Abstract
SUR1 is an ATP-binding cassette (ABC) transporter with a novel function. In contrast to other ABC proteins, it serves as the regulatory subunit of an ion channel. The ATP-sensitive (KATP) channel is an octameric complex of four pore-forming Kir6.2 subunits and four regulatory SUR1 subunits, and it links cell metabolism to electrical activity in many cell types. ATPase activity at the nucleotide-binding domains of SUR results in an increase in KATP channel open probability. Conversely, ATP binding to Kir6.2 closes the channel. Metabolic regulation is achieved by the balance between these two opposing effects. Precisely how SUR1 talks to Kir6.2 remains unclear, but recent studies have identified some residues and domains that are involved in both physical and functional interactions between the two proteins. The importance of these interactions is exemplified by the fact that impaired regulation of Kir6.2 by SUR1 results in human disease, with loss-of-function SUR1 mutations causing congenital hyperinsulinism and gain-of-function SUR1 mutations leading to neonatal diabetes. This paper reviews recent data on the regulation of Kir6.2 by SUR1 and considers the molecular mechanisms by which SUR1 mutations produce disease.
Keywords: ATP-binding cassette (ABC) transporter, sulphonylurea receptor, KATP channel, insulin secretion, diabetes
1. SUR1 is an ATP-binding cassette protein
ATP-binding cassette (ABC) transporters constitute a large group of membrane transport proteins (Dean 2002), which use the energy of ATP hydrolysis to mediate a variety of functions. In eukaryotes, ABC proteins are primarily involved in the transport of substrates out of the cell or into intracellular organelles, but in prokaryotes they also serve as importers (Hollenstein et al. 2007). The advent of high-resolution structures of various bacterial ABC proteins in recent years has suggested a mechanism by which ATP hydrolysis at the nucleotide-binding domains (NBDs) of ABC proteins is coupled to conformational changes that drive substrate transport (Locher 2009).
The sulphonylurea receptors SUR1 and SUR2 (ABCC8: GenBank accession number NM_000352.3 and ABCC9: GenBank accession number NM_005691.2 (SUR2A) and NM_020297.2 (SUR2B)) are unique among ABC transporters in that they serve as ion channel regulators. They belong to the ABCC subfamily of ABC proteins, which also includes the cystic fibrosis transmembrane conductance regulator (CFTR, ABCC7) and the multidrug resistance-associated protein 1 (MRP1, ABCC1). Despite their phylogenic similarity, these proteins have very diverse functions. MRP1 is a multidrug exporter of glutathione conjugates employing ATP hydrolysis to drive substrate transport, as in classical ABC transporters (Deeley et al. 2006). However, CFTR operates as an ion channel gated by ATP hydrolysis (Muallem & Vergani 2009), and SUR is an ion channel regulator in which ATP hydrolysis modulates the gating of a separate Kir6.2 channel pore (Nichols 2006).
All three types of protein are of clinical importance: MRP1 is overexpressed in certain tumour cells, conferring resistance to multiple anticancer drugs (Cole & Deeley 1998; Deeley et al. 2006); more than 1000 different mutations in the CFTR gene have been linked with cystic fibrosis (Gadsby et al. 2006); and mutations in SUR1 can cause insulin secretion disorders such as neonatal diabetes and hyperinsulinism (HI) (Nichols 2006; Ashcroft 2007). This review focuses on how SUR1 regulates the activity of Kir6.2, and how mutations in SUR1 that disrupt this regulation lead to disease.
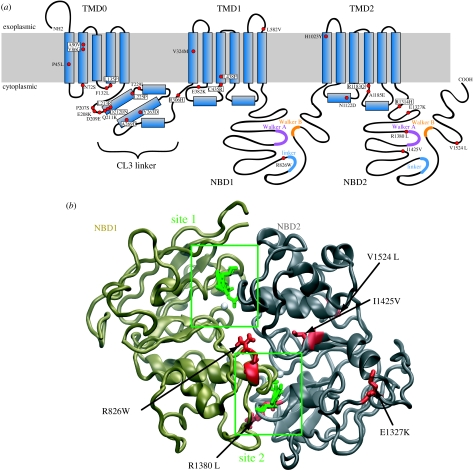
2. Structure of SUR1
ABC transporters are built in modular form. All possess four characteristic structural domains: two NBDs and two transmembrane domains (TMD1 and TMD2) each containing 6–10 transmembrane α-helices. SUR is unusual in having an additional set of five N-terminal transmembrane helices (TMD0) that is connected to TMD1 by a long cytosolic loop known as the CL3 linker (figure 1a). It shares this feature with some of the multidrug resistance proteins of the ABCC family (MRP1–3 and MRP6–7), but not with CFTR. It seems reasonable to postulate that TMD0 was once a separate protein which during evolution has been hijacked by SUR1 to serve a different function.
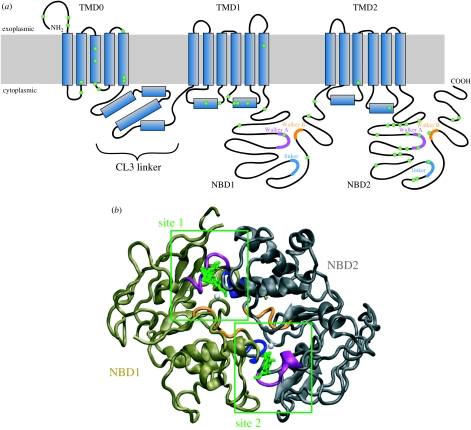
Figure 1.
SUR1 structure. (a) Membrane topology of SUR1. Residues mutated in HI are shown in green. The Walker A motifs are shown in purple, the Walker B motifs in orange and the ABC signature sequence (linker) motif in blue. (b) Homology model of the SUR1 NBD dimer. NBD1 is coloured bronze and NBD2 grey. Conserved motifs are colour coded as in (a). ATP is shown in green and Mg2+ in white.
Similar to the NBDs of other ABC proteins, those of SUR possess several highly conserved motifs (figure 1a,b). These include the Walker A and B motifs and an intervening linker known as the ABC signature sequence. Additional conserved residues include invariant glutamine (Q-loop), histidine (H-loop), aspartate (D-loop) and aromatic (A-loop) residues. The NBDs associate in a head-to-tail arrangement to form two nucleotide-binding sites, in which the nucleotide is sandwiched between the Walker A and B motifs of one NBD and the linker of the opposite NBD (Hollenstein et al. 2007). Thus, site 1 contains the Walker A and B motifs of NBD1 and the linker of NBD2 (figure 1b). Interestingly, members of the human ABCC subfamily show significant asymmetry in their NBDs: whereas all NBD2s have an Asp–Glu pair in their Walker B motif, virtually all NBD1s feature Asp–Asp. This is significant because the Walker B glutamate has been implicated in the catalytic activity of the NBDs (Linton & Higgins 2007). Furthermore, although the linker sequence (LSGGQ) of NBD1 is perfectly conserved, that of NBD2 is not. Thus, site 2 is better conserved than site 1 and more similar to consensus NBDs.
A further peculiarity of all ABCC NBDs except those of SUR is a 13-residue deletion between the NBD1 Walker A motif and the Q-loop (Ramaen et al. 2006). By contrast, SUR1 and SUR2 feature additional insertions of 13 and 4 residues, respectively. In the crystal structure of Sav1866, the 13 residues deleted in other ABCC proteins contact the coupling helix of the opposing TMD (Dawson & Locher 2006); this is presumably also the case for SUR.
