Abstract
Cells of most tissues require adhesion to a surface to grow. However, for hematopoietic cells, both stimulation and inhibition of proliferation by adhesion to extracellular matrix components have been described. Furthermore, it has been suggested that progenitor cells from chronic myelogenous leukemia show decreased β1 integrin-mediated adhesion to fibronectin, resulting in increased proliferation and abnormal trafficking. However, we show here that the chronic myelogenous leukemia-specific fusion protein p210bcr/abl stimulates the expression of α5β1 integrins and induces adhesion to fibronectin when expressed in the myeloid cell line 32D. Moreover, proliferation of both p210bcr/abl-transfected 32D (32Dp210) cells and untransfected 32D cells is stimulated by immobilized fibronectin. Cell cycle analysis revealed that nonadherent 32D and 32Dp210 cells are arrested in late G1 or early S phase, whereas the adherent fractions continue cycling. Although both adherent and nonadherent p210bcr/abl-transfected and parental 32D cells express equal amounts of cyclin A, a protein necessary for cell cycle progression at the G1/S boundary, cyclin A complexes immunoprecipitated from 32D cells cultured on immobilized fibronectin were found to be catalytically inactive in nonadherent but not in adherent cells. In addition, as compared with untransfected 32D cells, cyclin A immunoprecipitates from 32Dp210 cells exhibited a greatly elevated kinase activity and remained partially active irrespective of the adhesion status. The lack of cyclin A/cyclin-dependent kinase (CDK) 2 activity in nonadherent 32D cells appeared to result from increased expression and cyclin A complex formation of the CDK inhibitor p27Kip1. Taken together, our results indicate that adhesion stimulates cell cycle progression of hematopoietic cells by down-regulation of p27Kip1, resulting in activation of cyclin A/CDK2 complexes and subsequent transition through the G1/S adhesion checkpoint.
Cells of most tissues require adhesion to a surface to grow. However, there is conflicting evidence whether hematopoietic cells grow better in suspension or attached to a substrate. Both stimulation and inhibition of proliferation by adhesion to extracellular matrix components have been described. Several lines of evidence suggest the involvement of integrin-mediated adhesion in the control of hematopoietic progenitor cell proliferation. Miyake et al. (1) have shown in long-term bone marrow cultures that blocking anti-α4 antibodies completely abrogate lymphopoiesis and severely reduce myelopoiesis. In mice, blocking anti-β1-chain antibodies reduce in vivo colony formation in the spleen and medullar hematopoiesis from allogeneic hematopoietic progenitor cells (2). In cultures of purified CD34+ progenitors, fibronectin has been shown to stimulate, in cooperation with interleukin 3 (IL-3), the formation of colonies derived from colony-forming unit (CFU)-granulocyte, erythroid, monocyte, megakaryocyte, burst-forming unit-erythroid, CFU-erythroid, and CFU-macrophage, an effect reversed by RGDS-containing peptides that block fibronectin/α5β1-integrin interaction (3–5). In addition, cytokines such as IL-3, granulocyte-monocyte colony-stimulating factor, and stem cell factor are transient and selective activators of both α4β1 and α5β1 integrins expressed by normal CD34+ progenitor cells and cytokine-dependent myeloid cell lines MO7e and TF1, thus conferring on these cells a transient adherent phenotype to fibronectin (6). Moreover, recently, it has been demonstrated that the α4β1-integrin binding sequences of fibronectin stimulate the proliferation of CD34+ cells isolated from human cord blood (7). However, direct contact of normal or chronic myelogenous leukemia (CML) progenitor cells with bone marrow stroma or fibronectin may inhibit proliferation (8, 9).
CML is a myeloproliferative disorder associated with expression of the bcr/abl oncogene, a hybrid gene created by the Philadelphia translocation (10), juxtaposing the bcr and c-abl genes to generate the fusion protein p210bcr/abl. Altered integrin function may occur in CML cells, resulting in decreased adhesion of CML progenitors to bone marrow stroma and fibronectin (11, 12). Furthermore, it has been speculated that decreased integrin-mediated adhesion of CML progenitor cells may underlie not only the abnormal trafficking but also the increased proliferation of malignant CML cells (13). On the other hand, in vivo measurements in patients with CML have demonstrated a significantly higher percentage of S phase cells in bone marrow biopsy sections as compared with bone marrow aspirates and peripheral blood (14).
In contrast to hematopoietic cells, for fibroblasts the dependence of proliferation on adhesion is well established. The adhesion signal that regulates cell proliferation appears to be mediated through integrins (15). Recently, it has been shown that the adhesion requirement in mesenchymal cells is likely to reflect a cell cycle checkpoint in the late G1 phase of the cell cycle (16). Fibroblasts arrested by suspension fail to produce cyclin A (17). In addition, ectopic expression of cyclin A enabled normal rat kidney fibroblasts to bypass the adhesion requirement, implicating cyclin A as the major target of cell cycle control by the anchorage-signaling pathway.
Progression through the G1 phase of the cell cycle is controlled by the retinoblastoma protein (Rb) and the Rb-related proteins p107 and p130 (18). While underphosphorylated during G0 and the first half of G1, Rb becomes phosphorylated in the second half of the G1 phase, thereby releasing a number of cellular proteins, including the transcription factors E2F and CBP/cycA, from their bound to an active state (19, 20). Phosphorylation of Rb, and thereby control of cell cycle progression, is exerted by cyclin D-cyclin-dependent kinase (CDK) 4/6 (21) and cyclin E-CDK2 (22).
