Abstract
ATP-binding cassette (ABC) transporters constitute a large superfamily of integral membrane proteins that includes both importers and exporters. In recent years, several structures of complete ABC transporters have been determined by X-ray crystallography. These structures suggest a mechanism by which binding and hydrolysis of ATP by the cytoplasmic, nucleotide-binding domains control the conformation of the transmembrane domains and therefore which side of the membrane the translocation pathway is exposed to. A basic, conserved two-state mechanism can explain active transport of both ABC importers and ABC exporters, but various questions remain unresolved. In this article, I will review some of the crystal structures and the mechanistic insight gained from them. Future challenges for a better understanding of the mechanism of ABC transporters will be outlined.
Keywords: ATP-binding cassette (ABC) transporter, crystal structure, membrane transport proteins, mechanism, structure–function relationship
1. Introduction
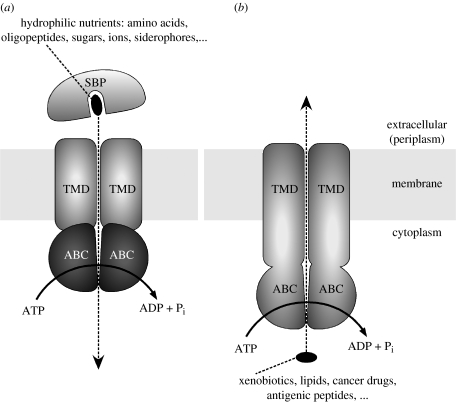
ATP-binding cassette (ABC) transporters are a large superfamily of membrane proteins with diverse functions (Holland et al. 2003). They convert the energy gained from ATP hydrolysis into trans-bilayer movement of substrates either into the cytoplasm (import) or out of the cytoplasm (export). In both cases, ATP hydrolysis is catalysed by a pair of cytoplasmic ABCs (also termed nucleotide-binding domains, NBDs), whereas the translocation of the substrate is facilitated by a pair of transmembrane domains (TMDs). The basic domain architecture and schematic mechanism are shown in figure 1. Note that importers have, to this date, only been found in prokaryotes, whereas exporter-type ABC transporters are expressed ubiquitously in all kingdoms of life. Considerable efforts have been directed at understanding the detailed mechanism of ABC transporters, which is in part motivated by the fact that there are several clinically relevant examples (Gottesman & Ambudkar 2001). Since the clustering of prokaryotic and eukaryotic ABC transporters into one protein family (Higgins 1992), various and sometimes contradictory mechanistic models have been presented and discussed. The advent of high-resolution structures of full length ABC transporters in recent years have led to a more unified picture (Hollenstein et al. 2007a). In particular, it appears that a conserved coupling mechanism may allow both ABC importers and exporters to convert ATP binding and hydrolysis to conformational changes that facilitate active transport (Dawson et al. 2007).
Figure 1.
Schematic of ABC transporter function. (a) ABC importers, which require a substrate binding protein (SBP) that feeds the hydrophilic substrates into the translocation pathway formed by the TMDs. The ABCs (or NBDs) are separate subunits. (b) ABC exporters, which typically have their TMDs fused to the ABCs.
2. A conserved coupling mechanism for ABC transporters
The NBD is the conserved domain of this protein family, and may be regarded as a common engine attached to diverse TMDs. NBDs contain two sub-domains, one resembling the functionally unrelated RecA protein, and another that has been dubbed the ‘helical sub-domain’. There are several conserved sequence motifs in the NBDs, all with specific functions (Jones & George 2004). The most important of these are the P-loops (Walker-A motifs), located in the RecA-like sub-domain, and the LSGGQ motif (denoting the amino acid sequence in single letter code), located in the helical sub-domain. In full transporters, the two NBDs assemble such that these conserved motifs are exposed at the shared interface in a head-to-tail arrangement (figure 2). This arrangement generates two ATP binding and hydrolysis sites between the P-loops of one NBD and the LSGGQ motif of the other, hence the name ‘head-to-tail’ (figure 2a). In the absence of a nucleotide, there is a gap at the domain interface, with water being able to access the nucleotide-binding sites. When ATP is bound, the interface closes and the nucleotides are sandwiched between the NBDs (Chen et al. 2003). It is generally believed that during a single transport cycle, two molecules of ATP are consumed, which is consistent with positive cooperativity observed for ATP hydrolysis in several ABC transporters (Senior & Bhagat 1998). Exceptions are possible where one of the ATP binding sites features mutations that prevent hydrolysis, as is the case with the cystic fibrosis transmembrane conductance regulator CFTR.