These structural asymmetries in the nucleotide-binding sites correlate with functional differences. Unlike typical ABC transporters, the NBDs of many ABCC proteins differ in their ATPase activity. Thus, site 2 of SUR1 (which more closely resembles that of bacterial ABC transporters) has greater ATPase activity than site 1 (Matsuo et al. 1999; de Wet et al. 2007a). Furthermore, it appears that it is occupancy of site 2 by MgADP that leads to changes in KATP channel activity (Reimann et al. 2000; Zingman et al. 2001).
No high-resolution structure yet exists for SUR1, and the lack of any ABCC high-resolution structures means that there are no suitable templates for homology modelling of the complete protein. However, several models of the NBDs, or of SUR1 lacking TMD0 and CL3, have been generated, based on the crystal structures of other ABC proteins (Campbell et al. 2003; Babenko 2008; de Wet et al. 2008). The structures of Sav1866 (Dawson & Locher 2006, 2007) and MsbA (Ward et al. 2007) reveal a domain-swapped topology in which the NBD of one monomer contacts the TMDs of the other monomer. If this topology also exists in SUR1, it would position NBD1 in contact with the cytosolic loop 7 of TMD2. This loop is thought to contribute to sulphonylurea binding (Ashfield et al. 1999) and therefore presumably lies in close proximity to both TMD0 and the N-terminus of Kir6.2, which are also involved in sulphonylurea binding (Hansen et al. 2005; Vila-Carriles et al. 2007).
3. Structure of the KATP channel
SUR1 is unique among ABC proteins in that it serves as a channel regulator, forming a tightly associated octameric KATP channel complex in which four Kir6.2 subunits form a central pore surrounded by four SUR1 subunits (Clement et al. 1997; Mikhailov et al. 2005). Gel filtration of purified SUR1 indicates that even in the absence of Kir6.2 the protein assembles as a tetramer (de Wet et al. 2007a). Likewise, the NBDs of SUR1 associate in ring-like structures of approximately eight monomers, even when Kir6.2 or the TMDs of SUR1 are not present (de Wet et al. 2007a). This suggests that SUR1 possesses some intrinsic capacity for stable association which may contribute to the formation of the octameric KATP channel complex; in other words, SUR1 does not simply assemble on a Kir6.2 scaffold. Interestingly, a human multidrug transporter of the ABCG subfamily (ABCG2) also purifies as a tetramer, with a hole in its centre (McDevitt et al. 2006).
Currently, the only published structure of the KATP channel complex is a cryonegatively stained electron microscopy map at 18 Å resolution. This shows a tightly packed complex approximately 18 nm in maximal diameter and 13 nm in height (Mikhailov et al. 2005). Obtaining high-resolution structural information on both the individual KATP subunits and the entire KATP channel complex is now essential, in order to better equate structure and function.
4. Regulation of Kir6.2 by SUR1, and vice versa
Kir6.2 is unable to reach the surface membrane in the absence of SUR1, owing to an endoplasmic reticulum retention tag (RKR) in the C-terminus of the protein that is screened by SUR1 (Zerangue et al. 1999). This makes it difficult to determine which KATP channel properties are intrinsic to Kir6.2 and which are conferred by SUR1. However, mutation of the RKR motif, or its deletion by truncation of Kir6.2 at residue 355 (Kir6.2ΔC), enables independent surface expression of Kir6.2 (Tucker et al. 1997; Zerangue et al. 1999). This allows the functional effects of ATP on Kir6.2 to be assessed in the absence of SUR1 (Tucker et al. 1997).
Such studies have shown that SUR1 has multiple effects on Kir6.2 (Proks & Ashcroft 1997; Tucker et al. 1997; Nichols 2006). It enhances the open probability (Po) from approximately 0.1 to 0.4. It increases the channel ATP sensitivity 10-fold, the ATP concentration required to half-maximally inhibit the channel (IC50) decreasing from approximately 100 μM to about 10 μM. It confers sensitivity to activation by Mg-nucleotides such as MgATP and MgADP. It also endows the channel with sensitivity to therapeutic drugs, which bind directly to SUR1 to modulate KATP channel activity: for example, sulphonylureas (e.g. glibenclamide, tolbutamide) inhibit and K-channel openers (e.g. diazoxide) activate the channel.
Physiologically, the KATP channel is regulated by changes in cytosolic adenine nucleotides, which thereby couple cell metabolism to channel activity. Thus, a key issue is to obtain a detailed understanding of the mechanism underlying this regulation. Because ATP binding to SUR1 requires Mg2+ whereas that to Kir6.2 does not (Gribble et al. 1998), the effects of ATP on Kir6.2 can be isolated by using Mg-free solutions. A more difficult task is to determine the effects of interactions of Mg-nucleotides with the NBDs of SUR1. One way to do this is to take advantage of mutations that abolish ATP binding to Kir6.2 without affecting any other channel properties: for example, G334D (Drain et al. 1998).
There is also evidence that Kir6.2 affects SUR1 function. One of the canonical functions of ABC proteins is that they hydrolyse MgATP. This is also true of SUR1 (Matsuo et al. 1999; Masia et al. 2005; de Wet et al. 2007a). However, the Km for ATP hydrolysis is increased when Kir6.2 is present (from 0.1 to 0.3 mM for SUR1 and KATP, respectively), indicating a lower affinity for the KATP channel complex (Mikhailov et al. 2005; de Wet et al. 2007a). The turnover rate of the purified KATP channel complex is also approximately 10-fold higher than that of purified SUR1. The activity of other ABC transporters, including the closely related MRP1 (Mao et al. 1999), is stimulated by their substrates, and it is possible that the mechanism by which Kir6.2 enhance the ATPase activity of SUR1 resembles this substrate activation. Interestingly, K-channel openers enhance the ATPase activity of SUR2A in cardiac membranes (Bienengraeber et al. 2000). However, we have not been able to observe modulation of the ATPase activity of purified SUR1 by either diazoxide or glibenclamide (H. de Wet and F.M. Ashcroft, unpublished).
5. How does SUR1 talk to Kir6.2?
As outlined above, SUR1 has many regulatory effects on Kir6.2 (and vice versa). Much uncertainty, however, still surrounds the molecular mechanisms underlying the interactions between these two proteins. The picture is complicated by the fact that there are several sites involved in both proteins and that different sites have been associated with different effects on channel function.
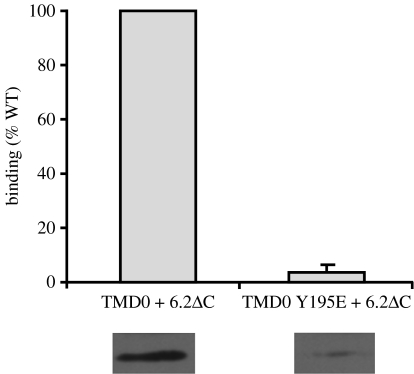
Physical interactions between SUR1 and Kir6.2 have been demonstrated both in co-immunoprecipitation studies (Lorenz et al. 1998; Chan et al. 2003) and by the requirement for both proteins for surface expression (Zerangue et al. 1999). Several regions of SUR1 and Kir6.2 appear to be involved. For example, interaction between the TMs of SUR1 and the first TM of Kir6.2 (Schwappach et al. 2000) are required for membrane trafficking, as are interactions of TMD0 with Kir6.2 (Chan et al. 2003). Co-immunoprecipitation (but not necessarily surface trafficking) is abolished by mutation of F132L in CL2 (Proks et al. 2006a) and of Y195E at the start of CL3 (figure 2).
Figure 2.
