Abstract
Rare, functional, non-synonymous variants in the human serotonin (5-hydroxytryptamine, 5-HT) transporter (hSERT) gene (SLC6A4) have been identified in both autism and obsessive–compulsive disorder (OCD). Within autism, rare hSERT coding variants associate with rigid–compulsive traits, suggesting both phenotypic overlap with OCD and a shared relationship with disrupted 5-HT signalling. Here, we document functional perturbations of three of these variants: Ile425Leu; Phe465Leu; and Leu550Val. In transiently transfected HeLa cells, the three variants confer a gain of 5-HT transport phenotype. Specifically, enhanced SERT activity was also observed in lymphoblastoid lines derived from mutation carriers. In contrast to previously characterized Gly56Ala, where increased transport activity derives from catalytic activation, the three novel variants exhibit elevated surface density as revealed through both surface antagonist-binding and biotinylation studies. Unlike Gly56Ala, mutants Ile425Leu, Phe465Leu and Leu550Val retain a capacity for acute PKG and p38 MAPK regulation. However, both Gly56Ala and Ile425Leu demonstrate markedly reduced sensitivity to PP2A antagonists, suggesting that deficits in trafficking and catalytic modulation may derive from a common basis in perturbed phosphatase regulation. When expressed stably from the same genomic locus in CHO cells, both Gly56Ala and Ile425Leu display catalytic activation, accompanied by a striking loss of SERT protein.
Keywords: serotonin, transporter, autism, genetics, kinase, phosphatase
1. Introduction
Autism is an increasingly recognized, juvenile (less than 3 years)-onset disorder characterized by deficits in language acquisition, an inability to infer meaning from normal social cues, and an insistence on sameness that often precipitates severe anxiety with minor changes in the environment (Lord et al. 2000). A male predominant (approx. 4 : 1 M/F) disorder, the prevalence of autism has been recently estimated to be as high as 1 : 150 (Van Naarden Braun et al. 2007). Such high prevalence recognizes the ‘autism spectrum’, which comprises subjects with typical autism, as well as those with a less severe expression of features, such as those with Asperger's syndrome. Significant risk for autism is thought to derive from heritable factors determined by multiple genes whose identity or variation may be distinct in different families (Veenstra-Vander Weele et al. 2004). Elucidation of the genetic variants and their biological impact offers the possibility of prenatal counselling, earlier identification of at-risk children, development of animal models and pursuit of biology-informed therapeutics. With these goals, we and others have pursued genome-wide linkage analyses of autism, identifying a strong linkage signal at 17q11.2–17q21 (Cook et al. 1997; McCauley et al. 2004; Stone et al. 2004; Cantor et al. 2005; Sutcliffe et al. 2005). Although the linkage signal we obtained (Sutcliffe et al. 2005) was already highly significant, particularly for a complex neurobehavioral disorder (HLOD∼5), linkage increased substantially when male-only subject families were included in the analysis (HLOD∼8). By contrast, no linkage signal was apparent with families in which at least one of the affected subjects was a female, suggesting not only that the 17q11.2–21 locus harbours one or more major risk determinants for autism, but also that it may contribute to the overall gender bias seen in autism.
The 17q11.2–21 locus includes, among other genes, the SLC6A4 gene that encodes the serotonin (5-hydroxytryptamine, 5-HT) transporter (hSERT, 5-HTT), the protein singularly responsible for antidepressant-sensitive, 5-HT inactivation in the brain and periphery (Ramamoorthy et al. 1993). SERT proteins are localized to the presynaptic terminals of 5-HT secreting neurons (Qian et al. 1995), and are also found in cells specializing in systemic 5-HT storage or inactivation, including specialized cells of the gut (Gordon & Barnes 2003), placenta (Balkovetz et al. 1989) and lung (Paczkowski et al. 1996), as well as in adrenal chromaffin cells (Schroeter et al. 1997), blood lymphocytes (Faraj et al. 1994; Gordon & Barnes 2003) and platelets (Carneiro & Blakely 2006; Carneiro et al. 2008). We were particularly drawn to hSERT as a candidate for autism risk as blood (or platelet) hyperserotonaemia is a long-standing and well-replicated finding in autism (Piven et al. 1991; Cook & Leventhal 1996). Platelet stores comprise virtually all of the 5-HT in the blood of both normal and autism subjects, though 5-HT is not synthesized in this cellular element. Rather, platelets express SERT proteins (the same gene produces SERT proteins in platelets and brain (Lesch et al. 1993)), to acquire 5-HT released into the circulation by enterochromaffin cells of the gut. Importantly, just as autism risk, blood 5-HT levels are heritable (Cross et al. 2008), suggesting that shared alleles at the SLC6A4 locus and other 5-HT determining genes may explain components of both traits in some autism subjects.
In conventional association studies using common SLC6A4 polymorphisms, we were unable to demonstrate strong association in the families from which our strong linkage signals arose (Sutcliffe et al. 2005). This prompted us to examine the SLC6A4 locus for the presence of rare variants that, collectively, could contribute to the linkage signals. Attempting to bias our efforts towards genetic variation with plausible contributions to functional disturbances, we concentrated our efforts on a search for alterations within exons that encode hSERT protein (as well as non-coding exon 1) from probands of affected male-only (MO) families that contributed most significantly to the linkage signals. Remarkably, in the initial 24 unrelated subjects analysed, we not only identified several instances of a previously reported coding variant (Gly56Ala; rs6355) but also multiple, novel non-synonymous variants at highly conserved amino acids. Ultimately, our study elaborated five hSERT-coding variants: Gly56Ala; Ile425Leu (rs28914832); Phe465Leu (rs28914833); Leu550Val (rs28914834); and Lys605Asn (rs6352). Ile425Leu, Phe465Leu and Leu550Val had not been previously described, and our own control studies, as well as independent explorations of hSERT-coding variation (Glatt et al. 2001), demonstrated each to represent rare genetic alterations, with a frequency of much less than 1 per cent. The Ile425Leu variant occurs at the same amino acid found to harbour a distinct variant (Ile425Val) associated with obsessive–compulsive disorder and Asperger's syndrome (Ozaki et al. 2003). The Lys605Asn variant was previously identified in a screen for SLC6A4 variants in a reference sample without available clinical features (Glatt et al. 2001).
Through genotyping studies on the full pedigrees, we found Gly56Ala to be elevated in frequency relative to controls, to be overtransmitted to affected probands and to be more likely associated with autism if the carrier was a female. Most of the subjects bearing a Gly56Ala variant were carriers, though we also identified three unrelated homozygous individuals in these families, comprising two affected males and a mother of two affected heterozygous males. The other variants that we identified, except for Lys605Asn, demonstrate selective transmission to affected males and the lack of transmission to unaffected females in the same family. In vitro functional studies of Gly56Ala in a parallel effort, where we characterized functional disturbances associated with all previously reported hSERT-coding variants (Prasad et al. 2005), established Gly56Ala and Lys605Asn as gain-of-function variants. Detailed studies of Gly56Ala revealed elevated basal 5-HT transport arising predominantly from a change in transport function (catalytic alteration), as opposed to elevated surface expression. Additionally, we found that Gly56Ala and Lys605Asn demonstrate a strikingly altered pattern of regulation by multiple Ser/Thr kinases known to regulate hSERT in native and transfected cells. Thus, both variants demonstrate insensitivity to activators of protein kinase G (PKG) and p38 mitogen-activated protein kinase (MAPK), stimuli that normally trigger elevated surface expression or enhancement of SERT catalytic rates (Blakely et al. 2005), respectively. Furthermore, we found that Gly56Ala demonstrates elevated basal phosphorylation relative to wild-type hSERT and also lacks enhanced phosphorylation with PKG activation by 8Br-cGMP. Finally, using lymphoblast lines from autism probands that were homozygous or heterozygous for Gly56 or Ala56 alleles, we demonstrated both hypermorphic status and lack of PKG/p38 MAPK regulation in a native expression context.