Figure 2.
Conserved coupling mechanism of ABC transporters. (a) The molecular motion triggered by binding of ATP triggers the closing of a gap between the ABCs. This moves the coupling helices, an architecturally conserved feature, closer together and flips the TMDs to an outward-facing conformation. Hydrolysis of ATP and release of the hydrolysis products revert the TMDs to adopt an inward-facing conformation. (b) Structural alignment of the coupling helices as observed in high-resolution crystal structures.
The crystal structures of full length ABC transporters have provided a plausible mechanism for coupling ATP hydrolysis to transport. Effective coupling requires the transmission of the molecular motion from the NBDs to the TMDs. At this interface, architecturally conserved α-helices, which are part of the TMDs, are present in all reported crystal structures. These ‘coupling helices’ interact with grooves formed at the boundaries of the two sub-domains of the NBDs, and mutational analysis suggests a similar coupling interface in ABC transporters without known crystal structures (Zolnerciks et al. 2007). Figure 2b shows a structure-based sequence alignment of the coupling helices of ABC transporters with known high-resolution structures (i.e. available coordinate sets that include side chains). The alignment suggests that even though the coupling helix may be an architecturally conserved motif, its sequence is not conserved. This is not surprising since its interaction with the NBD within a given ABC transporter relies on specific side-chain contacts.
A comparison of the structures of Sav1866, a multi-drug ABC transporter trapped in a conformation reflecting the ATP-bound state, with the molybdate/tungstate transporter ModBC, trapped in a nucleotide-free state, has suggested a basic mechanism of coupling ATP binding and hydrolysis to transport (Dawson et al. 2007). Upon binding of ATP, the gap between the NBD closes, bringing the coupling helices closer together. As a consequence, the TMDs flip from facing inward to facing outward. ABC importers and exporters make use of this mechanism to expose binding and extrusion sites, located in the TMDs, to opposite sides of the membrane, thus moving substrates across the bilayer unidirectionally. Even though the general coupling mechanism may be conserved, the folds of the TMDs in different transporters are not. In the following discussion, I will therefore subdivide ABC transporters into three classes.
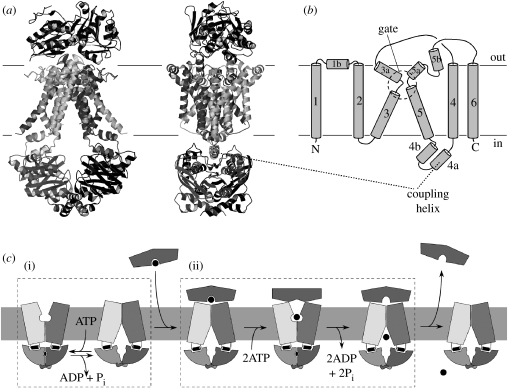
3. ABC exporters
ABC exporters are found in the genomes of all organisms sequenced to date. Many organisms have multiple different transporters with diverse physiological functions; for example, the human genome codes for 48 or 49 distinct ABC transporters (Dean 2005). For several bacterial and eukaryotic ABC exporters, the physiological substrates are unknown or uncertain. However, many are involved in multi-drug extrusion of toxic substances, which can lead to resistance of cancer cells against drugs used in chemotherapy (Gottesman et al. 2002). Even though ABC exporters can recognize diverse substrates, they all share a common core architecture that consists of 12 transmembrane helices. These extend well beyond the cytoplasmic boundary of the lipid bilayer, and as a consequence, the ABC domains of ABC exporters are spaced approximately 25 Å away from the membrane. The TMDs of exporters are invariably fused to the NBDs, typically with the TMDs preceding the NBDs (but there are exceptions). The first high-resolution crystal structure of an ABC exporter, the Sav1866 protein from Staphylococcus aureus, revealed the fold and topology, which is remarkably intricate (Dawson & Locher 2006). In particular, the individual TMDs are not simply aligned side by side as independent helical bundles. Rather, they embrace each other and have a significant twist (figure 3). Sav1866 