Abstract
Plants need nitrate for growth and store the major part of it in the central vacuole of cells from root and shoot tissues. Based on few studies on the two model plants Arabidopsis thaliana and rice, members of the large ChLoride Channel (CLC) family have been proposed to encode anion channels/transporters involved in nitrate homeostasis. Proteins from the Arabidopsis CLC family (AtClC, comprising seven members) are present in various membrane compartments including the vacuolar membrane (AtClCa), Golgi vesicles (AtClCd and AtClCf) or chloroplast membranes (AtClCe). Through a combination of electrophysiological and genetic approaches, AtClCa was shown to function as a 2NO3−/1H+ exchanger that is able to accumulate specifically nitrate into the vacuole, in agreement with the main phenotypic trait of knockout mutant plants that accumulate 50 per cent less nitrate than their wild-type counterparts. The set-up of a functional complementation assay relying on transient expression of AtClCa cDNA in the mutant background opens the way for studies on structure–function relationships of the AtClCa nitrate transporter. Such studies will reveal whether important structural determinants identified in bacterial or mammalian CLCs are also crucial for AtClCa transport activity and regulation.
Keywords: plant chloride channels, anion transport, nitrate, vacuole, transporter/channel, Arabidopsis thaliana
1. Anion transport in plant cells
In plant cells as in animal cells, anion channels/transporters appear as key players in the control of metabolism, in the maintenance of electrochemical gradients and in signalling pathways leading to plant adaptation to abiotic and biotic environmental stresses. They contribute to various physiological functions such as control of stomatal movements regulating gas exchanges in leaves, plant–pathogen interaction, root xylem loading, compartmentation of metabolites and coupling with proton gradients (reviewed in Barbier-Brygoo et al. 2000; De Angeli et al. 2007). Anion channel activities and associated regulation mechanisms have been characterized primarily using electrophysiological techniques. They were reported in all plant membranes including the plasma membrane, tonoplast, endoplasmic reticulum, mitochondria and chloroplasts, plasma membrane channels being by far the best characterized compared to those located on other membranes. By contrast, the identification of the corresponding genes is still in its infancy.
The most abundant anions in plants are malate and nitrate. Malate plays a major role in plant carbon metabolism and has many functions in plant cells, as charge balancing solute in the vacuole, in the carboxylate and glyoxylate cycle, in CO2 temporary storage in C4 plants and in stomata and pulvini movements. In many of these processes, malate is accumulated into the vacuole (Martinoia & Ratajcsak 1997). The transport of malate to the vacuolar lumen has been shown to occur via specific transporters such as AttDT (Arabidopsis thaliana tonoplast dicarboxylate transporter; Hurth et al. 2005) and via specific channels as well (Hafke et al. 2003). The molecular identity of malate channels has been revealed only recently with the identification of the AtALMT9 protein (A. thaliana aluminium-activated malate transporter; Kovermann et al. 2007). The other major anion in plants is nitrate that serves both as a nutrient and as a signal transducer and has profound effects on plant metabolism and growth. Plants have evolved intricate mechanisms to detect its presence in the soil and to integrate its assimilation with photosynthesis and the overall metabolism of nitrogen and carbon. These mechanisms allow plants to control growth rates, root architecture and carbon/nitrogen ratios under diverse environmental conditions.
The first step in nitrate assimilation is its uptake from the soil solution by cortical epidermal cells of the root, via high-affinity (Km=10–100 μM) or low-affinity (Km≈0.5 mM) transport systems allowing plants to cope with a wide range of external nitrate concentrations without severe deficiency or toxicity (reviewed in Miller & Smith 2007; Tsay et al. 2007). Once in the root, nitrate can be stored in situ or loaded into the xylem to undergo long distance transport to the shoots where it can be either stored or assimilated in organic molecules. At the cellular level, cytosolic nitrate follows two major routes: one is to be assimilated in organic compounds via the nitrate reductase (NR) pathway and the second one is to be stored in the vacuole up to concentrations of 50–80 mM (Martinoia & Wiemken 1981). The balance between the uptake from extracellular media, NR activity and vacuole storage/remobilization determines the homeostasis of nitrate into the cytosol (Miller & Smith 2007). Although several authors reported the presence of proton/nitrate exchange activity in isolated tonoplast vesicles (Blumwald & Poole 1985; Schumaker & Sze 1986), the molecular identity of the protein(s) responsible for the accumulation and the release of this anion into/from the vacuole was unknown until very recently (see below).
A key issue in the field thus remains to connect orphan anion transport activities to the corresponding proteins and genes, for a better understanding of their integrated function in the plant. After the proteins of the chloride channel (CLC) family were discovered in A. thaliana and Nicotiana tabacum (Hechenberger et al. 1996; Lurin et al. 1996), it was hypothesized that these proteins might be involved in anion transport across plant membranes.
