Abstract
Proteins belonging to the ATP-binding cassette superfamily couple ATP binding and hydrolysis at conserved nucleotide-binding domains (NBDs) to diverse cellular functions. Most superfamily members are transporters, while cystic fibrosis transmembrane conductance regulator (CFTR), alone, is an ion channel. Despite this functional difference, recent results have suggested that CFTR shares a common molecular mechanism with other members. ATP binds to partial binding sites on the surface of the two NBDs, which then associate to form a NBD dimer, with complete composite catalytic sites now buried at the interface. ATP hydrolysis and γ-phosphate dissociation, with the loss of molecular contacts linking the two sides of the composite site, trigger dimer dissociation. The conformational signals generated by NBD dimer formation and dissociation are transmitted to the transmembrane domains where, in transporters, they drive the cycle of conformational changes that translocate the substrate across the membrane; in CFTR, they result in opening and closing (gating) of the ion-permeation pathway.
Keywords: CFTR, ion channel, ATP-binding cassette transporter, ATP binding and hydrolysis, gating
1. Introduction
Cystic fibrosis is one of the most common lethal autosomal recessive disorders in Caucasian populations, affecting approximately 60 000 people worldwide (Guggino & Stanton 2006). The molecular basis of the disease was identified in 1989 (Riordan et al. 1989): patients had a mutation in the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR), which resulted in abnormal electrolyte permeability in various exocrine epithelia (Quinton 2007). The sequence clearly identified CFTR as a member of the superfamily of ATP-binding cassette (ABC) proteins (Dean & Annilo 2005; Hollenstein et al. 2007a). Most of these are integral transmembrane proteins that harness the chemical potential of ATP to transport very diverse compounds (referred to as allocrites) across membranes, often against steep electrochemical potential gradients. However, purified reconstituted CFTR was shown to act as a channel (Bear et al. 1992), providing a permeation pathway for anions to cross membranes, driven by their electrochemical potential difference. Recent research has suggested that the CFTR channel employs the molecular mechanism of its transporter relatives in its own individual way: ATPase cycles do not power uphill allocrite transport but simply the opening and closing of the channel's gates. In this paper, we will review the evidence supporting this view.
2. Structure and mechanism of ABC transporters
The minimal functional unit of ABC transporters is thought to comprise two transmembrane domains (TMDs), which line the path across the membrane for transported allocrites, and two nucleotide binding domains (NBDs), which bind and hydrolyse ATP (Hollenstein et al. 2007a). In most eukaryotic transporters, as in CFTR, these four domains are encoded by a single gene comprising an N-terminal TMD1 and NBD1 and a C-terminal TMD2 and NBD2.
The numerous NBD structures solved to date reveal a conserved fold (Hung et al. 1998; Hopfner et al. 2000; Gaudet & Wiley 2001; Karpowich et al. 2001; Yuan et al. 2001; Locher et al. 2002; Smith et al. 2002; Chen et al. 2003; Schmitt et al. 2003; Verdon et al. 2003a,b; Lewis et al. 2004; Thibodeau et al. 2005; Zaitseva et al. 2005, 2006; Dawson & Locher 2006, 2007; Procko et al. 2006; Ramaen et al. 2006; Hollenstein et al. 2007b; Oldham et al. 2007; Pinkett et al. 2007; Ward et al. 2007), comprising a RecA-like subdomain (‘head’), and a helical subdomain, unique to ABC transporters (‘tail’). In the head subdomain, conserved motifs include the Walker A and B motifs involved in nucleotide coordination and hydrolysis, while the conserved ABC signature sequence (LSGGQXXR) is in the tail subdomain.
NBDs can assume a dimeric ‘head-to-tail’ arrangement. In ATP-bound crystals, NBDs form tight dimers so that two composite ATP-binding sites are formed at the interface, each ATP molecule contacting the conserved motifs from the head of one NBD and the signature sequence from the tail of the partner (Smith et al. 2002; Chen et al. 2003; Zaitseva et al. 2005; Dawson & Locher 2007; Oldham et al. 2007; Ward et al. 2007). At the NBD–NBD interface, the two ATP molecules mediate a large part of the intersubunit interactions (acting as ‘molecular glue’, Karpowich et al. 2001), and indeed biochemical experiments carried out with isolated prokaryotic NBDs confirmed the importance of the ATP molecules, and in particular of the γ-phosphates, in stabilizing the dimeric conformation (Hopfner et al. 2000; Moody et al. 2002; Horn et al. 2003; Janas et al. 2003; Verdon et al. 2003a). These observations led to the hypothesis that an ATPase cycle involving formation and dissociation of tight NBD dimers (ATP binding → tight NBD dimerization → hydrolysis → loss of the γ-phosphate → opening of the NBD–NBD interface) could drive the cyclical conformational changes in the TMDs resulting in uphill allocrite transport (Hopfner et al. 2000).
High resolution structures of several full length ABC transporters, including both importers and exporters, have been solved recently (Dawson & Locher 2006, 2007; Hollenstein et al. 2007b; Oldham et al. 2007; Pinkett et al. 2007; Ward et al. 2007). The overall architecture of the TMDs is not conserved and in some the central cavity is accessible from the cytoplasm (inward facing) and in others from the extracellular space (outward facing). However, in all the crystals, most of the NBD/TMD contacts are seen to occur via a pair of structurally conserved α-helices, the ‘coupling’ helices. It has been suggested that the conformational signal generated by the binding of ATP and NBD dimerization could be transmitted to the TMDs via the movement of the coupling helices closer to each other, leading to a flipping of the TMD conformation from inward facing to outward facing (Hollenstein et al. 2007a).
3. ATP binding to CFTR's NBDs precedes channel opening
Recent results have suggested that a similar cycle of ATP binding, NBD dimerization and ATP hydrolysis also plays an important part in controlling CFTR channel gating. In addition, CFTR requires phosphorylation by cAMP-dependent protein kinase (PKA) (Tabcharani et al. 1991; Csanády et al. 2000; Hegedus et al. 2008). The target serines are within the regulatory or ‘R’ domain, which is not present in other ABC transporters. Once the channels are phosphorylated, interaction with ATP controls the channel gates (figure 1). The molecular mechanism underlying the regulation of gating by ATP in pre-phosphorylated channels will be our focus here, since its structural underpinnings are common in CFTR and transporters.
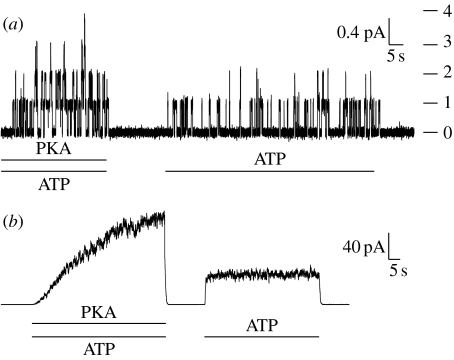
Figure 1.