was captured in a conformation that reflects the ATP-bound state (Dawson & Locher 2007), with a central cavity between the TMDs facing outward. This cavity is relatively hydrophilic and was interpreted to represent an extrusion pocket, with little or no affinity for the hydrophobic substrates. This is in agreement with biochemical data (Ramachandra et al. 1998; van Veen et al. 2000) and with the coupling mechanism discussed above. Binding of ATP would convert a presumed inward-facing conformation (exposing a binding site to the inside) to the outward-facing conformation observed in Sav1866. Upon extrusion of the substrate, hydrolysis of ATP and dissociation of the hydrolysis products from the ATP-binding pockets complete the transport cycle. The Sav1866 structure is in agreement with many biochemical studies of homologous transporters, and it has also served as a useful template for modelling human ABC transporters (Mendoza & Thomas 2007; O'Mara & Tieleman 2007).
Figure 3.
Topology and structure of the multi-drug-type ABC transporter Sav1866. (a) The topology of the TMD of a single TMD is shown, with an emphasis on the pseudo-twofold symmetry between the sets of TM helices 1–3 and 4–6. (b) The structure of the homodimeric Sav1866 is shown in ribbon representation, with the monomers in light and dark grey. (c) Sections through the Sav1866 are shown after rotating the protein by 90° from the view shown in (b), i.e. from the external side. TM helices of one monomer are numbered.
4. Type I ABC importers
These mediate the uptake of ions, sugars, amino acids and other substrates, which are all captured by specific binding proteins that deliver them to the transporters (Davidson & Chen 2004). The TMDs generally contain 12 TM helices, and the fold of this subfamily has been established by the crystal structure of the molybdate/tungstate transporter ModBC from Archaeoglobus fulgidus (figure 4; Hollenstein et al. 2007b). The core topology contains 10 TM helices, and an additional, N-terminal TM helix wraps around the partner TMD, but is not present in all type I importers. For example, the molybdate transporter ModBC from Escherichia coli does not contain this helix. Two ABC importers of type I, the HisPQM system specific for histidine and the MalFGK system specific for maltose, have been studied extensively using biochemical techniques. The structure of ModBC is in agreement with all the data assembled from these systems. ModBC was captured in a nucleotide-free, inward-facing conformation. Soon after its publication, the structure of the maltose transporter MalFGK was also determined, revealing the same TMD fold but a distinct conformation which reflected an intermediate of the transport cycle (Oldham et al. 2007). MalFGK was similar in conformation to Sav1866 in that it was ATP bound and outward facing. The opening to the outside, however, was covered by the attached binding protein MalE, which prevented the substrate (maltose) from escaping to the periplasm.
Figure 4.
Structure, topology and mechanism of type I ABC importers. (a) The structure of the molybdate/tungstate transporter ModBC from A. fulgidus in a complex with the cognate, substrate-bound binding protein is shown in ribbon representation. (b) The topology of a single ModB subunit (TMD) is shown, with TM helices numbered and critical regions indicated. (c) The proposed mechanism of molybdate transport is shown schematically. (i) The futile hydrolysis cycle observed in vitro and in the absence of substrate or binding protein. (ii) For a productive transport cycle, two conformations are required. While the inward-facing state was visualized by the ModBC-A structure, that of the outward-facing intermediate was observed in the crystal structure of the MalFGK-E complex. The transient binding pocket in the translocation pathway, only exposed to the external side, is indicated as a round opening.