Binding of TMD0 to Kir6.2ΔC—effect of the Y195E mutation. Co-immunoprecipitation from oocytes of FLAG-tagged TMD0 by HA-tagged Kir6.2. Oocytes were injected with cRNA for the relevant constructs and lysed after 2 days of incubation. Anti-HA antibodies were used to immunoprecipitate expressed Kir6.2ΔC. Precipitates were resolved on SDS-PAGE and transferred to nitrocellulose by western blotting. TMD0 bound to Kir6.2 was detected with anti-FLAG antibody. The extent of binding was quantified with densitometry and normalized to expression levels of both Kir6.2ΔC and TMD0. Data are mean±s.e.m. of three experiments. Bands below are typical western blot results.
TMD0 and CL3 of SUR1 are sufficient to regulate the intrinsic (ligand-independent) gating of Kir6.2, as demonstrated by the fact that the coexpression of Kir6.2ΔC with TMD0 modulates gating, increasing the channel open probability (Po) from approximately 0.1 to approximately 0.6 (Babenko & Bryan 2003; Proks et al. 2007). TMD0 is also responsible for the different kinetics of channels containing different SUR isoforms (Babenko et al. 1999). There are clearly several different interactions between Kir6.2 and TMD0/CL3 that mediate the modulation of Kir6.2 gating by SUR1. For example, the F132L mutation in SUR1 disrupts the physical binding of Kir6.2 and TMD0 (Proks et al. 2007). However, it also increases Po. This indicates that the F132L mutation must disrupt an inhibitory interaction between Kir6.2 and TMD0, leaving a stimulatory interaction intact. Similar findings have been reported for CL3: coexpression of Kir6.2ΔC with TMD0 plus increasing lengths of CL3 first increases and then decreases Po (Babenko & Bryan 2003). This implies that there are multiple binding sites within CL3 that have different effects on channel gating. Whether or not Mg-nucleotides and/or drugs that interact with SUR1 also mediate their effects on KATP channel Po via interaction of TMD0/CL3 and Kir6.2 is unknown.
There also appear to be many sites on Kir6.2 that interact with SUR1. Deletion of as few as five residues in the N-terminus of Kir6.2 enhances the intrinsic Po, but only in the presence of SUR1, suggesting that these residues may interact with SUR1 (Reimann et al. 1999; Koster et al. 2000). Single-point mutations in both the N-terminus (F35V, Q52R) and the C-terminus (Y330C) of Kir6.2 have the same effect (Tammaro et al. 2005, 2006; Proks et al. 2006b). These residues lie close to one another in a structural model of Kir6.2 (Haider et al. 2005), which raises the possibility that these regions of Kir6.2 interact with SUR1.
6. When regulation fails: SUR1 mutations and disease
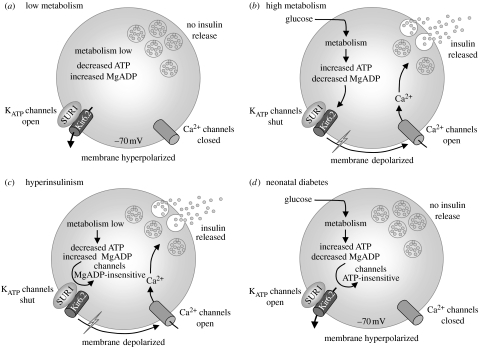
The importance of SUR1 as a regulator of KATP channel activity is exemplified by the fact that loss- and gain-of-function mutations result in congenital HI and neonatal diabetes, respectively (Ashcroft 2005, 2007; Gloyn et al. 2006). KATP channels play a key role in insulin secretion both in response to glucose, the main physiological stimulus, and to sulphonylurea drugs that are used to treat type 2 diabetes (figure 3a,b). Loss-of-function mutations reduce KATP channel activity, producing a persistent membrane depolarization that leads to the activation of voltage-gated Ca2+ influx and continuous insulin secretion, irrespective of the blood glucose level (figure 3c). Conversely, gain-of-function mutations prevent the channel from closing in response to metabolically generated changes in adenine nucleotides. Thus the β-cell remains hyperpolarized even when blood glucose levels rise, thereby keeping voltage-gated Ca2+ channels closed and preventing Ca2+ influx and insulin secretion (figure 3d).
Figure 3.
Role of KATP channels in insulin secretion. (a) When metabolism is low, KATP channels are open, keeping the membrane hyperpolarized and voltage-gated Ca2+ channels closed, so that [Ca2+]i remains low and insulin secretion is prevented. (b) When metabolism increases, ATP increases and MgADP falls, closing KATP channels. This triggers depolarization of the β-cell, opening voltage-gated Ca2+ channels, and initiating Ca2+ influx and insulin release. (c) Loss-of-function mutations in SUR1 cause HI by producing permanent KATP channel closure, continuous membrane depolarization and Ca2+ influx, and thus persistent insulin secretion. (d) Gain-of-function mutations in SUR1 result in a failure of KATP channel closure when metabolism rises, so that the β-cell remains hyperpolarized even when blood glucose levels are elevated, keeping voltage-gated Ca2+ channels closed and preventing insulin secretion. This leads to diabetes.
(a) Hyperinsulinism of infancy
Congenital HI is characterized by abnormally high levels of insulin secretion despite severe hypoglycaemia (Dunne et al. 2004). It presents at birth or in early childhood and is a potentially serious condition as lack of treatment can result in irreversible brain damage. In most populations, the disease affects approximately 1 in 50 000 live births but the incidence is higher in some communities (Gloyn et al. 2006). Although HI is a heterogeneous disorder, it is most commonly caused by SUR1 mutations, and almost 100 have been reported (Gloyn et al. 2006; figure 4). There is no precise genotype–phenotype correlation and the same mutation can cause HI with different degrees of severity in different people.
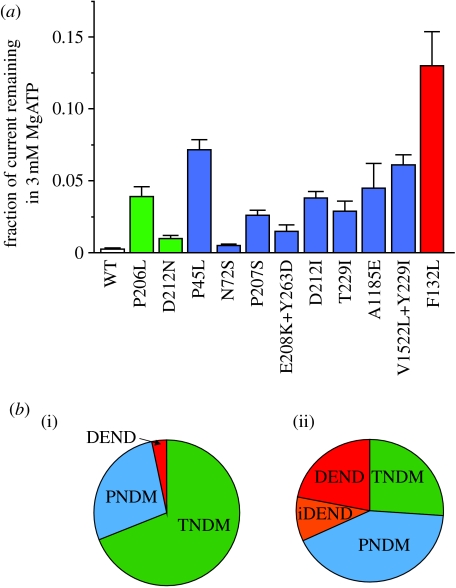
Figure 4.
SUR1 mutations enhance KATP currents and cause neonatal diabetes. (a) Disease severity correlates with the extent of unblocked KATP current measured in inside-out patches at 3 mM MgATP. The number of patches varies from five to nine. Data are taken from Proks et al. (2006a), Ellard et al. (2007) and Shield et al. (2008). (b) Relative incidence of ND subtypes caused by (i) SUR1 and (ii) Kir6.2 mutations.
The functional effects of HI mutations have only been studied in detail in a few cases (Ashcroft 2005). One class of mutations leads to a reduction or total loss of KATP channels in the plasma membrane due to abnormalities in gene expression, protein synthesis, maturation, assembly or membrane trafficking (e.g. Taschenberger et al. 2002). Such mutations are distributed throughout the protein (figure 1). Interestingly, surface expression of a subset of trafficking mutants within TMD0 could be rescued by sulphonylureas such as glibenclamide, suggesting that the drug may act as a chaperone to ensure the correct folding of the protein (Yan et al. 2004, 2006). Following glibenclamide removal, the channels were activated by metabolic inhibition, raising the possibility that they might be able to respond to hypoglycaemia in vivo.
