Abstract
KCNJ11-encoded Kir6.2 assembles with ATP-binding cassette sulphonylurea receptors to generate ATP-sensitive K+ (KATP) channel complexes. Expressed in tissues with dynamic metabolic flux, these evolutionarily conserved yet structurally and functionally unique heteromultimers serve as high-fidelity rheostats that adjust membrane potential-dependent cell functions to match energetic demand. Genetic defects in channel subunits disrupt the cellular homeostatic response to environmental stress, compromising organ tolerance in the adult. As maladaptation characterizes malignant KATP channelopathies, establishment of platforms to examine progression of KATP channel-dependent adaptive behaviour is warranted. Chimeras provide a powerful tool to assay the contribution of genetic variance to stress intolerance during prenatal or post-natal development. Here, KCNJ11 KATP channel gene knockout↔wild-type chimeras were engineered through diploid aggregation. Integration of wild-type embryonic stem cells into zona pellucida-denuded morula derived from knockout embryos achieved varying degrees of incorporation of stress-tolerant tissue within the KATP channel-deficient background. Despite the stress-vulnerable phenotype of the knockout, ex vivo derived mosaic blastocysts tolerated intrauterine transfer and implantation, followed by full-term embryonic development in pseudopregnant surrogates to produce live chimeric offspring. The development of adult chimerism from the knockout↔wild-type mosaic embryo offers thereby a new paradigm to probe the ecogenetic control of the KATP channel-dependent stress response.
Keywords: ATP-sensitive K+ channel, chimerism, ecogenetics, genetic variance, Kir6.2, stress
1. Introduction
From conception to senescence, environmental challenges pose ongoing threats to organismal integrity (Seley 1955; McEwen 2007). Decoding of the continuous influx of stress signals is integral to the initiation and execution of the adaptive, cytoprotective response that secures stress tolerance and promotes evolutionary survival (Chien 1999; Degterev & Yuan 2008). Biosensors have been recognized as essential components in distress resolution, matching demand and ensuring safeguard of organ function (Barki-Harrington & Rockman 2003; Zingman et al. 2003). Failure to respond to stress load, in the context of a genetic defect and malfunction in sensor proteins, results in maladaptation and poor outcome underlying the centrality of ecogenetic homeostasis in disease avoidance and species preservation (Zingman et al. 2002a; Ashcroft 2007; Olson et al. 2007).
The ATP-sensitive K+ (KATP) channel complex, a unique combination of an inward rectifier K+ channel and an ATP-binding cassette protein, is a prototypic metabolism-gated biosensor (Miki & Seino 2005; Nichols 2006; Zingman et al. 2007). KATP channels operate as high-fidelity molecular rheostats adjusting membrane potential-dependent functions to match cellular energetic demands (Terzic et al. 1995; Alekseev et al. 2005). Underscoring the critical role for KATP channels in coupling metabolic dynamics with electrical activity is the recognition that disruption of channel function is life threatening (Ashcroft 2005; Reyes et al. 2007). Dysfunction in KATP channel gating has been linked to insulin secretory disorders, namely congenital hyperinsulinism and neonatal diabetes (Thomas et al. 1995; Dunne et al. 2004; Gloyn et al. 2004; Babenko et al. 2006; Pearson et al. 2006; Ashcroft 2007; Lin et al. 2008). Beyond the isolated failure of pancreatic β-cells, mutations in KCNJ11, the gene encoding the pore-forming Kir6.2 subunit of KATP channels (Aguilar-Bryan et al. 1995; Inagaki et al. 1995), are pathogenic in a syndrome that encompasses diabetes, developmental delay and epilepsy (Proks et al. 2004; Hattersley & Ashcroft 2005; Gloyn et al. 2006; Ashcroft 2007).
Kir6.2 is also integral to the make-up of myocardial KATP channels (Inagaki et al. 1996), and targeted disruption of KCNJ11 generates Kir6.2-deficient mice that lack functional KATP channels in ventricular myocytes (Suzuki et al. 2001). Intact Kir6.2 is required in cardiac adaptation to physiological and pathophysiological stress (Zingman et al. 2002a, 2003; Kane et al. 2006a; Tong et al. 2006; Yamada et al. 2006; Gumina et al. 2007). Moreover, KATP channel malfunction has been implicated in the development and progression of heart disease (Hodgson et al. 2003; Kane et al. 2005). Originally discovered in cardiomyocytes (Noma 1983), KATP channels are abundant in the sarcolemma where they assemble as heteromultimers of the Kir6.2 pore and SUR2A, the ATP-binding cassette regulatory sulphonylurea receptor subunit (Inagaki et al. 1996; Lorenz & Terzic 1999; Nichols 2006; Bryan et al. 2007; Dupuis et al. 2008; Karger et al. 2008). Integrated with cellular metabolic pathways (Dzeja & Terzic 1998; Carrasco et al. 2001; Abraham et al. 2002; Selivanov et al. 2004; Dhar-Chowdhury et al. 2005; Jovanovic et al. 2005), SUR2A contains nucleotide-binding domains and intrinsic ATPase activity, endowing this regulatory KATP channel subunit with the ability to process energetic signals of distress under conditions of increased workload (Bienengraeber et al. 2000; Zingman et al. 2001; Alekseev et al. 2005; Park et al. 2008). The tandem function of nucleotide-binding domains confers Kir6.2-gating competence to SUR2A (Zingman et al. 2002b), leading to regulation of pore opening in response to stress challenge (Zingman et al. 2002a; Liu et al. 2004; Nichols 2006). A deficit in KATP channels impairs tolerance to various systemic stressors that may be imposed by a sympathetic surge (Zingman et al. 2002a), endurance challenge (Kane et al. 2004) or haemodynamic load (Kane et al. 2006a,b; Yamada et al. 2006). Genetic disruption of KATP channels compromises the protective benefits of preconditioning (Suzuki et al. 2002; Gumina et al. 2003), while overexpression of channel subunits generates a resistant phenotype (Du et al. 2006). In fact, mutations in KATP channel proteins have been linked to cardiac pathology in patient populations (Bienengraeber et al. 2004; Kane et al. 2005; Olson et al. 2007). Thus, molecular medicine has advanced our understanding of KATP channels as conserved regulators of homeostasis (Nichols 2006; Ashcroft 2007; Sattiraju et al. 2008).