CFTR gating is regulated by PKA-mediated phosphorylation and by interaction with ATP. Current records from excised, inside-out patches, from Xenopus oocytes expressing WT CFTR. (a) Patch containing a small number of channels. Transitions between the closed and the open state of individual channels can be detected. On the right, horizontal lines show current levels reached by the indicated number of simultaneously open channels. Bars beneath traces indicate changes in solution composition on the cytosolic side of the patch. (b) Patch containing hundreds of WT channels. Pre-phosphorylated channels can be opened by exposure to ATP alone. After ATP removal, all channels close within seconds.
As with bacterial ABC transporters, the N-terminal NBD1 and the C-terminal NBD2 of CFTR are thought to associate to form an intramolecular NBD1/NBD2 ‘heterodimer’ with two composite binding sites formed at the interface (Kidd et al. 2004; Vergani et al. 2005; Mense et al. 2006). We here refer to the composite site comprising NBD1 Walker motifs and NBD2 signature as site 1, while site 2 will comprise NBD2 Walker motifs and NBD1 signature. In CFTR, as in several other eukaryotic ABC transporters (all the other members of the human ABCC subfamily, MRP1-9, SUR1 and SUR2 (Dean et al. 2001), as well as TAP1/TAP2 (Procko et al. 2006)), only interfacial site 2 presents conserved, consensus residues in all the motifs involved in ATP binding and hydrolysis. By contrast, on both faces of site 1 there are non-conservative substitutions at key residues. These result in the degenerate site 1 binding tightly, but not efficiently hydrolysing the bound ATP (Aleksandrov et al. 2001, 2002; Basso et al. 2003). When CFTR is reacted with the photoactive nucleotide 8-azido ATP, labelled on the γ-phosphate (8-azido [γ 32P] ATP), radioactivity is retained at site 1, even after prolonged (30 min) incubation at 30°C (Cui et al. 2006). If, after incubation with 8-azido [α 32P] ATP, CFTR is washed extensively at 30°C in nucleotide-free solution before photocrosslinking, nucleotide is seen to dissociate from site 1 with a time constant of approximately 15 min (Basso et al. 2003).
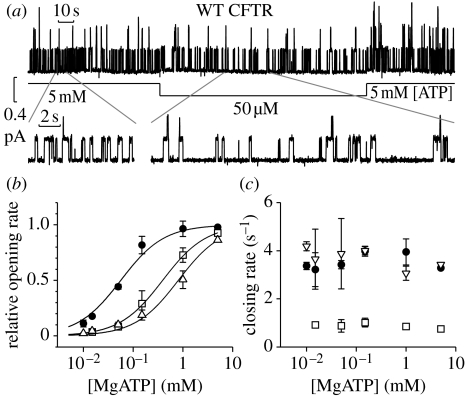
The [ATP] dependence of single-channel kinetic parameters was investigated to determine at which stage of the gating cycle ATP binding is required (figure 2). Reducing [ATP] does not affect average open burst duration. However, at low [ATP], the closed dwell time, the average time spent in the closed state between openings, is increased (Gunderson & Kopito 1994; Venglarik et al. 1994; Winter et al. 1994; Zeltwanger et al. 1999; Csanády et al. 2000; Vergani et al. 2003). For wild-type (WT), a simple hyperbolic equation with an apparent dissociation constant of approximately 50 μM ATP describes the dose–response curve of opening rate as a function of [ATP] (figure 2b, filled circles). Thus, at sub-saturating [ATP], a binding step appears to rate-limit channel opening, indicating that ATP binding occurs on closed channels and is required for channel opening.
Figure 2.
Rate of channel opening is [ATP] dependent. (a) Sample WT trace. Segments with expanded time scale are shown below. (b) Hyperbolic relationship between [ATP] and opening rates (apparent dissociation constants are 56±5, 807±185, 391±118 μM for WT (filled circles), K464A (open triangles) and D1370N (open squares), respectively). (c) Closing rates are [ATP] independent. Modified from Vergani et al. (2003).
To determine whether one or both composite sites in the NBD1/NBD2 dimer are involved in channel opening, we introduced mutations at residues belonging to the conserved Walker motifs seen to interact directly with the bound nucleotide in solved crystal structures. For both mutants (K464A in site 1 and D1370N in site 2), opening rate was reduced at low [ATP], but fast opening could be restored by increasing [ATP] (figure 2b, open symbols). These results led us to conclude that binding at either sites can be made rate limiting for channel opening, suggesting that only after ATP binds to both head subdomains does the transition to the open state become energetically favourable (Vergani et al. 2003). However, Zhou et al. (2006), using a similar experimental strategy, obtained very different results. Sites seen to interact with the adenine moiety of ATP were mutated. The dose–response curve of channel opening rate as a function of [ATP] was seen to shift dramatically to the right when the consensus site 2 was mutated (Y1219G or Y1219I) but not when the degenerate site 1 was altered (W401G). These authors conclude that ATP binding at site 2 initiates channel opening (Chen & Hwang 2008). It is likely that the rate of opening for channels with no ATP bound at site 1 (monoliganded channels) can be altered by mutations at the NBD interface around site 1. Possibly W401G channels open frequently as monoliganded, while WT, with its high-affinity site 1, only rarely would. More research is needed to clarify the role played by ATP binding at site 1 in channel gating.
In any case, ATP binding—at one or both NBDs—occurs on closed channels and is required for channel opening. In the light of recent advances in our structural understanding of ABC transporters, this electrophysiological and biochemical evidence is consistent with the hypothesis that after ATP binding, formation of a tight NBD1/NBD2 dimer is coupled to conformational changes in the TMDs opening the diffusion pathway for anions.