A second class of HI mutations do not affect KATP channel density but instead impair the ability of SUR1 to regulate channel activity: in particular, they reduce or abolish channel activation by MgADP and/or MgATP (e.g. Huopio et al. 2002). Consequently, KATP channels are always closed, independent of the metabolic state of the cell. These mutations tend to cluster within NBD2 and presumably act by impairing nucleotide binding/hydrolysis. However, they have also been reported in other regions of SUR1, including TMD0 (Abdulhadi-Atwan et al. 2008): one can speculate that these mutations either affect coupling to Kir6.2, or interfere with MgATP binding/hydrolysis allosterically. In general, this class of mutations result in a less severe phenotype due to a partial response to Mg-nucleotides, and some patients can be treated by the K-channel opener diazoxide (Dunne et al. 2004).
(b) Neonatal diabetes
Activating mutations in SUR1 cause neonatal diabetes (Hattersley & Ashcroft 2005). Some of these mutations lead to more severe syndromes that also include developmental delay and muscle weakness (iDEND syndrome) or to developmental delay, epilepsy muscle weakness, dysmorphic features and neonatal diabetes (DEND syndrome) (Hattersley & Ashcroft 2005). All mutations studied to date decrease the ability of ATP to inhibit channel activity (at Kir6.2) and/or enhance the ability of Mg-nucleotides to stimulate channel activity (at SUR1), thus increasing the magnitude of the KATP current (Ashcroft 2005). This is expected to produce β-cell hyperpolarization, thereby inhibiting electrical activity, calcium influx and insulin secretion. Currents of larger magnitude are considered to hyperpolarize other cells in which Kir6.2 is expressed, such as muscle and brain, accounting for the DEND phenotype. Consistent with this idea, the single SUR1 mutation that is associated with neurological problems results in a greater KATP current than those that cause only neonatal diabetes (Proks et al. 2006a, 2007; figure 4a).
In general, the increase in the current produced by SUR1 mutations is smaller than that caused by Kir6.2 mutations, reflecting the smaller number of severe phenotypes and the relatively higher incidence of relapsing–remitting (transient) diabetes (TNDM) than permanent neonatal diabetes (PNDM) or DEND syndrome (figure 4b). It is important to recognize, however, that even very small changes in the KATP currents may have large effects on the β-cell membrane potential when the input resistance of the β-cell is very high.
(i) Mechanism of action
Gain-of-function mutations in SUR are dispersed throughout the protein sequence, but are particularly concentrated in the first five transmembrane helices and their connecting loops, in CL3 and in NBD2 (figure 5a,b). They mediate their effects in two principal ways. Some mutations lead to a reduction in the inhibition produced by ATP binding at Kir6.2 (Proks et al. 2006a, 2007), whereas others enhance channel activation by Mg-nucleotides. Both effects lead to an increase in KATP current at a given MgATP concentration (Babenko et al. 2006; de Wet et al. 2007a, 2008; Masia et al. 2007).
Figure 5.
Location of ND mutations. (a) Schematic of the membrane topology of SUR1 showing the location of ND mutations. Regions predicted to be helices are indicated as boxes. Residues causing neonatal diabetes are indicated in red. (b) Location of ND mutations in site 2.
How might mutations in SUR1 reduce the extent of ATP block at Kir6.2? One possibility is that they reduce ATP binding. The presence of SUR1 enhances ATP inhibition at Kir6.2 suggesting that SUR1 may contribute to the ATP-binding site or influence it allosterically (Tucker et al. 1997). Disruption of this effect could reduce ATP binding directly: no mutation has yet been shown to act this way, but this may reflect the fact that few SUR1 mutations have been analysed mechanistically to date. Other mutations could disrupt ATP inhibition indirectly, by increasing the channel Po. Only one mutation (F132L) has been shown to act this way to date (Proks et al. 2006a, 2007). This lies within TMD0, a region of SUR1 known to be involved in modulating the channel Po (Babenko & Bryan 2003). The F132L mutation increases the duration of the bursts of the KATP channel openings and reduces the frequency and duration of the interburst closed states. This shift in gating equilibrium towards channel opening in the absence of ATP will produce a similar shift in the presence of the nucleotide, and thus indirectly reduce ATP inhibition. Interestingly, not all mutations in TMD0 mediate their effects via a change in Po (Masia et al. 2007).
Because the KATP channel activity is determined by the balance between ATP block at Kir6.2 and MgATP activation at SUR1, SUR1 mutations may also reduce the overall extent of ATP inhibition by enhancing MgATP and/or MgADP activation. Mutations in the nucleotide-binding sites may be expected to act this way.
Although many SUR1 mutations that lead to neonatal diabetes are found in NBD2, it is striking that only one is found in NBD1, and that this mutation lies in the linker that forms part of nucleotide-binding site 2 (figure 5b). As predicted from their location, both R826W (in NBD1) and R1380L (in NBD2) influence ATPase activity. The fact that R826W reduces ATPase activity, whereas R1380L enhances it, yet both increase MgATP activation of the channel, may at first seem surprising. However, the conundrum is resolved by the fact that both mutations appear to increase the probability of SUR being in a MgADP-bound state, thereby enhancing channel activity. In the case of R826W, this is because the mutation slows the rate at which Pi dissociates following ATP hydrolysis (de Wet et al. 2008). The R1380L mutation seems to speed up the catalytic cycle, so that the protein spends less time in the pre-hydrolytic ATP-bound state (de Wet et al. 2007b). The I1425V mutation in NBD2 also shows greater Mg-nucleotide-dependent stimulation of the channel activity (Babenko et al. 2006; I1424V in their notation). While other NBD mutations have not been analysed in detail, their location suggests that they may also influence ATPase activity and/or Mg-nucleotide activation.
Finally, some mutations (e.g. H1023Y in TM12, Babenko et al. 2006; L225P in CL3, Masia et al. 2007) enhance Mg-nucleotide activation by unknown mechanisms. Such naturally occurring mutations provide fresh insights into KATP channel function and are especially valuable when structural information is lacking.
(ii) Implications for therapy
Prior to the discovery that neonatal diabetes could be caused by SUR1 and Kir6.2 mutations, many patients were treated from diagnosis with insulin. Recognition of the genetic basis of their disease rapidly led to a switch to sulphonylurea therapy. Sulphonylurea tablets have been used safely for many years to treat type 2 diabetes, so no clinical trials were required. Many patients with Kir6.2 mutations have now successfully transferred to sulphonylurea therapy (Pearson et al. 2006). Although fewer studies have been reported to date, sulphonylureas also appear to be effective in many patients with SUR1 mutations (Masia et al. 2007; Babenko 2008).
SUR1 mutations that dramatically enhance Po are likely to result in a reduced block by sulphonylureas, as is the case for Kir6.2 mutations that increase Po. Patients carrying such mutations are unlikely to be able to transfer to sulphonylurea therapy (Pearson et al. 2006). It is also possible that some SUR1 mutations may affect drug binding/transduction, as well as nucleotide sensitivity, although this has not yet been described.
Finally, a word of caution. Several SUR1 mutations have been identified in patients with neonatal diabetes, but on closer inspection it transpired that they were not the cause of the disease. In some cases, the parents were non-symptomatic carriers of the mutation, and in other cases no functional effects were found. This emphasizes the need to confirm that the mutant protein has a functional effect before attributing it to be the cause of the disease.