2. The plant CLC family
The first protein of the CLC family to be identified is ClC-0, a CLC present in the electric organ of torpedo fishes (Miller & White 1980). The cloning of the ClC-0 gene (Jentsch et al. 1990) led to the identification of a wide family of related proteins whose members are present in many bacteria and in all eukaryotic organisms analysed up to now (Miller 2006). There are nine members of the CLC family in humans. Seven CLC genes have been identified in each of the complete genome sequences of the model plants A. thaliana (AtClCa–AtClCg; Marmagne et al. 2007) and rice (Oryza sativa, OsClC1–OsClC7; Diédhiou & Golldack 2005). Combining studies of gene expression patterns, subcellular localization of these proteins and characterization of the phenotypes of knockout mutants, the first clues about the physiological roles of CLCs in plants have emerged.
Arabidopsis CLC genes are ubiquitously expressed all over the plant (Genevestigator reference expression database, https://www.genevestigator.ethz.ch/gv/index.jsp). More detailed studies of organ expression patterns by semi-quantitative RT-PCR and real-time PCR methods did not reveal strong differences between the seven genes, AtClCa and AtClCe showing the strongest and the weakest expression levels, respectively (D. Monachello & G. Ephritikhine 2007, personal communication). AtClCa transcript levels were upregulated by nitrate in both roots and shoots (Geelen et al. 2000). The question of the subcellular localization of AtClC proteins was addressed using protein fusions with the green fluorescent protein (GFP) transiently expressed in protoplasts from Arabidopsis cell suspensions (figure 1). Confocal microscopy observations of CLC–GFP expressing cells revealed that AtClCa was targeted to the vacuolar membrane (De Angeli et al. 2006), while AtClCe and AtClCf co-localized with chloroplast and Golgi markers, respectively (Marmagne et al. 2007). Western blot analyses using AtClC-specific antibodies on purified membrane protein fractions confirmed the tonoplast targeting of AtClCa and showed that AtClCe was specifically associated with thylakoid membranes. For AtClCf, a refined analysis combining different markers of Golgi vesicles showed that AtClCf is targeted mainly to the early cis-Golgi subcompartment, and to some extent to trans-Golgi cisternae (Marmagne et al. 2007). Using the same approaches, Von der Fecht-Bartenbach et al. (2007) demonstrated that AtClCd resides in the trans-Golgi network (TGN). The subcellular localization of AtClCb, AtClCc and AtClCg is still unknown. In rice, OsClC1 transcripts were found in leaves and roots and their abundance was regulated by salt stress (Diédhiou & Golldack 2005). OsClC1 and OsClC2 proteins, both closely related with AtClCc, were localized at the vacuolar membrane using both immunochemical detection methods and transient expression of GFP fusions (Nakamura et al. 2006).
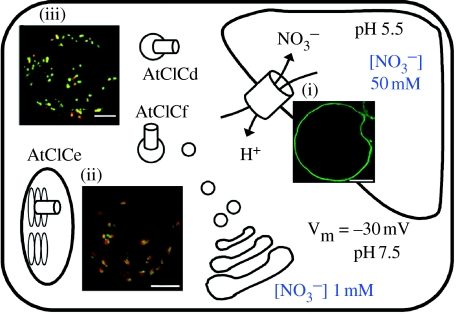
Figure 1.
Schematic representation of the subcellular localization of AtClC proteins. Using transient expression of CLC–GFP fusion proteins and confocal microscopy, combined with protein immunodetection by specific antibodies, AtClCa was localized in the vacuolar membrane (De Angeli et al. 2006), AtClCe in the thylakoid membrane (Marmagne et al. 2007), AtClCf mainly in cis-Golgi vesicles (Marmagne et al. 2007) and AtClCd in the trans-Golgi network (Von der Fecht-Bartenbach et al. 2007). The subcellular localization of AtClCb, AtClCc and AtClCg is still unknown and therefore not reported in the figure. Vm, electrical potential at the vacuolar membrane (Bertl et al. 1992). (i)–(iii) Laser-scanning images of GFP fluorescence of protoplasts from Arabidopsis cell suspensions transformed with AtClC–GFP constructs. (i) AtClCa–GFP (green fluorescence), white bar represents 16 μm; (ii) AtClCe–GFP (green fluorescence) partially co-localizes with chlorophyll fluorescence (red), white bar represents 16 μm; (iii) AtClCf––DsRed2 (red fluorescence) partially co-localizes with β-1,4 FucT–GFP (green fluorescence), white bar represents 8 μm.