Recently, we have shown that the activation of cyclin A transcription by adhesion in the late G1 phase is mediated by CBP/cycA, a novel, CCAAT-binding transcription factor (23–25). During G0, CBP/cycA, like E2F, is inactivated by binding to Rb or an Rb family member. In addition, we and others have demonstrated that, whereas the expression of cyclin E is unaffected by suspension, cyclin E-associated kinase is only active in adherent fibroblasts (26, 27). The lack of cyclin E/CDK2 activity in suspended fibroblasts appears to result from increased expression of the CDK inhibitors p21Cip1 and p27Kip1 (27). Hence, in fibroblasts an adhesion signal leads to activation of the cyclin E-CDK2 complex. This activation results in the release of CBP/cycA from binding to an Rb family member and enables the induction of cyclin A expression and progression through the G1/S adhesion checkpoint.
In this study, we have determined the effect of adhesion to fibronectin on the proliferation of the hematopoietic mouse cell line 32D. In addition, the effect of p210bcr/abl on adhesion to immobilized fibronectin and proliferation of 32D cells was analyzed. The results presented here indicate that expression of p210bcr/abl is associated with increased adhesion of 32D cells to fibronectin. Proliferation of both 32Dp210 cells and untransfected parental 32D cells is stimulated by immobilized fibronectin. Furthermore, we demonstrate that adhesion stimulates cell cycle progression of hematopoietic cells by down-regulation of p27Kip1, resulting in activation of preformed cyclin A/CDK2 complexes and subsequent transition through the G1/S adhesion checkpoint.
MATERIALS AND METHODS
Cell Lines and Cell Culture.
The murine myeloid progenitor cell line 32Dcl3 (32D) depends on IL-3 for growth and viability and was cultured in RPMI medium 1640 supplemented with 20% fetal calf serum (FCS), and 10% WEHI-3B-conditioned medium as a source of murine IL-3. 32Dp210 cells were generated from 32D cells by stable transfection with the p210bcr/abl expression vector pGDp210Bcr/Abl (28) as described (29). Bcr/abl makes the 32D cell line leukemic in syngeneic mice where it is rapidly lethal (30). 32Dp210 cells, which grow IL-3 independent, were cultured in RPMI medium 1640 supplemented with 20% FCS. K562 cells were cultured in RPMI medium 1640 supplemented with 10% FCS.
RNA Extraction and Reverse Transcriptase–PCR.
Conditions for RNA extraction, reverse transcription, and PCR for p210bcr/abl have been described (31).
Adhesion Assays.
Cell adhesion to fibronectin was carried out as described (32). Briefly, 1 × 106 32D or 32Dp210 cells were seeded on fibronectin-coated 35-mm dishes (Becton Dickinson). Control cells were cultured on 35-mm plastic tissue culture dishes (Becton Dickinson). At the indicated time points, nonadherent and loosely attached cells were removed by three consecutive washings with RPMI medium 1640 after standardized horizontal shaking of the dishes (1 min, 50 excursions per min). Adherent cells were harvested by using trypsin, followed by two additional washings with RPMI medium 1640. Percent adhesion was calculated as (number of cells in the adherent fraction/number of total cells) × 100.
Proliferation Assays.
To determine the proliferation of 32D and 32Dp210 cells in the presence or absence of immobilized fibronectin, 1 × 105 32D or 32Dp210 cells were seeded either on fibronectin-coated 35-mm dishes or on 35-mm plastic tissue culture dishes. At the indicated time points, nonadherent cells were removed and adherent cells were harvested by using trypsin, followed by two additional washings with RPMI medium 1640.
Fluorescence-Activated Cell Sorting (FACS).
For surface staining of antigens, adherent and nonadherent 32D or 32Dp210 cells were incubated for 40 min at 4°C with rat anti-mouse mAbs directed against the following epitopes: integrin α4 chain (CD49d), integrin α5 chain (CD49e), and integrin β1 chain (CD29). Subsequently, cells were washed twice in PBS and incubated for 40 min at 4°C with a fluorescein isothiocyanate-conjugated mouse anti-rat mAb. This secondary antibody also served as a control. Cells were analyzed within 1 hr after staining. Acquisition and analysis were performed on a FACSCalibur flow cytometer (Becton Dickinson) by using cell quest software (Becton Dickinson). Ten thousand cells were acquired from each sample. Subsequent gates were set to exclude debris identified in the forward scatter versus side scatter dot blot.
Cell Cycle Analysis.
Analysis of DNA content of adherent and nonadherent 32D or 32Dp210 cells was done by FACS using the Cycle test plus DNA reagent kit (Becton Dickinson) according to the manufacturer’s instructions. Cells were analyzed within 1 hr after staining. Acquisition and analysis were performed on a FACSCalibur flow cytometer by using cell quest software. Ten thousand cells were acquired from each sample. Subsequent gates were set to exclude debris identified in the forward scatter versus side scatter dot blot. A doublet discrimination module was used to discriminate between cells in the G2/M phase and doublets. Only single cells were included in the analysis. Cell cycle phase distribution was calculated by using modfit lt software (Verity Software House, Topsham, ME).
Antibodies.
The antibodies against the integrin chains α4 (9C10), α5 (5H10–27), and β1 (9EG7), p27Kip1 (G173–524), and the secondary fluorescein isothiocyanate-conjugated mouse anti-rat mAb were obtained from PharMingen. The anti-cyclin A antibody (C-19), the antibody against p21Cip1 (C-19), and the horseradish peroxidase-conjugated anti-rabbit IgG antibody were purchased from Santa Cruz Biotechnology. The secondary horseradish peroxidase-conjugated anti-mouse IgG antibody was purchased from Jackson ImmunoResearch.