While focus has been placed on adult KATP channel-dependent phenotypes in health and disease, available tools limit the analysis of gene–environment interactions during embryonic development. Here, in a KCNJ11 null mutant background, we have established a mosaic developmental platform based on diploid aggregation with wild-type embryonic stem cells that produced chimeric offspring. Engineering knockout↔wild-type chimeras provide a previously unavailable tool to examine the contribution of genetic variance in stress tolerance, and anticipate the fitness of the adaptive response during prenatal and post-natal development.
2. Material and methods
(a) Kir6.2 knockout
KATP channel knockout mice were generated by targeted disruption of the KCNJ11 gene that encodes the Kir6.2 channel pore, and backcrossed for five generations into a C57BL/6 background (Miki et al. 1998). Owing to proximity of the mutated KCNJ11 gene with the gene encoding for albino hair colour in the SV129 embryonic stem cells used to create the null mutant, Kir6.2 knockout mice remain white upon backbreeding into the black C57BL/6 line (Kane et al. 2004). Mice were kept under a 12 L : 12 D cycle and allowed free access to tap water and standard chow.
(b) Timed pregnancy of superovulating knockout donors
Female KCNJ11 gene knockout mice were treated with reproductive hormones to maximize the isolation of stage-specific embryos (Eakin & Hadjantonakis 2006). In brief, superovulation was achieved in three to four-week-old females at the final stage of pre-pubescent development. On day 1 at 14.00 h, female donors received a single intraperitoneal (i.p.) injection (5 units in 0.1 ml) of pregnant mare serum gonadotrophin (PMSG) using a 27-gauge needle (figure 1). Two days later at 13.00 h, donors received an i.p. injection (5 units in 0.1 ml) of human chorionic gonadotrophin (HCG). Knockout females were immediately paired with knockout studs to achieve timed mating that occurred during the night on day 3 according to circadian rhythm dictated by the light/dark cycle. Superovulated females were removed from studs the following morning, and allowed to proceed through normal pregnancy. Knockout embryos, at 2.5 days post-coitum (d.p.c.), were harvested by retrograde flushing from the distal oviduct through the infundibulum using a 32-gauge needle. Superovulated donors produced up to 30 synchronized embryos in a single oviduct.
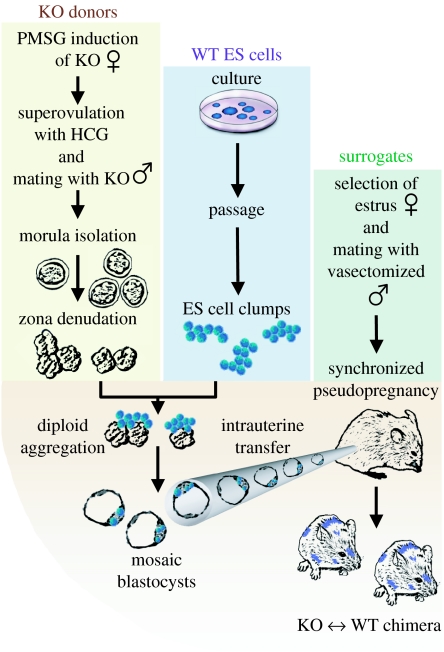
Figure 1.
Morula-stage embryos re-engineered through diploid aggregation. Flowchart of chimeric offspring derivation from random incorporation of embryonic stem cells into early stage embryos achieved through imposed diploid aggregation. Yellow box: Initial embryos are generated from timed pregnant KCNJ11 gene-deficient donors previously superovulated through hormonal activation using sequential i.p. injections of PMSG and HCG. Knockout embryos are harvested at 2.5 d.p.c. to collect at the morula stage. Zona pellucida is removed using acid Tyrode's solution to prepare aggregation competent early embryos. Blue box: Simultaneously, embryonic stem cells are grown for two passages to produce low-density cultures that when digested are able to generate clumps of 8–15 pluripotent stem cells. Green box: Synchronized pseudopregnant surrogates are produced by appropriate selection of females in oestrus, and mated with vasectomized studs. Bottom: Mosaic morula generated after aggregation of KNCJ11 gene-deficient embryos with wild-type embryonic stem cells. Following intrauterine surgical transfer, surrogate females support normal embryonic development and give birth to chimeric offspring.
(c) Collection of zona pelucida-denuded knockout morulae
Morula-stage embryos (figure 1) were washed in EmbryoMax M2 medium (Millipore, Billerica, MA) to remove cellular debris associated with oviduct flushing (Eakin & Hadjantonakis 2006). Glycoproteinaceous zona pellucida was removed to produce denuded morulae, competent for stem cell integration. A 35 mm culture dish was prepared with a drop of M2 and a drop of acid Tyrode's solution at room temperature. Embryos, in groups of 20–30, were transferred with as little M2 medium as possible into the acid Tyrode solution, and continuously irrigated to keep neighbouring embryos separated until zona pelucida dissolved within 30–40 s. Once stripped of their zona pellucida, denuded morulae were washed in five drops of M2 followed by five drops of EmbryoMax KSOM (Millipore, Billerica, MA), preparing them for subsequent in vitro manipulation.
(d) Selection of wild-type embryonic stem cell clumps
Murine embryonic stem cells (R1-derived line) containing a single copy of the constitutively expressed β-galactosidase gene were maintained in Glasgow's Minimum Essential Medium (BioWhittaker-Cambrex, Walkersville, MD) supplemented with pyruvate and l-glutamine (Cellgro, Mediatech, Inc. Herndon, VA), non-essential amino acids (Cellgro, Mediatech, Inc. Herndon, VA), β-mercaptoethanol (Sigma-Aldrich, St Louis, MO), 15 per cent foetal calf serum (FCS, Invitrogen Corporation, Carlsbad, CA) and leukaemia inhibitory factor (LIF; ESGRO, Chemicon International, Inc, Temecula, CA) and cocultured with inactivated mouse embryonic fibroblast feeders in a six-well plate on day 1 (Nelson et al. 2006, 2008). Embryonic stem cells were split 1/3, 1/6 and 1/12 on day 3 in order to ensure proper density for diploid aggregation (Nelson et al. 2004). On day 6, embryonic stem cells at approximately 60 per cent confluence were digested with 1 ml of trypsin for 4 min until the cells were loosely associated with each other. Gentle mechanical disruption was required to produce small clumps of the cells before adding 5 ml of growth medium to inactivate trypsin solution (Eakin & Hadjantonakis 2006). Care was taken to avoid producing single cell suspensions. The mixture was pre-plated on tissue culture plates to allow feeder cells to attach before collection of embryonic stem cell clumps. Selected clumps were washed in five drops of M2 medium followed by five drops of KSOM medium for subsequent diploid aggregation (figure 1).