Whereas loss-of-function mutants for OsClC1 and 2 genes exhibit reduced growth at all life stages (Nakamura et al. 2006), no developmental or growth phenotype was observed for AtClCa or AtClCe knockout mutant plants. However, detailed phenotypic analyses showed that clca-1 (Geelen et al. 2000) and clca-2 (D. Monachello & G. Ephritikhine 2007, personal communication) mutant plants have a nitrate concentration 50 per cent lower than that of wild-type in root and shoot tissues. The same plants did not display any difference in the contents of other anions such as sulphate or organic acids (Geelen et al. 2000). In agreement with the chloroplast localization of AtClCe, the clce mutants showed altered photosynthetic activity (Marmagne et al. 2007), but their nitrate content is also reduced as observed in clca mutants (D. Monachello & G. Ephritikhine 2007, personal communication). Quantitative trait loci analysis of nitrate storage in Arabidopsis combined with the physiological characterization of a knockout mutant in the AtClCc gene showed that the AtClCc protein was also involved in the regulation of nitrate levels in planta (Harada et al. 2004).
On one hand, these findings on the involvement of AtClC genes in nitrate metabolism and storage suggest that the corresponding proteins might mediate nitrate fluxes across plant cell membranes. On the other hand, the ability of some Arabidopsis and rice CLCs, upon expression in CLC-depleted yeast mutant cells, to rescue their altered growth phenotype (Hechenberger et al. 1996; Gaxiola et al. 1998; Nakamura et al. 2006; Marmagne et al. 2007) suggests that plant CLCs exert a similar function as the yeast CLC. Nevertheless, so far, all the experiments that aimed at demonstrating a transport activity of the AtClCa protein (as well as other plant ClCs) using heterologous expression systems, such as Xenopus oocytes, insect cells or mammalian cells, have failed. This has represented a strong limitation in the understanding of the role of ClC proteins at cellular and whole-plant levels. The localization of the AtClCa protein on the vacuolar membrane, a membrane amenable for in situ electrophysiology studies on isolated vacuoles, opened new perspectives to investigate the anion transport properties of AtClCa in its native membrane.
3. AtClCa, a nitrate/proton antiporter mediating nitrate accumulation in plant vacuoles
In patch-clamp experiments performed on isolated mesophyll vacuoles under bi-ionic conditions ( in the vacuolar lumen, Cl− on the cytosolic side), an outward rectifying anion current was identified (De Angeli et al. 2006). The current densities and reversal potentials measured on wild-type vacuoles are shown in table 1. Substituting the vacuolar with an equal amount of Cl− decreased the total current density and shifted the reversal potential to less negative values, indicating that the anion channel/transporter is highly selective for nitrate over chloride from the luminal side. The selectivity sequence, determined by exchanging external Cl− with various anions, was ∼I−>Br−>Cl−>>glutamate−.
Table 1.
Characteristics of anion currents measured by the patch-clamp technique on vacuoles isolated from wild-type or AtClCa knockout mutant plants (clca-1 and clca-2 alleles). (Average values±s.e. of current density (measured at +43 or −80 mV) and reversal potential (Erev) for various combinations of experimentally imposed concentrations of cytosolic and vacuolar anions. n.d., not determined.)
| genotype | cytosolic anion (mM) | vacuolar anion (mM) | current at +43 mV (pA pF−1) | Erev (mV) |
|---|---|---|---|---|
| wild-type | Cl− 19.2 | NO3− 200 | +8.7±1.1 | −43.3±1.4 |
| Cl− 19.2 | Cl− 200 | +1.1±0.1 | −4.0±6.0 | |
| clca-1 | Cl− 19.2 | 200 | +1.6±0.1 | −16.2±2.0 |
| clca-2 | Cl− 19.2 | 200 | +2.8±0.8 | −11.0±2.0 |
| current at −80 mV (pA pF−1) | ||||
| wild-type | malate2− 100 | malate2− 10 | +6.7±1.4 | n.d. |
| clca-2 | malate2− 100 | malate2− 10 | +6.8±1.9 | n.d. |
To test whether the nitrate selective current is mediated by AtClCa, vacuoles from two independent clca knockout lines were analysed. None of the mutant vacuoles displayed a current similar to the wild-type current (table 1). At +43 mV, the total current density was reduced by more than 60 per cent in clca-2 and by approximately 80 per cent in clca-1. In both mutant alleles, reversal potentials were different from wild-type, in agreement with the absence of a transport system in mutant vacuoles that would impose its equilibrium potential. The lack of AtClCa did not affect vacuolar malate currents (Hafke et al. 2003; Hurth et al. 2005) that were similar in wild-type and clca-2 mutant vacuoles (table 1), thus confirming that AtClCa specifically determines the nitrate current. At physiological membrane potentials (i.e. from −40 to −20 mV), AtClCa-mediated current is able to transport into the vacuole (negative current), when cytosolic [] is in a physiological range (4.2–19.2 mM). Nevertheless, there is a clear discrepancy between the measured reversal potential and the Nernst potential for , suggesting that other ions are transported by AtClCa. Previous studies showed that some members of the CLC family actually function as H+/Cl− antiporters (Accardi & Miller 2004; Picollo & Pusch 2005; Scheel et al. 2005). Monitoring reversal potentials of AtClCa current when cytosolic was changed in a fixed pH gradient, or when cytosolic pH varied in a fixed gradient, led to the conclusion that AtClCa functions as a antiporter (De Angeli et al. 2006) that is in agreement with the 2Cl−/1H+ ratio reported for CLCec-1 (Accardi & Miller 2004). Thus, AtClCa is the first anion/proton antiporter belonging to the CLC family, identified and functionally characterized in plant cells. Considering AtClCa within the plant cell context, with a gradient of 2 pH units (pHvac 5.5, pHcyt 7.5) and a transmembrane potential of −30 mV between the cytosol and the vacuole (figure 1), the exchange mechanism allows an accumulation of nitrate in the vacuole by a factor of 50, whereas a channel could only drive a threefold accumulation. Interestingly, the measured concentration gradient between vacuole and cytosol in A. thaliana mesophyll cells (Coockson et al. 2005) falls within the same range as that potentially built up by the AtClCa /H+ exchanger.