Immunoblotting.
For immunoblotting, 1.5 × 106 adhererent and nonadherent 32D or 32Dp210 cells were lysed with 200 μl of RIPA buffer (10 mM Tris⋅HCl, pH 8.0/150 mM NaCl/0.5% sodium deoxycholate/0.1% SDS/1 μg/ml aprotinin/2 μg/ml leupeptin/0.1 μg/ml phenylmethylsulfonyl fluoride/0.1 mM NaVO4/1 mM NaF), passed three times through a 25-gauge needle, left on ice for 30 min, and cleared by centrifugation at 12,000 g for 10 min. Cleared lysates were separated on polyacrylamide gels and transferred to nitrocellulose membranes (Immobilon-P, Millipore) by semidry transfer. Membranes were blocked by incubation with 5% dry milk in Tris-buffered saline (10 mM Tris-HCl, pH 8.0/150 mM NaCl) and probed with 1 μg/ml of the primary antibody in Tris-buffered saline for 1 hr. After incubation with a horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG secondary antibody, horseradish peroxidase activity was detected by enhanced chemiluminescence by using the ECL kit (Amersham) following the manufacturer’s instructions.
Immunoprecipitations.
For immunoprecipitations, 5 × 106 adherent and nonadherent 32D or 32Dp210 cells were harvested 20 hr after seeding on fibronectin-coated dishes by lysis in 1 ml of ice-cold lysis buffer (50 mM Tris-HCl, pH 7.4/250 mM NaCl/5 mM EDTA/0.1% Triton X-100/0.4% Nonidet P-40/10 μg/ml aprotinin/10 μg/ml leupeptin/0.1 μg/ml phenylmethylsulfonyl fluoride). After addition of the lysis buffer, cells were passed four times through a 21-gauge needle, and the lysates were cleared by centrifugation for 15 min at 12,000 g. Protein complexes were immunoprecipitated from cleared lysates by addition of 1 μg of primary antibody and incubation for 2.5 hr at 4°C. Immunoprecipitated protein antibody complexes then were collected by addition of 20 μl of protein A-Sepharose beads (Amersham Pharmacia) and incubation at 4°C with occasional mixing for 2.5 hr.
Kinase Assays.
Histone H1 kinase assays were performed as described (33). Briefly, immunoprecipitates recovered as described above were washed once in lysis buffer and three times in kinase buffer (50 mM Tris⋅HCl, pH 7.4/10 mM MgCl2/1 mM DTT). For kinase reactions, the immunoprecipitates were resuspended in 50 μl of kinase buffer supplemented with 1 μg of histone H1 (GIBCO/BRL), 30 μM ATP, and 5 μCi [γ-32P]ATP and incubated for 30 min at 37°C. Phosphorylated proteins were separated on denaturing polyacrylamide gels and detected by autoradiography.
RESULTS
Adhesion of 32D and 32Dp210 Cells to Fibronectin.
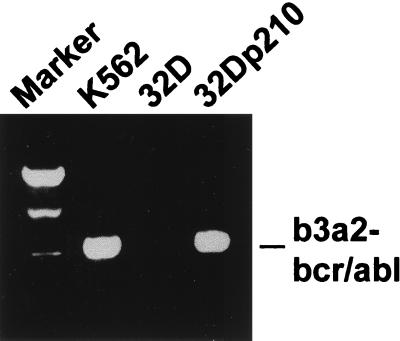
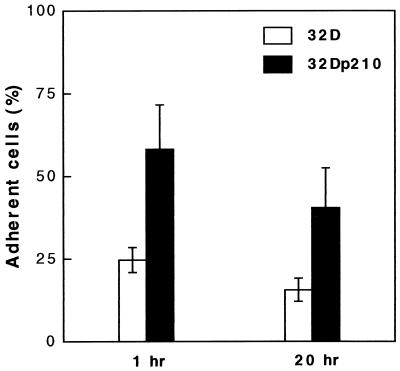
Adhesion to fibronectin was examined by using the hematopoietic mouse cell line 32D and the p210bcr/abl-transfected subclone 32Dp210. Expression of p210bcr/abl mRNA in transfected cells was confirmed by reverse transcription–PCR (Fig. 1). As compared with parental 32D cells, adhesion of the bcr/abl transfectants was greatly elevated at both time points tested (Fig. 2). One hour as well as 20 hr after seeding, 32Dp210 cells showed approximately 2.5-fold higher adhesion to fibronectin than untransfected cells. In contrast, neither 32D nor 32Dp210 cells adhered to collagen I, collagen IV, laminin, or poly-d-lysine (data not shown).
Figure 1.
Expression of p210bcr/abl in 32D cells. Total RNA was isolated from untransfected and p210bcr/abl-transfected 32D cells. p210bcr/abl mRNA expression was detected by reverse transcription–PCR.
Figure 2.
p210bcr/abl stimulates adhesion of 32D cells to immobilized fibronectin. Untransfected (open bar) and p210bcr/abl-transfected (filled bar) 32D cells were cultured for 1 and 20 hr in fibronectin-coated dishes. Results are mean ± SD of a representative experiment performed in triplicate.