(e) Synchronized pseudopregnancy of surrogate females
Surrogate mothers are required for proper in vivo development of embryos re-engineered outside of the natural environment. CD-1 females, at least six to eight weeks old, were maintained in a colony of 50–70 animals (Eakin & Hadjantonakis 2006). On day 4, females in oestrus were identified by careful examination of vaginal changes indicated by dry, pink and swollen external mucosa. Selected females provided the most reliable source for successful mating when paired with vasectomized studs (figure 1). The oestrous cycle in mice is 3–4 days long with ovulation occurring at approximately the mid-point of the dark period (mid-night) of a light/dark cycle. Females caged together without a male tend to cycle in unison. Pseudopregnant females were identified the following morning (day 5) upon visualization of a vaginal plug.
(f) Diploid aggregation of KCNJ11 knockout embryos with wild-type embryonic stem cells
Integration of embryonic stem cells with competent morula-stage embryos produced mosaic blastocytes in vitro that were surgically transferred into the uterus of pseudopregnant females for subsequent embryonic development. Using a KSOM-filled syringe, 12 microdrops (approx. 3 mm in diameter) were placed into a 35 mm tissue culture dish and covered with sterile mineral oil using aseptic procedures. Drops were incubated overnight at 37°C in 5 per cent CO2 to buffer the medium to appropriate pH. The aggregation needle (Type DN-09, BLS Ltd., Hungary) was washed with 70 per cent EtOH, and used to make five wells/drop under mineral oil by firmly pressing into the plastic tissue culture plate. Aggregation competent morulae devoid of zona pellucida were micropipetted with a capillary needle. Each well containing two morulae also received a single clump of 8–15 embryonic stem cells initiating coerced aggregation. The aggregation partners were incubated for 24 hours in KSOM medium in a table-top incubator with continuous flow of a humidified gas mixture (5% CO2/5% O2/90% N2). Cellular integration of embryonic stem cells with endogenous blastomeres of the knockout morula developed into a mosaic blastocyte displaying characteristic morphology indicated by progressive cavitation. Cultured blastocytes were collected, washed in M2 medium and loaded into a glass capillary with a diameter slightly larger than an individual embryo for intrauterine transfer.
(g) Intrauterine blastocyte transfer
Pseudopregnant surrogates were surgically prepared under general anaesthesia (2–3% inhaled isoflurane) for intrauterine blastocyte transfer. The uterus was dissected out through a small incision in the flank of a pseudopregnant surrogate and exposed with the ovary, oviduct and distal portion of the uterus pulled outside of the peritoneum and secured with a microtissue clamp attached to the ovarian fat pad. Blunt forceps were used to hold the oviduct in order to position and stabilize the transfer site without direct manipulation of the uterus. A 30-gauge needle was used to puncture an entry hole in the distal portion of the uterus using a low-power dissection microscope, followed by immediate insertion of glass capillary and transfer of blastocyte-stage embryos. Pseudopregnant surrogates tolerated the invasive surgical procedure without complications and were capable of full-term pregnancy. Chimeric offspring were identified by heterogenous coat colour derived from a mixture of wild-type embryonic stem cells that produce black coat colour and embryonic tissues in the white knockout background (figure 1).
3. Results
(a) KCNJ11 gene-deficient morulae tolerate ex utero manipulation
Chimera blastocytes resulting from integration of embryonic stem cells with morula have provided a powerful tool to study developmental biology from early embryonic stages to adult phenotypes (Wood et al. 1993; Tam & Rossant 2003). Compact morulae were here harvested from KCNJ11 gene knockout donors after undergoing superovulation and mating with knockout studs. The optimal age of female donors was three weeks to maximize the number of appropriate morula-stage embryos with 20 donors producing 200–300 viable embryos for each experiment. Traditionally, the wild-type CD-1 mouse strain is used as a donor at this age due to robust capacity to produce large number of embryos following a superovulation generating 30–40 high-quality embryos in a single uterus. The efficiency of morula production in KCNJ11 gene knockout donors was significantly less, with the average uterus producing 5–15 appropriate embryos, despite optimization of the protocol. This was in part due to a high degree of unfertilized single cells and atypical eight-cell embryos in KCNJ11 gene knockout donors. Early morula-stage embryos were collected, washed and processed through acid Tyrode's solution to remove the zona pellucida (figure 2a and 2b). Again, compared with traditional wild-type CD-1 embryos, recovery of KCNJ11 gene knockout embryos after in vitro removal of zona pellucida was less efficient. The zona denudation with acid washing resulted in the destruction of approximately a third of knockout embryos compared with less than 5 per cent of CD-1 embryos. A significant percentage of knockout embryos were destroyed as individual cells of the morula were completely dissociated from each other following acid wash, which eliminated the structure of the embryo required for aggregation and normal development. Morulae that tolerated the stress of denudation were selected for subsequent embryonic stem cell aggregation. Embryonic stem cells labelled with β-galactosidase gene allowed lacZ staining to detect wild-type embryonic stem cell-derived progeny at subsequent stages of development. An embryonic stem cell clump of 8–15 cells was collected upon careful enzymatic digestion (figure 2c), and placed into a well containing two aggregation competent knockout embryos (figure 2d). The complementation assay allowed integration of embryonic stem cells into KCNJ11 gene knockout embryos at the early morula stage. The efficiency of blastocyte formation from knockout morula re-engineered with wild-type embryonic stem cell aggregation was also decreased compared with CD-1 counterparts, and moreover the knockout morula required longer observation for an additional approximately 4–6 hours to achieve full maturation of blastocyte cavitation. Thus, KCNJ11 gene-deficient embryos were vulnerable to stressful in vitro manipulation; however, a sufficient number of KCNJ11 gene-deficient morulae were able to incorporate embryonic stem cells and advance beyond the morula stage.
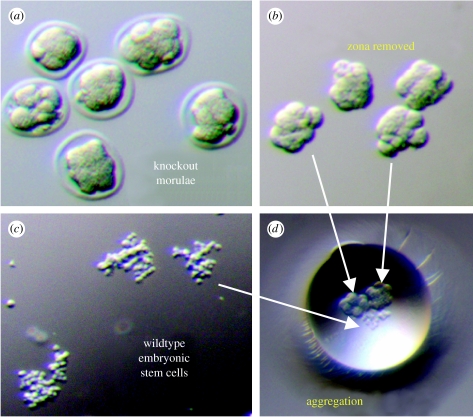
Figure 2.