The studies noted above led us to conclude that rare, functional variants in hSERT may contribute to autism in some pedigrees. Additionally, we showed that the rigid–compulsive trait domain is particularly influenced by these variants (either Gly56Ala alone or Gly56Ala, Ile425Leu, Phe465Leu, Leu550Val analysed as a group; Sutcliffe et al. 2005), consistent with the evidence that hSERT-targeting selective serotonin reuptake inhibitor (SSRI) medications provide therapeutic relief for anxiety features (versus language or social communication skills; Kolevzon et al. 2006). Whether the gain-of-function and altered regulation properties of Gly56Ala and Lys605Asn are shared by other autism-associated hSERT variants remains to be determined. In the present study, we examined the functional properties of Ile425Leu, Phe465Leu and Leu550Val, comparing the function with that of wild-type and Gly56Ala hSERT. Remarkably, we find that all variants confer elevated hSERT activity whether assessed in transfected or natively expressing (lymphocytes) cells, though they appear to do so via (at least) two distinct mechanisms. We discuss our findings with respect to endogenous pathways that support basal and receptor-mediated regulation of hSERT activity, and how disruption of these pathways may contribute additional determinants of autism risk.
2. Material and methods
(a) Genotyping and lymphocyte studies
Lymphocyte lines were derived from autistic pedigrees recruited by Susan E. Folstein at Tufts University (TUFTS), from the Autism Genetics Resource Exchange (AGRE) collection (http://www.agre.org/) and from the NIMH Human Genetics Initiative Repository (http://www.nimhgenetics.org/) at Rutgers University. DNA from human Epstein–Barr virus (EBV)-transformed lymphoblastoid cell lines was genotyped to identify individuals carrying the variant allele via TaqMan-based allelic discrimination, using an Applied Biosystems (Foster City, CA, USA) Assay-by-Design, developed with intra-exonic sequences. Controls were selected to match for 5HTTLPR and second intron VNTR. Genotypes were independently confirmed by PCR and direct sequence analysis of the corresponding products. The Leu550Val lines derive from AGRE 254-04 (affected male) and was compared with AGRE 254-02 (unaffected father, non-carrier). The Phe465Leu lines derive from TUFTS 4001-4 (affected male) and 4001-5 (affected female) and was compared with 4001-01 (affected male sibling, non-carrier) and 4001-03 (unaffected mother, non-carrier). The Ile425Leu line derives from AGRE 509-10 (affected male) and was compared with AGRE 509-02 (unaffected father, non-carrier). Genotyped lymphocytes were cultured in suspension in RPMI 1640 medium prior to assay, supplemented with 15 per cent foetal bovine serum, 2 mM l-glutamine, 100 units ml−1 penicillin and 100 μg ml−1 streptomycin at 37°C in a humidified incubator at 5 per cent CO2 and maintained in uniform growth conditions.
(b) HeLa cell culture and transfection
HeLa cells, maintained at 37°C in a 5 per cent CO2 humidified incubator, were grown in complete medium (Dulbecco's Modified Eagle's Medium (DMEM, Invitrogen, Carlsbad, CA, USA), 10 per cent foetal bovine serum, 2 mM l-glutamine, 100 units ml−1 penicillin and 100 μg ml−1 streptomycin). Transfections (0.05 μg/10 000 cells/24 well plate) were performed using FuGENE 6 reagent (Roche) in Opti-MEM I (Invitrogen). hSERT cDNA (Ramamoorthy et al. 1993) was expressed via the mammalian expression vector pcDNA3.1 (Invitrogen). Mutations in SERT producing the following amino acid changes were generated using the QuikChange mutagenesis kit (Stratagene): Gly56Ala; Ile425Leu; Phe465Leu; and Leu550V. All mutations were confirmed by fluorescent dideoxynucleotide sequencing (Center for Molecular Neuroscience Neurogenomics Core) and purified in parallel (Qiagen) prior from multiple transformations to preclude ascription of non-specific phenotypes in transfected cells. hSERT cDNAs were incubated with the FuGENE-Opti-MEM I reagent (1 : 3 DNA/lipid ratio) at ambient temperature for 30 min before adding to plated cells. The transfected cells were cultured as above for 36 h prior to 5-HT transport and biochemical assays.
(c) CHO-Flp-In stable cell generation
To evaluate wild-type hSERT versus hSERT mutants in a stable cell model where expression derives from the same genomic locus, cDNAs were subcloned into pcDNA5/FRT plasmid (Invitrogen). Seeded Flp-In-CHO cells (Invitrogen) were then transfected with plasmid constructs using TransIT transfection reagent (Mirus Bio, Madison, WI, USA) using 100 ng hSERT/pcDNA5/FRT and 900 ng pOG44 (Invitrogen). Post-transfection (48 hours), the cells were split into hygromycin B-containing media and allowed to select for approximately 10 days with media changes throughout. Resistant cells were screened for Zeocin (Invitrogen) sensitivity and propagated in the same medium prior to 5-HT uptake studies assessment of SERT protein expression.
(d) Assessment of basal and regulated SERT activity
Transport of [3H]5-HT (5-hydroxy[3H]tryptamine trifluoroacetate; Amersham Biosciences, 20 nM final concentration) in transfected HeLa and stable CHO cells was conducted in a total assay volume of 500 μl Krebs–Ringer HEPES (KRH) assay buffer containing 130 mM NaCl, 1.3 mM KCl, 2.2 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, 1.8 g l−1 glucose and 10 mM HEPES (pH 7.4). Specific 5-HT uptake was determined by subtracting the amount of [3H]5-HT accumulated in the presence (10 μM) of the SSRI paroxetine (SmithKline Beecham). Saturation analyses were performed using serial dilutions of a labelled/unlabelled 5-HT mix. For lymphocyte assays, the cells were pelleted at 1500g for 5 min and washed with KRH assay buffer. A total of 1×106 cells in triplicate were pre-warmed (37°C) in a shaking water bath (10 min) in a 12×75 polypropylene tube in KRH buffer containing 100 μM pargyline and 100 μM ascorbic acid with or without modifiers. After 5 min incubation with [3H]5-HT (20 nM) at 37°C, uptake assays were terminated by immersion on ice, and uptake in pelleted, SDS (1%)-extracted cells quantitated by scintillation spectrometry. Specific 5-HT uptake was determined by subtracting the amount of [3H]5-HT accumulated in the presence of 10 μM paroxetine.
(e) Analysis of SERT surface expression
For estimation of SERT total and surface density in transfected HeLa cells, we measured [125I]RTI-55 binding (5 nM) to intact cells on ice, using the membrane-impermeant competitor 5-HT (100 μM) and the membrane-permeant competitor paroxetine (1 μM) to define surface and total specific binding, respectively. To establish the levels and biosynthetic progression of hSERT protein produced from mutant cDNAs, HeLa cells were plated onto 24-well dishes at 10 000 cells per well and transfected 12 h later. Twenty-four hours after transfection, the cells were washed twice with PBS/CM and incubated for 30 min at 4°C with shaking in 100 μl/well RIPA solubilization buffer (10 mM Tris (pH 7.4), 150 mM NaCl, 0.1% SDS, 1% Triton X-100, 1% sodium deoxycholate) containing protease inhibitors (1.0 μg ml−1 soya bean trypsin inhibitor, 1.0 μg ml−1 leupeptin, 10 units ml−1 aprotinin, 250 μM phenylmethylsulphonyl fluoride) and blotted for hSERT (1 : 1000; mAb Technologies, Inc.) using enhanced chemiluminescence (ECL, Amersham) detection. Altered density of SERT surface proteins was validated using immunoblotting of biotinylated whole-cell extracts produced using the lysine-directed, membrane-impermeant biotinylating reagent sulpho-NHS-SS-biotin (Pierce).