4. Hydrolysis at the consensus site 2 allows fast channel closure
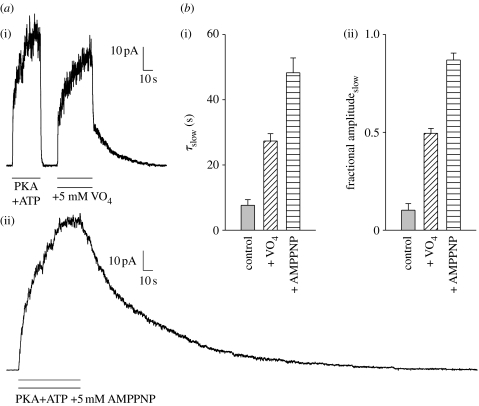
Closing rate of WT CFTR and mutants is not strongly affected by [ATP] (figure 2c, open symbols, Gunderson & Kopito 1994; Venglarik et al. 1994; Winter et al. 1994; Csanády et al. 2000; Vergani et al. 2003, but cf. Zeltwanger et al. 1999). However, CFTR, like other ABC proteins, is an active ATPase (Li et al. 1996), and blocking the hydrolytic cycle, through addition of either inorganic vanadate (Baukrowitz et al. 1994; Gunderson & Kopito 1994) or non-hydrolysable nucleotide analogues (Gunderson & Kopito 1994; Hwang et al. 1994), slows channel closing (figure 3). The effect of such compounds can be mimicked by introducing mutations at key catalytic positions on both sides of composite site 2: in the head of NBD2 (Carson et al. 1995; Gunderson & Kopito 1995; Ramjeesingh et al. 1999; Zeltwanger et al. 1999; Vergani et al. 2003) and in the tail of NBD1 (Vergani et al. 2005). Equivalent mutations at the corresponding residues in site 1 (which in CFTR has lost catalytic activity, see §3) do not significantly affect closing rate (Carson & Welsh 1995; Gunderson & Kopito 1995; Ramjeesingh et al. 1999; Powe et al. 2002; Vergani et al. 2003), except in particular non-hydrolytic conditions (Powe et al. 2002; Vergani et al. 2003). These experiments suggest that hydrolysis of the nucleotide bound at site 2 triggers the rapid channel closure seen in WT (figures 1 and 3a: fast current decay after removal of ATP in control conditions).
Figure 3.
Blocking the hydrolytic cycle slows channel closing. (a) Because opening rate falls to virtually zero after ATP removal, time course of current decay measures channel closing rate. (i) Time constant for current decay in control conditions is less than 1 s, but a slow component (τslow=21 s) appears if opening occurs in the presence of orthovanadate (VO4). In other ABC transporters VO4 inhibits ATPase activity by acting as an analogue of the pentacovalent transition state intermediate of hydrolysis. (ii) Adenosine 5′-(β,γ-imido)triphosphate (AMPPNP) can also ‘lock’ channels open. (b,c) Mean (±s.e.m., n≥15) (i) time constant (τslow) and (ii) fractional amplitude of the slow component.
Again, the evidence is consistent with the idea of NBD dimerization controlling CFTR's gates: fast channel closing would follow dissociation of the tight NBD dimer caused by hydrolysis of the nucleotide bound at site 2. The equilibrium between the closed and open conformations for the channel complex with two bound ATP molecules would be shifted towards the open channel conformation, so that closing through reversal of the opening transition (non-hydrolytic pathway) would occur rarely. For the majority of openings, hydrolysis, triggering release of the γ-phosphate and weakening of inter-NBD contacts would provide an alternative rapid pathway for channel closure.
Minor changes in closing rate have been reported caused by manipulations not expected to alter the hydrolytic rate. For example, the W401G mutant, with a weakened contact with the adenine of the ATP molecule bound at site 1, presented a less than twofold reduction in mean open time, interpreted by the authors as suggesting that separation of the NBD dimer at site 1 can constitute the rate-limiting step in channel closing (Zhou et al. 2006). It is likely that the destabilization of the NBD dimer caused by a weaker interaction with ATP at site 1 would speed up dimer dissociation, and therefore closure, following ATP hydrolysis at site 2.
5. CFTR opening and tight NBD dimerization
Can we link the opening of the CFTR gate to the formation of an NBD1/NBD2 dimer more directly? To do this, we studied the energetic coupling between two residues on opposite sides of the consensus site 2, before, during and after channel opening.
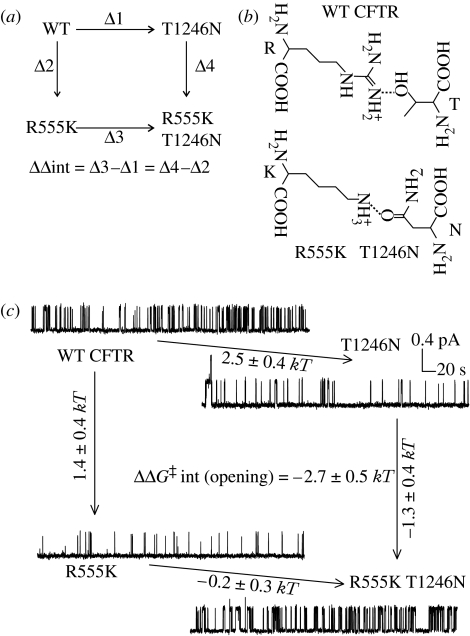
R555 is in the signature sequence in the NBD1 tail; T1246 is in the Walker motif in the NBD2 head. The corresponding residues are seen to form a hydrogen bond in ATP bound, dimeric NBD crystals (Smith et al. 2002; Chen et al. 2003; Zaitseva et al. 2005, 2006). In addition, multiple sequence analysis (Lockless & Ranganathan 1999) on a sequence alignment comprising more than 10 000 NBD sequences (http://www.sanger.ac.uk/cgi-bin/Pfam/getacc?PF00005) indicated that this pair of positions had co-evolved. Changes in side chain at one position had been compensated by changes at the other, so that most existing sequences contain two side chains capable of forming a hydrogen bond that positions the two α-carbons at an approximately 10 Å distance: either an arginine–serine (or an equivalent arginine–threonine, as in WT CFTR), or a lysine–asparagine pair (see figure 4b).
Figure 4.
R555 and T1246 become coupled as the channel opens. (a) Schematic of the thermodynamic mutant cycle. ‘Δ’ beside arrows represents a mutation-linked change in any path-independent measurement. (b) Representation of hydrogen-bonded arginine–threonine and lysine–asparagine pairs. (c) Mutant cycle using changes in activation free energy (ΔΔG‡) for the opening reaction reveal that R555 and T1246 become energetically coupled as channels approach the open state. For each mutation introduced (arrow) the mutation-linked change in ΔG‡ (mean±s.d.) is indicated. Modified from Vergani et al. (2005).
To study energetic coupling we applied mutant cycle analysis (Serrano et al. 1990; Fersht 1999). The WT, the two single mutants (R555K and T1246N) and the double mutant (R555K T1246N) form the corners of a thermodynamic cycle (figure 4a). If the two residues are independent, then the effects of mutations in WT and in mutant background (on parallel sides of the mutant cycle) will be the same. Any non-zero difference between mutation-linked changes on parallel sides of the cycle signifies—and can be used to quantify—energetic coupling between the two target residues (ΔΔint in figure 4a; see also supplementary notes to Vergani et al. 2005).
