-C., Hanlon R.T. Cuttlefish camouflage: visual perception of size, contrast and number of white squares on artificial chequerboard substrata initiates disruptive coloration. J. Exp. Biol. 2001a;204:2119–2125. doi: 10.1242/jeb.204.12.2119. [DOI] [PubMed] [Google Scholar]
- Chiao C.-C., Hanlon R.T. Cuttlefish cue visually on area—not shape or aspect ratio—of light objects on the substrate to produce disruptive body patterns for camouflage. Biol. Bull. 2001b;201:269–270. doi: 10.2307/1543359. doi:10.2307/1543359 [DOI] [PubMed] [Google Scholar]
- Chiao C.-C., Kelman E.J., Hanlon R.T. Disruptive body pattern of cuttlefish (Sepia officinalis) requires visual information regarding edges and contrast of objects in natural substrate backgrounds. Biol. Bull. 2005;208:7–11. doi: 10.2307/3593095. doi:10.2307/3593095 [DOI] [PubMed] [Google Scholar]
- Chiao C.-C., Chubb C., Hanlon R.T. Interactive effects of size, contrast, intensity and configuration of background objects in evoking disruptive camouflage in cuttlefish. Vision Res. 2007;47:2223–2235. doi: 10.1016/j.visres.2007.05.001. doi:10.1016/j.visres.2007.05.001 [DOI] [PubMed] [Google Scholar]
- Chiao, C.-C., Chubb, C., Buresch, K. & Hanlon, R. T. Submitted. The scaling effects of substrate texture on camouflage patterning in cuttlefish. [DOI] [PubMed]
- Clarke, M. R. (ed.) 1996 The role of cephalopods in the world's oceans. Phil. Trans. R. Soc. B351, 979–983. (doi:10.1098/rstb.1996.0088)
- Cott H.B. Methuen & Co Ltd; London, UK: 1940. Adaptive coloration in animals; p. 508. [Google Scholar]
- Cuthill I.C., Stevens M., Sheppard J., Maddocks T., Parraga C.A., Troscianko T.S. Disruptive coloration and background pattern matching. Nature. 2005;434:72–74. doi: 10.1038/nature03312. doi:10.1038/nature03312 [DOI] [PubMed] [Google Scholar]
- Cuthill I.C., Stevens M., Windsor A.M.M., Walker H.J. The effects of pattern symmetry on detection of disruptive and background matching coloration. Behav. Ecol. 2006;17:828–832. doi:10.1093/beheco/arl015 [Google Scholar]
- Endler J.A. Progressive background matching in moths, and a quantitative measure of crypsis. Biol. J. Linn. Soc. 1984;22:187–231. doi:10.1111/j.1095-8312.1984.tb01677.x [Google Scholar]
- Endler J.A. Disruptive and cryptic coloration. Proc. R. Soc. B. 2006;273:2425–2426. doi: 10.1098/rspb.2006.3650. doi:10.1098/rspb.2006.3650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe J.W., Hanlon R.T. Behavior, body patterning and reproductive biology of Octopus biamaculoides from California. Malacologia. 1988;29:41–55. [Google Scholar]
- Fraser S., Callahan A., Klassen D., Sherratt T.N. Empirical tests of the role of disruptive coloration in reducing detectability. Proc. R. Soc. B. 2007;274:1325–1331. doi: 10.1098/rspb.2007.0153. doi:10.1098/rspb.2007.0153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon R.T. Cephalopod dynamic camouflage. Curr. Biol. 2007;17:R400–R404. doi: 10.1016/j.cub.2007.03.034. doi:10.1016/j.cub.2007.03.034 [DOI] [PubMed] [Google Scholar]
- Hanlon R.T., Messenger J.B. Adaptive coloration in young cuttlefish (Sepia officinalis L.): the morphology and development of body patterns and their relation to behaviour. Phil. Trans. R. Soc. B. 1988;320:437–487. doi:10.1098/rstb.1988.0087 [Google Scholar]
- Hanlon R.T., Messenger J.B. Cephalopod behaviour. Cambridge University Press; Cambridge, UK: 1996. p. 232. [Google Scholar]