(f) Data analyses
All data were derived from three or more independent replicates of the conditions employed. Data were graphed and analysed statistically using Prism (GraphPad). Specific statistical tests and p values are provided in the figure legends. Kinetic constants (KM and Vmax) from saturation 5-HT transport studies were determined in Prism from nonlinear least-squares curve fits of data using the general Michaelis–Menten equation.
3. Results
A topological, 12-transmembrane (TM) domain model, organizing the five hSERT-coding variants identified in autism subjects, is shown in figure 1a. The three novel hSERT variants uncovered in autism probands, Ile425Leu, Phe465Leu and Leu550Val, are located within predicted TM domains, whereas the previously reported Gly56Ala and Lys605Asn substitutions occur in the cytoplasmic amino and carboxy termini of hSERT, respectively. Previously, we demonstrated that the lymphoblasts homozygous for the Ala56 allele demonstrate elevated SERT activity, relative to Gly56 homozygous lines (Prasad et al. 2005; Sutcliffe et al. 2005). Here, we extended these studies to the lines derived from the autism subjects that carry single alleles of Ile425Leu, Phe465Leu or Leu550Val. We measured SERT activity in these lines and, in parallel, measured l-glutamate transport activity as a measure of specificity. As shown in figure 1b, SERT activity was elevated in all three variant lines relative to the activity found in the cells bearing wild-type alleles at each of the mutated sites. All 5-HT transport in the variant-expressing cells' activity was citalopram sensitive, diminishing the possibility of induction of other transport systems capable of transporting 5-HT such as PMAT (Zhou et al. 2007) or OCT3 (Kekuda et al. 1998). More importantly, no change was observed in l-glutamate transport, indicating a measure of specificity for the transport alterations to hSERT, as well as stability of cellular bioenergetics, such as ion gradients or membrane potential that could indirectly impact SERT. Rather, these initial findings suggest an intrinsic, gain-of-function property produced by each of the hSERT variants.
Figure 1.
Location and 5-HT transport activity of autism-associated SERT-coding variants. (a) Autism-associated variants are overlaid on a 12-TM model of a single SERT subunit, with NH2 and COOH termini oriented inside the cell. Variants in extramembrane domains are shaded blue, whereas those in membrane domains are shaded white. (b) Altered activity of SERT variants is evident in native lymphocytes. Lymphocytes were genotyped and cultured as described in §2 and assessed for 5-HT uptake. Data presented derive from n=3 individual assays on lymphocyte lines of determined genotype. Findings were replicated in a separate set of pre-genotyped samples with equivalent results. Transport activities were analysed by a one-way ANOVA with post hoc Dunnett's tests, with *p<0.05 taken as significant. (c) 5-HT transport activity of autism-associated SERT-coding variants in transfected HeLa cells. All variants were transfected in parallel with reference hSERT cDNA into HeLa cells and assayed for 5-HT transport activity as described in §2. Data reflect mean±s.e.m. of three separate experiments. Means were compared with hSERT cDNA using a one-way ANOVA followed by Dunnett's test of individual means against hSERT values, with *p<0.05 taken as significant.
In order to establish the impact of Ile425Leu, Phe465Leu and Leu550Val on SERT activity in a model system devoid of other influences arising from the cells of the affected subjects, we transiently expressed the five cDNAs individually into HeLa cells for comparison with wild-type hSERT. Regulation of hSERT activity by intracellular signalling pathways can be lost or altered if the transporter expression level greatly exceeds that found in natively expressing cells. Thus, we titrated hSERT plasmids used for transfection to achieve a level of expression comparable to that found in cultured lymphoblast lines and mast cells. With saturation kinetic studies (table 1), we observe significant reductions in 5-HT KM for all variants; Ile425Leu, Phe465Leu and Leu550Val also exhibit significantly elevated transport capacity (Vmax). The 5-HT transport activity of all variants retains sensitivity to the highly specific hSERT antagonist paroxetine, and thus we attribute the gain-of-function properties to the introduced constructs, rather than ectopic stimulation of non-specific uptake systems.
Table 1.
Kinetic impact of autism-associated SERT-coding variants. (Values are expressed as the mean of at least three experiments±s.e.m. *p<0.05, **p<0.01 versus hSERT control, Student's t-test.)
| variant | Vmax | KM |
|---|---|---|
| hSERT | 406.7±13 | 0.98±0.12 |
| G56A | 404.3±10 | 0.59±0.17* |
| I425L | 773.9±23** | 0.45±0.11** |
| F465L | 667.2±12** | 0.68±0.09* |
| L550V | 597.7±11* | 0.58±0.16* |
Analysis of whole-cell radioligand-binding experiments with hSERT-transfected cells reveals a striking difference for the three novel variants in relative surface expression (figure 2a,b). Thus, whereas Ile425Leu, Phe465Leu and Leu550Val display equivalent levels of total SERT protein, as inferred from paroxetine-sensitive [125I]RTI-55 binding to transfected cells (figure 2a), these variants exhibit significantly enhanced binding of the radioligand under conditions (5-HT displacement, 4°C) adopted to reveal the surface pool of transporters (figure 2b). These data suggest that a significant component of the elevation of SERT activity seen in transport studies for these variants arises from altered surface trafficking.
Figure 2.
Analysis of protein expression of autism-associated SERT-coding variants. Impact of SERT-coding variants on total and cell surface [125I]RTI-55 binding. HeLa cells transiently transfected with hSERT or one of the SERT-coding variants were subjected to intact cell-binding assays at 4°C with the cocaine analogue [125I]RTI-55 (5 nM) as described in §2. (a) Total binding values as defined with paroxetine (10 μM) as displacer. (b) Surface labelling by [125I]RTI-55 as defined with 5-HT (100 μM) as displacer. In vehicle-treated cells, hSERT total binding (fmol×10−5) was 583.7±20.6, and the surface binding was 175.7±11.7. Results for (a,b) reflect mean±s.e.m. of three separate experiments normalized to hSERT (100%). Binding levels were analysed via a one-way ANOVA followed by post hoc Dunnett's tests comparing mutant means to hSERT, with *p<0.05 taken as significant. (c) Immunoblots of total cell extracts prepared from HeLa cells transfected with hSERT or one of the variants described in the study. (d) Cell surface expression alterations in hSERT Gly56Ala, Ile425Leu, Phe465Leu and Leu550Val. Variants were transfected in parallel with hSERT into HeLa cells and cell surface transporters identified by immunoblotting of biotinylated samples, captured as described in §2. Quantitative estimations of relative (e) total and (f) surface density of hSERT, Gly56Ala, Ile425Leu, Phe465Leu and Leu550Val based on densitometry of biotinylation immunoblots. Data reflect mean values of three separate experiments ±s.e.m. Means were compared with a one-way ANOVA followed by Dunnett's test to compare variant surface expression with that achieved with hSERT, with p<0.05 taken as significant (*significantly elevated versus hSERT, p<0.05; NT, non-transfected.)