We used mutation-linked changes in activation free energy for the opening reaction to follow energetic coupling between the two target residues as the channel opens. Both single mutations slowed channel opening, but in the double mutant fast opening was partially restored (figure 4c). The effects on parallel sides of the mutant cycle are therefore very different, yielding a significant energetic coupling (ΔΔG‡int(opening) =−2.7±0.5 kT), consistent with the formation of a stabilizing interaction in the transition state for the opening reaction that was not present in the closed ground state.
We next used open probability (Po) measurements in non-hydrolytic conditions to infer how energetic coupling changes as the channel gates between closed and open states. For channels containing a mutation impairing hydrolysis, the gating scheme is reduced to a simple closed-to-open equilibrium at saturating [ATP]. In these conditions, measurements of channel Po can be used to calculate the free energy difference between the closed and open states. Introducing the T1246N mutation in a non-hydrolytic background (mutated at a crucial lysine, K1250R; Lerner-Marmarosh et al. 1999) strongly destabilized the open state with respect to the closed one. However, the same T to N mutation, when introduced in the R555K K1250R non-hydrolytic background, did not significantly alter the closed-to-open equilibrium. The sign and magnitude of the calculated coupling energy (ΔΔGint(open–closed)=−2.4±1.0 kT) are consistent with the two residues forming a stabilizing interaction, most likely a hydrogen bond, in the open state that is absent in the ATP-bound closed state (Vergani et al. 2005).
To examine coupling in the closed state preceding opening, we analysed the energetics of ATP binding. The apparent dissociation constant obtained from [ATP] dependence of opening rate is, for CFTR (Vergani et al. 2005), a reasonable estimate for the real dissociation constant for the ATP binding reaction at site 2. The R555K mutation did not significantly affect apparent affinity, while the T1246N mutation reduced it to the same degree whether the residue at position 555 was WT R or mutant K. The effects of the T to N mutation in WT and mutant background are thus similar, yielding a negligible energetic coupling between the two target residues (ΔΔGint(unbound–bound)=0.3±0.5 kT). This indicates that any coupling is similar in the closed states before and after ATP binding. Because in all solved ATP bound structures the residue corresponding to the T1246 acceptor also acts as a donor in a hydrogen bond to a γ-phosphate oxygen, it is highly unlikely that binding of ATP would not alter the interdomain hydrogen bond involving T1246. It is more likely that there is no significant energetic coupling between T1246 and R555 in either closed state, neither before nor after ATP binding, with the NBD arrangement more similar to the nucleotide free dimeric crystals (Locher et al. 2002; Chen et al. 2003; Hollenstein et al. 2007b; Pinkett et al. 2007).
In summary, we have followed the interaction between two sites on opposite sides of CFTR's composite site 2: the target residues are not coupled in closed states that bind ATP, but become coupled as the channel opens. The state dependence of energetic coupling that we observe and the conservation of NBD sequences among all ABC proteins, in particular of the residues involved in the hydrogen bond described here, provide strong experimental support to the idea that NBD dimerization drives CFTR channel opening using the same molecular mechanism used by its transporter relatives.
6. ATPase driven gating in CFTR's TMDs
Although we do not have atomic resolution structures for CFTR's TMDs, low resolution structures of CFTR and of P-glycoprotein, a eukaryotic ABC exporter, revealed a striking structural similarity (Rosenberg et al. 2004), and a CFTR homology model built on the crystal structure of the bacterial exporter Sav1866 could be validated biochemically (Serohijos et al. 2008). So it is likely that CFTR's TMDs are structurally similar to those of more conformist ABC transporters. But what subtle structural changes could turn a transporter into a channel?
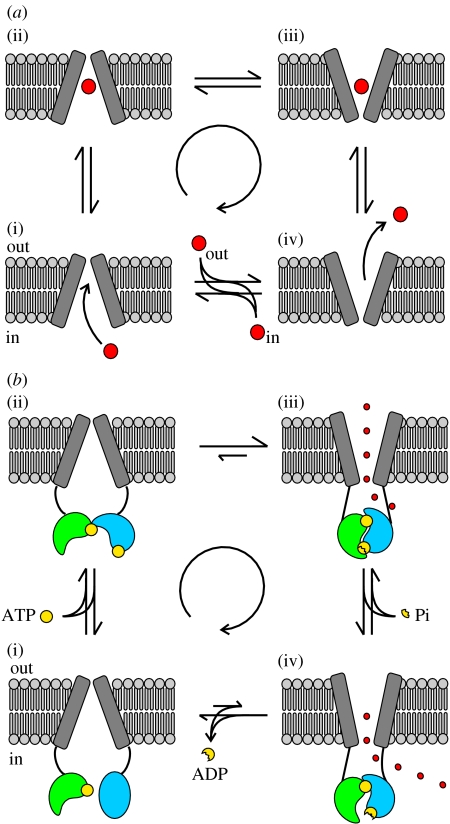
At no time point along the reaction coordinate of an active transporter can there be a pore open to both sides of the membrane, since this would allow dissipation of hard-won electrochemical gradients (Läuger 1980). Thus, a transporter needs to have the allocrite binding site, within the lipid bilayer, flanked on both sides by a gate: one gate limiting access to/from the cytoplasm and one gate limiting access to/from the external solution. As an example, an exporter binds the allocrite from the cytosolic side of the membrane (outer gate closed, figure 5a(i)), then, following a conformational change, releases it to the extracellular side (inner gate closed, figure 5a(iv)), and then in the unloaded state undergoes a further conformational change returning to its initial outward-facing conformation (figure 5a(iv)–(i)).
Figure 5.
Conformational changes occurring in the TMDs of an exporter and in CFTR. (a(i–iv)) Cartoon representing an exporter actively transporting an allocrite (large red circle), through an alternating access and release mechanism. After a sufficient period of time the transporter will have cycled around the four conformations resulting in the net transport of allocrite. The coupled free energy releasing process is not shown. (b(i–iv)) Schematic of ATPase-driven CFTR gating. After ATP (yellow circle) binding, formation of a tight NBD1(green)/NBD2(blue) dimer is coupled to a conformational change in the TMDs, that opens the (external) channel gate. Although each step is reversible, ATP hydrolysis drives cyclic conformational changes resulting in a time asymmetry in single channel current records (Gunderson & Kopito 1995; Hennager et al. 2001). The number of anions (small red circles) crossing the membrane during each cycle is not stoichiometrically related to the number of ATP molecules hydrolysed: throughout the time the gate is open, the rate of anion flow will depend on the electrochemical potential gradient. However, on average, more than 106 Cl− ions cross the membrane during one CFTR gating cycle (i.e. more than 106 ions/1ATP), far more than the 1 (or half) allocrite per ATP molecule moved by the transporter relatives.