7. Conclusions and future challenges
The sulphonylurea receptor is an ABC protein that has been ‘captured’ by an inward rectifier channel (Kir6.2) and press-ganged into serving a novel regulatory role. Conformational changes caused by ATP binding/hydrolysis at the NBDs are now used to drive the opening of the tetrameric Kir6.2 channel rather than power substrate efflux. Precisely, how this is achieved remains a mystery. We still do not really know what, if any, is the role of NBD1 and why mutations in NBD1 impair both nucleotide binding and Mg-nucleotide-induced channel activation (Gribble et al. 1997). Nor is it certain how many SUR1 subunits must bind MgADP in order to cause channel opening. Where therapeutic drugs bind, and how this binding modulates KATP channel gating is important to resolve at a mechanistic level. What other functions SUR1 may have (see box 1) and whether it has any (as yet unidentified) transporter activity require further investigation. It also remains largely unclear exactly how the observed sequence diversity between ABCC proteins leads to their marked functional differences, i.e. what structural features determine why MRP1 is a transporter, CFTR an ion channel and SUR a channel regulator.
Box 1. Other roles for SUR1.
The fact that SUR1 is an ABC protein raises the question of whether in addition to its role as a channel regulator, it also acts as a transporter. To date, this has not been demonstrated. Although the KATP channel has a relatively slow rate of ATP hydrolysis (100 nmol Pi min−1 mg−1 protein; Mikhailov et al. 2005) compared with other ABC proteins, it nevertheless lies within the range of that reported for MRP1 (from 5 to 470 nmol Pi min−1 mg−1 protein; Chang et al. 1998; Mao et al. 1999). Thus, it is not inconceivable that it serves as a transporter of an as yet unidentified substrate.
There is accumulating evidence, however, that SUR1 also plays a role in insulin granule exocytosis. First, KATP channels are found at much higher density on insulin granules than in the plasma membrane (Geng et al. 2003). Second, insulin granule fusion is reduced in SUR1 knockout mice (Kikuta et al. 2005). Third, sulphonylureas enhance exocytosis as measured by an increase in β-cell membrane capacitance (Hoy et al. 2000). Fourth, the ability of cyclic AMP to potentiate insulin exocytosis in a PKA-independent manner is impaired in SUR1 knockout mice (Nakazaki et al. 2002). This may be related to the fact that Epac (cAMP–GEFII) specifically binds to NBD1 of SUR1 in a cAMP-dependent manner (Shibasaki et al. 2004a). SUR1 and Epac form part of a large complex involved in exocytosis, which includes piccolo (Shibasaki et al. 2004a,b), and RIM2, a Rab-effector protein thought to be a scaffold on which many exocytotic proteins bind (Sudhof 2004). Whether SUR1 serves merely as a scaffold protein or if it has some more active role in insulin granule exocytosis remains to be determined. The fact that the binding of Epac to SUR1 does not alter KATP channel function favours the former possibility. However, syntaxin binding to the NBDs of SUR1 (Pasyk et al. 2004) inhibits KATP channel activity (Cui et al. 2004). Another possibility is that SUR1 might be involved in granule trafficking, as we recently found that SUR1 binds α- and β-tubulin.
A number of proteins involved in glucose metabolism and ATP handling have been shown to physically interact with SUR2, including GAPDH (Jovanovic et al. 2005), LDH (Crawford et al. 2002a) and creatine kinase (Crawford et al. 2002b). It would be of interest to explore if this is also the case for SUR1.
Mechanistic insight into these problems will require both high-resolution structural information and more detailed functional analyses. Analysis of naturally occurring mutations in SUR1 (and indeed Kir6.2) may be valuable in pinpointing key residues. However, as shown here, to understand how these mutations work requires not only electrophysiological studies, but also biochemical measurements of ATPase activity, ATP binding, etc. Furthermore, because these properties differ for the isolated NBDs and are influenced by the presence of Kir6.2, ultimately biochemical studies will need to be carried out on the intact KATP channel complex. This will not be easy to achieve. Neither will it be simple to obtain a high-resolution structure of the KATP channel complex. Nevertheless, the rewards are such that substantial work in this area can be expected in the next years.
Acknowledgements
We thank the Wellcome Trust, the Royal Society, Diabetes UK and the European Union for support. F.M.A. is a Royal Society Research Professor.
Footnotes
One contribution of 16 to a Discussion Meeting Issue ‘Membrane transport in flux: the ambiguous interface between channels and pumps’.
References
- Abdulhadi-Atwan M., Bushman J.D., Tornovsky-Babaey S., Perry A., Abu-Libdeh A., Glaser B., Shyng S.L., Zangen D.H. Novel de novo mutation in SUR1 presenting as HI in infancy followed by overt diabetes in early adolescence. Diabetes. 2008;57:1935–1940. doi: 10.2337/db08-0159. doi:10.2337/db08-0159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft F.M. ATP-sensitive potassium channelopathies: focus on insulin secretion. J. Clin. Invest. 2005;115:2047–2058. doi: 10.1172/JCI25495. doi:10.1172/JCI25495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft F.M. The Walter B. cannon physiology in perspective lecture, 2007 ATP-sensitive K+ channels and disease: from molecule to malady. Am. J. Physiol. Endocrinol. Metab. 2007;293:E880–E889. doi: 10.1152/ajpendo.00348.2007. doi:10.1152/ajpendo.00348.2007 [DOI] [PubMed] [Google Scholar]
- Ashfield R., Gribble F.M., Ashcroft S.J., Ashcroft F.M. Identification of the high-affinity tolbutamide site on the SUR1 subunit of the KATP channel. Diabetes. 1999;48:1341–1347. doi: 10.2337/diabetes.48.6.1341. doi:10.2337/diabetes.48.6.1341 [DOI] [PubMed] [Google Scholar]
- Babenko A.P. A novel ABCC8 (SUR1)-dependent mechanism of metabolism-excitation uncoupling. J. Biol. Chem. 2008;283:8778–8782. doi: 10.1074/jbc.C700243200. doi:10.1074/jbc.C700243200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babenko A.P., Bryan J. Sur domains that associate with and gate KATP pores define a novel gatekeeper. J. Biol. Chem. 2003;278:41 577–41 580. doi: 10.1074/jbc.C300363200. doi:10.1074/jbc.C300363200 [DOI] [PubMed] [Google Scholar]
- Babenko A.P., Gonzalez G., Bryan J. Two regions of sulfonylurea receptor specify the spontaneous bursting and ATP inhibition of KATP channel isoforms. J. Biol. Chem. 1999;274:11 587–11 592. doi: 10.1074/jbc.274.17.11587. doi:10.1074/jbc.274.17.11587 [DOI] [PubMed] [Google Scholar]