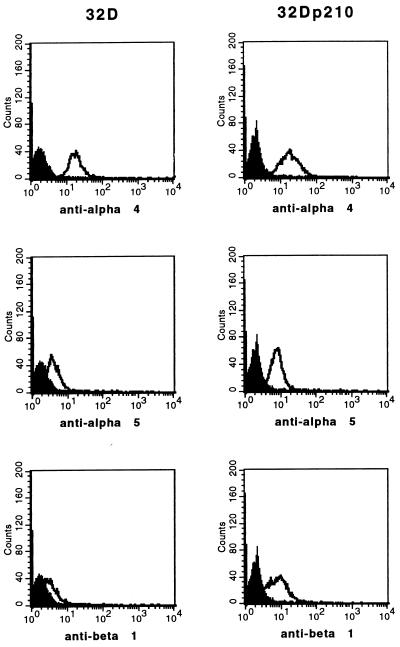
Expression of Integrin Chains α4, α5, and β1 on 32D and 32Dp210 Cells.
To analyze the mechanism of bcr/abl-stimulated adhesion of 32D cells to fibronectin, the expression of the integrin chains α4, α5, and β1 on 32D and 32Dp210 cells was examined by FACS analysis. α4 chains were expressed at comparable levels at the surface of both parental and p210bcr/abl-transfected 32D cells with median fluorescence intensities (MFI) of 16.4 and 16.7, respectively (Fig. 3). In contrast, on 32Dp210 cells we detected higher levels of α5 and β1 chains than on untransfected 32D cells. MFI for α5 chains were 3.7 on 32D and 7.4 on 32Dp210 cells. For β1 chains MFI were 2.9 and 7.3 on 32D and 32Dp210 cells, respectively.
Figure 3.
p210bcr/abl stimulates the expression of α5β1 integrins in 32D cells. Untransfected and p210bcr/abl-transfected 32D cells were stained with an irrelevant antibody (filled curve) and with mAbs directed against the α4, α5, and β1 integrin subunits (solid lines). Expression of α4, α5, and β1 integrins was analyzed by FACS.
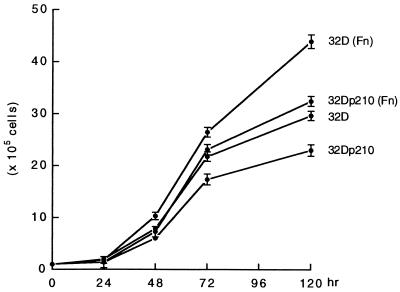
Effect of Immobilized Fibronectin on the Proliferation of 32D and 32Dp210 Cells.
To examine the influence of immobilized fibronectin on the proliferation of parental and p210bcr/abl-transfected 32D cells, 32D and 32Dp210 cells were seeded either on fibronectin-coated dishes or on plastic tissue culture dishes. After incubation for 24, 48, 72, and 120 hr, the proliferation of both 32D and 32Dp210 cells was significantly enhanced when cultured in the presence of immobilized human fibronectin as compared with incubation on uncoated plastic tissue culture dishes (Fig. 4).
Figure 4.
Immobilized fibronectin stimulates the proliferation of parental and p210bcr/abl-transfected 32D cells. 32D and 32Dp210 cells were cultured on fibronectin (Fn)-coated dishes and harvested at 24, 48, 72, and 120 hr.
Effect of Adhesion to Immobilized Fibronectin on Cell Cycle Progression of 32D and 32Dp210 Cells.
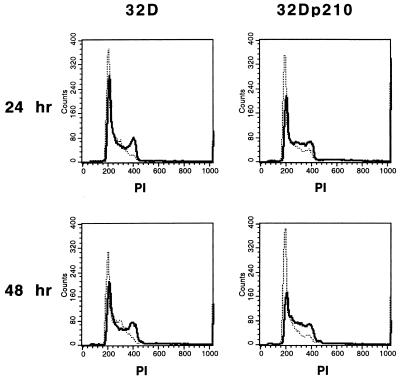
To analyze the effect of adhesion to immobilized human fibronectin on cell cycle progression of parental and p210bcr/abl-transfected 32D cells, the DNA content of adherent and nonadherent cell fractions was examined by FACS. After 24 and 48 hr, adhesion to immobilized fibronectin reduced the number of 32D cells in G0/G1 phase from 51.4% to 32.7% and 39.3% to 28.2%, respectively (Fig. 5). Similarly, the number of 32Dp210 cells in G0/G1 phase was reduced from 52.0% to 29.1% and 58.4% to 22.0% by adhesion. Conversely, the number of 32D cells in S-G2/M phase was increased from 48.6% to 67.3% and 60.7% to 71.8% by adhesion to immobilized fibronectin after 24 and 48 hr. Similarly, the number of 32Dp210 cells in S-G2/M phase increased from 48.0% to 70.9% and 41.6% to 78.0%, respectively.
Figure 5.
Adhesion to fibronectin induces G1/S phase progression in 32D and 32Dp210 cells. 32D and 32Dp210 cells were cultured on fibronectin-coated dishes and harvested at 24 and 48 hr. Their DNA content was measured by propidium iodide (PI) staining and FACS. Shown here are the fluorescence histograms for adherent (solid lines) and nonadherent (dashed lines) 32D and 32Dp210 cells incubated for 24 or 48 hr. The two major peaks observed in adherent cells represent, from left to right, G1 and G2/M. Adhesion reduces the number of G0/G1 and increases the number of S-G2/M phase 32D as well as 32Dp210 cells.
Expression and Associated Kinase Activity of Cyclin A in Adherent and Nonadherent 32D and 32Dp210 Cells.