A transient transformation system of mesophyll protoplasts extracted from mutant plants was set up with a dual objective: (i) to demonstrate that the knockout mutants were functionally complemented by AtClCa cDNA, and (ii) to set up an expression system suitable for electrophysiological experiments to investigate AtClCa structure–function relationships. The AtClCa cDNA was cloned into the pFunct+Tag vector (Hosy et al. 2005) harbouring two independent cassettes controlled by two separate (but identical) EN50PMA promoters. One of the two cassettes is needed for the cloning of the gene of interest (AtClCa) while the other drives the expression of a soluble GFP serving as a transformation marker. Transformation by the AKT1 plasma membrane potassium channel cDNA was used as a negative control. Once the transformation was done, protoplasts were incubated for 12 hours at 18°C and the pFunct+Tag-transformed protoplasts were selected individually in the patch-clamp recording chamber on the basis of GFP fluorescence. The rate of transformed protoplasts varied from 25 to 50%. AtClCa-transformed clca-2 vacuoles displayed currents identical to those measured on wild-type vacuoles (figure 2) with both time-dependent and instantaneous currents and comparable reversal potential (−39.6±2.4 and −43±1.0 mV, in transformed and WT vacuoles, respectively). The measured current density was between 20 and 50 pA pF−1 at +43 mV, which is two to five times higher than wild-type current density (8.7±1.1 pA pF−1) in the same experimental conditions (table 1). This is probably due to the strong activity of the EN50PMA promoter compared with that of the endogenous AtClCa promoter. By contrast, vacuoles from the non-fluorescent clca-2 protoplasts (which do not express the AtClCa gene) or from protoplasts transformed with the AKT1 cDNA displayed clca-2-type anion currents, with current densities below 3 pA pF−1 at +43 mV (figure 2) and a reversal potential of −11 mV (table 1). These complementation experiments demonstrate that AtClCa cDNA is able to functionally complement knockout vacuoles by restoring an anion current equivalent to that measured in wild-type vacuoles. Since all trials to express AtClCa in heterologous systems have failed, this homologous expression system in the mutant clca-2 background appears as a powerful tool opening the way to structure–function studies of the AtClCa transporter via site-directed mutagenesis.
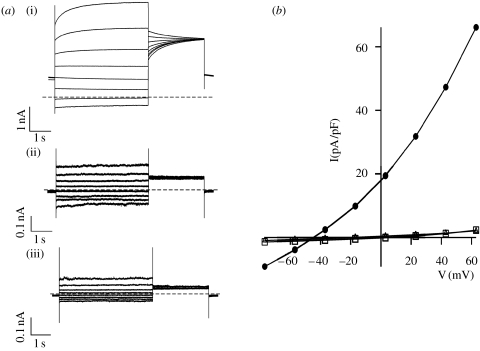
Figure 2.
Transient expression of AtClCa cDNA functionally complements clca-2 knockout mutant protoplasts. (a) Current traces recorded by the patch-clamp technique in the whole-vacuole configuration on vacuoles extracted from clca-2 mutant plants (i) transiently transformed with wild-type AtClCa (ii) transiently transformed with the plasma membrane potassium channel AKT1 or (iii) untransformed. The applied voltage is intended to be the cytoplasmic–lumenal potential (Bertl et al. 1992). Note that the current scale is 1 nA for AtClCa and 0.1 nA for AKT1 and untransformed. (b) Corresponding I/V curves (circles, AtClCa; squares, AKT1; and triangles, untransformed). In AtClCa-transformed vacuoles, the current density is dramatically larger than in the two negative controls. Cytosolic solution in mM: 7.5 BisTrisPropane, 15 HCl, 0.1 CaCl2, 2 MgCl2, 15 MES (pH=7). Vacuolar solution in mM: 100 BTP, 200 HNO3, 1 CaCl2, 5 MgCl2, 5 MES (pH=5.5). Osmotic pressures were adjusted with sorbitol to 640 mOsm and 590 mOsm for the cytosolic and vacuolar solutions, respectively.