- Babenko A.P., et al. Activating mutations in the ABCC8 gene in neonatal diabetes mellitus. N. Engl. J. Med. 2006;355:456–466. doi: 10.1056/NEJMoa055068. doi:10.1056/NEJMoa055068 [DOI] [PubMed] [Google Scholar]
- Bienengraeber M., Alekseev A.E., Abraham M.R., Carrasco A.J., Moreau C., Vivaudou M., Dzeja P.P., Terzic A. ATPase activity of the sulfonylurea receptor: a catalytic function for the KATP channel complex. FASEB J. 2000;14:1943–1952. doi: 10.1096/fj.00-0027com. doi:10.1096/fj.00-0027com [DOI] [PubMed] [Google Scholar]
- Campbell J.D., Sansom M.S., Ashcroft F.M. Potassium channel regulation. EMBO Rep. 2003;4:1038–1042. doi: 10.1038/sj.embor.7400003. doi:10.1038/sj.embor.7400003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K.W., Zhang H., Logothetis D.E. N-terminal transmembrane domain of the SUR controls trafficking and gating of Kir6 channel subunits. EMBO J. 2003;22:3833–3843. doi: 10.1093/emboj/cdg376. doi:10.1093/emboj/cdg376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang X.B., Hou Y.X., Riordan J.R. Stimulation of ATPase activity of purified multidrug resistance-associated protein by nucleoside diphosphates. J. Biol. Chem. 1998;273:23 844–23 848. doi: 10.1074/jbc.273.37.23844. doi:10.1074/jbc.273.37.23844 [DOI] [PubMed] [Google Scholar]
- Clement J.P., Kunjilwar K., Gonzalez G., Schwanstecher M., Panten U., Aguilar-Bryan L., Bryan J. Association and stoichiometry of KATP channel subunits. Neuron. 1997;18:827–838. doi: 10.1016/s0896-6273(00)80321-9. doi:10.1016/S0896-6273(00)80321-9 [DOI] [PubMed] [Google Scholar]
- Cole S.P., Deeley R.G. Multidrug resistance mediated by the ATP-binding cassette transporter protein MRP. Bioessays. 1998;20:931–940. doi: 10.1002/(SICI)1521-1878(199811)20:11<931::AID-BIES8>3.0.CO;2-J. doi:10.1002/(SICI)1521-1878(199811)20:11<931::AID-BIES8>3.0.CO;2-J [DOI] [PubMed] [Google Scholar]
- Crawford R.M., Budas G.R., Jovanovic S., Ranki H.J., Wilson T.J., Davies A.M., Jovanovic A. M-LDH serves as a sarcolemmal KATP channel subunit essential for cell protection against ischemia. EMBO J. 2002a;21:3936–3948. doi: 10.1093/emboj/cdf388. doi:10.1093/emboj/cdf388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford R.M., Ranki H.J., Botting C.H., Budas G.R., Jovanovic A. Creatine kinase is physically associated with the cardiac ATP-sensitive K+ channel in vivo. FASEB J. 2002b;16:102–104. doi: 10.1096/fj.01-0466fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui N., et al. H3 domain of syntaxin 1A inhibits KATP channels by its actions on the sulfonylurea receptor 1 nucleotide-binding folds-1 and -2. J. Biol. Chem. 2004;279:53 259–53 265. doi: 10.1074/jbc.M410171200. doi:10.1074/jbc.M410171200 [DOI] [PubMed] [Google Scholar]
- Dawson R.J., Locher K.P. Structure of a bacterial multidrug ABC transporter. Nature. 2006;443:180–185. doi: 10.1038/nature05155. doi:10.1038/nature05155 [DOI] [PubMed] [Google Scholar]
- Dawson R.J., Locher K.P. Structure of the multidrug ABC transporter Sav1866 from Staphylococcus aureus in complex with AMP-PNP. FEBS Lett. 2007;581:935–938. doi: 10.1016/j.febslet.2007.01.073. doi:10.1016/j.febslet.2007.01.073 [DOI] [PubMed] [Google Scholar]
- de Wet H., Mikhailov M.V., Fotinou C., Dreger M., Craig T.J., Venien-Bryan C., Ashcroft F.M. Studies of the ATPase activity of the ABC protein SUR1. FEBS J. 2007a;274:3532–3544. doi: 10.1111/j.1742-4658.2007.05879.x. doi:10.1111/j.1742-4658.2007.05879.x [DOI] [PubMed] [Google Scholar]
- de Wet H., et al. Increased ATPase activity produced by mutations at arginine-1380 in nucleotide-binding domain 2 of ABCC8 causes neonatal diabetes. Proc. Natl Acad. Sci. USA. 2007b;104:18 988–18 992. doi: 10.1073/pnas.0707428104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wet, H., Proks, P., Lafond, M., Aittoniemi, J., Sansom, M. S. P., Flanagan, S. E., Pearson, E. R., Hattersley, A. T. & Ashcroft, F. M. 2008 A mutation (R826W) in nucleotide-binding domain 1 of ABCC8 reduces ATPase activity and causes transient neonatal diabetes. EMBO Rep 9, 648–654. (doi:10.1038/embor.2008.71) [DOI] [PMC free article] [PubMed]
- Dean, M. 2002 The human ATP-binding cassette (ABC) transporter superfamily. National Library of Medicine (US), NCBI. See http://www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=mono_001.chapter.137
- Deeley R.G., Westlake C., Cole S.P. Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiol. Rev. 2006;86:849–899. doi: 10.1152/physrev.00035.2005. doi:10.1152/physrev.00035.2005 [DOI] [PubMed] [Google Scholar]
- Drain P., Li L., Wang J. KATP channel inhibition by ATP requires distinct functional domains of the cytoplasmic C terminus of the pore-forming subunit. Proc. Natl Acad. Sci. USA. 1998;95:13 953–13 958. doi: 10.1073/pnas.95.23.13953. doi:10.1073/pnas.95.23.13953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne M.J., Cosgrove K.E., Shepherd R.M., Aynsley-Green A., Lindley K.J. Hyperinsulinism in infancy: from basic science to clinical disease. Physiol. Rev. 2004;84:239–275. doi: 10.1152/physrev.00022.2003. doi:10.1152/physrev.00022.2003 [DOI] [PubMed] [Google Scholar]
- Ellard S., et al. Permanent neonatal diabetes caused by dominant, recessive, or compound heterozygous SUR1 mutations with opposite functional effects. Am. J. Hum. Genet. 2007;81:375–382. doi: 10.1086/519174. doi:10.1086/519174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby D.C., Vergani P., Csanady L. The ABC protein turned chloride channel whose failure causes cystic fibrosis. Nature. 2006;440:477–483. doi: 10.1038/nature04712. doi:10.1038/nature04712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng X., Li L., Watkins S., Robbins P.D., Drain P. The insulin secretory granule is the major site of KATP channels of the endocrine pancreas. Diabetes. 2003;52:767–776. doi: 10.2337/diabetes.52.3.767. doi:10.2337/diabetes.52.3.767 [DOI] [PubMed] [Google Scholar]
- Gloyn A.L., Siddiqui J., Ellard S. Mutations in the genes encoding the pancreatic beta-cell KATP channel subunits Kir6.2 (KCNJ11) and SUR1 (ABCC8) in diabetes mellitus and hyperinsulinism. Hum. Mutat. 2006;27:220–231. doi: 10.1002/humu.20292. doi:10.1002/humu.20292 [DOI] [PubMed] [Google Scholar]