- Hanlon R.T., Forsythe J.W., Joneschild D.E. Crypsis, conspicuousness, mimicry and polyphenism as antipredator defences of foraging octopuses on Indo-Pacific coral reefs, with a method of quantifying crypsis from video tapes. Biol. J. Linn. Soc. 1999;66:1–22. doi:10.1111/j.1095-8312.1999.tb01914.x [Google Scholar]
- Hanlon R.T., Naud M.-J., Forsythe J.W., Hall K., Watson A.C., McKechnie J. Adaptable night camouflage by cuttlefish. Am. Nat. 2007;169:543–551. doi: 10.1086/512106. doi:10.1086/512106 [DOI] [PubMed] [Google Scholar]
- Hill A.V., Solandt D.Y. Myograms from the chromatophores of Sepia. J. Physiol. (Lond.) 1935;83:13P–14P. [Google Scholar]
- Holmes W. The colour changes and colour patterns of Sepia officinalis L. Proc. Zool. Soc. A. 1940;110:2–35. [Google Scholar]
- Kelman E.J., Baddeley R., Shohet A., Osorio D. Perception of visual texture and the expression of disruptive camouflage by the cuttlefish, Sepia officinalis. Proc. R. Soc. B. 2007;274:1369–1375. doi: 10.1098/rspb.2007.0240. doi:10.1098/rspb.2007.0240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelman E.J., Osorio D., Baddeley R.J. A review of cuttlefish camouflage and object recognition and evidence for depth perception. J. Exp. Biol. 2008;211:1757–1763. doi: 10.1242/jeb.015149. doi:10.1242/jeb.015149 [DOI] [PubMed] [Google Scholar]
- Lythgoe J.N. The ecology of vision. Oxford University Press; Oxford, UK: 1979. p. 244. [Google Scholar]
- Marshall N.J., Messenger J.B. Colour-blind camouflage. Nature. 1996;382:408–409. doi:10.1038/382408b0 [Google Scholar]
- Marshall N.J., Jennings K.J., McFarland W.N., Loew E.R., Losey G.S. Visual biology of Hawaiian coral reef fishes. III. Environmental light and an integrated approach to the ecology of reef fish vision. Copeia. 2003;3:467–480. doi:10.1643/01-056 [Google Scholar]
- Mather J.A., Mather D.L. Skin colours and patterns of juvenile Octopus vulgaris (Mollusca, Cephalopoda) in Bermuda. Vie Milieu. 1994;44:267–272. [Google Scholar]
- Mäthger L.M., Barbosa A., Miner S., Hanlon R.T. Color blindness and contrast perception in cuttlefish (Sepia officinalis) determined by a visual sensorimotor assay. Vision Res. 2006;46:1746–1753. doi: 10.1016/j.visres.2005.09.035. doi:10.1016/j.visres.2005.09.035 [DOI] [PubMed] [Google Scholar]
- Mäthger L.M., Chiao C., Barbosa A., Buresch K., Kaye S., Hanlon R.T. Disruptive coloration elicited on controlled natural substrates in cuttlefish, Sepia officinalis. J. Exp. Biol. 2007;210:2657–2666. doi: 10.1242/jeb.004382. doi:10.1242/jeb.004382 [DOI] [PubMed] [Google Scholar]
- Mäthger L.M., Chiao C.-C., Barbosa A., Hanlon R.T. Color matching on natural substrates in cuttlefish, Sepia officinalis. J. Comp. Physiol. A. 2008;194:577–585. doi: 10.1007/s00359-008-0332-4. doi:10.1007/s00359-008-0332-4 [DOI] [PubMed] [Google Scholar]
- Merilaita S. Crypsis through disruptive coloration in an isopod. Proc. R. Soc. B. 1998;265:1059–1064. doi:10.1098/rspb.1998.0399 [Google Scholar]
- Merilaita S., Lind J. Background-matching and disruptive coloration, and the evolution of cryptic coloration. Proc. R. Soc. B. 2005;272:665–670. doi: 10.1098/rspb.2004.3000. doi:10.1098/rspb.2004.3000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messenger J.B. Cephalopod chromatophores: neurobiology and natural history. Biol. Rev. 2001;76:473–528. doi: 10.1017/s1464793101005772. [DOI] [PubMed] [Google Scholar]