4. Towards structure–function analyses of plant CLCs
The first question to be addressed concerns the molecular basis of the difference in selectivity observed between AtClCa (∼I−>Br−>Cl−>>glutamate−, De Angeli et al. 2006) and bacterial ClC transporters (SCN−>Cl−>Br−∼> for ClCec-1, Accardi & Miller 2004). The positions of nitrate and chloride in the two selectivity sequences are inverted, and in both cases the divalent anions are not transported. It has been shown that in ClCec-1 the coupling of the exchange reaction depends on the transported anions (Nguitragool & Miller 2006), with Cl− being more tightly coupled to protons than . Instead, in AtClCa, is the anion more tightly coupled to protons; this appears to be physiologically relevant since nitrate is the major anion in plant cells. The three-dimensional structure of ClCec-1 (Dutzler et al. 2002), together with protein sequence alignments, provide clues for finding the structural determinants of the selectivity of this protein. In ClCec-1, the amino acids of the selectivity filter, involved in the interaction with Cl− anions, are E148, S107, Y445 and F357. Sequence comparison with AtClCa shows that in equivalent positions the residues are E203, P160, Y564 and F480. The major difference between the two selectivity filters is the presence of a proline instead of a serine. From a physicochemical point of view, serine has a polar lateral chain, while proline is a non-polar amino acid presenting fixed ψ and Φ torsion angles in a protein structure. This proline–serine substitution could be responsible for the observed differences in anion selectivity. Thus it will be worthwhile to mutate this proline residue into a serine to see whether it could turn the nitrate selectivity of AtClCa into a Cl− selectivity.
The question of the structural frontier between H+/anion− antiporter and H+-gated anion channels, which has not been yet understood (reviewed in Miller 2006; Jentsch 2008; Zifarelli & Pusch 2008), represents another challenging issue. The first ClC that was demonstrated to function as an antiporter is the bacterial ClCec-1 (Accardi & Miller 2004), for which the crystallographic structure has been solved (Dutzler et al. 2002). In eukaryotic cells, the antiporter behaviour was found in endocellular mammalian proteins ClC-4, ClC-5 and ClC-7 (Picollo & Pusch 2005; Scheel et al. 2005; Graves et al. 2008) and in plant AtClCa (De Angeli et al. 2006), whereas ClC-0 and ClC-1 are H+-gated channels. In both channel and antiporter types, a glutamate residue is present in a position equivalent to position 148 in ClCec-1. In CLC channels, this glutamate residue is important for gating, whereas in exchangers the same residue is fundamental for the coupling between H+ and Cl−. It has been shown that CLC exchangers present an additional glutamate in a position equivalent to position 203 of CLCec-1, which is not present in the channel subtype (Accardi et al. 2005). Electrophysiological experiments have shown that the mutation of such glutamate abolishes the coupling with chloride (Accardi et al. 2005). Sequence comparison of AtClCa with ClCec-1 and other CLCs shows that glutamates are present in both equivalent positions (E203 and E270). The clca-2 expression system opens the door to site-directed mutagenesis of these residues too, to understand whether the coupling of H+/anions in AtClCa relies on the same structural determinants as those identified in bacterial and mammalian CLCs.
Several studies have addressed the question of the interaction of nucleotides with proteins of the CLC family, such as hClC-1 and hClC-5 (Bennetts et al. 2007; Meyer et al. 2007; Tseng et al. 2007; Zifarelli & Pusch 2008). On hClC-5, crystallographic and calorimetric studies demonstrated that the C-terminal domain of the protein is able to bind ATP, ADP and AMP (Wellhauser et al. 2006; Meyer et al. 2007), and that the interaction with ATP takes place at the interface of the two CBS (cystathionine-β-synthetase) motifs (Meyer et al. 2007). As all known eukaryotic CLCs, AtClCa possesses two CBS domains in its C-terminus that might as well bind intracellular nucleotides and exert a regulatory role on the transport activity of the protein. Interestingly, the orientation of AtClCa in the vacuolar membrane exposes the C-terminus containing the CBS domains to the bath solution (cytosolic side), thus enabling accurate studies of functional effects following from binding of cytosolic nucleotides to AtClCa. The understanding of such regulation is necessary to obtain an integrated view of the role of AtClCa in whole cell physiology. In particular, nucleotide modulation of AtClCa activity could enlighten a possible coordinated regulation with the vacuolar H+-ATPase.