To gain further support for surface trafficking alterations, we biotinylated transfected cells using the membrane-impermeant reagent NHS-SS biotin and compared the level of SERT proteins in total and biotinylated (surface) fractions by immunoblotting with a human SERT-specific antibody. Our studies demonstrate that as with total SERT binding, total protein levels for all hSERT variants were equivalent to wild-type hSERT (figure 2c,e), whereas surface proteins were significantly elevated for Ile425Leu, Phe465Leu and Leu550Val (figure 2d,f). In these experiments, we actually observed diminished Gly56Ala SERT protein in surface membranes when compared with wild-type SERT. These findings reinforce our contention that the gain-of-function transport property of the Gly56Ala variant arises from catalytic activation and that gain of catalytic activity can, unexpectedly, accompany a reduction in surface expression. Additionally, they ascribe to Ile452Leu, Phe465Leu and Leu550Val a mechanistic perturbation distinct from that impacting Gly56Ala, which leads to elevated transporter surface expression.
Previously, we demonstrated that the hSERT variants Gly56Ala and Lys605Asn not only display a gain of transport activity but also exhibit a striking loss of regulation through PKG and p38 MAPK pathways (Prasad et al. 2005). To determine whether this is a common property of other autism-associated hSERT variants, we returned to the HeLa model to explore sensitivity of variants to 8Br-cGMP or anisomycin, which activate PKG or p38 MAPK, respectively. As described previously, wild-type SERT displays an elevation of activity in response to these agents that can be attenuated by PKG or p38 MAPK inhibitors, whereas hSERT Gly56Ala is insensitive to these agents (figure 3a,b). In contrast to Gly56Ala, the cells expressing Ile425Leu, Phe465Leu and Leu550Val exhibit significant PKG- and p38 MAPK-stimulated SERT activity, though percentage stimulation was not as great as that seen with wild-type SERT, possibly a consequence of their elevated basal function. Regardless, these findings are consistent with a distinct mechanism supporting the functional perturbations leading to the gain-of-function alterations exhibited by Ile425Leu, Phe465Leu and Leu550Val.
Figure 3.
Impact of 8Br-cGMP and p38 MAPK on SERT activity of autism-associated hSERT-coding variants. (a) Activity modulation. HeLa cells transfected with hSERT or autism-associated hSERT-coding variants were examined for 5-HT transport activities as described in §2 following pretreatments of cells with either 100 μM 8Br-cGMP or vehicle for 1 hour. Parallel wells were treated with the PKG inhibitor H8 (10 μM) to validate specificity. (b) Altered p38 MAPK-dependent regulation of hSERT in transfected HeLa cells. The cells transfected with hSERT or autism-associated hSERT-coding variants were examined for 5-HT transport activities as described in §2 following pretreatments of cells with either 1 μM anisomycin or vehicle for 10 min. Parallel wells were treated with the p38 MAPK inhibitor SB203580 (1 μM) to validate specificity. Results reflect mean±s.e.m. of three separate experiments normalized to each mutant's control measured under vehicle-treated conditions (100%). Results in (a,b) reflect mean ±s.e.m. of three separate experiments normalized to each mutant's level under vehicle-treated conditions (100%). Data were analysed by a one-way ANOVA with post hoc Bonferroni tests comparing variant with hSERT 8Br-cGMP/anisomycin responses, with *p<0.05 taken as significant.
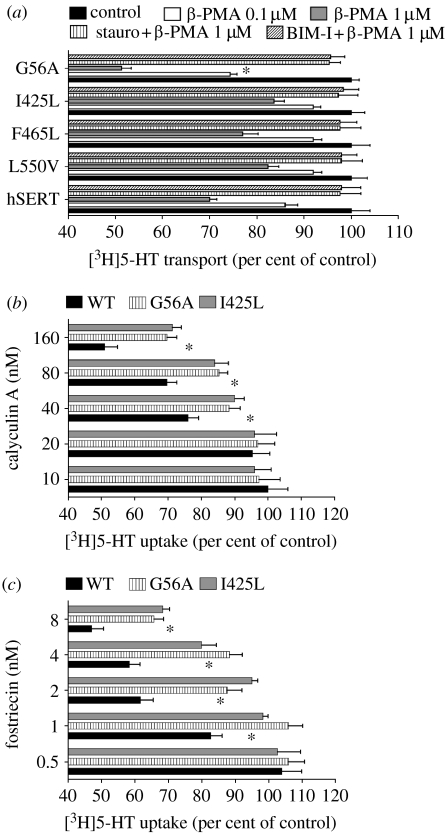
Activation of protein kinase C (PKC) with phorbol esters such as β-PMA leads to a rapid reduction in 5-HT transport capacity and surface expression in hSERT-transfected cells (Qian et al. 1997), a sensitivity significantly enhanced with the Gly56Ala and Lys605Asn mutants (Prasad et al. 2005). To examine whether the novel autism coding variants display similar or distinct patterns of regulation to PKC activators as seen with Gly56Ala, wild-type- and mutant-transfected cells were treated with β-PMA (0.1–1 μM, 30 min). As shown in figure 4a, wild-type (WT) hSERT displays under these conditions a dose-dependent sensitivity to β-PMA that can be blocked by either staurosporine or bisindolylmaleimide (BIM). Under these conditions, 1 μM β-PMA induces an approximately 30 per cent loss of SERT activity. As described previously (Prasad et al. 2005), the Gly56Ala variant exhibits increased sensitivity to β-PMA, with an approximately 50 per cent loss of function evident under the same conditions. By contrast, the Ile425Leu, Phe465Leu and Leu550Val variants displayed comparable or even slightly reduced sensitivity to β-PMA when compared with wild-type hSERT.
Figure 4.
Impact of PKC and PP2A on SERT activity of autism-associated hSERT-coding variants. (a) Activity modulation. HeLa cells transfected with hSERT or autism-associated hSERT-coding variants were examined for 5-HT transport activities as described in §2 following pretreatments of cells with either vehicle or 0.1 or 1 μM β-PMA for 30 min. Parallel wells were treated with the PKC inhibitors staurosporine (stauro; 10 μM) or bisindolylmaleimide (BIM, 10 μM). Altered PP2A-dependent regulation of hSERT in transfected HeLa cells. (c) The cells transfected with hSERT or autism-associated hSERT-coding variants were examined for 5-HT transport activities as described in §2 following pretreatments of cells with increasing concentrations of (b) calyculin A or (c) fostriecin for 10 min. Results reflect mean±s.e.m. of three separate experiments normalized to each mutant's control measured under vehicle-treated conditions (100%). Results in (a–c) reflect mean±s.e.m. of three separate experiments normalized to each mutant's level under vehicle-treated conditions (100%). Data were analysed by a one-way ANOVA with post hoc Bonferroni tests comparing the variant with hSERT responses, with *p<0.05 taken as significant.