Combined, the structures of ModBC-A and MalFGK-E define the two basic states of the transport mechanism of type I ABC importers (shown in figure 4c). For several transporters, basal ATPase activity is observed in vitro and in the absence of substrate or binding protein, but the physiological relevance of this futile ATPase cycle is unclear. Some type I ABC importers are tightly coupled and do not exhibit futile ATP hydrolysis, which made it possible to unequivocally determine the reaction stoichiometry as two ATPs per transported substrate (Patzlaff et al. 2003). Notably, futile ATP hydrolysis has recently been observed in the yeast multi-drug ABC transporter Pdr5 (Ernst et al. 2008). The relevant point for a productive transport cycle through type I ABC importers is that upon binding of ATP, a transient-binding pocket for substrate is formed within the translocation pathway, but is only accessible from the outside. This is coupled to the release of the substrate from the binding protein by means of spreading of its lobes. The substrate may then travel into the translocation pathway and bind to the transient binding site, as observed in the maltose transporter structure (Oldham et al. 2007). Hydrolysis of ATP and flipping of the TMDs to the inward-facing conformation occlude the transient binding site and eject the substrate into the cytoplasm.
5. Type II ABC importers
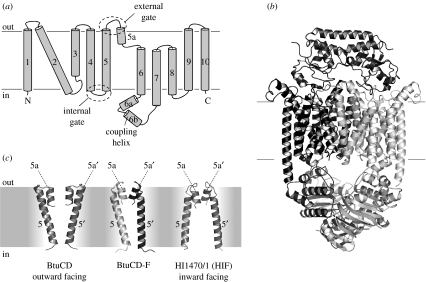
Importers of type II facilitate the uptake of metal chelates that are generally larger than the substrates of type I ABC importers. Examples of substrates are cobalamine (vitamin B12) or haem (DeVeaux & Kadner 1985; Griffiths & Williams 1999). The acquisition of haem-bound iron from the host organism is medically relevant because it has been associated with virulence of certain pathogenic bacteria (Pattery et al. 1999; Janakiraman & Slauch 2000). Type II importers have a distinct TMD architecture from those of type I, with 10 helices in each TMD for a total of 20 transmembrane segments in the assembled transporter (figure 5a). The architecture was revealed in the crystal structure of the E. coli vitamin B12 transporter BtuCD (Locher et al. 2002). Since then, two more crystal structures have been determined, one of the homologous HIF protein (HI1470/71) from Haemophilus influenzae (Pinkett et al. 2007), and that of the BtuCD protein in complex with the binding protein BtuF (Hvorup et al. 2007; figure 5b). The structures of BtuCD, HIF and BtuCD-F demonstrated that a subset of TM helices (3–5a) can adopt one of two conformations (figure 5c). In BtuCD, both the TMDs adopt conformation 1, which results in an outward-facing translocation pathway. In HIF, they adopt conformation 2, resulting in an inward-facing translocation pathway. In BtuCD-F, one TMD adopts conformation 1, whereas the other adopts conformation 2, resulting in an occluded translocation pathway. In none of the structures is there a substrate bound in the translocation pathway, and the surfaces of the cavities in the TMDs bear no resemblance to binding pockets for B12 or haem. This has led to the notion that type II ABC importers provide inert (‘Teflon’) translocation pathways with little or no affinities for their substrates. The specificity of the transport reaction therefore appears to depend exclusively on the cognate binding protein and its interface with the TMDs. An intriguing finding was that there was no apparent correlation of the TMD conformations with those of the NBDs, as all three crystal structures were nucleotide free. There are two equally likely explanations: one is that the purification and crystallization conditions (in particular the presence of detergents) have induced partial uncoupling of the TMD and NBD conformations. The other explanation is that the transport mechanism of type II ABC importers may operate on a modified scheme when compared with that of ABC exporters or type I importers. Further structural and functional studies are required to clarify this point.
Figure 5.
Topology, structure, and conformations of distinct states of type II ABC importers. (a) The topology of the BtuC subunit (TMD) is shown, with TM helices numbered and critical regions indicated. (b) Ribbon diagram of the BtuCD-F complex probably reflecting a post-translocation intermediate. (c) Comparison of the conformations of the TM helices 5 and 5a in distinct crystal structures. The conformation of these helices in BtuCD-F is a hybrid between those of BtuCD (outward facing) and those of HIF (inward facing).