Because adhesion-dependent cell cycle progression at the G1/S boundary is known to be eventually mediated by the activity of cyclin A/CDK2 (34), parental and bcr/abl-transfected 32D cells were seeded into fibronectin-coated dishes. Cell extracts from adherent and nonadherent fractions were prepared 20 hr after seeding. In these cell extracts cyclin A was detected by immunoblotting. As shown in Fig. 6, nonadherent 32D and 32Dp210 cells express cyclin A at comparable levels to those of their adherent counterparts. However, although cyclin A expression was unaffected by the adhesion status, cyclin A immunoprecipitates from nonadherent 32D cells, but not from adherent 32D cells, failed to display histone H1 kinase activity above the nonspecific background (Fig. 7). To test for the hypothesis that bcr/abl is involved in the adhesion signal transduction cascade that controls cell cycle progression, cyclin A-associated kinase activity also was determined in 32Dp210 cells. In comparison to adherent 32D cells, cyclin A immunoprecipitates from adherent 32Dp210 cells exhibited a greatly elevated histone H1 kinase activity. In addition, cyclin A immunoprecipitates from 32Dp210 cells remained partially active irrespective of the adhesion status.
Figure 6.
Expression of cyclin A, p27Kip1, and p21Cip1 in adherent and nonadherent 32D or 32Dp210 cells. Cells were seeded on fibronectin-coated dishes. Cell extracts from adherent and nonadherent fractions were prepared 20 hr after seeding. Extracts containing 12.5 μg of protein were separated on a 12.5% polyacrylamide gel, and cyclin A, p27Kip1, and p21Cip1 were detected by immunoblotting and enhanced chemiluminescence.
Figure 7.
Adhesion dependence of cyclin A-associated kinase activity in 32D and 32Dp210 cells. Kinase activity associated with cyclin A immunoprecipitates was detected by using histone H1 as substrate in the presence of 32P-labeled ATP. Labeled histone H1 was separated by PAGE and detected by autoradiography. Cyclin A was immunoprecipitated from cell extracts prepared 20 hr after seeding of 32D or 32Dp210 cells on fibronectin-coated dishes.
Expression of p21Cip1 and p27Kip1 and Association of Cyclin A with p27Kip1 in Adherent and Nonadherent 32D and 32Dp210 Cells.
Cyclin A/CDK2 complexes have been shown to be inhibited by the CDK inhibitors p21Cip1 (35) and p27Kip1 (34, 36). Because p21Cip1 and p27 have been described to mediate suspension-induced cell cycle arrest at the G1/S boundary in fibroblasts by inhibiting cyclin E/CDK2 activity (27), the effect of the adhesion status of parental and bcr/abl-transfected 32D cells on p21Cip1 and p27Kip1 expression was determined. As can be seen in Fig. 6, the pattern of p27Kip1 expression in adherent and nonadherent 32D and 32Dp210 cells is complementary to the cyclin A-associated histone H1 kinase activity (Fig. 7) with nonadherent 32Dp210 cells expressing elevated amounts of p27Kip1 in comparison to nonadherent 32D. In contrast, expression of p21Cip1 appeared to be unaffected by the adhesion status of 32D as well as 32Dp210 cells (Fig. 6). We then immunoprecipitated cyclin A complexes from extracts of adherent and nonadherent 32D and 32Dp210 cells to determine the associated levels of p27Kip1. The amounts of p27Kip1 in the immunoprecipitates were measured by immunoblotting. Higher levels of p27Kip1 associated with cyclin A immunoprecipitates from nonadherent versus adherent 32D and 32Dp210 cells could be detected (data not shown).
DISCUSSION
In the present study we have analyzed the effect of adhesion to fibronectin on the proliferation of normal and p210bcr/abl-transfected hematopoietic cells. We have observed that (i) p210bcr/abl strongly enhances adhesion of hematopoietic 32D cells to fibronectin, (ii) the presence of p210bcr/abl is associated with the expression of α5β1 integrins on 32D cells, (iii) adhesion to immobilized fibronectin stimulates the proliferation of both p210bcr/abl-transfected as well as parental 32D cells whereas the nonadherent counterparts appear to be arrested at the G1/S boundary, (iv) adhesion controls G1/S phase progression of normal and p210bcr/abl-transfected 32D cells by modulating the kinase activity of cyclin A/CDK2, (v) cyclin A/CDK2 activity seems to be regulated by adhesion-dependent expression of p27Kip1, and (vi) as compared with untransfected 32D cells, cyclin A immunoprecipitates from 32Dp210 cells exhibited a greatly elevated kinase activity and remained partially active irrespective of the adhesion status.
All experiments were carried out by using the parental and p210bcr/abl-transfected 32D mouse hematopoietic cell lines, thus allowing definitive conclusions regarding the effects of p210bcr/abl expression. In contrast, primary human CML cells are heterogenous with respect to lineage and stage of differentiation, and it is difficult to define an ideal normal cell population for comparison. In addition, it is difficult to control for possible cytokine secretion by stromal cells, macrophages, T lymphocytes, or even the leukemic cells themselves in these heterologous primary cultures. For similar reasons, fibronectin instead of a stromal layer was used as a well-defined adhesive substrate.
A study by Verfaillie et al. (12) suggests that adhesion of human CML bone marrow progenitor cells to fibronectin is reduced compared with the adhesion of progenitor cells from normal donors. However, in this study, adhesion was evaluated indirectly by counting the number of colonies initiated by adherent cells after 3 and 5 weeks of bone marrow culture. At these time points, the number of colonies might reflect expansion of initiator cells, rather than differences in the initial adhesion. Consistent with our observations, Bazzoni et al. (37) have published results indicating that in MO7e, BaF/3, and 32D cells the expression of p210bcr/abl leads to an enhanced adhesion to fibronectin. In contrast to our findings, however, they did not find differences in the expression of the integrin chains α4, α5, and β1 on parental and p210bcr/abl-transfected MO7e and 32D cells. Instead, they report activation of integrin α5β1 by bcr/abl expression as the cause of increased adhesion to fibronectin.