- Packard A. Cephalopods and fish: the limits of convergence. Biol. Rev. 1972;47:241–307. doi:10.1111/j.1469-185X.1972.tb00975.x [Google Scholar]
- Poulton E.B. The International Series. Kegan Paul, Trench Trubner & Co; London, UK: 1890. The colours of animals: their meaning and use. Especially considered in the case of insects. [Google Scholar]
- Ruxton G.D., Sherratt T.N., Speed M.P. Oxford University Press; Oxford, UK: 2004. Avoiding attack: the evolutionary ecology of crypsis, warning signals, and mimicry. [Google Scholar]
- Schaefer H., Stobbe N. Disruptive coloration provides camouflage independent of background matching. Proc. R. Soc. B. 2006;273:2427–2432. doi: 10.1098/rspb.2006.3615. doi:10.1098/rspb.2006.3615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohet A.J., Baddeley R.J., Anderson J.C., Kelman E.J., Osorio D. Cuttlefish responses to visual orientation of substrates, water flow and a model of motion camouflage. J. Exp. Biol. 2006;209:4717–4723. doi: 10.1242/jeb.02580. doi:10.1242/jeb.02580 [DOI] [PubMed] [Google Scholar]
- Shohet A.J., Baddeley R.J., Anderson J.C., Osorio D. Cuttlefish camouflage: a quantitative study of patterning. Biol. J. Linn. Soc. 2007;92:335–345. doi:10.1111/j.1095-8312.2007.00842.x [Google Scholar]
- Stevens M. Predator perception and the interrelation between different forms of protective coloration. Proc. R. Soc. B. 2007;274:1457–1464. doi: 10.1098/rspb.2007.0220. doi:10.1098/rspb.2007.0220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M., Cuthill I.C. Disruptive coloration, crypsis and edge detection in early visual processing. Proc. R. Soc. B. 2006;273:2141–2147. doi: 10.1098/rspb.2006.3556. doi:10.1098/rspb.2006.3556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M., Merilaita S. Defining disruptive coloration and distinguishing its functions. Phil. Trans. R. Soc. B. 2009a;364:481–488. doi: 10.1098/rstb.2008.0216. doi:10.1098/rstb.2008.0216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M., Merilaita S. Introduction. Animal camouflage: current issues and new perspectives. Phil. Trans. R. Soc. B. 2009b;364:423–427. doi: 10.1098/rstb.2008.0217. doi:10.1098/rstb.2008.0217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M., Cuthill I., Windsor A., Walker H. Disruptive contrast in animal camouflage. Proc. R. Soc. B. 2006a;273:2433–2438. doi: 10.1098/rspb.2006.3614. doi:10.1098/rspb.2006.3614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens, M., Cuthill, I. C., Parraga, C. A. & Troscianko, T. 2006b The effectiveness of disruptive coloration as a concealment strategy. In Visual perception, part 2: fundamentals of awareness: multi-sensory integration and high-order perception (eds S. M. S. Martinez-Conde, L. Martinez, J.-M. Aloso & P. Tse). Progress in brain research, no. 155, pp. 49–64. Amsterdam, The Netherlands: Elsevier. [DOI] [PubMed]
- Stobbe N., Schaefer H.M. Enhancement of chromatic contrast increases predation risk for striped butterflies. Proc. R. Soc. B. 2008;275:1535–1541. doi: 10.1098/rspb.2008.0209. doi:10.1098/rspb.2008.0209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer A.H. The law which underlies protective coloration. Auk. 1896;13:124–129. [Google Scholar]
- Thayer G.H. The Macmillan Company; New York, NY: 1909. Concealing-coloration in the animal kingdom. An exposition of the laws of disguise through color and pattern: being a summary of Abbott H. Thayer's discoveries. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.