The catalytic subunit of the serine/threonine phosphatase PP2A (PP2Ac) has been found to physically associate with SERTs in brain and transfected cells (Bauman et al. 2000). PP2A inhibitors that bind to and inhibit PP2Ac, including okadaic acid, calyculin A and fostriecin, also trigger hSERT phosphorylation, reduced surface expression and loss of SERT activity (Blakely & Bauman 2000), presumably due to loss of SERT-associated phosphatase activity that can attenuate SERT phosphorylation and internalization triggered by kinases such as PKC. Stimulatory phosphorylation, such as that triggered by PKG and p38 MAPK, may also depend upon SERT-associated PP2A activity to suppress phosphorylation at inhibitory sites (Samuvel et al. 2005; Ramamoorthy et al. 2007; Zhang et al. 2007). To determine whether PP2A dysregulation might contribute commonly or uniquely to one or more of the autism-associated SERT variants, we treated transfected cells with increasing concentrations of the two most selective PP2Ac inhibitors available: calyculin A and fostriecin. As shown in figure 4b,c, both drugs demonstrate dose-dependent inhibition of 5-HT transport activity for wild-type hSERT. This effect is significantly enhanced over the more modest sensitivity displayed by either Gly56Ala or Ile425Val.
hSERTs that are transiently transfected into HeLa cells express transporters in a reduced, heterologous system, where the changes in function, pharmacology and regulation can be rapidly assessed. Although this is a powerful paradigm, it is not without its limitations, chiefly the lack of sustained expression that can allow for the impact of chronically expressed hSERT variants to be evaluated. Typical stable cell models have the disadvantage of integration of transfected DNAs at different genomic loci for each cell line produced, introducing clone-to-clone variability in the levels of expression dictated by cis-acting transcriptional mechanisms unique to each site of integration. We therefore created stable cell lines that express the wild-type, Gly56Ala and Ile425Leu hSERT alleles at a common genomic locus (CHO-Flp-In cells). As observed with transient expression studies, stably expressing Gly56Ala cells demonstrate elevated function versus the 5-HT transport achieved by wild-type hSERT (figure 5a). Surprisingly, we found Ile425Leu cells to show a small reduction in 5-HT transport function. Exploration of the basis for these changes via western blots of total and surface proteins yielded even more remarkable results. In total extracts, we found that both Gly56Ala and Ile425Leu expression to be significantly reduced relative to wild-type hSERT (figure 5b). Despite elevated transport activity, Gly56Ala total protein levels were actually only approximately 50 per cent of wild-type, whereas Ileu425Leu expression was only approximately 25 per cent of wild-type. Surface expression of hSERT proteins was similarly reduced for both variants relative to wild-type SERT (figure 4c), and the losses here were even more profound. Thus, Gly56Ala and Ile425Leu reached only approximately 45 per cent and approximately 15 per cent of wild-type SERT surface expression, respectively. When we normalized 5-HT transport for the levels of surface expression in the same cells, we demonstrated as expected that Gly56Ala proteins exhibit enhanced catalytic function, with a turnover rate of approximately 250 per cent that of wild-type SERT (figure 4g). Ile425Leu was also strikingly elevated in its catalytic rate by this measure, achieving turnover rates equal to or slightly above those of Gly56Ala.
Figure 5.
Altered activity of SERT variants is differently exhibited in CHO-Flp-In stable cells. (a) 5-HT transport activity of autism-associated SERT-coding variants in CHO-Flp-In stable cells. All variants were assayed for 5-HT transport activity as described in §2. Data reflect mean±s.e.m. of three separate experiments. Means for variants were compared with hSERT using a one-way ANOVA followed by Dunnett's test of individual means against hSERT values, with *p<0.05 taken as significant. (b) Immunoblots of total cell extracts prepared from CHO-Flp-In stable cells expressing hSERT or one of the variants described in the study. (c) Cell surface expression alterations in hSERT, Gly56Ala and Ile425Leu. Cell surface transporters were identified by immunoblotting of biotinylated samples, captured as described in §2. Quantitative estimations of relative (d) total and (e) surface density of hSERT, Gly56Ala and Ile425Leu based on densitometry of biotinylation immunoblots. Data reflect mean values of three separate experiments±s.e.m. Means were compared with a one-way ANOVA followed by Dunnett's test to compare variant surface expression with that achieved with hSERT, with *p<0.05 taken as significant. (f) Gly56Ala and Ile425leu proteins exhibit enhanced catalytic function, with a turnover rate of approximately 250% or more than that of wild-type SERT. Data reflect mean values of three separate experiments±s.e.m. Means were compared with a one-way ANOVA followed by Dunnett's test.
4. Discussion
In this report, we extend our initial analyses of autism-associated rare SERT variants (Prasad et al. 2005; Sutcliffe et al. 2005) and reveal for the first time a shared cellular phenotype among them, elevated 5-HT transport function. This gain of function is evident in both natively expressing lymphocytes derived from autism subjects and in transfected cells, particularly when mutants are expressed transiently. Although the Gly56Ala and Ile425Leu variants continue to demonstrate elevated catalytic function in stably transfected CHO-Flp-In cells, where targeting occurs to the same genomic locus, both mutants demonstrate a striking reduction in steady-state protein levels and surface expression that may indicate a detrimental effect of chronically elevated hSERT function. In the CHO-Flp-In model, expression is driven at high levels by an integrated viral promoter and either the high level of expression or the distinct CHO environment could contribute to an overall negative impact of mutant expression. Studies with integrated variants at natively expressing sites, such as those afforded with knock-in mice, are now needed to provide an assessment of how the different cellular environments known to harbour SERT react to these mutants. Such models will also permit the analysis of mutant impact on brain development, neurochemistry or function, which is exceedingly difficult, if not impossible, with human carriers.
As SERT is expressed in the midgestation embryo in rodents and primates (Schroeter & Blakely 1996; Sodhi & Sanders-Bush 2004), the consequences of heritable changes in SERT structure would probably arise during development, though they may also contribute to ongoing behavioural deficits in older subjects as changes were evident in lymphocyte lines prepared from the affected subjects. Indeed, the use of SSRI medications for the treatment of some facets of autism indicate that ongoing dysfunction in 5-HT networks may be an enduring facet of idiopathic autism and gives hope that further inspection of signalling deficits attributable to rare SERT variants can provide a meaningful advance in autism therapeutics. Rodent studies demonstrate a significant effect on brain structure (Persico et al. 2003; Altamura et al. 2007) and behaviour (Holmes et al. 2002, 2003) following genetic or pharmacological manipulations of SERT and/or 5-HT during development (Whitaker-Azmitia et al. 1996; Bengel et al. 1998). In this regard, recent studies examining a common SERT promoter polymorphism (5HTTLPR) have linked autism-associated changes in brain size to altered SERT expression (Wassink et al. 2007). Possibly, the gain of function changes in SERT activity could be compounded by the purportedly higher functioning l/l genotype of the 5HTTLPR. However, the impact of the 5HTTLPR is significantly affected by environment (Caspi et al. 2003) and the 5HTTLPR also carries additional allelic variation recently demonstrated to modify penetrance of the l allele (Hu et al. 2006). Also, both the l and s alleles of the 5HTTLPR have been associated with autism (Veenstra-VanderWeele et al. 2004), reinforcing a need for phenotypic studies of pedigrees carrying highly penetrant, functional SERT alleles since these may be less susceptible to moderation by environmental factors.