The absence of growth factors or adhesion leads to cell cycle arrest in G0 or late G1, respectively (16). Addition of growth factors to G0-arrested fibroblasts brings the cell to an adhesion-responsive portion of the cell cycle (17). Integrin-mediated adhesion then initiates proliferation by induction of G1/S phase progression in several mesenchymal and epithelial cell types. Transformation of mesenchymal and epithelial cells results in loss of growth factor and/or adhesion requirements (38–46). For hematopoietic cells Lévesque and coworkers (47) report that the activation state of fibronectin receptors expressed by these cells is strongly correlated with their proliferative state. They propose a two-step model with an initial activation of α4β1- and α5β1-integrins by cytokines followed by a secondary signal resulting from integrin-fibronectin binding that may cooperate with those generated by cytokine receptors. We have reported previously that, in contrast to human marrow-adherent CD34+ cells, the majority of peripheral blood CD34+ cells mobilized after granulocyte-colony-stimulating factor-supported chemotherapy is arrested in the late G1 phase of the cell cycle and fibronectin-mediated activation via α4 and α5 integrins seems to be a major pathway that enables G1/S transition (48). These findings are in agreement with the data presented here, because adhesion to fibronectin stimulated cell cycle progression of both parental and p210bcr/abl-transfected 32D cells at the G1/S boundary. Adhesion reduced the proportion of p210bcr/abl-transfected and untransfected 32D cells in G0/G1 phase and increased the proportion of S-G2/M phase cells.
Hurley and coworkers (49) report a different model of cell cycle regulation and propose that direct adhesive interactions between normal hematopoietic progenitor cells and stromal components inhibit their proliferation. In addition, antibody-induced α5β1 integrin-mediated adhesion to fibronectin has been described to decrease proliferation of CML progenitors and K562 cells (13). However, in these studies CD34+/HLA-DR+ cells selected from bone marrow were used as a source for hematopoietic progenitor cells. Because these primary cultures are heterologous, down-regulation of proliferation might have been caused by cytokines released from more mature progeny. In the study by Lundell et al. (13), α5β1 integrin-mediated adhesion to fibronectin has been induced by the β1 activating antibody 8A2. However, recent studies have indicated that distinct integrin functions can be attributed to the single and combined effects of ligand occupancy and receptor clustering (50). In addition, it appears that several cell membrane-reactive antibodies act as signal transduction agonists and cause cell cycle arrest in late G1 phase (51).
For Chinese hamster ovary cells, it is known that overexpression of the α5β1 integrin suppresses the transformed phenotype of the cells and leads to loss of their anchorage-independent growth (15). Recently, a similar finding has been described for the CML blast crisis cell line K562 (52). In contrast to parental K562 cells, α5β1 integrin-overexpressing K562 cells are unable to grow in suspension. Addition of GRGDS, a peptide ligand of the α5β1 integrin, to α5β1 integrin-overexpressing K562 cells leads to G1/S phase progression in these cells. These observations are in agreement with our results reported here.
For fibroblasts, expression of cyclin A has been shown to be adhesion dependent (17). In these cells, we and others previously have demonstrated that an adhesion signal results in the activation of the cyclin E/CDK2 complex by down-regulation of the CDK inhibitors p21Cip1 and p27Kip1 (26, 27). As a consequence the CCAAT-binding transcription factor CBP/cycA is released from binding to an Rb family member and enables the induction of cyclin A expression and progression through the G1/S adhesion checkpoint (23–25). For NIH 3T3 and normal human fibroblasts as well as for the B-lineage acute lymphoblastic leukemia cell line BLIN-2, adhesion is even required for expression of cyclin D1 early in G1 phase (53–55). Here we demonstrate that, analogous to the situation in fibroblasts, the hematopoietic cell line 32D also requires an adhesion signal for cell cycle progression at the G1/S boundary. In these cells, however, adhesion seems to directly regulate the activity of a preexisting cyclin A/CDK2 complex via modulation of p27Kip1 expression. These results are consistent with our recent observation that significantly less cyclin A-expressing CD34+ cells were found in the S/G2-M phase in mobilized peripheral blood compared with steady-state bone marrow (48). Also, recent reports confirm that p27Kip1 is expressed in circulating but not bone marrow CD34+ progenitor cells (56). Consistent with our observations, Symington (52) has published results indicating that in α5β1 integrin-overexpressing K562 cells, α5β1 receptor occupation by the peptide ligand GRGDS promotes G1/S phase progression by inducing cyclin A-associated kinase activity. The adhesion-dependent regulation of cyclin A/CDK2 activity as the most downstream G1/S checkpoint control might allow for a more rapid response to an adhesion signal of hematopoietic cells as compared with fibroblasts.
A hallmark of neoplastic transformation is the loss of adhesion dependence that usually accompanies the loss of growth factor requirement. The CML-specific p210bcr/abl tyrosine kinase recently has been shown to abrogate growth factor and/or adhesion requirements dependent on the cell context (42, 43). In addition, it recently has been described that p210bcr/abl prevents cell cycle arrest at the G1/S boundary and down-regulation of CDK activity after growth factor deprivation of hematopoietic progenitor cells (57). Consistently, we have found that, as compared with untransfected 32D cells, cyclin A immunoprecipitates from 32Dp210 cells exhibited a greatly elevated kinase activity and remained partially active irrespective of the adhesion status. These findings may explain, in part, the proliferative advantage of 32Dp210 cells over their untransfected counterpart.