- Gribble F.M., Tucker S.J., Ashcroft F.M. The essential role of the Walker A motifs of SUR1 in K-ATP channel activation by Mg-ADP and diazoxide. EMBO J. 1997;16:1145–1152. doi: 10.1093/emboj/16.6.1145. doi:10.1093/emboj/16.6.1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribble F.M., Proks P., Corkey B.E., Ashcroft F.M. Mechanism of cloned ATP-sensitive potassium channel activation by oleoyl-CoA. J. Biol. Chem. 1998;273:26 383–26 387. doi: 10.1074/jbc.273.41.26383. doi:10.1074/jbc.273.41.26383 [DOI] [PubMed] [Google Scholar]
- Haider S., Antcliff J.F., Proks P., Sansom M.S., Ashcroft F.M. Focus on Kir6.2: a key component of the ATP-sensitive potassium channel. J. Mol. Cell. Cardiol. 2005;38:927–936. doi: 10.1016/j.yjmcc.2005.01.007. doi:10.1016/j.yjmcc.2005.01.007 [DOI] [PubMed] [Google Scholar]
- Hansen A.M., Hansen J.B., Carr R.D., Ashcroft F.M., Wahl P. Kir6.2-dependent high-affinity repaglinide binding to beta-cell KATP channels. Br. J. Pharmacol. 2005;144:551–557. doi: 10.1038/sj.bjp.0706082. doi:10.1038/sj.bjp.0706082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattersley A.T., Ashcroft F.M. Activating mutations in Kir6.2 and neonatal diabetes: new clinical syndromes, new scientific insights, and new therapy. Diabetes. 2005;54:2503–2513. doi: 10.2337/diabetes.54.9.2503. doi:10.2337/diabetes.54.9.2503 [DOI] [PubMed] [Google Scholar]
- Hollenstein K., Dawson R.J., Locher K.P. Structure and mechanism of ABC transporter proteins. Curr. Opin. Struct. Biol. 2007;17:412–418. doi: 10.1016/j.sbi.2007.07.003. doi:10.1016/j.sbi.2007.07.003 [DOI] [PubMed] [Google Scholar]
- Hoy M., Olsen H.L., Bokvist K., Buschard K., Barg S., Rorsman P., Gromada J. Tolbutamide stimulates exocytosis of glucagon by inhibition of a mitochondrial-like ATP-sensitive K+ (KATP) conductance in rat pancreatic A-cells. J. Physiol. 2000;527(Pt 1):109–120. doi: 10.1111/j.1469-7793.2000.00109.x. doi:10.1111/j.1469-7793.2000.00109.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huopio H., Shyng S.L., Otonkoski T., Nichols C.G. KATP channels and insulin secretion disorders. Am. J. Physiol. Endocrinol. Metab. 2002;283:E207–E216. doi: 10.1152/ajpendo.00047.2002. [DOI] [PubMed] [Google Scholar]
- Jovanovic S., Du Q., Crawford R.M., Budas G.R., Stagljar I., Jovanovic A. Glyceraldehyde 3-phosphate dehydrogenase serves as an accessory protein of the cardiac sarcolemmal KATP channel. EMBO Rep. 2005;6:848–852. doi: 10.1038/sj.embor.7400489. doi:10.1038/sj.embor.7400489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuta T., Ohara-Imaizumi M., Nakazaki M., Nishiwaki C., Nakamichi Y., Tei C., Aguilar-Bryan L., Bryan J., Nagamatsu S. Docking and fusion of insulin secretory granules in SUR1 knock out mouse beta-cells observed by total internal reflection fluorescence microscopy. FEBS Lett. 2005;579:1602–1606. doi: 10.1016/j.febslet.2005.01.074. doi:10.1016/j.febslet.2005.01.074 [DOI] [PubMed] [Google Scholar]
- Koster J.C., Marshall B.A., Ensor N., Corbett J.A., Nichols C.G. Targeted overactivity of beta cell KATP channels induces profound neonatal diabetes. Cell. 2000;100:645–654. doi: 10.1016/s0092-8674(00)80701-1. doi:10.1016/S0092-8674(00)80701-1 [DOI] [PubMed] [Google Scholar]
- Linton K.J., Higgins C.F. Structure and function of ABC transporters: the ATP switch provides flexible control. Pflugers Arch. 2007;453:555–567. doi: 10.1007/s00424-006-0126-x. doi:10.1007/s00424-006-0126-x [DOI] [PubMed] [Google Scholar]
- Locher K.P. Structure and mechanism of ATP-binding cassette transporters. Phil. Trans. R. Soc. B. 2009;364:239–245. doi: 10.1098/rstb.2008.0125. doi:10.1098/rstb.2008.0125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz E., Alekseev A.E., Krapivinsky G.B., Carrasco A.J., Clapham D.E., Terzic A. Evidence for direct physical association between a K+ channel (Kir6.2) and an ATP-binding cassette protein (SUR1) which affects cellular distribution and kinetic behavior of an ATP-sensitive K+ channel. Mol. Cell. Biol. 1998;18:1652–1659. doi: 10.1128/mcb.18.3.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Q., Leslie E.M., Deeley R.G., Cole S.P. ATPase activity of purified and reconstituted multidrug resistance protein MRP1 from drug-selected H69AR cells. Biochim. Biophys. Acta. 1999;1461:69–82. doi: 10.1016/s0005-2736(99)00150-9. doi:10.1016/S0005-2736(99)00150-9 [DOI] [PubMed] [Google Scholar]
- Masia R., Enkvetchakul D., Nichols C.G. Differential nucleotide regulation of KATP channels by SUR1 and SUR2A. J. Mol. Cell. Cardiol. 2005;39:491–501. doi: 10.1016/j.yjmcc.2005.03.009. doi:10.1016/j.yjmcc.2005.03.009 [DOI] [PubMed] [Google Scholar]
- Masia R., De Leon D.D., MacMullen C., McKnight H., Stanley C.A., Nichols C.G. A mutation in the TMD0-L0 region of sulfonylurea receptor-1 (L225P) causes permanent neonatal diabetes mellitus (PNDM) Diabetes. 2007;56:1357–1362. doi: 10.2337/db06-1746. doi:10.2337/db06-1746 [DOI] [PubMed] [Google Scholar]
- Matsuo M., Kioka N., Amachi T., Ueda K. ATP binding properties of the nucleotide-binding folds of SUR1. J. Biol. Chem. 1999;274:37 479–37 482. doi: 10.1074/jbc.274.52.37479. doi:10.1074/jbc.274.52.37479 [DOI] [PubMed] [Google Scholar]
- McDevitt C.A., Collins R.F., Conway M., Modok S., Storm J., Kerr I.D., Ford R.C., Callaghan R. Purification and 3D structural analysis of oligomeric human multidrug transporter ABCG2. Structure. 2006;14:1623–1632. doi: 10.1016/j.str.2006.08.014. doi:10.1016/j.str.2006.08.014 [DOI] [PubMed] [Google Scholar]
- Mikhailov M.V., Campbell J.D., de Wet H., Shimomura K., Zadek B., Collins R.F., Sansom M.S., Ford R.C., Ashcroft F.M. 3-D structural and functional characterization of the purified KATP channel complex Kir6.2-SUR1. EMBO J. 2005;24:4166–4175. doi: 10.1038/sj.emboj.7600877. doi:10.1038/sj.emboj.7600877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muallem D., Vergani P. ATP hydrolysis-driven gating in cystic fibrosis transmembrane conductance regulator. Phil. Trans. R. Soc. B. 2009;364:247–255. doi: 10.1098/rstb.2008.0191. doi:10.1098/rstb.2008.0191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazaki M., Crane A., Hu M., Seghers V., Ullrich S., Aguilar-Bryan L., Bryan J. cAMP-activated protein kinase-independent potentiation of insulin secretion by cAMP is impaired in SUR1 null islets. Diabetes. 2002;51:3440–3449. doi: 10.2337/diabetes.51.12.3440. doi:10.2337/diabetes.51.12.3440 [DOI] [PubMed] [Google Scholar]
- Nichols C.G. KATP channels as molecular sensors of cellular metabolism. Nature. 2006;440:470–476. doi: 10.1038/nature04711. doi:10.1038/nature04711 [DOI] [PubMed] [Google Scholar]
- Pasyk E.A., Kang Y., Huang X., Cui N., Sheu L., Gaisano H.Y. Syntaxin-1A binds the nucleotide-binding folds of sulphonylurea receptor 1 to regulate the KATP channel. J. Biol. Chem. 2004;279:4234–4240. doi: 10.1074/jbc.M309667200. doi:10.1074/jbc.M309667200 [DOI] [PubMed] [Google Scholar]