KCNJ11 gene-deficient embryos aggregated with embryonic stem cells. (a) Stage-specific embryos were harvested from KCNJ11 gene deficient donors. (b) Zona pellucida was removed with acid Tyrode washes to produce aggregation competent embryos. (c) Embryonic stem cells are prepared in 8–15 cell clumps. (d) Diploid aggregation was coerced between two morula-stage embryos and embryonic stem cell clumps.
(b) Diploid aggregation creates mosaic knockout↔wild-type embryos
Embryonic stem cell integration into developing embryonic tissue through diploid aggregation is typically achieved using wild-type donor embryos aggregated with a variety of mutant embryonic stem cells (Eakin & Hadjantonakis 2006). Despite the lower overall efficiency of KCNJ11 gene knockout embryo production, aggregation competent progeny enabled the generation of mosaic embryos using wild-type embryonic stem cells labelled with a constitutively expressed β-galactosidase (lacZ) gene from the elongation promoter (Nelson et al. 2006). Blastocytes resulting from the aggregation between knockout donors and wild-type embryonic stem cells were collected from in vitro culturing media on day 7 of the procedure. Normal morphology with proper cavitation and inner cell mass formation was observed (figure 3(i)). These engineered early stage embryos were indistinguishable from traditional wild-type CD-1 embryos at the 3.5 d.p.c. developmental stage. Re-engineered embryos were stained for lacZ expression and demonstrated robust expression of wild-type embryonic stem cell progeny in the majority of embryos at day 7 (figure 3(ii)). Thus, engraftment of wild-type embryonic stem cells was maintained in KCNJ11 gene knockout embryos during in vitro blastocyte formation and provided the opportunity to examine chimeric embryo formation between Kir6.2-deficient embryos and wild-type embryonic stem cells. Upon intrauterine transplantation and proper development, mosaic blastocytes differentiated into morphologically normal, age-appropriate embryos at 9.5 d.p.c. (figure 3b(i)). Staining for wild-type embryonic stem cell-derived tissue expressing lacZ demonstrated embryonic stem cell incorporation throughout embryonic tissues including the heart, brain, somites, pharyngeal arches and primordial liver (figure 3b(ii)). Chimeric KCNJ11 gene knockout embryos incorporated wild-type embryonic stem cells during early stages of embryonic organogenesis, demonstrating functional chimerism in the embryo and justifying the experimental approach to generate adult chimeras.
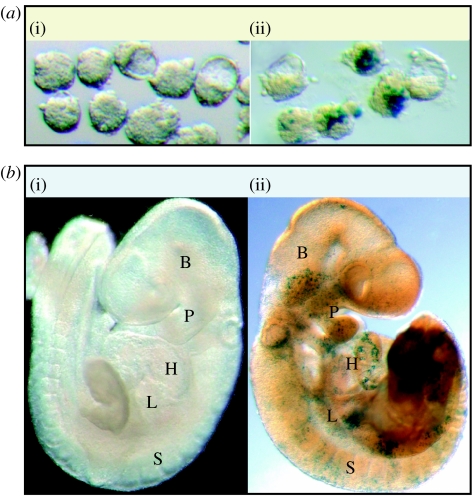
Figure 3.
Mosaic blastocytes derived from labelled embryonic stem cells differentiate as components of multiple organs. (a) 3.5 d.p.c. Mosaic blastocytes: (i) (WT↔WT) normal morphology of blastocyte stage embryos with proper cavitation and inner cell mass formation was observed with (ii) (KO↔WT) lacZ stain highlighting the presence of wild-type embryonic stem cell progeny in the majority of late stage morula: or blastocytes. (b) 9.5 d.p.c. Chimeric blastocytes (i) (WT↔WT) upon intrauterine transplantation and proper development, mosaic blastocytes differentiated into morphologically normal 9.5 d.p.c. with (ii) (KO↔WT) lacZ stain revealing wild-type embryonic stem cell-derived tissue. Expression of lacZ demonstrated wild-type embryonic stem cell-derived progeny throughout the embryo in tissues such as the heart (H), brain (B), somites (S), pharyngeal arches (P) and primordial liver (L).
(c) Assortment of knockout and wild-type tissues in viable adult chimera
Surrogate mothers of the CD-1 background were used to support proper in utero development for chimeric embryos (figure 3). High-throughput diploid aggregation was required to produce sufficient numbers of chimeric blastocytes due to the stress intolerance of the KCNJ11 gene-deficient background. Further vulnerability of this background mouse strain was realized when the majority of born pups were unexpectedly destroyed by CD-1 surrogate mothers within 3 post-natal days. This suggests a selection process by the surrogate mothers to identify unfit offspring and eliminate pups according to perceived maladaptive behaviours, which is less common when chimeras are derived from the traditional CD-1 background strain. The white coat colour of the KCNJ11 gene-deficient background allowed chimeric animals to be identified based on dark hair colour derived from embryonic stem cell contribution. Of note, three-week-old pups derived from the KCNJ11 gene-deficient background using the diploid aggregation platform produced offspring with varying degrees of chimeric coat colours, indicating a spectrum of wild-type embryonic stem cell incorporation. Diploid aggregation-derived chimeric pups were larger than non-chimeric littermates, suggesting a disparity between the two cohorts (figure 4). The overall efficiency of adult chimera production using KCNJ11 gene-deficient morula was less than 0.01 per cent initially, and has been increased to more than 1 per cent after optimization to minimize unnecessary stress induced at each stage of the production process. Despite the low number of viable offspring using the KCNJ11 gene-deficient donors, the high percentage of chimeric offspring according to coat colour identification may suggest an overall survival advantage conferred by wild-type embryonic stem cell-derived tissues during prenatal and perinatal development of the KCNJ11 gene-deficient background.
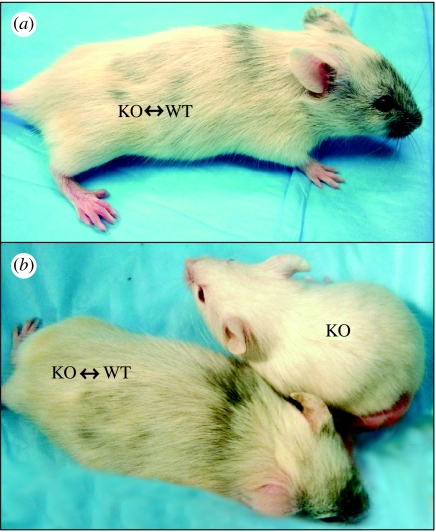
Figure 4.