The four SERT variants we have investigated in this report localize to two distinct domains. Gly56Ala and Lys605Asn (Prasad et al. 2005) localize to cytoplasmic amino and carboxy termini. By contrast, the Ile425Leu, Phe465Leu and Leu550Val variants are found within TM domains. Ile425Leu is found in TM8, which contributes to structural elements of the 5-HT binding pocket (Henry et al. 2006). Phe465Leu (TM9) and Leu550Val (TM11), though highly conserved across the SLC6 gene family, are localized to domains without, as yet, ascribed function. As such, perturbations of trafficking evident with these variants may, collectively, derive from stabilized conformations that dictate intracellular trafficking decisions. Regulation of SERT proteins through cytoplasmic domains might be expected to impact both regulation of transport by cellular signalling pathways and transporter trafficking, whereas the TM domain mutants might appear to be more likely to support catalytic modulation. The evidence from the studies we present here suggests a more complex picture. Whereas the cytoplasmic domain mutants clearly perturb PKG, p38 MAPK and PKC regulation, these are complex regulatory stimuli that engage both catalytic modulation and trafficking pathways. We (Zhu et al. 2004b, 2007) and others (Zhu et al. 2005; Ramamoorthy et al. 2007; Zhang et al. 2007) have demonstrated that basal and activated PKG supports transporter trafficking to the plasma membrane, whereas p38 MAPK and PKC support both trafficking and catalytic modulation (Jayanthi et al. 2005; Samuvel et al. 2005; Zhu et al. 2005). Since we have shown that PKG activation leads to p38 MAPK-dependent catalytic modulation of SERTs (Zhu et al. 2004a), these pathways are not independent. Interestingly, our studies with PP2Ac antagonist sensitivity suggest that this SERT-associated phosphatase may hold a key to how mutations in either TM or cytoplasmic domains can impact 5-HT transport KM but have differential effects on membrane trafficking. For example, altered PP2Ac associations, established through direct or indirect conformational alterations in PP2Ac-binding sites (as yet undefined), could lead to the observed elevated constitutive SERT phosphorylation at a SERT catalytic regulatory site controlled by PKG indirectly through p38 MAPK, also predicting the observed lack of further PKG phosphorylation (Prasad et al. 2005). Such a deficit could also lead to unattenuated phorbol ester-dependent, hSERT regulation by PKC which we document in the Gly56Ala and Lys605Asn mutants (Prasad et al. 2005). For the hSERT TM mutants, loss of PP2Ac associations would presumably arise through indirect conformational adjustments that indirectly contribute to enhanced basal PKG/p38 MAPK modulation of SERT trafficking.
Autism is a disorder diagnosed through behavioural assessments and it is reasonable to seek causative explanations for the contributions of the hSERT variants we have described to altered brain function. However, SERTs are widely distributed, and thus, at this time, it is not possible to ascribe behavioural changes to a specific cellular insult (such as altered 5-HT signalling in the brain). Indeed, we view the contributions of the studies presented here not as much as a guide to how altered SERT function may disrupt the developmental roles of 5-HT in the developing CNS, but rather as an opportunity to elucidate a mechanistically linked gene network where other disease risk alleles may be identified and where therapeutic opportunities may reside. As one example, we have recently gained evidence that autism subjects harbour at least one novel, hyperactive variant in the A3 adenosine receptor (ADORA3), a receptor whose stimulation leads to activation of PKG and p38 MAPK in the brain and periphery as well as SERT stimulation (Zhu et al. 2007). NOS1 proteins have recently been found to associate with SERTs (Chanrion et al. 2007), and we have shown that NOS activation is a critical step between A3 adenosine receptor activation and SERT stimulation; further focus on NOS1 structural and regulatory pathways seem worthy of investment for additional insights into autism risk. Finally, we recently documented the physical association and regulation of SERT by integrin β3 (Carneiro et al. 2008). Integrin β3 has been associated with whole blood hyperserotonaemia (Weiss et al. 2004) and with autism (Weiss et al. 2006). Notably, coexpression of a rare, hyperfunctional integrin β3 variant stimulates hSERT function in co-transfected cells (Carneiro et al. 2008). Together, these examples reinforce the use of network-oriented variant analyses (NOVA) to ascribe cumulative genetic risk for complex behavioural disorders such as autism.
Acknowledgments
The authors thank Tammy Jessen, Jane Wright, Qiao Han, Angela Steele and Tracy Moore-Jarrett for their excellent technical support. This research was supported by a NARSAD YI Award to H.C.P., and NIH Awards NS049261 to J.S.S. and DA07390 and MH078028 to R.D.B.
Footnotes
One contribution of 16 to a Discussion Meeting Issue ‘Membrane transport in flux: the ambiguous interface between channels and pumps’.
References
- Altamura C., Dell'Acqua M.L., Moessner R., Murphy D.L., Lesch K.P., Persico A.M. Altered neocortical cell density and layer thickness in serotonin transporter knockout mice: a quantitation study. Cereb. Cortex. 2007;17:1394–1401. doi: 10.1093/cercor/bhl051. doi:10.1093/cercor/bhl051 [DOI] [PubMed] [Google Scholar]
- Balkovetz D.F., Tiruppathi C., Leibach F.H., Mahesh V.B., Ganapathy V. Evidence for an imipramine-sensitive serotonin transporter in human placental brush-border membranes. J. Biol. Chem. 1989;264:2195–2198. [PubMed] [Google Scholar]
- Bauman A.L., Apparsundaram S., Ramamoorthy S., Wadzinski B.E., Vaughan R.A., Blakely R.D. Cocaine and antidepressant-sensitive biogenic amine transporters exist in regulated complexes with protein phosphatase 2A. J. Neurosci. 2000;20:7571–7578. doi: 10.1523/JNEUROSCI.20-20-07571.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengel D., Murphy D.L., Andrews A.M., Wichems C.H., Feltner D., Heils A., Mossner R., Westphal H., Lesch K.P. Altered brain serotonin homeostasis and locomotor insensitivity to 3,4-methylenedioxymetamphetamine (“ecstasy”) in serotonin transporter-deficient mice. Mol. Pharmacol. 1998;53:649–655. doi: 10.1124/mol.53.4.649. [DOI] [PubMed] [Google Scholar]
- Blakely R.D., Bauman A.L. Biogenic amine transporters: regulation in flux. Curr. Opin. Neurobiol. 2000;10:328–336. doi: 10.1016/s0959-4388(00)00088-x. doi:10.1016/S0959-4388(00)00088-X [DOI] [PubMed] [Google Scholar]
- Blakely R.D., DeFelice L.J., Galli A. Biogenic amine neurotransmitter transporters: just when you thought you knew them. Physiology (Bethesda) 2005;20:225–231. doi: 10.1152/physiol.00013.2005. [DOI] [PubMed] [Google Scholar]