It has been proposed that CLC proteins of endosomal membranes, such as mammalian ClC3−7, participate in the establishment and regulation of an acidic intra-organellar pH (Jentsch et al. 2005; Jentsch 2008). A very recent study, based on ion flux measurements on isolated lysosomes from rat liver and knockdown experiments in HeLa cells, demonstrated that ClC7 is a Cl−/H+ antiporter that plays an essential role in lysosomal acidification (Graves et al. 2008). In yeast, the disruption of the only CLC homologue, gef1p, induces a defect in mitochondrial function that results from an alteration in intra-Golgi pH regulation (Gaxiola et al. 1998). Interestingly, the expression of the plant AtClCd and AtClCf proteins, both residing in Golgi vesicles, complements the gef1 yeast mutant phenotype (Hechenberger et al. 1996; Gaxiola et al. 1998; Marmagne et al. 2007), suggesting functional similarity with gef1p. The localization of AtClCd coincides with that of the V-type ATPase VHA1a, in agreement with a functional link between these two proteins (Von der Fecht-Bartenbach et al. 2007). A possible role in proton gradient maintenance remains to be tested for AtClCf which also resides in Golgi vesicles, or for AtClCe present in thylakoid membranes. On a more general basis, it would be interesting to test the hypothesis that plant CLCs participate in the maintenance of subcellular proton gradients by directly measuring organelle pH in AtClC knockout mutants. As pH is an intermediate in many signalling pathways in plant cells, a possible connection between the functions of anion channel/transporter in signalling and their role in pH control would appear physiologically relevant.
Acknowledgments
This work was supported by the EU-RTN Project VaTEP ‘Vacuolar transport equipment for growth regulation in plants’ contract MRTN-CT-2006 035833 and the CNR–CNRS bilateral project ‘Biophysical properties of the AtClCa antiporter in A. thaliana’.
Footnotes
One contribution of 16 to a Discussion Meeting Issue ‘Membrane transport in flux: the ambiguous interface between channels and pumps’.
References
- Accardi A., Miller C. Secondary active transport mediated by a prokaryotic homologue of ClC Cl− channels. Nature. 2004;427:803–807. doi: 10.1038/nature02314. doi:10.1038/nature02314 [DOI] [PubMed] [Google Scholar]
- Accardi A., Walden M., Nguitragool W., Jayaram H., Williams C., Miller C. Separate ion pathways in a Cl−/H+ exchanger. J. Gen. Physiol. 2005;126:563–570. doi: 10.1085/jgp.200509417. doi:10.1085/jgp.200509417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbier-Brygoo H., Vinauger M., Colcombet J., Ephritikhine G., Frachisse J., Maurel C. Anion channels in higher plants: functional characterization, molecular structure and physiological role. Biochim. Biophys. Acta. 2000;1465:199–218. doi: 10.1016/s0005-2736(00)00139-5. doi:10.1016/S0005-2736(00)00139-5 [DOI] [PubMed] [Google Scholar]
- Bennetts B., Parker M.W., Cromer B.A. Inhibition of skeletal muscle ClC-1 chloride channels by low intracellular pH and ATP. J. Biol. Chem. 2007;282:32 780–32 791. doi: 10.1074/jbc.M703259200. doi:10.1074/jbc.M703259200 [DOI] [PubMed] [Google Scholar]
- Bertl A., et al. Electrical measurements on endomembranes. Science. 1992;258:873–874. doi: 10.1126/science.1439795. doi:10.1126/science.1439795 [DOI] [PubMed] [Google Scholar]
- Blumwald E., Poole R.J. Nitrate storage and retrival in Beta vulgaris: effects of nitrate and chloride on proton gradients in tonoplast vesicles. Proc. Natl Acad. Sci. USA. 1985;82:3683–3687. doi: 10.1073/pnas.82.11.3683. doi:10.1073/pnas.82.11.3683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coockson S.J., Williams L.E., Miller A.J. Light-dark changes in cytosolic nitrate pools depend on nitrate reductase activity in Arabidopsis leaf cells. Plant Physiol. 2005;138:1097–1105. doi: 10.1104/pp.105.062349. doi:10.1104/pp.105.062349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Angeli A., Monachello D., Ephritikhine G., Frachisse J.M., Thomine S., Gambale F., Barbier-Brygoo H. The nitrate/proton antiporter AtCLCa mediates nitrate accumulation in plant vacuoles. Nature. 2006;442:939–942. doi: 10.1038/nature05013. doi:10.1038/nature05013 [DOI] [PubMed] [Google Scholar]