Chimeric animals produced from KCNJ11-deficient embryos and wild-type embryonic stem cells. Surrogate mothers support normal development and give birth to live (three-week-old) chimeric offspring. (a) KCNJ11 knockout wild-type chimera animals develop combination of white and dark coat colours. (b) Three-week-old litter mates: chimera animals are larger than non-chimera animals.
4. Discussion
An emerging body of evidence implicates, in the adult, the KATP channel as a unifying molecular coordinator of metabolic well-being under stress, ensuring energetic homeostasis in health and disease (Zingman et al. 2003; Nichols 2006; Ashcroft 2007). Less is, however, known regarding the contribution of KATP channel-dependent adaptation during development. Yet the activity of ion channels and pumps, and their molecular regulators, is an increasingly recognized contributor to embryonic development as initial mapping unravels complex functional roles in non-mammalian phylogeny (Cheng et al. 2002; Akasaka et al. 2006). In mammalian systems, a traditional approach to probe the genetic control of prenatal and post-natal development has included engineering chimeric constructs (Eakin & Hadjantonakis 2006). Here, we provide the first KATP channel knockout↔wild-type chimera as a platform to monitor the outcome of gene–environment interactions from conception to senescence.
Specifically, in the KCNJ11 null mutant background, diploid aggregation with wild-type embryonic stem cells added wild-type blastomeres to the KATP channel knockout morula yielding a mosaic blastocyst. Diploid aggregation allows the integration, in the context of the host morula, of an independent source of pluripotent progenitors which is in principle equally competent to mature into all lineages (Wood et al. 1993). Although genetic modifications have been typically studied in the milieu of wild-type background providing the foundation for developmental biology and lineage allocation (Tam & Rossant 2003), diploid aggregation using a mutant host—as developed herein—allows the study of putative disparity between KATP channel knockout versus wild-type blastomeres in a cell-autonomous (environment-independent) as well as non-cell-autonomous (environment-dependent) paradigm. In this way, the study of embryonic ecogenetics is enabled through direct manipulation of either the progenitor cell or its environment.
The KATP channel knockout morula displayed a fragile phenotype on removal of the zona pellucida as demonstrated by frequent dispersion of blastomeres and ensuing embryo destruction, in contrast to the wild-type counterparts, which maintained functional and structural integrity under equivalent stress load. While stress intolerance associated with KATP channel-deficiency is well documented in the adults (Kane et al. 2005; Miki & Seino 2005; Nichols 2006; Ashcroft 2007), the vulnerability observed here at the morula stage indicates that ablation of the Kir6.2 channel pore causes disruption of the KATP channel-dependent cytoprotection early in development. Such inherent maladaptation to stress is in line with primordial KATP channel-dependent functions in the regulation of proliferation, cell cycle and cell migration (Cheng et al. 2002). In the context of the stress-vulnerable KATP channel knockout morula, aggregation-derived knockout↔wild-type mosaic blastocysts tolerated intrauterine transfer and implantation, followed by full-term embryonic development in pseudopregnant surrogates to produce live chimeric offspring. Longitudinal analysis of chimerism throughout pre- and post-natal development thereby allows the dissection of KATP channel-mediated adaptive behaviour for each developing tissue and organ beyond the cell source in the antecedent morula.
In summary, this study re-engineers the KATP channel knockout morula into a knockout↔wild-type chimera offering a new technological platform to probe the disparity between KATP channel-dependent and independent pathways underlying the adaptive stress response. Chimerism generated through the competing fitness in the adaptive response of Kir6.2-rich versus Kir6.2-depleted progenitors opens a unique window to deconvolute the process of evolutionary selection, according to KATP channel functionality.
Acknowledgements
The investigation conformed to the National Institutes of Health guidelines regulating the care and use of laboratory animals and was approved by the Institutional Animal Care and Use Committee.The authors are particularly grateful to Dr Takashi Miki and Dr Susumu Seino for the initial derivation of the Kir6.2 knockout mice. We acknowledge support by grants from the National Institutes of Health, Marriott Heart Disease Research Program and Marriott Foundation. T.J.N. is supported by Mayo Clinic Clinician-Investigator Program, and A.M.F. by La Caixa Foundation Graduate Program. A.T. holds the Marriott Family Professorship in Cardiovascular Research at Mayo Clinic.
Footnotes
One contribution of 16 to a Discussion Meeting Issue ‘Membrane transport in flux: the ambiguous interface between channels and pumps’.
References
- Abraham M.R., Selivanov V.A., Hodgson D.M., Pucar D., Zingman L.V., Wieringa B., Dzeja P.P., Alekseev A.E., Terzic A. Coupling of cell energetics with membrane metabolic sensing: integrative signaling through creatine kinase phosphotransfer disrupted by M-CK gene knock-out. J. Biol. Chem. 2002;277:24 427–24 434. doi: 10.1074/jbc.M201777200. doi:10.1074/jbc.M201777200 [DOI] [PubMed] [Google Scholar]
- Aguilar-Bryan L., et al. Cloning of the beta cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science. 1995;268:423–426. doi: 10.1126/science.7716547. doi:10.1126/science.7716547 [DOI] [PubMed] [Google Scholar]