6. Future challenges
The progress in revealing the basic folds and conformations of ABC transporters by X-ray crystallography will allow various unanswered questions to be addressed. As was the case with other biological problems, bacterial model systems have paved the way for a mechanistic understanding of the transport cycle, and will probably continue to be useful in the coming years. In particular, they will be useful to understand how multi-drug ABC transporters recognize diverse drugs, or how inward-facing conformations of ABC exporters may bind substrates. Eukaryotic ABC transporters are also involved in other processes such as gated chloride flux through CFTR (Sheppard & Welsh 1999; Riordan 2005; Gadsby et al. 2006), the regulation of potassium channels by SUR1 (Campbell et al. 2003) or the loading of antigenic peptides onto MHC class I molecules by Tap1/2 and tapasin (Abele & Tampe 2004). These processes cannot be modelled readily using bacterial homologues, suggesting that direct structure determination is required to understand their mechanisms.
A better understanding of ABC transporters will require a combination of structural, biophysical, biochemical and physiological studies. As with any membrane protein, structural characterization at high resolution requires crystallization, which in turn requires the proteins to be extracted from the membrane using detergents. Our experience has shown that ABC transporters are extremely dynamic molecules that are not easily coerced into an ordered lattice of a three-dimensional crystal. In addition, there is always the risk that the detergents not only remove the membrane lipids, but also simultaneously alter the conformation of the protein. Thus, the validity of crystal structures of full ABC transporters must be evaluated on whether the conformations are mandatory intermediates of a transport cycle. Despite this caveat, structures are indispensable for a detailed understanding of ABC transporter function.
Acknowledgements
The work described in this article is supported by the Swiss National Science Foundation (SNSF), the Roche Research Fund (RRF), the National Center for Excellence in Research (NCCR) Structural Biology, Zurich, and the Swiss Cancer League Oncosuisse.
Footnotes
One contribution of 16 to a Discussion Meeting Issue ‘Membrane transport in flux: the ambiguous interface between channels and pumps’.
References
- Abele R., Tampe R. The ABCs of immunology: structure and function of TAP, the transporter associated with antigen processing. Physiology. 2004;19:216–224. doi: 10.1152/physiol.00002.2004. doi:10.1152/physiol.00002.2004 [DOI] [PubMed] [Google Scholar]
- Campbell J.D., Sansom M.S., Ashcroft F.M. Potassium channel regulation. EMBO Rep. 2003;4:1038–1042. doi: 10.1038/sj.embor.7400003. doi:10.1038/sj.embor.7400003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Lu G., Lin J., Davidson A.L., Quiocho F.A. A tweezers-like motion of the ATP-binding cassette dimer in an ABC transport cycle. Mol. Cell. 2003;12:651–661. doi: 10.1016/j.molcel.2003.08.004. doi:10.1016/j.molcel.2003.08.004 [DOI] [PubMed] [Google Scholar]
- Davidson A.L., Chen J. ATP-binding cassette transporters in bacteria. Annu. Rev. Biochem. 2004;73:241–268. doi: 10.1146/annurev.biochem.73.011303.073626. doi:10.1146/annurev.biochem.73.011303.073626 [DOI] [PubMed] [Google Scholar]
- Dawson R.J.P., Locher K.P. Structure of a bacterial multidrug ABC transporter. Nature. 2006;443:180–185. doi: 10.1038/nature05155. doi:10.1038/nature05155 [DOI] [PubMed] [Google Scholar]