- De Angeli A., Thomine S., Frachisse J.M., Ephritikhine G., Gambale F., Barbier-Brygoo H. Anion channels and transporters in plant cell membranes. FEBS Lett. 2007;581:2367–2374. doi: 10.1016/j.febslet.2007.04.003. doi:10.1016/j.febslet.2007.04.003 [DOI] [PubMed] [Google Scholar]
- Diédhiou C.J., Golldack D. Salt-dependent regulation of chloride channel transcripts in rice. Plant Sci. 2005;170:793–800. doi:10.1016/j.plantsci.2005.11.014 [Google Scholar]
- Dutzler R., Campbell E.B., Cadene M., Chait B.T., MacKinnon R. X-ray structure of a ClC chloride channel at 3.0 Å reveals the molecular basis of anion selectivity. Nature. 2002;415:287–294. doi: 10.1038/415287a. doi:10.1038/415287a [DOI] [PubMed] [Google Scholar]
- Gaxiola R.A., Yuan D.S., Klausner R.D., Fink G.R. The yeast CLC chloride channel functions in cation homeostasis. Proc. Natl Acad. Sci. USA. 1998;95:4046–4050. doi: 10.1073/pnas.95.7.4046. doi:10.1073/pnas.95.7.4046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geelen D., Lurin C., Bouchez D., Frachisse J.M., Lelievre F., Courtial B., Barbier-Brygoo H., Maurel C. Disruption of putative anion channel gene AtCLC-a in Arabidopsis suggests a role in the regulation of nitrate content. Plant J. 2000;21:259–267. doi: 10.1046/j.1365-313x.2000.00680.x. doi:10.1046/j.1365-313x.2000.00680.x [DOI] [PubMed] [Google Scholar]
- Graves A.R., Curran P.K., Smith C.L., Mindell J.A. The Cl−/H+ antiporter ClC-7 is the primary chloride permeation pathway in lysosomes. Nature. 2008;453:788–792. doi: 10.1038/nature06907. doi:10.1038/nature06907 [DOI] [PubMed] [Google Scholar]
- Hafke J.B., Hafke Y., Smith J.A., Lüttge U., Thiel G. Vacuolar malate uptake is mediated by an anion-selective inward rectifier. Plant J. 2003;35:116–128. doi: 10.1046/j.1365-313x.2003.01781.x. doi:10.1046/j.1365-313X.2003.01781.x [DOI] [PubMed] [Google Scholar]
- Harada H., Kuromori T., Hirayama T., Shinozaki K., Leigh R.A. Quantitative trait loci analysis of nitrate storage in Arabidopsis leading to an investigation of the contribution of the anion channel gene, AtCLC-c, to variation in nitrate levels. J. Exp. Bot. 2004;55:2005–2014. doi: 10.1093/jxb/erh224. doi:10.1093/jxb/erh224 [DOI] [PubMed] [Google Scholar]
- Hechenberger M., Schwappach B., Fischer W.N., Frommer W.B., Jentsch T.J., Steinmeyer K. A family of putative chloride channels from Arabidopsis and functional complementation of a yeast strain with a CLC gene disruption. J. Biol. Chem. 1996;271:33 632–33 638. doi: 10.1074/jbc.271.52.33632. doi:10.1074/jbc.271.52.33632 [DOI] [PubMed] [Google Scholar]
- Hosy E., Buby Y., Véry A.-A., Costa A., Sentenac H., Thibaud J.-B. A procedure for localisation and electrophysiological characterisation for ion channels heterologously expressed in a plant context. Plant Methods. 2005;19:1–14. doi: 10.1186/1746-4811-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurth A., Suh S., Kretzscmar T., Geis T., Bregante M., Gambale F., Martinoia E., Neuhaus E. Impaired pH homeostasis in Arabidopsis lacking the vacuolar dicarboxylate transporter and analysis of carboxylic acid transport across the tonoplast. Plant Physiol. 2005;137:901–910. doi: 10.1104/pp.104.058453. doi:10.1104/pp.104.058453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch T.J. Chloride and the endosomal–lysosomal pathway: emerging role of CLC chloride transporters. J. Physiol. 2008;578:633–640. doi: 10.1113/jphysiol.2006.124719. doi:10.1113/jphysiol.2006.124719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch T.J., Steinmeyer K., Schwarz G. Primary structure of Torpedo marmorata chloride channel isolated by expression cloning in Xenopus oocytes. Nature. 1990;348:510–514. doi: 10.1038/348510a0. doi:10.1038/348510a0 [DOI] [PubMed] [Google Scholar]
- Jentsch T.J., Poet M., Fuhrmann J.C., Zdebik A.A. Physiological functions of CLC Cl− channels gleaned from human genetic disease and mouse models. Annu. Rev. Physiol. 2005;67:779–807. doi: 10.1146/annurev.physiol.67.032003.153245. doi:10.1146/annurev.physiol.67.032003.153245 [DOI] [PubMed] [Google Scholar]
- Kovermann P., Meyer S., Hortensteiner S., Picco C., Scholz-Starke J., Ravera S., Lee Y., Martinoia E. The Arabidopsis vacuolar malate channel is a member of the ALMT family. Plant J. 2007;52:1169–1180. doi: 10.1111/j.1365-313X.2007.03367.x. [DOI] [PubMed] [Google Scholar]