For a protein to function as an ion channel it must provide an aqueous pore through which the permeant ions flow. Because in this case ions move down their electrochemical gradient, and not against it, access to the permeation pathway need not be controlled by two gates. Since in crystals of the Sav1866 bacterial exporter, which have the NBDs in a tight dimer conformation, the TMDs have an ‘outward-facing’ conformation (Dawson & Locher 2006, 2007), it is simplest to imagine that CFTR's present working gate might be equivalent to a transporter's external gate (figure 5b). The formation of a tight NBD dimer would flip the TMDs into an outward facing conformation in which the structures homologous to a transporter's internal gate no longer prevent anion flow between the diffusion pathway and the cytosol (figure 5b(iii) and (iv)). Hydrolysis of the ATP bound at site 2, by triggering NBD dimer dissociation, would flip the TMDs to an inward facing conformation in which a functional external gate acts as an effective barrier between the external solution and the permeation pathway (figure 5b(i)).
Further observations suggest that conformational changes in CFTR's TMDs retain evidence of transporter ancestors. For the example exporter in figure 5a, the cycle of conformational changes must occur more frequently in the clockwise direction (central arrow). If one could look at these TMD conformational changes in isolation, an apparent violation of microscopic reversibility would be detected, suggesting coupling to a free energy releasing process. For an ABC exporter, this free energy is provided by the ATP hydrolysis cycle at the NBDs. It turns out that such a violation of microscopic reversibility can be detected by following single-channel CFTR current traces. Using heavily filtered traces three distinct conductances were observed, one closed (baseline current, C) and two open (a lower O1 and a higher O2) conductance levels (Gunderson & Kopito 1995). Following the interconversions between these levels, a time asymmetry was detected: the cycle C-O1-O2-C (57%) occurred much more frequently than the reverse C-O2-O1-C (4%). The remaining openings apparently followed linear pathways (C-O1-C, 27%, and C-O2-C, 12%), but likely many of these included non-resolved missed events. In various non-hydrolytic conditions, the O1–O2 transition virtually disappeared. Using a broader bandwidth, it was later shown that the two open conductance levels were caused by higher (O1) or lower (O2) frequencies of a fast flickery closure (Hennager et al. 2001). Analysis of ‘burst type’ distribution in saturating [ATP] revealed an apparent violation of microscopic reversibility, closely reproducing the previous findings. In the absence of ATP, with openings occurring approximately 150-fold less frequently than in saturating ATP, no time asymmetry was detected. Thus, in the presence of ATP, the conformational states C, O1 and O2 are not at thermodynamic equilibrium, but are kept at a steady state in which clockwise transitions (figure 5b) are more frequent than anticlockwise ones. The simplest interpretation of these observations is that the free energy needed to maintain the system away from equilibrium is, as in our example ABC exporter, derived from the ATP hydrolysis cycle occurring at the NBDs.
7. Conclusion
The current evidence suggests that ATP hydrolysis drives CFTR channel gating. Why would a channel use a free energy source to drive conformational changes of its gates? Is this simply a vestigial, redundant aspect of CFTR channel function? Or could modulation of CFTR hydrolysis provide another sensitive level of regulation for activity, as has been shown for GTPases (Sprang et al. 2007)? Clearly we still have a long way to go before we really understand.
Acknowledgements
We thank the Medical Research Council UK [G0501200]; the Wellcome Trust [081298/Z/06/Z]; and the NIH USA [DK51767 to David C. Gadsby] for funding, as well as László Csanády for helpful discussion.
Footnotes
One contribution of 16 to a Discussion Meeting Issue ‘Membrane transport in flux: the ambiguous interface between channels and pumps’.
References
- Aleksandrov L., Mengos A., Chang X.B., Riordan J.R. Differential interactions of nucleotides at the two nucleotide binding domains of the cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 2001;276:12 918–12 923. doi: 10.1074/jbc.M100515200. doi:10.1074/jbc.M100515200 [DOI] [PubMed] [Google Scholar]
- Aleksandrov L., Aleksandrov A.A., Chang X.B., Riordan J.R. The first nucleotide binding domain of cystic fibrosis transmembrane conductance regulator is a site of stable nucleotide interaction, whereas the second is a site of rapid turnover. J. Biol. Chem. 2002;277:15 419–15 425. doi: 10.1074/jbc.M111713200. doi:10.1074/jbc.M111713200 [DOI] [PubMed] [Google Scholar]
- Basso C., Vergani P., Nairn A.C., Gadsby D.C. Prolonged nonhydrolytic interaction of nucleotide with CFTR's NH2-terminal nucleotide binding domain and its role in channel gating. J. Gen. Physiol. 2003;122:333–348. doi: 10.1085/jgp.200308798. doi:10.1085/jgp.200308798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baukrowitz T., Hwang T.C., Nairn A.C., Gadsby D.C. Coupling of CFTR Cl− channel gating to an ATP hydrolysis cycle. Neuron. 1994;12:473–482. doi: 10.1016/0896-6273(94)90206-2. doi:10.1016/0896-6273(94)90206-2 [DOI] [PubMed] [Google Scholar]
- Bear C.E., Li C.H., Kartner N., Bridges R.J., Ramjeesingh M., Riordan J.R. Purification and functional reconstitution of the cystic fibrosis transmembrane conductance regulator (CFTR) Cell. 1992;68:809–818. doi: 10.1016/0092-8674(92)90155-6. doi:10.1016/0092-8674(92)90155-6 [DOI] [PubMed] [Google Scholar]