- Cantor R., Kono N., Duvall J., Alvarez-Retuerto A., Stone J., Alarcon J., Nelson S., Geschwind D. Replication of autism linkage: fine-mapping peak at 17q21. Am. J. Hum. Genet. 2005;76:1050–1056. doi: 10.1086/430278. doi:10.1086/430278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro A.M., Blakely R.D. Serotonin, protein kinase C and HIC-5 associated redistribution of the platelet serotonin transporter. J. Biol. Chem. 2006;281:24 769–24 780. doi: 10.1074/jbc.M603877200. doi:10.1074/jbc.M603877200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro A.M., Cook E.H., Murphy D.L., Blakely R.D. Interactions between integrin alphaIIbbeta3 and the serotonin transporter regulate serotonin transport and platelet aggregation in mice and humans. J. Clin. Invest. 2008;118:1544–1552. doi: 10.1172/JCI33374. doi:10.1172/JCI33374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A., et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. doi:10.1126/science.1083968 [DOI] [PubMed] [Google Scholar]
- Chanrion B., Mannoury la Cour C., Lerner-Natoli M., Freissmuth M., Millan M., Bockaert J., Marin P. Physical interaction between the serotonin transporter and neuronal nitric oxide synthase underlies reciprocal modulation of their activity. Proc. Natl Acad. Sci. USA. 2007;104:8119–8124. doi: 10.1073/pnas.0610964104. doi:10.1073/pnas.0610964104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook E.H., Jr, Leventhal B.L. The serotonin system in autism. Curr. Opin. Pediatr. 1996;8:348–354. doi: 10.1097/00008480-199608000-00008. doi:10.1097/00008480-199608000-00008 [DOI] [PubMed] [Google Scholar]
- Cook E.H., Jr, Courchesne R., Lord C., Cox N.J., Yan S., Lincoln A., Haas R., Courchesne E., Leventhal B.L. Evidence of linkage between the serotonin transporter and autistic disorder. Mol. Psychiatry. 1997;2:247–250. doi: 10.1038/sj.mp.4000266. doi:10.1038/sj.mp.4000266 [DOI] [PubMed] [Google Scholar]
- Cross S., Kim S., Weiss L., Delahanty R., Sutcliffe J., Levental B., Cook E.J., Veenstra-VanderWeele J. Molecular genetics of the platelet serotonin system in first-degree relatives of patients with autism. Neuropsychopharmacology. 2008;33:353–360. doi: 10.1038/sj.npp.1301406. doi:10.1038/sj.npp.1301406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraj B.A., Olkowski Z.L., Jackson R.T. Expression of a high-affinity serotonin transporter in human lymphocytes. Int. J. Immunopharmacol. 1994;16:561–567. doi: 10.1016/0192-0561(94)90107-4. doi:10.1016/0192-0561(94)90107-4 [DOI] [PubMed] [Google Scholar]
- Glatt C.E., DeYoung J.A., Delgado S., Service S.K., Giacomini K.M., Edwards R.H., Risch N., Freimer N.B. Screening a large reference sample to identify very low frequency sequence variants: comparisons between two genes. Nat. Genet. 2001;27:435–438. doi: 10.1038/86948. doi:10.1038/86948 [DOI] [PubMed] [Google Scholar]
- Gordon J., Barnes N.M. Lymphocytes transport serotonin and dopamine: agony or ecstasy? Trends Immunol. 2003;24:438–443. doi: 10.1016/s1471-4906(03)00176-5. doi:10.1016/S1471-4906(03)00176-5 [DOI] [PubMed] [Google Scholar]
- Henry L.K., DeFelice L.J., Blakely R.D. Getting the message across: a recent transporter structure shows the way. Neuron. 2006;49:791–796. doi: 10.1016/j.neuron.2006.03.002. doi:10.1016/j.neuron.2006.03.002 [DOI] [PubMed] [Google Scholar]
- Holmes A., Murphy D.L., Crawley J.N. Reduced aggression in mice lacking the serotonin transporter. Psychopharmacology (Berl.) 2002;161:160–167. doi: 10.1007/s00213-002-1024-3. doi:10.1007/s00213-002-1024-3 [DOI] [PubMed] [Google Scholar]
- Holmes A., Murphy D.L., Crawley J.N. Abnormal behavioral phenotypes of serotonin transporter knockout mice: parallels with human anxiety and depression. Biol. Psychiatry. 2003;54:953–959. doi: 10.1016/j.biopsych.2003.09.003. doi:10.1016/j.biopsych.2003.09.003 [DOI] [PubMed] [Google Scholar]
- Hu X.Z., et al. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am. J. Hum. Genet. 2006;78:815–826. doi: 10.1086/503850. doi:10.1086/503850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayanthi L.D., Samuvel D.J., Blakely R.D., Ramamoorthy S. Evidence for biphasic effects of protein kinase C on serotonin transporter function, endocytosis, and phosphorylation. Mol. Pharmacol. 2005;67:2077–2087. doi: 10.1124/mol.104.009555. doi:10.1124/mol.104.009555 [DOI] [PubMed] [Google Scholar]
- Kekuda R., Prasad P.D., Wu X.A., Wang H.P., Fei Y.J., Leibach F.H., Ganapathy V. Cloning and functional characterization of a potential-sensitive, polyspecific organic cation transporter (OCT3) most abundantly expressed in placenta. J. Biol. Chem. 1998;273:15 971. doi: 10.1074/jbc.273.26.15971. doi:10.1074/jbc.273.26.15971 [DOI] [PubMed] [Google Scholar]
- Kolevzon A., Mathewson K., Hollander E. Selective serotonin reuptake inhibitors in autism: a review of efficacy and tolerability. J. Clin. Psychiatry. 2006;67:407–414. doi: 10.4088/jcp.v67n0311. [DOI] [PubMed] [Google Scholar]
- Lesch K.P., Wolozin B.L., Murphy D.L., Riederer P. Primary structure of the human platelet serotonin uptake site: identity with the brain serotonin transporter. J. Neurochem. 1993;60:2319–2322. doi: 10.1111/j.1471-4159.1993.tb03522.x. doi:10.1111/j.1471-4159.1993.tb03522.x [DOI] [PubMed] [Google Scholar]
- Lord C., Cook E.H., Leventhal B.L., Amaral D.G. Autism spectrum disorders. Neuron. 2000;28:355–363. doi: 10.1016/s0896-6273(00)00115-x. doi:10.1016/S0896-6273(00)00115-X [DOI] [PubMed] [Google Scholar]
- McCauley J.L., Olson L.M., Dowd M., Amin T., Steele A., Blakely R.D., Folstein S.E., Haines J.L., Sutcliffe J.S. Linkage and association analysis at the serotonin transporter (SLC6A4) locus in a rigid compulsive subset of autism. Am. J. Med. Genet. 2004;127B:104–112. doi: 10.1002/ajmg.b.20151. doi:10.1002/ajmg.b.20151 [DOI] [PubMed] [Google Scholar]
- Ozaki N., Goldman D., Kaye W.H., Plotnicov K., Greenberg B.D., Lappalainen J., Rudnick G., Murphy D.L. Serotonin transporter missense mutation associated with a complex neuropsychiatric phenotype. Mol. Psychiatry. 2003;8:933–936. doi: 10.1038/sj.mp.4001365. doi:10.1038/sj.mp.4001365 [DOI] [PubMed] [Google Scholar]
- Paczkowski N.J., Vuocolo H.E., Bryan-Lluka L.J. Conclusive evidence for distinct transporters for 5-hydroxytryptamine and noradrenaline in pulmonary endothelial cells of the rat. Naunyn Schmiedebergs Arch. Pharmacol. 1996;353:423–430. doi: 10.1007/BF00261439. [DOI] [PubMed] [Google Scholar]
- Persico A.M., Baldi A., Dell'Acqua M.L., Moessner R., Murphy D.L., Lesch K.P., Keller F. Reduced programmed cell death in brains of serotonin transporter knockout mice. Neuroreport. 2003;14:341–344. doi: 10.1097/00001756-200303030-00009. doi:10.1097/00001756-200303030-00009 [DOI] [PubMed] [Google Scholar]
- Piven J., Tsai G.C., Nehme E., Coyle J.T., Chase G.A., Folstein S.E. Platelet serotonin, a possible marker for familial autism. J. Autism. Dev. Disord. 1991;21:51–59. doi: 10.1007/BF02206997. doi:10.1007/BF02206997 [DOI] [PubMed] [Google Scholar]