- Akasaka T., Klinedinst S., Ocorr K., Bustamante E.L., Kim S.K., Bodmer R. The ATP-sensitive potassium (KATP) channel-encoded dSUR gene is required for Drosophila heart function and is regulated by tinman. Proc. Natl Acad. Sci. USA. 2006;103:11 999–12 004. doi: 10.1073/pnas.0603098103. doi:10.1073/pnas.0603098103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alekseev A.E., Hodgson D.M., Karger A.B., Park S., Zingman L.V., Terzic A. ATP-sensitive K+ channel channel/enzyme multimer: metabolic gating in the heart. J. Mol. Cell. Cardiol. 2005;38:895–905. doi: 10.1016/j.yjmcc.2005.02.022. doi:10.1016/j.yjmcc.2005.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft F.M. ATP-sensitive potassium channelopathies: focus on insulin secretion. J. Clin. Invest. 2005;115:2047–2058. doi: 10.1172/JCI25495. doi:10.1172/JCI25495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft F.M. ATP-sensitive K+ channels and disease: from molecule to malady. Am. J. Physiol. Endocrinol. Metab. 2007;293:E880–E889. doi: 10.1152/ajpendo.00348.2007. doi:10.1152/ajpendo.00348.2007 [DOI] [PubMed] [Google Scholar]
- Babenko A.P., et al. Activating mutations in the ABCC8 gene in neonatal diabetes mellitus. N. Engl. J. Med. 2006;355:456–466. doi: 10.1056/NEJMoa055068. doi:10.1056/NEJMoa055068 [DOI] [PubMed] [Google Scholar]
- Barki-Harrington L., Rockman H.A. Sensing heart stress. Nat. Med. 2003;9:19–20. doi: 10.1038/nm0103-19. doi:10.1038/nm0103-19 [DOI] [PubMed] [Google Scholar]
- Bienengraeber M., Alekseev A.E., Abraham M.R., Carrasco A.J., Moreau C., Vivaudou M., Dzeja P.P., Terzic A. ATPase activity of the sulfonylurea receptor: a catalytic function for the KATP channel complex. FASEB J. 2000;14:1943–1952. doi: 10.1096/fj.00-0027com. doi:10.1096/fj.00-0027com [DOI] [PubMed] [Google Scholar]
- Bienengraeber M., et al. ABCC9 mutations identified in human dilated cardiomyopathy disrupt catalytic KATP channel gating. Nat. Genet. 2004;36:382–387. doi: 10.1038/ng1329. doi:10.1038/ng1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan J., Muñoz A., Zhang X., Düfer M., Drews G., Krippeit-Drews P., Aguilar-Bryan L. ABCC8 and ABCC9: ABC transporters that regulate K+ channels. Pflugers Arch. 2007;453:703–718. doi: 10.1007/s00424-006-0116-z. doi:10.1007/s00424-006-0116-z [DOI] [PubMed] [Google Scholar]
- Carrasco A.J., et al. Adenylate kinase phosphotransfer communicates cellular energetic signals to ATP-sensitive potassium channels. Proc. Natl Acad. Sci. USA. 2001;98:7623–7628. doi: 10.1073/pnas.121038198. doi:10.1073/pnas.121038198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S.-M., Chen I., Levin M. KATP channel activity is required for hatching in Xenopus embryos. Dev. Dyn. 2002;225:588–591. doi: 10.1002/dvdy.10183. doi:10.1002/dvdy.10183 [DOI] [PubMed] [Google Scholar]
- Chien K.R. Stress pathways and heart failure. Cell. 1999;98:555–558. doi: 10.1016/s0092-8674(00)80043-4. doi:10.1016/S0092-8674(00)80043-4 [DOI] [PubMed] [Google Scholar]
- Degterev A., Yuan J. Expansion and evolution of cell death programmes. Nat. Rev. Mol. Cell. Biol. 2008;9:378–390. doi: 10.1038/nrm2393. doi:10.1038/nrm2393 [DOI] [PubMed] [Google Scholar]
- Dhar-Chowdhury P., et al. The glycolytic enzymes, glyceraldehyde-3-phosphate dehydrogenase, triose-phosphate isomerase, and pyruvate kinase are components of the KATP channel macromolecular complex and regulate its function. J. Biol. Chem. 2005;280:38 464–38 470. doi: 10.1074/jbc.M508744200. doi:10.1074/jbc.M508744200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Q., Jovanovic S., Clelland A., Sukhodub A., Budas G., Phelan K., Murray-Tait V., Malone L., Jovanovic A. Overexpression of SUR2A generates a cardiac phenotype resistant to ischemia. FASEB J. 2006;20:1131–1141. doi: 10.1096/fj.05-5483com. doi:10.1096/fj.05-5483com [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne M.J., Cosgrove K.E., Shepherd R.M., Aynsley-Green A., Lindley K.J. Hyperinsulinism in infancy: from basic science to clinical disease. Physiol. Rev. 2004;84:239–275. doi: 10.1152/physrev.00022.2003. doi:10.1152/physrev.00022.2003 [DOI] [PubMed] [Google Scholar]
- Dupuis J.P., Revilloud J., Moreau C.J., Vivaudou M. Three C-terminal residues from SUR contribute to the functional coupling between the KATP channel subunits SUR2A and Kir6.2. J. Physiol. 2008;586:3075–3085. doi: 10.1113/jphysiol.2008.152744. doi:10.1113/jphysiol.2008.152744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzeja P.P., Terzic A. Phosphotransfer reactions in the regulation of ATP-sensitive K+ channels. FASEB J. 1998;12:523–529. doi: 10.1096/fasebj.12.7.523. [DOI] [PubMed] [Google Scholar]
- Eakin G.S., Hadjantonakis A.K. Production of chimeras by aggregation of embryonic stem cells with diploid or tetraploid mouse embryos. Nat. Protoc. 2006;1:1145–1153. doi: 10.1038/nprot.2006.173. doi:10.1038/nprot.2006.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloyn A.L., et al. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N. Engl. J. Med. 2004;350:1838–1849. doi: 10.1056/NEJMoa032922. doi:10.1056/NEJMoa032922 [DOI] [PubMed] [Google Scholar]
- Gloyn A.L., Siddiqui J., Ellard S. Mutations in the genes encoding the pancreatic beta-cell KATP channel subunits Kir6.2 (KCNJ11) and SUR1 (ABCC8) in diabetes mellitus and hyperinsulinism. Hum. Mutat. 2006;27:220–231. doi: 10.1002/humu.20292. doi:10.1002/humu.20292 [DOI] [PubMed] [Google Scholar]
- Gumina R.J., Pucar D., Bast P., Hodgson D.M., Kurtz C.E., Dzeja P.P., Miki T., Seino S., Terzic A. Knockout of Kir6.2 negates ischemic preconditioning-induced protection of myocardial energetics. Am. J. Physiol. Heart Circ. Physiol. 2003;284:H2106–H2113. doi: 10.1152/ajpheart.00057.2003. [DOI] [PubMed] [Google Scholar]