- Carson M.R., Welsh M.J. Structural and functional similarities between the nucleotide-binding domains of CFTR and GTP-binding proteins. Biophys. J. 1995;69:2443–2448. doi: 10.1016/S0006-3495(95)80113-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson M.R., Travis S.M., Welsh M.J. The two nucleotide-binding domains of cystic fibrosis transmembrane conductance regulator (CFTR) have distinct functions in controlling channel activity. J. Biol. Chem. 1995;270:1711–1717. doi: 10.1074/jbc.270.4.1711. doi:10.1074/jbc.270.4.1711 [DOI] [PubMed] [Google Scholar]
- Chen T.Y., Hwang T.C. CLC-0 and CFTR: chloride channels evolved from transporters. Physiol. Rev. 2008;88:351–387. doi: 10.1152/physrev.00058.2006. doi:10.1152/physrev.00058.2006 [DOI] [PubMed] [Google Scholar]
- Chen J., Sharma S., Quiocho F.A., Davidson A.L. A tweezers-like motion of the ATP-binding cassette dimer in an ABC transport cycle. Mol. Cell. 2003;12:651–661. doi: 10.1016/j.molcel.2003.08.004. doi:10.1016/j.molcel.2003.08.004 [DOI] [PubMed] [Google Scholar]
- Csanády L., Chan K.W., Seto-Young D., Kopsco D.C., Nairn A.C., Gadsby D.C. Severed channels probe regulation of gating of cystic fibrosis transmembrane conductance regulator by its cytoplasmic domains. J. Gen. Physiol. 2000;116:477–500. doi: 10.1085/jgp.116.3.477. doi:10.1085/jgp.116.3.477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L., Aleksandrov L., Hou Y.X., Gentzch M., Chen J.H., Riordan J.R., Aleksandrov A.A. The role of cystic fibrosis transmembrane conductance regulator phenylalanine 508 side chain in ion channel gating. J. Physiol. 2006;572:347–358. doi: 10.1113/jphysiol.2005.099457. doi:10.1113/jphysiol.2005.099457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson R.J.P., Locher K.P. Structure of a bacterial multidrug ABC transporter. Nature. 2006;443:180–185. doi: 10.1038/nature05155. doi:10.1038/nature05155 [DOI] [PubMed] [Google Scholar]
- Dawson R.J.P., Locher K.P. Structure of the multidrug ABC transporter Sav1866 from Staphylococcus aureus in complex with AMP-PNP. FEBS Lett. 2007;581:935–938. doi: 10.1016/j.febslet.2007.01.073. doi:10.1016/j.febslet.2007.01.073 [DOI] [PubMed] [Google Scholar]
- Dean M., Annilo T. Evolution of the ATP-binding cassette (ABC) transporter superfamily in vertebrates. Annu. Rev. Genomics Hum. Genet. 2005;6:123–142. doi: 10.1146/annurev.genom.6.080604.162122. doi:10.1146/annurev.genom.6.080604.162122 [DOI] [PubMed] [Google Scholar]
- Dean M., Rzhetsky A., Allikmets R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 2001;11:1156–1166. doi: 10.1101/gr.184901. doi:10.1101/gr.GR-1649R [DOI] [PubMed] [Google Scholar]
- Fersht A. WH Freeman and Company; New York, NY: 1999. Structure and mechanism in protein science. A guide to enzyme catalysis and protein folding. [Google Scholar]
- Gaudet R., Wiley D.C. Structure of the ABC ATPase domain of human TAP1, the transporter associated with antigen processing. EMBO J. 2001;20:4964–4972. doi: 10.1093/emboj/20.17.4964. doi:10.1093/emboj/20.17.4964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guggino W.B., Stanton B.A. New insights into cystic fibrosis: molecular switches that regulate CFTR. Nat. Rev. Mol. Cell. Biol. 2006;7:426–436. doi: 10.1038/nrm1949. doi:10.1038/nrm1949 [DOI] [PubMed] [Google Scholar]
- Gunderson K.L., Kopito R.R. Effects of pyrophosphate and nucleotide analogs suggest a role for ATP hydrolysis in cystic fibrosis transmembrane regulator channel gating. J. Biol. Chem. 1994;269:19 349–19 353. [PubMed] [Google Scholar]
- Gunderson K.L., Kopito R.R. Conformational states of CFTR associated with channel gating: the role of ATP binding and hydrolysis. Cell. 1995;82:231–239. doi: 10.1016/0092-8674(95)90310-0. doi:10.1016/0092-8674(95)90310-0 [DOI] [PubMed] [Google Scholar]
- Hegedus T., Serohijos A.W.R., Dokholyan N.V., He L., Riordan J.R. Computational studies reveal phosphorylation-dependent changes in the unstructured R domain of CFTR. J. Mol. Biol. 2008;378:1052–1063. doi: 10.1016/j.jmb.2008.03.033. doi:10.1016/j.jmb.2008.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennager D.J., Ikuma M., Hoshi T., Welsh M.J. A conditional probability analysis of cystic fibrosis transmembrane conductance regulator gating indicates that ATP has multiple effects during the gating cycle. Proc. Natl Acad. Sci. USA. 2001;98:3594–3599. doi: 10.1073/pnas.051633298. doi:10.1073/pnas.051633298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenstein K., Dawson R.J., Locher K.P. Structure and mechanism of ABC transporter proteins. Curr. Opin. Struct. Biol. 2007a;17:412–418. doi: 10.1016/j.sbi.2007.07.003. doi:10.1016/j.sbi.2007.07.003 [DOI] [PubMed] [Google Scholar]
- Hollenstein K., Frei D.C., Locher K.P. Structure of an ABC transporter in complex with its binding protein. Nature. 2007b;446:213–216. doi: 10.1038/nature05626. doi:10.1038/nature05626 [DOI] [PubMed] [Google Scholar]
- Hopfner K.P., Karcher A., Shin D.S., Craig L., Arthur L.M., Carney J.P., Tainer J.A. Structural biology of Rad50 ATPase: ATP-driven conformational control in DNA double-strand break repair and the ABC-ATPase superfamily. Cell. 2000;101:789–800. doi: 10.1016/s0092-8674(00)80890-9. doi:10.1016/S0092-8674(00)80890-9 [DOI] [PubMed] [Google Scholar]
- Horn C., Bremer E., Schmitt L. Nucleotide dependent monomer/dimer equilibrium of OpuAA, the nucleotide-binding protein of the osmotically regulated ABC transporter OpuA from bacillus subtilis. J. Mol. Biol. 2003;334:403–419. doi: 10.1016/j.jmb.2003.09.079. doi:10.1016/j.jmb.2003.09.079 [DOI] [PubMed] [Google Scholar]
- Hung L.W., Wang I.X., Nikaido K., Liu P.Q., Ames G.F., Kim S.H. Crystal structure of the ATP-binding subunit of an ABC transporter. Nature. 1998;396:703–707. doi: 10.1038/25393. doi:10.1038/25393 [DOI] [PubMed] [Google Scholar]