Taken together, our results indicate that adhesion stimulates cell cycle progression of hematopoietic cells by down-regulation of p27Kip1, resulting in activation of cyclin A/CDK2 complexes and subsequent transition through the G1/S adhesion checkpoint. We conclude that adhesion to fibronectin stimulates cell cycle progression at the G1/S transition of the hematopoietic cell line 32D as well as of its p210bcr/abl-transfected counterpart. Because p210bcr/abl expression leads to increased adhesion to fibronectin, the proliferative advantage of CML cells might be the result of an increased access to adhesion signals.
Acknowledgments
We are grateful to Dr. Carsten-Peter Carstens (Stratagene) for valuable technical advice. This work was supported by the Deutsche Krebshilfe (Grant No. 10-1179-Kr1) and the Forschungsfonds, Fakultät für Klinische Medizin Mannheim, Universität Heidelberg, Germany (Grant No. 0022/97).
ABBREVIATIONS
- Rb
retinoblastoma protein
- CDK
cyclin-dependent kinase
- CML
chronic myelogenous leukemia
- IL-3
interleukin-3
- FACS
fluorescence-activated cell sorting
References
- 1.Miyake K, Weissman I L, Greenberger J S, Kincade P W. J Exp Med. 1991;173:599–607. doi: 10.1084/jem.173.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams D A, Rios M, Stephens C, Patel V P. Nature (London) 1991;352:438–441. doi: 10.1038/352438a0. [DOI] [PubMed] [Google Scholar]
- 3.Weinstein R, Riordan M A, Wenc K, Kreczko S, Dainiak N. Blood. 1989;73:111–116. [PubMed] [Google Scholar]
- 4.Campbell A D, Long M W, Wicha M S. J Clin Invest. 1985;75:2085–2092. doi: 10.1172/JCI111928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou Y Q, Lévesque J P, Hatzfeld A, Cardoso A A, Li M L, Sansilvestri P, Hatzfeld J. Blood. 1993;82:800–806. [PubMed] [Google Scholar]
- 6.Lévesque J P, Leavesley D I, Niutta S, Vadas M, Simmons P J. J Exp Med. 1995;181:1805–1815. doi: 10.1084/jem.181.5.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schofield K P, Humphries M J, de Wynter E, Testa N, Gallagher J T. Blood. 1998;91:3230–3238. [PubMed] [Google Scholar]
- 8.Verfaillie C M. Blood. 1992;79:2821–2826. [PubMed] [Google Scholar]
- 9.Hurley R W, McCarthy J B, Verfaillie C M. J Clin Invest. 1995;96:511–519. doi: 10.1172/JCI118063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Klein A, van Kessel A G, Grosveld G, Bartram C R, Hagemeijer A, Bootsma D, Spurr N K, Heisterkamp N, Groffen J, Stephenson J R. Nature (London) 1982;300:765–767. doi: 10.1038/300765a0. [DOI] [PubMed] [Google Scholar]
- 11.Gordon M Y, Dowding C R, Riley G P, Goldman J M, Greaves M F. Nature (London) 1987;328:342–344. doi: 10.1038/328342a0. [DOI] [PubMed] [Google Scholar]
- 12.Verfaillie C M, McCarthy J B, McClave P B. J Clin Invest. 1992;90:1232–1241. doi: 10.1172/JCI115985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lundell B I, McCarthy J B, Kovach N L, Verfaillie C M. Blood. 1996;87:2450–2458. [PubMed] [Google Scholar]
- 14.Hedge U, Kotelnikov V M, Handa H, Burke P, Preisler H D. Leuk Lymphoma. 1996;22:431–437. doi: 10.3109/10428199609054781. [DOI] [PubMed] [Google Scholar]
- 15.Giancotti F G, Ruoslahti E. Cell. 1990;60:849–859. doi: 10.1016/0092-8674(90)90098-y. [DOI] [PubMed] [Google Scholar]
- 16.Guadagno T M, Assoian R K. J Cell Biol. 1991;115:1419–1425. doi: 10.1083/jcb.115.5.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guadagno T M, Ohtsubo M, Roberts J M, Assoian R K. Science. 1993;262:1572–1575. doi: 10.1126/science.8248807. [DOI] [PubMed] [Google Scholar]
- 18.Weinberg R A. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 19.Chen P L, Scully P, Shew J Y, Wang J Y J, Lee W H. Cell. 1989;58:1193–1198. doi: 10.1016/0092-8674(89)90517-5. [DOI] [PubMed] [Google Scholar]
- 20.DeCaprio J A, Ludlow J W, Lynch D, Furukawa Y, Griffin J, Piwnica-Worms H, Huang C M, Livingston D M. Cell. 1989;58:1085–1095. doi: 10.1016/0092-8674(89)90507-2. [DOI] [PubMed] [Google Scholar]
- 21.Kato J, Matsushime H, Hiebert S W, Ewen M E, Sherr C J. Genes Dev. 1993;7:331–342. doi: 10.1101/gad.7.3.331. [DOI] [PubMed] [Google Scholar]
- 22.Hinds P W, Mittnacht S, Dulic V, Arnold A, Reed S I, Weinberg R A. Cell. 1992;70:993–1006. doi: 10.1016/0092-8674(92)90249-c. [DOI] [PubMed] [Google Scholar]
- 23.Krämer A, Carstens C-P, Fahl W E. J Biol Chem. 1996;271:6579–6582. doi: 10.1074/jbc.271.12.6579. [DOI] [PubMed] [Google Scholar]