- Dawson R.J.P., Locher K.P. Structure of the multidrug ABC transporter Sav1866 from Staphylococcus aureus in complex with AMP-PNP. FEBS Lett. 2007;581:935–938. doi: 10.1016/j.febslet.2007.01.073. doi:10.1016/j.febslet.2007.01.073 [DOI] [PubMed] [Google Scholar]
- Dawson R.J.P., Hollenstein K., Locher K.P. Uptake or extrusion: crystal structures of full ABC transporters suggest a common mechanism. Mol. Microbiol. 2007;65:250–257. doi: 10.1111/j.1365-2958.2007.05792.x. doi:10.1111/j.1365-2958.2007.05792.x [DOI] [PubMed] [Google Scholar]
- Dean M. The genetics of ATP-binding cassette transporters. Methods Enzymol. 2005;400:409–429. doi: 10.1016/S0076-6879(05)00024-8. doi:10.1016/S0076-6879(05)00024-8 [DOI] [PubMed] [Google Scholar]
- DeVeaux L.C., Kadner R.J. Transport of vitamin-B12 in E. coli—cloning of the Btucd region. J. Bacteriol. 1985;162:888–896. doi: 10.1128/jb.162.3.888-896.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst R., Kueppers P., Klein C.M., Schwarzmueller T., Kuchler K., Schmitt L. A mutation of the H-loop selectively affects rhodamine transport by the yeast multidrug ABC transporter Pdr5. Proc. Natl Acad. Sci. USA. 2008;105:5069–5074. doi: 10.1073/pnas.0800191105. doi:10.1073/pnas.0800191105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby D.C., Vergani P., Csanady L. The ABC protein turned chloride channel whose failure causes cystic fibrosis. Nature. 2006;440:477–483. doi: 10.1038/nature04712. doi:10.1038/nature04712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman M.M., Ambudkar S.V. Overview: ABC transporters and human disease. J. Bioenerg. Biomembr. 2001;33:453–458. doi: 10.1023/a:1012866803188. doi:10.1023/A:1012866803188 [DOI] [PubMed] [Google Scholar]
- Gottesman M.M., Fojo T., Bates S.E. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat. Rev. Cancer. 2002;2:48–58. doi: 10.1038/nrc706. doi:10.1038/nrc706 [DOI] [PubMed] [Google Scholar]
- Griffiths E., Williams P. The iron-uptake systems of pathogenic bacteria, fungi and protozoa. In: Bullen J.J., Griffiths E., Bullen D.J., editors. Iron and infection: molecular, physiological and clinical aspects. John Wiley; Chichester, UK: 1999. pp. 87–212. [Google Scholar]
- Higgins C.F. ABC transporters—from microorganisms to man. Annu. Rev. Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. doi:10.1146/annurev.cb.08.110192.000435 [DOI] [PubMed] [Google Scholar]
- Holland I.B., Cole S.P.C., Kuchler K., Higgins C.F. ABC proteins: from bacteria to man. Academic Press; London, UK: 2003. [Google Scholar]
- Hollenstein K., Dawson R.J.P., Locher K.P. Structure and mechanism of ABC transporter proteins. Curr. Opin. Struct. Biol. 2007a;17:412–418. doi: 10.1016/j.sbi.2007.07.003. doi:10.1016/j.sbi.2007.07.003 [DOI] [PubMed] [Google Scholar]
- Hollenstein K., Frei D.C., Locher K.P. Structure of an ABC transporter in complex with its binding protein. Nature. 2007b;446:213–216. doi: 10.1038/nature05626. doi:10.1038/nature05626 [DOI] [PubMed] [Google Scholar]
- Hvorup R.N., Goetz B.A., Niederer M., Hollenstein K., Perozo E., Locher K.P. Asymmetry in the structure of the ABC transporter-binding protein complex BtuCD-BtuF. Science. 2007;317:1387–1390. doi: 10.1126/science.1145950. doi:10.1126/science.1145950 [DOI] [PubMed] [Google Scholar]
- Janakiraman A., Slauch J.M. The putative iron transport system SitABCD encoded on SPI1 is required for full virulence of Salmonella typhimurium. Mol. Microbiol. 2000;35:1146–1155. doi: 10.1046/j.1365-2958.2000.01783.x. doi:10.1046/j.1365-2958.2000.01783.x [DOI] [PubMed] [Google Scholar]