- Hwang T.C., Nagel G., Nairn A.C., Gadsby D.C. Regulation of the gating of cystic fibrosis transmembrane conductance regulator Cl channels by phosphorylation and ATP hydrolysis. Proc. Natl Acad. Sci. USA. 1994;91:4698–4702. doi: 10.1073/pnas.91.11.4698. doi:10.1073/pnas.91.11.4698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janas E., Hofacker M., Chen M., Gompf S., van der Does C., Tampe R. The ATP hydrolysis cycle of the nucleotide-binding domain of the mitochondrial ATP-binding cassette transporter Mdl1p. J. Biol. Chem. 2003;278:26 862–26 869. doi: 10.1074/jbc.M301227200. doi:10.1074/jbc.M301227200 [DOI] [PubMed] [Google Scholar]
- Karpowich N., Martsinkevich O., Millen L., Yuan Y.R., Dai P.L., MacVey K., Thomas P.J., Hunt J.F. Crystal structures of the MJ1267 ATP binding cassette reveal an induced-fit effect at the ATPase active site of an ABC transporter. Structure. 2001;9:571–586. doi: 10.1016/s0969-2126(01)00617-7. doi:10.1016/S0969-2126(01)00617-7 [DOI] [PubMed] [Google Scholar]
- Kidd J.F., Ramjeesingh M., Stratford F., Huan L.-J., Bear C.E. A heteromeric complex of the two nucleotide binding domains of cystic fibrosis transmembrane conductance regulator (CFTR) mediates ATPase activity. J. Biol. Chem. 2004;279:41 664–41 669. doi: 10.1074/jbc.M407666200. doi:10.1074/jbc.M407666200 [DOI] [PubMed] [Google Scholar]
- Läuger P. Kinetic properties of ion carriers and channels. J. Membr. Biol. 1980;57:163–178. doi: 10.1007/BF01869585. doi:10.1007/BF01869585 [DOI] [PubMed] [Google Scholar]
- Lerner-Marmarosh N., Gimi K., Urbatsch I.L., Gros P., Senior A.E. Large scale purification of detergent-soluble P-glycoprotein from Pichia pastoris cells and characterization of nucleotide binding properties of wild-type, Walker A, and Walker B mutant proteins. J. Biol. Chem. 1999;274:34 711–34 718. doi: 10.1074/jbc.274.49.34711. doi:10.1074/jbc.274.49.34711 [DOI] [PubMed] [Google Scholar]
- Lewis H., et al. Structure of nucleotide-binding domain 1 of the cystic fibrosis transmembrane conductance regulator. EMBO J. 2004;23:282–293. doi: 10.1038/sj.emboj.7600040. doi:10.1038/sj.emboj.7600040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Ramjeesingh M., Wang W., Garami E., Hewryk M., Lee D., Rommens J.M., Galley K., Bear C.E. ATPase activity of the cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 1996;271:28 463–28 468. doi: 10.1074/jbc.271.45.28463. doi:10.1074/jbc.271.45.28463 [DOI] [PubMed] [Google Scholar]
- Locher K.P., Lee A.T., Rees D.C. The E. coli BtuCD structure: a framework for ABC transporter architecture and mechanism. Science. 2002;296:1091–1098. doi: 10.1126/science.1071142. doi:10.1126/science.1071142 [DOI] [PubMed] [Google Scholar]
- Lockless S.W., Ranganathan R. Evolutionarily conserved pathways of energetic connectivity in protein families. Science. 1999;286:295–299. doi: 10.1126/science.286.5438.295. doi:10.1126/science.286.5438.295 [DOI] [PubMed] [Google Scholar]
- Mense M., Vergani P., White D.M., Altberg G., Nairn A.C., Gadsby D.C. In vivo phosphorylation of CFTR promotes formation of a nucleotide-binding. EMBO J. 2006;25:4728–4739. doi: 10.1038/sj.emboj.7601373. doi:10.1038/sj.emboj.7601373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody J.E., Millen L., Binns D., Hunt J.F., Thomas P.J. Cooperative, ATP-dependent association of the nucleotide binding cassettes during the catalytic cycle of ATP-binding cassette transporters. J. Biol. Chem. 2002;277:21 111–21 114. doi: 10.1074/jbc.C200228200. doi:10.1074/jbc.C200228200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham M.L., Khare D., Quiocho F.A., Davidson A.L., Chen J. Crystal structure of a catalytic intermediate of the maltose transporter. Nature. 2007;450:515–521. doi: 10.1038/nature06264. doi:10.1038/nature06264 [DOI] [PubMed] [Google Scholar]
- Pinkett H.W., Lee A.T., Lum P., Locher K.P., Rees D.C. An inward-facing conformation of a putative metal–chelate-type ABC transporter. Science. 2007;315:373–377. doi: 10.1126/science.1133488. doi:10.1126/science.1133488 [DOI] [PubMed] [Google Scholar]
- Powe A.C.J., Al-Nakkash L., Li M., Hwang T.C. Mutation of Walker-A lysine 464 in cystic fibrosis transmembrane conductance regulator reveals functional interaction between its nucleotide-binding domains. J. Physiol. 2002;539:333–346. doi: 10.1113/jphysiol.2001.013162. doi:10.1113/jphysiol.2001.013162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procko E., Ferrin-O'Connell I., Ng S.-L., Gaudet R. Distinct structural and functional properties of the ATPase sites in an asymmetric ABC transporter. Mol. Cell. 2006;24:51–62. doi: 10.1016/j.molcel.2006.07.034. doi:10.1016/j.molcel.2006.07.034 [DOI] [PubMed] [Google Scholar]
- Quinton P.M. Cystic fibrosis: lessons from the sweat gland. Physiology. 2007;22:212–225. doi: 10.1152/physiol.00041.2006. doi:10.1152/physiol.00041.2006 [DOI] [PubMed] [Google Scholar]
- Ramaen O., Leulliot N., Sizun C., Ulryck N., Pamlard O., Lallemand J.-Y., van Tilbeurgh H., Jacquet E. Structure of the human multidrug resistance protein 1 nucleotide binding domain 1 bound to Mg2+/ATP reveals a non-productive catalytic site. J. Mol. Biol. 2006;359:940–949. doi: 10.1016/j.jmb.2006.04.005. doi:10.1016/j.jmb.2006.04.005 [DOI] [PubMed] [Google Scholar]
- Ramjeesingh M., Li C., Garami E., Huan L.J., Galley K., Wang Y., Bear C.E. Walker mutations reveal loose relationship between catalytic and channel-gating activities of purified CFTR (cystic fibrosis transmembrane conductance regulator) Biochemistry. 1999;38:1463–1468. doi: 10.1021/bi982243y. doi:10.1021/bi982243y [DOI] [PubMed] [Google Scholar]
- Riordan J.R., et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. doi:10.1126/science.2475911 [DOI] [PubMed] [Google Scholar]
- Rosenberg M.F., Kamis A.B., Aleksandrov L.A., Ford R.C., Riordan J.R. Purification and crystallization of the cystic fibrosis transmembrane conductance regulator (CFTR) J. Biol. Chem. 2004;279:39 051–39 057. doi: 10.1074/jbc.M407434200. doi:10.1074/jbc.M407434200 [DOI] [PubMed] [Google Scholar]