- 24.Krämer A, Carstens C-P, Wasserman W W, Fahl W E. Cancer Res. 1997;57:5117–5121. [PubMed] [Google Scholar]
- 25.Carstens C-P, Krämer A, Fahl W E. Radiat Oncol Invest. 1996;3:307–314. [Google Scholar]
- 26.Carstens C-P, Krämer A, Fahl W E. Exp Cell Res. 1996;229:86–92. doi: 10.1006/excr.1996.0346. [DOI] [PubMed] [Google Scholar]
- 27.Fang F, Orend G, Watanabe N, Hunter T, Ruoslahti E. Science. 1996;271:499–502. doi: 10.1126/science.271.5248.499. [DOI] [PubMed] [Google Scholar]
- 28.Daley G Q, Van Etten R A, Baltimore D. Science. 1990;247:824–830. doi: 10.1126/science.2406902. [DOI] [PubMed] [Google Scholar]
- 29.Danhauser-Riedl S, Warmuth M, Druker B J, Emmerich B, Hallek M. Cancer Res. 1996;56:3589–3596. [PubMed] [Google Scholar]
- 30.Matulonis U A, Dosiou C, Lamont C, Freeman G J, Mauch P, Nadler L M, Griffin J D. Blood. 1995;85:2507–2515. [PubMed] [Google Scholar]
- 31.Cross N C P, Hughes T P, Feng L, O’Shea P, Bungey J, Marks D I, Ferrant A, Martiat P, Goldman J M. Br J Haematol. 1993;84:67–74. doi: 10.1111/j.1365-2141.1993.tb03026.x. [DOI] [PubMed] [Google Scholar]
- 32.Bhatia R, McCarthy J B, Verfaillie C M. Blood. 1996;87:3883–3891. [PubMed] [Google Scholar]
- 33.Koff A, Cross F, Fisher A, Schumacher J, Leguellec K, Philippe M, Roberts J M. Cell. 1991;66:1217–1228. doi: 10.1016/0092-8674(91)90044-y. [DOI] [PubMed] [Google Scholar]
- 34.Resnitzky D, Hengst L, Reed S I. Mol Cell Biol. 1995;15:4347–4352. doi: 10.1128/mcb.15.8.4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harper J W, Adami G R, Wei N, Keyomarsi K, Elledge S J. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 36.Toyoshima H, Hunter T. Cell. 1994;78:67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- 37.Bazzoni G, Carlesso N, Griffin J D, Hemler M E. J Clin Invest. 1996;98:521–528. doi: 10.1172/JCI118820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hurlin P J, Fry D G, Maher V M, McCormick J J. Cancer Res. 1987;47:5752–5757. [PubMed] [Google Scholar]
- 39.Johnson P J, Coussens P M, Danko A V, Shalloway D. Mol Cell Biol. 1985;5:1073–1083. doi: 10.1128/mcb.5.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stevens C W, Brondyk W H, Burgess J A, Manoharan T H, Hane B G, Fahl W E. Mol Cell Biol. 1988;8:2089–2096. doi: 10.1128/mcb.8.5.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Renshaw M W, Kipreos E T, Albrecht M R, Wang J Y J. EMBO J. 1992;11:3941–3951. doi: 10.1002/j.1460-2075.1992.tb05488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Renshaw M W, McWhirter J R, Wang J Y J. Mol Cell Biol. 1995;15:1286–1293. doi: 10.1128/mcb.15.3.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lugo T G, Pendergast A M, Witte O N. Science. 1990;247:1079–1082. doi: 10.1126/science.2408149. [DOI] [PubMed] [Google Scholar]
- 44.McClure D B. Cell. 1983;32:999–1006. doi: 10.1016/0092-8674(83)90084-3. [DOI] [PubMed] [Google Scholar]
- 45.Montell C, Courtois G, Eng C, Berk A. Cell. 1984;36:951–961. doi: 10.1016/0092-8674(84)90045-x. [DOI] [PubMed] [Google Scholar]
- 46.Chesters P M, Vousden K H, Edmonds C, McCance D J J. J Gen Virol. 1990;71:449–453. doi: 10.1099/0022-1317-71-2-449. [DOI] [PubMed] [Google Scholar]
- 47.Lévesque J P, Haylock D N, Simmons P J. Blood. 1996;88:1168–1176. [PubMed] [Google Scholar]
- 48.Fruehauf S, Veldwijk M R, Krämer A, Haas R, Zeller W J. Stem Cells. 1998;16:271–279. doi: 10.1002/stem.160271. [DOI] [PubMed] [Google Scholar]
- 49.Hurley R, McCarthy J B, Verfaillie C M. J Clin Invest. 1995;96:511–519. doi: 10.1172/JCI118063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miyamoto S, Akiyama S K, Yamada K M. Science. 1995;267:883–885. doi: 10.1126/science.7846531. [DOI] [PubMed] [Google Scholar]
- 51.Marches R, Scheuermann R H, Uhr J W. Cancer Res. 1998;58:691–697. [PubMed] [Google Scholar]
- 52.Symington B E. J Biol Chem. 1992;267:25744–25747. [PubMed] [Google Scholar]
- 53.Zhu X, Ohtsubo M, Böhmer R M, Roberts J M, Assoian R K. J Cell Biol. 1996;133:391–403. doi: 10.1083/jcb.133.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shah N, LeBien T W. Blood. 1997;90:492a. (abstr.). [Google Scholar]
- 55.Assoian R K. J Cell Biol. 1997;136:1–4. doi: 10.1083/jcb.136.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Teofili L, Larocca L M, Chiusolo P, Urbano R, Leone G. Exp Hematol. 1997;25:799. (abstr.). [Google Scholar]
- 57.Cortez D, Reuther G, Pendergast A M. Oncogene. 1997;15:2333–2342. doi: 10.1038/sj.onc.1201400. [DOI] [PubMed] [Google Scholar]