- Jones P.M., George A.M. The ABC transporter structure and mechanism: perspectives on recent research. Cell. Mol. Life Sci. 2004;61:682–699. doi: 10.1007/s00018-003-3336-9. doi:10.1007/s00018-004-4272-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locher K.P., Lee A.T., Rees D.C. The E. coli BtuCD structure: a framework for ABC transporter architecture and mechanism. Science. 2002;296:1091–1098. doi: 10.1126/science.1071142. doi:10.1126/science.1071142 [DOI] [PubMed] [Google Scholar]
- Mendoza J.L., Thomas P.J. Building an understanding of cystic fibrosis on the foundation of ABC transporter structures. J. Bioenerg. Biomembr. 2007;39:499–505. doi: 10.1007/s10863-007-9117-7. doi:10.1007/s10863-007-9117-7 [DOI] [PubMed] [Google Scholar]
- Oldham M.L., Khare D., Quiocho F.A., Davidson A.L., Chen J. Crystal structure of a catalytic intermediate of the maltose transporter. Nature. 2007;450:515–521. doi: 10.1038/nature06264. doi:10.1038/nature06264 [DOI] [PubMed] [Google Scholar]
- O'Mara M.L., Tieleman D.P. P-glycoprotein models of the apo and ATP-bound states based on homology with Sav1866 and MalK. FEBS Lett. 2007;581:4217–4222. doi: 10.1016/j.febslet.2007.07.069. doi:10.1016/j.febslet.2007.07.069 [DOI] [PubMed] [Google Scholar]
- Pattery T., Hernalsteens J.P., De Greve H. Identification and molecular characterization of a novel Salmonella enteritidis pathogenicity islet encoding an ABC transporter. Mol. Microbiol. 1999;33:791–805. doi: 10.1046/j.1365-2958.1999.01526.x. doi:10.1046/j.1365-2958.1999.01526.x [DOI] [PubMed] [Google Scholar]
- Patzlaff J.S., van der Heide T., Poolman B. The ATP/substrate stoichiometry of the ATP-binding cassette (ABC) transporter OpuA. J. Biol. Chem. 2003;278:29 546–29 551. doi: 10.1074/jbc.M304796200. doi:10.1074/jbc.M304796200 [DOI] [PubMed] [Google Scholar]
- Pinkett H.W., Lee A.T., Lum P., Locher K.P., Rees D.C. An inward-facing conformation of a putative metal-chelate-type ABC transporter. Science. 2007;315:373–377. doi: 10.1126/science.1133488. doi:10.1126/science.1133488 [DOI] [PubMed] [Google Scholar]
- Ramachandra M., Ambudkar S.V., Chen D., Hrycyna C.A., Dey S., Gottesman M.M., Pastan I. Human P-glycoprotein exhibits reduced affinity for substrates during a catalytic transition state. Biochemistry. 1998;37:5010–5019. doi: 10.1021/bi973045u. doi:10.1021/bi973045u [DOI] [PubMed] [Google Scholar]
- Riordan J.R. Assembly of functional CFTR chloride channels. Annu. Rev. Physiol. 2005;67:701–718. doi: 10.1146/annurev.physiol.67.032003.154107. doi:10.1146/annurev.physiol.67.032003.154107 [DOI] [PubMed] [Google Scholar]
- Senior A.E., Bhagat S. P-glycoprotein shows strong catalytic cooperativity between the two nucleotide sites. Biochemistry. 1998;37:831–836. doi: 10.1021/bi9719962. doi:10.1021/bi9719962 [DOI] [PubMed] [Google Scholar]
- Sheppard D.N., Welsh M.J. Structure and function of the CFTR chloride channel. Physiol. Rev. 1999;79:S23–S45. doi: 10.1152/physrev.1999.79.1.S23. [DOI] [PubMed] [Google Scholar]
- van Veen H.W., Margolles A., Muller M., Higgins C.F., Konings W.N. The homodimeric ATP-binding cassette transporter LmrA mediates multidrug transport by an alternating two-site (two-cylinder engine) mechanism. EMBO J. 2000;19:2503–2514. doi: 10.1093/emboj/19.11.2503. doi:10.1093/emboj/19.11.2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolnerciks J.K., Wooding C., Linton K.J. Evidence for a Sav1866-like architecture for the human multidrug transporter P-glycoprotein. FASEB J. 2007;21:3937–3948. doi: 10.1096/fj.07-8610com. doi:10.1096/fj.07-8610com [DOI] [PubMed] [Google Scholar]