- Prasad H.C., Zhu C.B., McCauley J.L., Samuvel D.J., Ramamoorthy S., Shelton R.C., Hewlett W.A., Sutcliffe J.S., Blakely R.D. Human serotonin transporter variants display altered sensitivity to protein kinase G and p38 mitogen-activated protein kinase. Proc. Natl Acad. Sci. USA. 2005;102:11 545–11 550. doi: 10.1073/pnas.0501432102. doi:10.1073/pnas.0501432102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y., Melikian H.E., Rye D.B., Levey A.I., Blakely R.D. Identification and characterization of antidepressant-sensitive serotonin transporter proteins using site-specific antibodies. J. Neurosci. 1995;15:1261–1274. doi: 10.1523/JNEUROSCI.15-02-01261.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y., Galli A., Ramamoorthy S., Risso S., DeFelice L.J., Blakely R.D. Protein kinase C activation regulates human serotonin transporters in HEK-293 cells via altered cell surface expression. J. Neurosci. 1997;17:45–47. doi: 10.1523/JNEUROSCI.17-01-00045.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthy S., Bauman A.L., Moore K.R., Han H., Yang-Feng T., Chang A.S., Ganapathy V., Blakely R.D. Antidepressant- and cocaine-sensitive human serotonin transporter: molecular cloning, expression, and chromosomal localization. Proc. Natl Acad. Sci. USA. 1993;90:2542–2546. doi: 10.1073/pnas.90.6.2542. doi:10.1073/pnas.90.6.2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthy S., Samuvel D.J., Buck E.R., Rudnick G., Jayanthi L.D. Phosphorylation of threonine residue 276 is required for acute regulation of serotonin transporter by cyclic GMP. J. Biol. Chem. 2007;282:11 639–11 647. doi: 10.1074/jbc.M611353200. doi:10.1074/jbc.M611353200 [DOI] [PubMed] [Google Scholar]
- Samuvel D.J., Jayanthi L.D., Bhat N.R., Ramamoorthy S. A role for p38 mitogen-activated protein kinase in the regulation of the serotonin transporter: evidence for distinct cellular mechanisms involved in transporter surface expression. J. Neurosci. 2005;25:29–41. doi: 10.1523/JNEUROSCI.3754-04.2005. doi:10.1523/JNEUROSCI.3754-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter S., Blakely R.D. Drug targets in the embryo. Studies on the cocaine- and antidepressant-sensitive serotonin transporter. Ann. NY Acad. Sci. 1996;801:239–255. doi: 10.1111/j.1749-6632.1996.tb17446.x. doi:10.1111/j.1749-6632.1996.tb17446.x [DOI] [PubMed] [Google Scholar]
- Schroeter S., Levey A.I., Blakely R.D. Polarized expression of the antidepressant-sensitive serotonin transporter in epinephrine-synthesizing chromaffin cells of the rat adrenal gland. Mol. Cell. Neurosci. 1997;9:170–184. doi: 10.1006/mcne.1997.0619. doi:10.1006/mcne.1997.0619 [DOI] [PubMed] [Google Scholar]
- Sodhi M.S.K., Sanders-Bush E. Serotonin and brain development. Int. Rev. Neurobiol. 2004;59:111–174. doi: 10.1016/S0074-7742(04)59006-2. doi:10.1016/S0074-7742(04)59006-2 [DOI] [PubMed] [Google Scholar]
- Stone J.L., Merriman B., Cantor R.M., Yonan A.L., Gilliam T.C., Geschwind D.H., Nelson S.F. Evidence for sex-specific risk alleles in autism spectrum disorder. Am. J. Hum. Genet. 2004;75:1117–1123. doi: 10.1086/426034. doi:10.1086/426034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J.S., Delahanty R.J., Prasad H.C., McCauley J.L., Han Q., Jiang L., Li C., Folstein S.E., Blakely R.D. Allelic heterogeneity at the serotonin transporter locus (SLC6A4) confers susceptibility to autism and rigid-compulsive behaviors. Am. J. Hum. Genet. 2005;77:265–279. doi: 10.1086/432648. doi:10.1086/432648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Naarden Braun K., et al. Evaluation of a methodology for a collaborative multiple source surveillance network for autism spectrum disorders-autism and developmental disabilities monitoring network, 14 sites, United States, 2002. MMWR Surveill Summ. 2007;56:29–40. [PubMed] [Google Scholar]
- Veenstra-VanderWeele J., Christian S., Cook E.J. Autism as a paradigmatic complex genetic disorder. Annu. Rev. Genomics Hum. Genet. 2004;5:379–405. doi: 10.1146/annurev.genom.5.061903.180050. doi:10.1146/annurev.genom.5.061903.180050 [DOI] [PubMed] [Google Scholar]
- Wassink T.H., Hazlett H.C., Epping E.A., Arndt S., Dager S.R., Schellenberg G.D., Dawson G., Piven J. Cerebral cortical gray matter overgrowth and functional variation of the serotonin transporter gene in autism. Arch. Gen. Psychiatry. 2007;64:709–717. doi: 10.1001/archpsyc.64.6.709. doi:10.1001/archpsyc.64.6.709 [DOI] [PubMed] [Google Scholar]
- Weiss L.A., et al. Genome-wide association study identifies ITGB3 as a QTL for whole blood serotonin. Eur. J. Hum. Genet. 2004;12:949–954. doi: 10.1038/sj.ejhg.5201239. doi:10.1038/sj.ejhg.5201239 [DOI] [PubMed] [Google Scholar]
- Weiss L.A., Kosova G., Delahanty R.J., Jiang L., Cook E.H., Ober C., Sutcliffe J.S. Variation in ITGB3 is associated with whole-blood serotonin level and autism susceptibility. Eur. J. Hum. Genet. 2006;14:923–931. doi: 10.1038/sj.ejhg.5201644. doi:10.1038/sj.ejhg.5201644 [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia P.M., Druse M., Walker P., Lauder J.M. Serotonin as a developmental signal. Behav. Brain Res. 1996;73:19–29. doi: 10.1016/0166-4328(96)00071-x. doi:10.1016/0166-4328(96)00071-X [DOI] [PubMed] [Google Scholar]
- Zhang Y.W., Gesmonde J., Ramamoorthy S., Rudnick G. Serotonin transporter phosphorylation by cGMP-dependent protein kinase is altered by a mutation associated with obsessive compulsive disorder. J. Neurosci. 2007;27:10 878–10 886. doi: 10.1523/JNEUROSCI.0034-07.2007. doi:10.1523/JNEUROSCI.0034-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M., Engel K., Wang J. Evidence for significant contribution of a newly identified monoamine transporter (PMAT) to serotonin uptake in the human brain. Biochem. Pharmacol. 2007;73:147–154. doi: 10.1016/j.bcp.2006.09.008. doi:10.1016/j.bcp.2006.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C., Hewlett W., Feoktistov I., Biaggioni I., Blakely R.D. Adenosine receptor, protein kinase G, and p38 mitogen-activated protein kinase-dependent up-regulation of serotonin transporters involves both transporter trafficking and activation. Mol. Pharmacol. 2004a;65:1462–1474. doi: 10.1124/mol.65.6.1462. doi:10.1124/mol.65.6.1462 [DOI] [PubMed] [Google Scholar]
- Zhu C.B., Hewlett W.A., Feoktistov I., Biaggioni I., Blakely R.D. Adenosine receptor, protein kinase G, and p38 mitogen-activated protein kinase-dependent up-regulation of serotonin transporters involves both transporter trafficking and activation. Mol. Pharmacol. 2004b;65:1462–1474. doi: 10.1124/mol.65.6.1462. doi:10.1124/mol.65.6.1462 [DOI] [PubMed] [Google Scholar]
- Zhu C.B., Carneiro A.M., Dostmann W.R., Hewlett W.A., Blakely R.D. p38 MAPK activation elevates serotonin transport activity via a trafficking-independent, protein phosphatase 2A-dependent process. J. Biol. Chem. 2005;280:15 649–15 658. doi: 10.1074/jbc.M410858200. doi:10.1074/jbc.M410858200 [DOI] [PubMed] [Google Scholar]
- Zhu C.B., Steiner J.A., Munn J.L., Daws L.C., Hewlett W.A., Blakely R.D. Rapid stimulation of presynaptic serotonin transport by A3 adenosine receptors. J. Pharmacol. Exp. Ther. 2007;322:332–340. doi: 10.1124/jpet.107.121665. doi:10.1124/jpet.107.121665 [DOI] [PubMed] [Google Scholar]