- Gumina R.J., et al. KATP channel knockout worsens myocardial calcium stress load in vivo and impairs recovery in stunned heart. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H1706–H1713. doi: 10.1152/ajpheart.01305.2006. doi:10.1152/ajpheart.01305.2006 [DOI] [PubMed] [Google Scholar]
- Hattersley A.T., Ashcroft F.M. Activating mutations in Kir6.2 and neonatal diabetes: new clinical syndromes, new scientific insights, and new therapy. Diabetes. 2005;54:2503–2513. doi: 10.2337/diabetes.54.9.2503. doi:10.2337/diabetes.54.9.2503 [DOI] [PubMed] [Google Scholar]
- Hodgson D.M., et al. Cellular remodeling in heart failure disrupts KATP channel-dependent stress tolerance. EMBO J. 2003;22:1732–1742. doi: 10.1093/emboj/cdg192. doi:10.1093/emboj/cdg192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki N., Gonoi T., Clement J.P., Namba N., Inazawa J., Gonzalez G., Aguilar-Bryan L., Seino S., Bryan J. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science. 1995;270:1166–1170. doi: 10.1126/science.270.5239.1166. doi:10.1126/science.270.5239.1166 [DOI] [PubMed] [Google Scholar]
- Inagaki N., Gonoi T., Clement J.P., Wang C.Z., Aguilar-Bryan L., Bryan J., Seino S. A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron. 1996;16:1011–1017. doi: 10.1016/s0896-6273(00)80124-5. doi:10.1016/S0896-6273(00)80124-5 [DOI] [PubMed] [Google Scholar]
- Jovanovic S., Du Q., Crawford R.M., Budas G.R., Stagljar I., Jovanovic A. Glyceraldehyde-3 phosphate dehydrogenase serves as an accessory protein of the cardiac sarcolemmal KATP channel. EMBO Rep. 2005;6:848–852. doi: 10.1038/sj.embor.7400489. doi:10.1038/sj.embor.7400489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane G.C., et al. ATP-sensitive K+ channel knockout compromises the metabolic benefit of exercise training, resulting in cardiac deficits. Diabetes. 2004;53:S169–S175. doi: 10.2337/diabetes.53.suppl_3.s169. doi:10.2337/diabetes.53.suppl_3.S169 [DOI] [PubMed] [Google Scholar]
- Kane G.C., Liu X.K., Yamada S., Olson T.M., Terzic A. Cardiac KATP channels in health and disease. J. Mol. Cell Cardiol. 2005;38:937–943. doi: 10.1016/j.yjmcc.2005.02.026. doi:10.1016/j.yjmcc.2005.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane G.C., et al. KCNJ11 gene knockout of the Kir6.2 KATP channel causes maladaptive remodeling and heart failure in hypertension. Hum. Mol. Genet. 2006;15:2285–2297. doi: 10.1093/hmg/ddl154. doi:10.1093/hmg/ddl154 [DOI] [PubMed] [Google Scholar]
- Kane G.C., et al. Gene knockout of the KCNJ8-encoded Kir6.1 KATP channel imparts fatal susceptibility to endotoxemia. FASEB J. 2006;20:2271–2280. doi: 10.1096/fj.06-6349com. doi:10.1096/fj.06-6349com [DOI] [PubMed] [Google Scholar]
- Karger A.B., Park S., Reyes S., Bienengraeber M., Dyer R.B., Terzic A., Alekseev A.E. Role for SUR2A ED domain in allosteric coupling within the KATP channel complex. J. Gen. Physiol. 2008;131:185–196. doi: 10.1085/jgp.200709852. doi:10.1085/jgp.200709852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.W., Bushman J.D., Yan F.F., Haidar S., MacMullen C., Ganguly A., Stanley C.A., Shyng S.L. Destabilization of ATP-sensitive potassium channel activity by novel KCNJ11 mutations identified in congenital hyperinsulinism. J. Biol. Chem. 2008;283:9146–9156. doi: 10.1074/jbc.M708798200. doi:10.1074/jbc.M708798200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.K., et al. Genetic disruption of Kir6.2, the pore-forming subunit of ATP-sensitive K+ channel, predisposes to catecholamine-induced ventricular dysrhythmia. Diabetes. 2004;53:S165–S168. doi: 10.2337/diabetes.53.suppl_3.s165. doi:10.2337/diabetes.53.suppl_3.S165 [DOI] [PubMed] [Google Scholar]
- Lorenz E., Terzic A. Physical association between recombinant cardiac ATP-sensitive K+ channel subunits Kir6.2 and SUR2A. J. Mol. Cell. Cardiol. 1999;31:425–434. doi: 10.1006/jmcc.1998.0876. doi:10.1006/jmcc.1998.0876 [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Physiology and neurobiology of stress and adaptation. Physiol. Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. doi:10.1152/physrev.00041.2006 [DOI] [PubMed] [Google Scholar]
- Miki T., Seino S. Roles of KATP channels as metabolic sensors in acute metabolic changes. J. Mol. Cell. Cardiol. 2005;38:917–925. doi: 10.1016/j.yjmcc.2004.11.019. doi:10.1016/j.yjmcc.2004.11.019 [DOI] [PubMed] [Google Scholar]
- Miki T., et al. Defective insulin secretion and enhanced insulin action in KATP channel-deficient mice. Proc. Natl Acad. Sci. USA. 1998;95:10 402–10 406. doi: 10.1073/pnas.95.18.10402. doi:10.1073/pnas.95.18.10402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson T.J., Duncan S.A., Misra R.P. Conserved enhancer in the serum response factor promoter controls expression during early coronary vasculogenesis. Circ. Res. 2004;94:1059–1066. doi: 10.1161/01.RES.0000125296.14014.17. doi:10.1161/01.RES.0000125296.14014.17 [DOI] [PubMed] [Google Scholar]
- Nelson T.J., et al. Improved cardiac function in infarcted mice after treatment with pluripotent embryonic stem cells. Anat. Rec. A. 2006;288:1216–1224. doi: 10.1002/ar.a.20388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson T.J., Faustino R.S., Chiriac A., Crespo-Diaz R., Behfar A., Terzic A. CXCR4+/Flk-1+ biomarkers select a cardiopoietic lineage from embryonic stem cells. Stem Cells. 2008;26:1464–1473. doi: 10.1634/stemcells.2007-0808. doi:10.1634/stemcells.2007-0808 [DOI] [PubMed] [Google Scholar]
- Nichols C.G. KATP channels as molecular sensors of cellular metabolism. Nature. 2006;440:470–476. doi: 10.1038/nature04711. doi:10.1038/nature04711 [DOI] [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–148. doi: 10.1038/305147a0. doi:10.1038/305147a0 [DOI] [PubMed] [Google Scholar]