- Schmitt L., Benabdelhak H., Blight M.A., Holland I.B., Stubbs M.T.U. Crystal structure of the nucleotide-binding domain of the ABC-transporter haemolysin B: identification of a variable region within ABC helical domains. J. Mol. Biol. 2003;330:333–342. doi: 10.1016/s0022-2836(03)00592-8. doi:10.1016/S0022-2836(03)00592-8 [DOI] [PubMed] [Google Scholar]
- Serohijos A.W.R., Hegedus T., Aleksandrov A.A., He L., Cui L., Dokholyan N.V., Riordan J.R. Phenylalanine-508 mediates a cytoplasmic-membrane domain contact in the CFTR 3D structure crucial to assembly and channel function. Proc. Natl Acad. Sci. USA. 2008;105:3256–3261. doi: 10.1073/pnas.0800254105. doi:10.1073/pnas.0800254105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano L., Horovitz A., Avron B., Bycroft M., Fersht A.R. Estimating the contribution of engineered surface electrostatic interactions to protein stability by using double-mutant cycles. Biochemistry. 1990;29:9343–9352. doi: 10.1021/bi00492a006. doi:10.1021/bi00492a006 [DOI] [PubMed] [Google Scholar]
- Smith P.C., Karpowich N., Millen L., Moody J.E., Rosen J., Thomas P.J., Hunt J.F. ATP binding to the motor domain from an ABC transporter drives formation of a nucleotide sandwich dimer. Mol. Cell. 2002;10:139–149. doi: 10.1016/s1097-2765(02)00576-2. doi:10.1016/S1097-2765(02)00576-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprang S.R., Chen Z., Du X. Structural basis of effector regulation and signal termination in heterotrimeric Gα proteins. Adv. Prot. Chem. 2007;74:1–65. doi: 10.1016/S0065-3233(07)74001-9. doi:10.1016/S0065-3233(07)74001-9 [DOI] [PubMed] [Google Scholar]
- Tabcharani J.A., Chang X.B., Riordan J.R., Hanrahan J.W. Phosphorylation-regulated Cl− channel in CHO cells stably expressing the cystic fibrosis gene. Nature. 1991;352:628–631. doi: 10.1038/352628a0. doi:10.1038/352628a0 [DOI] [PubMed] [Google Scholar]
- Thibodeau P.H., Brautigam C.A., Machius M., Thomas P.J. Side chain and backbone contributions of Phe508 to CFTR folding. Nat. Struct. Mol. Biol. 2005;12:10–16. doi: 10.1038/nsmb881. doi:10.1038/nsmb881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venglarik C.J., Schultz B.D., Frizzell R.A., Bridges R.J. ATP alters current fluctuations of cystic fibrosis transmembrane conductance regulator: evidence for a three-state activation mechanism. J. Gen. Physiol. 1994;104:123–146. doi: 10.1085/jgp.104.1.123. doi:10.1085/jgp.104.1.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdon G., Albers S.-V., van Oosterwijk N., Dijkstra B.W., Driessen A.J.M., Thunnissen A.-M. W. Formation of the productive ATP-Mg2+-bound dimer of GlcV, an ABC-ATPase from Sulfolobus solfataricus. J. Mol. Biol. 2003;334:255–267. doi: 10.1016/j.jmb.2003.08.065. doi:10.1016/j.jmb.2003.08.065 [DOI] [PubMed] [Google Scholar]
- Verdon G., Albers S.V., Dijkstra B.W., Driessen A.J.M., Thunnissen A.-M.W. Crystal structures of the ATPase subunit of the glucose ABC transporter from Sulfolobus solfataricus: nucleotide-free and nucleotide-bound conformations. J. Mol. Biol. 2003b;330:343–358. doi: 10.1016/s0022-2836(03)00575-8. doi:10.1016/S0022-2836(03)00575-8 [DOI] [PubMed] [Google Scholar]
- Vergani P., Nairn A.C., Gadsby D.C. On the mechanism of MgATP-dependent gating of CFTR Cl− channels. J. Gen. Physiol. 2003;121:17–36. doi: 10.1085/jgp.20028673. doi:10.1085/jgp.20028673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergani P., Lockless S.W., Nairn A.C., Gadsby D.C. CFTR channel opening by ATP-driven tight dimerization of its nucleotide-binding domains. Nature. 2005;433:876–880. doi: 10.1038/nature03313. doi:10.1038/nature03313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward A., Reyes C.L., Yu J., Roth C.B., Chang G. Flexibility in the ABC transporter MsbA: alternating access with a twist. Proc. Natl Acad. Sci. USA. 2007;104:19 005–19 010. doi: 10.1073/pnas.0709388104. doi:10.1073/pnas.0709388104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter M.C., Sheppard D.N., Carson M.R., Welsh M.J. Effect of ATP concentration on CFTR Cl− channels: a kinetic analysis of channel regulation. Biophys. J. 1994;66:1398–1403. doi: 10.1016/S0006-3495(94)80930-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y.R., Blecker S., Martsinkevich O., Millen L., Thomas P.J., Hunt J.F. The crystal structure of the MJ0796 ATP-binding cassette. Implications for the structural consequences of ATP hydrolysis in the active site of an ABC transporter. J. Biol. Chem. 2001;276:32 313–32 321. doi: 10.1074/jbc.M100758200. doi:10.1074/jbc.M100758200 [DOI] [PubMed] [Google Scholar]
- Zaitseva J., Jenewein S., Jumpertz T., Holland I., Schmitt L. H662 is the linchpin of ATP hydrolysis in the nucleotide-binding domain of the ABC transporter HlyB. EMBO J. 2005;24:1901–1910. doi: 10.1038/sj.emboj.7600657. doi:10.1038/sj.emboj.7600657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitseva J., Oswald C., Jumpertz T., Jenewein S., Wiedenmann A., Holland B., Schmitt L. A structural analysis of asymmetry required for catalytic activity of an ABC-ATPase domain dimer. EMBO J. 2006;25:3432–3443. doi: 10.1038/sj.emboj.7601208. doi:10.1038/sj.emboj.7601208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeltwanger S., Wang F., Wang G.T., Gillis K.D., Hwang T.C. Gating of cystic fibrosis transmembrane conductance regulator chloride channels by adenosine triphosphate hydrolysis. Quantitative analysis of a cyclic gating scheme. J. Gen. Physiol. 1999;113:541–554. doi: 10.1085/jgp.113.4.541. doi:10.1085/jgp.113.4.541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Wang X., Liu H.-Y., Zou X., Li M., Hwang T.-C. The two ATP binding sites of cystic fibrosis transmembrane conductance regulator (CFTR) play distinct roles in gating kinetics and energetics. J. Gen. Physiol. 2006;128:413–422. doi: 10.1085/jgp.200609622. doi:10.1085/jgp.200609622 [DOI] [PMC free article] [PubMed] [Google Scholar]