- Lurin C., Geelen D., Barbier-Brygoo H., Guern J., Maurel C. Cloning and functional expression of a plant voltage-dependent chloride channel. Plant Cell. 1996;8:701–711. doi: 10.1105/tpc.8.4.701. doi:10.1105/tpc.8.4.701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmagne A., et al. Two members of the Arabidopsis CLC (chloride channel) family, AtCLCe and AtCLCf, are associated with thylakoid and Golgi membranes, respectively. J. Exp. Bot. 2007;58:3385–3393. doi: 10.1093/jxb/erm187. doi:10.1093/jxb/erm187 [DOI] [PubMed] [Google Scholar]
- Martinoia E., Ratajcsak R. Transport of organic molecules across the tonoplast. Adv. Bot. Res. 1997;25:365–400. doi:10.1016/S0065-2296(08)60158-5 [Google Scholar]
- Martinoia E., Wiemken A. Vacuoles as storage compartments for nitrate in barley leaves. Nature. 1981;289:292–294. doi:10.1038/289292a0 [Google Scholar]
- Meyer S., Savaresi S., Forster I.C., Dutzler R. Nucleotide recognition by the cytoplasmic domain of the human chloride transporter ClC-5. Nat. Struct. Mol. Biol. 2007;14:60–67. doi: 10.1038/nsmb1188. doi:10.1038/nsmb1188 [DOI] [PubMed] [Google Scholar]
- Miller C. ClC chloride channels viewed through a transporter lens. Nature. 2006;440:484–489. doi: 10.1038/nature04713. doi:10.1038/nature04713 [DOI] [PubMed] [Google Scholar]
- Miller A.J., Smith J.S. Cytosolic nitrate ion homeostasis: could it have a role in sensing nitrogen status? Ann. Bot. 2007;101:1590–1602. doi: 10.1093/aob/mcm313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C., White M.M. A voltage-dependent chloride conductance channel from Torpedo electroplax membrane. Ann. N Y Acad. Sci. 1980;341:534–551. doi: 10.1111/j.1749-6632.1980.tb47197.x. doi:10.1111/j.1749-6632.1980.tb47197.x [DOI] [PubMed] [Google Scholar]
- Nakamura A., Fukuda A., Sakai S., Tanaka Y. Molecular cloning, functional expression and subcellular localization of two putative vacuolar voltage-gated chloride channels in rice (Oryza sativa L.) Plant Cell. Physiol. 2006;47:32–42. doi: 10.1093/pcp/pci220. doi:10.1093/pcp/pci220 [DOI] [PubMed] [Google Scholar]
- Nguitragool W., Miller C. Uncoupling of a CLC Cl−/H+ exchange transporter by polyatomic anions. J. Mol. Biol. 2006;362:682–690. doi: 10.1016/j.jmb.2006.07.006. doi:10.1016/j.jmb.2006.07.006 [DOI] [PubMed] [Google Scholar]
- Picollo A., Pusch M. Chloride/proton antiporter activity of mammalian CLC proteins ClC-4 and ClC-5. Nature. 2005;436:420–423. doi: 10.1038/nature03720. doi:10.1038/nature03720 [DOI] [PubMed] [Google Scholar]
- Scheel O., Zdebik A.A., Lourdel S., Jentsch T.J. Voltage-dependent electrogenic chloride/proton exchange by endosomal CLC proteins. Nature. 2005;436:424–427. doi: 10.1038/nature03860. doi:10.1038/nature03860 [DOI] [PubMed] [Google Scholar]
- Schumaker K.S., Sze H. Decrease of pH gradients in tonoplast vesicles by NO3− and Cl−: evidence for H+-coupled anion transport. Plant Physiol. 1986;83:490–496. doi: 10.1104/pp.83.3.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsay Y.F., Chiu C.C., Tsai C.B., Ho C.H., Hsu P.K. Nitrate transporters and peptide transporters. FEBS Lett. 2007;581:2290–2300. doi: 10.1016/j.febslet.2007.04.047. doi:10.1016/j.febslet.2007.04.047 [DOI] [PubMed] [Google Scholar]
- Tseng P.Y., Bennetts B., Chen T.Y. Cytoplasmic ATP inhibition of CLC-1 is enhanced by low pH. J. Gen. Physiol. 2007;130:217–221. doi: 10.1085/jgp.200709817. doi:10.1085/jgp.200709817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von der Fecht-Bartenbach J., Bogner M., Krebs M., Stierhof Y.-D., Schumacher K., Ludewig U. Function of the anion transporter AtClC-d in the trans Golgi network. Plant J. 2007;50:466–474. doi: 10.1111/j.1365-313X.2007.03061.x. doi:10.1111/j.1365-313X.2007.03061.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellhauser L., Kuo H.H., Stratford F.L., Ramjeesingh M., Huan L.J., Luong W., Li C., Deber C.M., Bear C.E. Nucleotides bind to the C-terminus of ClC-5. Biochem. J. 2006;398:289–294. doi: 10.1042/BJ20060142. doi:10.1042/BJ20060142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zifarelli G., Pusch M. The muscle chloride channel ClC-1 is not directly regulated by intracellular ATP. J. Gen. Physiol. 2008;13:109–116. doi: 10.1085/jgp.200709899. doi:10.1085/jgp.200709899 [DOI] [PMC free article] [PubMed] [Google Scholar]