- Olson T.M., et al. KATP channel mutation confers risk for vein of Marshall adrenergic atrial fibrillation. Nat. Clin. Pract. Cardiovasc. Med. 2007;4:110–116. doi: 10.1038/ncpcardio0792. doi:10.1038/ncpcardio0792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Lim B.B., Perez-Terzic C., Mer G., Terzic A. Interaction of asymmetric ABCC9-encoded nucleotide binding domains determines KATP channel SUR2A catalytic activity. J. Proteome Res. 2008;7:1721–1728. doi: 10.1021/pr7007847. doi:10.1021/pr7007847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson E.R., et al. Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N. Engl. J. Med. 2006;355:467–477. doi: 10.1056/NEJMoa061759. doi:10.1056/NEJMoa061759 [DOI] [PubMed] [Google Scholar]
- Proks P., Antcliff J.F., Lippiat J., Gloyn A.L., Hattersley A.T., Ashcroft F.M. Molecular basis of Kir6.2 mutations associated with neonatal diabetes or neonatal diabetes plus neurological features. Proc. Natl Acad. Sci. USA. 2004;101:17 539–17 544. doi: 10.1073/pnas.0404756101. doi:10.1073/pnas.0404756101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes S., Kane G.C., Miki T., Seino S., Terzic A. KATP channels confer survival advantage in cocaine overdose. Mol. Psychiatry. 2007;12:1060–1061. doi: 10.1038/sj.mp.4002083. doi:10.1038/sj.mp.4002083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattiraju S., Reyes S., Kane G.C., Terzic A. KATP channel pharmacogenomics: from bench to bedside. Clin. Pharmacol. Ther. 2008;83:354–357. doi: 10.1038/sj.clpt.6100378. doi:10.1038/sj.clpt.6100378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seley H. Stress and disease. Science. 1955;122:625–631. doi: 10.1126/science.122.3171.625. doi:10.1126/science.122.3171.625 [DOI] [PubMed] [Google Scholar]
- Selivanov V.A., Alekseev A.E., Hodgson D.M., Dzeja P.P., Terzic A. Nucleotide-gated KATP channels integrated with creatine and adenylate kinases: amplification, tuning and sensing of energetic signals in the compartmentalized cellular environment. Mol. Cell. Biochem. 2004;256:243–256. doi: 10.1023/b:mcbi.0000009872.35940.7d. doi:10.1023/B:MCBI.0000009872.35940.7d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M., et al. Functional roles of cardiac and vascular ATP-sensitive potassium channels clarified by Kir6.2-knockout mice. Circ. Res. 2001;88:570–577. doi: 10.1161/01.res.88.6.570. [DOI] [PubMed] [Google Scholar]
- Suzuki M., Sasaki N., Miki T., Sakamoto N., Ohmoto-Sekine Y., Tamagawa M., Seino S., Marban E., Nakaya H. Role of sarcolemmal KATP channels in cardioprotection against ischemia/reperfusion injury in mice. J. Clin. Invest. 2002;109:509–516. doi: 10.1172/JCI14270. doi:10.1172/JCI14270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam P.P., Rossant J. Mouse embryonic chimeras: tools for studying mammalian development. Development. 2003;130:6155–6163. doi: 10.1242/dev.00893. doi:10.1242/dev.00893 [DOI] [PubMed] [Google Scholar]
- Terzic A., Jahangir A., Kurachi Y. Cardiac ATP-sensitive K+ channels: regulation by intracellular nucleotides and K+ channel-opening drugs. Am. J. Physiol. 1995;269:C525–C545. doi: 10.1152/ajpcell.1995.269.3.C525. [DOI] [PubMed] [Google Scholar]
- Thomas P.M., Cote G.J., Wohllk N., Haddad B., Mathew P.M., Rabl W., Aguilar-Bryan L., Gagel R.F., Bryan J. Mutations in the sulfonylurea receptor gene in familial persistent hyperinsulinemic hypoglycemia of infancy. Science. 1995;268:426–429. doi: 10.1126/science.7716548. doi:10.1126/science.7716548 [DOI] [PubMed] [Google Scholar]
- Tong X.Y., et al. Consequences of cardiac myocyte-specific ablation of KATP channels in transgenic mice expressing dominant negative Kir6 subunits. Am. J. Physiol. 2006;291:H543–H551. doi: 10.1152/ajpheart.00051.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S.A., Allen N.D., Rossant J., Auerbach A., Nagy A. Non-injection methods for the production of embryonic stem cell–embryo chimaeras. Nature. 1993;365:87–89. doi: 10.1038/365087a0. doi:10.1038/365087a0 [DOI] [PubMed] [Google Scholar]
- Yamada S., Kane G.C., Behfar A., Liu X.K., Dyer R.B., Faustino R.S., Miki T., Seino S., Terzic A. Protection conferred by myocardial ATP-sensitive K+ channels in pressure overload-induced congestive heart failure revealed in KCNJ11 Kir6.2-null mutant. J. Physiol. 2006;577:1053–1065. doi: 10.1113/jphysiol.2006.119511. doi:10.1113/jphysiol.2006.119511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingman L.V., Alekseev A.E., Bienengraeber M., Hodgson D., Karger A.B., Dzeja P.P., Terzic A. Signaling in channel/enzyme multimers: ATPase transitions in SUR module gate ATP-sensitive K+ conductance. Neuron. 2001;31:233–245. doi: 10.1016/s0896-6273(01)00356-7. doi:10.1016/S0896-6273(01)00356-7 [DOI] [PubMed] [Google Scholar]
- Zingman L.V., et al. Kir6.2 is required for adaptation to stress. Proc. Natl Acad. Sci. USA. 2002;99:13 278–13 283. doi: 10.1073/pnas.212315199. doi:10.1073/pnas.212315199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingman L.V., Hodgson D.M., Bienengraeber M., Karger A.B., Kathmann E.C., Alekseev A.E., Terzic A. Tandem function of nucleotide binding domains confers competence to sulfonylurea receptor in gating ATP-sensitive K+ channels. J. Biol. Chem. 2002;277:14 206–14 210. doi: 10.1074/jbc.M109452200. doi:10.1074/jbc.M109452200 [DOI] [PubMed] [Google Scholar]
- Zingman L.V., Hodgson D.M., Alekseev A.E., Terzic A. Stress without distress: homeostatic role for KATP channels. Mol. Psychiatry. 2003;8:253–254. doi: 10.1038/sj.mp.4001323. doi:10.1038/sj.mp.4001323 [DOI] [PubMed] [Google Scholar]
- Zingman L.V., Alekseev A.E., Hodgson-Zingman D.M., Terzic A. ATP-sensitive potassium channels: metabolic sensing and cardioprotection. J. Appl. Physiol. 2007;103:1888–1893. doi: 10.1152/japplphysiol.00747.2007. doi:10.1152/japplphysiol.00747.2007 [DOI] [PubMed] [Google Scholar]