- Pearson E.R., et al. Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N. Engl. J. Med. 2006;355:467–477. doi: 10.1056/NEJMoa061759. doi:10.1056/NEJMoa061759 [DOI] [PubMed] [Google Scholar]
- Proks P., Ashcroft F.M. Phentolamine block of KATP channels is mediated by Kir6.2. Proc. Natl Acad. Sci. USA. 1997;94:11 716–11 720. doi: 10.1073/pnas.94.21.11716. doi:10.1073/pnas.94.21.11716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proks P., et al. A heterozygous activating mutation in the sulphonylurea receptor SUR1 (ABCC8) causes neonatal diabetes. Hum. Mol. Genet. 2006a;15:1793–1800. doi: 10.1093/hmg/ddl101. doi:10.1093/hmg/ddl101 [DOI] [PubMed] [Google Scholar]
- Proks P., Girard C., Baevre H., Njolstad P.R., Ashcroft F.M. Functional effects of mutations at F35 in the NH2-terminus of Kir6.2 (KCNJ11), causing neonatal diabetes, and response to sulfonylurea therapy. Diabetes. 2006b;55:1731–1737. doi: 10.2337/db05-1420. doi:10.2337/db05-1420 [DOI] [PubMed] [Google Scholar]
- Proks P., Shimomura K., Craig T.J., Girard C.A., Ashcroft F.M. Mechanism of action of a sulphonylurea receptor SUR1 mutation (F132L) that causes DEND syndrome. Hum. Mol. Genet. 2007;16:2011–2019. doi: 10.1093/hmg/ddm149. doi:10.1093/hmg/ddm149 [DOI] [PubMed] [Google Scholar]
- Ramaen O., Leulliot N., Sizun C., Ulryck N., Pamlard O., Lallemand J.Y., Tilbeurgh H., Jacquet E. Structure of the human multidrug resistance protein 1 nucleotide binding domain 1 bound to Mg2+/ATP reveals a non-productive catalytic site. J. Mol. Biol. 2006;359:940–949. doi: 10.1016/j.jmb.2006.04.005. doi:10.1016/j.jmb.2006.04.005 [DOI] [PubMed] [Google Scholar]
- Reimann F., Tucker S.J., Proks P., Ashcroft F.M. Involvement of the n-terminus of Kir6.2 in coupling to the sulphonylurea receptor. J. Physiol. 1999;518(Pt 2):325–336. doi: 10.1111/j.1469-7793.1999.0325p.x. doi:10.1111/j.1469-7793.1999.0325p.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann F., Gribble F.M., Ashcroft F.M. Differential response of KATP channels containing SUR2A or SUR2B subunits to nucleotides and pinacidil. Mol. Pharmacol. 2000;58:1318–1325. doi: 10.1124/mol.58.6.1318. [DOI] [PubMed] [Google Scholar]
- Schwappach B., Zerangue N., Jan Y.N., Jan L.Y. Molecular basis for KATP assembly: transmembrane interactions mediate association of a K+ channel with an ABC transporter. Neuron. 2000;26:155–167. doi: 10.1016/s0896-6273(00)81146-0. doi:10.1016/S0896-6273(00)81146-0 [DOI] [PubMed] [Google Scholar]
- Shibasaki T., Sunaga Y., Fujimoto K., Kashima Y., Seino S. Interaction of ATP sensor, cAMP sensor, Ca2+ sensor, and voltage-dependent Ca2+ channel in insulin granule exocytosis. J. Biol. Chem. 2004a;279:7956–7961. doi: 10.1074/jbc.M309068200. doi:10.1074/jbc.M309068200 [DOI] [PubMed] [Google Scholar]
- Shibasaki T., Sunaga Y., Seino S. Integration of ATP, cAMP, and Ca2+ signals in insulin granule exocytosis. Diabetes. 2004b;53(Suppl.3):S59–S62. doi: 10.2337/diabetes.53.suppl_3.s59. doi:10.2337/diabetes.53.suppl_3.S59 [DOI] [PubMed] [Google Scholar]
- Shield J.P., Flanagan S.E., Mackay D.J., Harries L.W., Proks P., Girard C., Ashcroft F.M., Temple I.K., Ellard S. Mosaic paternal uniparental isodisomy and an ABCC8 gene mutation in a patient with permanent neonatal diabetes and hemihypertrophy. Diabetes. 2008;57:255–258. doi: 10.2337/db07-0999. doi:10.2337/db07-0999 [DOI] [PubMed] [Google Scholar]
- Sudhof T.C. The synaptic vesicle cycle. Annu. Rev. Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. doi:10.1146/annurev.neuro.26.041002.131412 [DOI] [PubMed] [Google Scholar]
- Tammaro P., Girard C., Molnes J., Njolstad P.R., Ashcroft F.M. Kir6.2 mutations causing neonatal diabetes provide new insights into Kir6.2–SUR1 interactions. EMBO J. 2005;24:2318–2330. doi: 10.1038/sj.emboj.7600715. doi:10.1038/sj.emboj.7600715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tammaro P., Proks P., Ashcroft F.M. Functional effects of naturally occurring KCNJ11 mutations causing neonatal diabetes on cloned cardiac KATP channels. J. Physiol. 2006;571:3–14. doi: 10.1113/jphysiol.2005.099168. doi:10.1113/jphysiol.2005.099168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschenberger G., Mougey A., Shen S., Lester L.B., LaFranchi S., Shyng S.L. Identification of a familial hyperinsulinism-causing mutation in the sulfonylurea receptor 1 that prevents normal trafficking and function of KATP channels. J. Biol. Chem. 2002;277:17 139–17 146. doi: 10.1074/jbc.M200363200. doi:10.1074/jbc.M200363200 [DOI] [PubMed] [Google Scholar]
- Tucker S.J., Gribble F.M., Zhao C., Trapp S., Ashcroft F.M. Truncation of Kir6.2 produces ATP-sensitive K+ channels in the absence of the sulphonylurea receptor. Nature. 1997;387:179–183. doi: 10.1038/387179a0. doi:10.1038/387179a0 [DOI] [PubMed] [Google Scholar]
- Vila-Carriles W.H., Zhao G., Bryan J. Defining a binding pocket for sulfonylureas in ATP-sensitive potassium channels. FASEB J. 2007;21:18–25. doi: 10.1096/fj.06-6730hyp. doi:10.1096/fj.06-6730hyp [DOI] [PubMed] [Google Scholar]
- Ward A., Reyes C.L., Yu J., Roth C.B., Chang G. Flexibility in the ABC transporter MsbA: alternating access with a twist. Proc. Natl Acad. Sci. USA. 2007;104:19 005–19 010. doi: 10.1073/pnas.0709388104. doi:10.1073/pnas.0709388104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan F., Lin C.W., Weisiger E., Cartier E.A., Taschenberger G., Shyng S.L. Sulfonylureas correct trafficking defects of ATP-sensitive potassium channels caused by mutations in the sulfonylurea receptor. J. Biol. Chem. 2004;279:11 096–11 105. doi: 10.1074/jbc.M312810200. doi:10.1074/jbc.M312810200 [DOI] [PubMed] [Google Scholar]
- Yan F.F., Casey J., Shyng S.L. Sulfonylureas correct trafficking defects of disease-causing ATP-sensitive potassium channels by binding to the channel complex. J. Biol. Chem. 2006;281:33 403–33 413. doi: 10.1074/jbc.M605195200. doi:10.1074/jbc.M605195200 [DOI] [PubMed] [Google Scholar]
- Zerangue N., Schwappach B., Jan Y.N., Jan L.Y. A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane KATP channels. Neuron. 1999;22:537–548. doi: 10.1016/s0896-6273(00)80708-4. doi:10.1016/S0896-6273(00)80708-4 [DOI] [PubMed] [Google Scholar]
- Zingman L.V., Alekseev A.E., Bienengraeber M., Hodgson D., Karger A.B., Dzeja P.P., Terzic A. Signaling in channel/enzyme multimers: ATPase transitions in SUR module gate ATP-sensitive K+ conductance. Neuron. 2001;31:233–245. doi: 10.1016/s0896-6273(01)00356-7. doi:10.1016/S0896-6273(01)00356-7 [DOI] [PubMed] [Google Scholar]