Abstract
In principle, an ion channel needs no more than a single gate, but a pump requires at least two gates that open and close alternately to allow ion access from only one side of the membrane at a time. In the Na+,K+-ATPase pump, this alternating gating effects outward transport of three Na+ ions and inward transport of two K+ ions, for each ATP hydrolysed, up to a hundred times per second, generating a measurable current if assayed in millions of pumps. Under these assay conditions, voltage jumps elicit brief charge movements, consistent with displacement of ions along the ion pathway while one gate is open but the other closed. Binding of the marine toxin, palytoxin, to the Na+,K+-ATPase uncouples the two gates, so that although each gate still responds to its physiological ligand they are no longer constrained to open and close alternately, and the Na+,K+-ATPase is transformed into a gated cation channel. Millions of Na+ or K+ ions per second flow through such an open pump–channel, permitting assay of single molecules and allowing unprecedented access to the ion transport pathway through the Na+,K+-ATPase. Use of variously charged small hydrophilic thiol-specific reagents to probe cysteine targets introduced throughout the pump's transmembrane segments allows mapping and characterization of the route traversed by transported ions.
Keywords: ion transport, gating, cation/anion selectivity, cysteine scanning
1. Ion pumps versus ion channels
Life ultimately depends on the traffic of small ions across the membranes that surround cells and organelles. Because the phospholipids that make up those membranes represent an impenetrable barrier to ions, two general classes of membrane-spanning proteins have evolved to conduct the ion traffic: ion channels and ion pumps. An ion channel is a gated pore, selective for one kind of ion or another, through which when the gate is open the chosen ions flow, by electrodiffusion, in the direction dictated solely by the prevailing gradients of concentration of the ions (i.e. of their chemical potential), and of electrical potential, across the membrane (figure 1a). As a result, ion flow through ion channels dissipates the electrochemical potential gradients that sustain the flow. By contrast, an ion pump, although also selective for a particular kind of ion, can move the ion against its electrochemical potential gradient by coupling that ‘uphill’ movement to some energy-dissipating process. In the case of so-called primary pumps, the energy derives from the hydrolysis of ATP, while in secondary pumps (usually called transporters) the energy source is the electrochemical potential gradient of another ion (often Na+). So primary pumps generate electrochemical potential gradients that ion channels and transporters exploit.
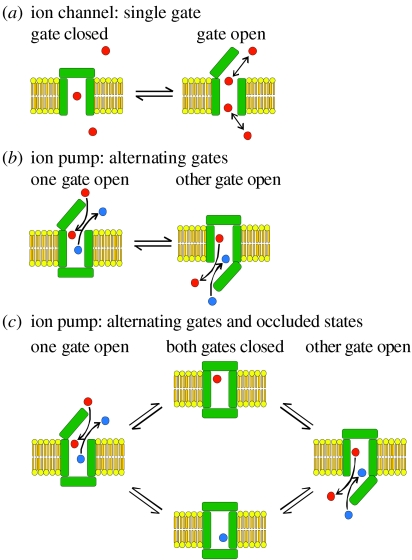
Figure 1.
Ion channels versus ion pumps. (a) Representation of minimal ion channel as transmembrane pore with single gate. (b) Representation of minimal ion pump, or transporter, as transmembrane pore with two gates that open and close alternately. An unspecified source of energy is harnessed to make the apparent binding affinity higher for red than blue ions in the left-hand state, and higher for blue than red ions in the right-hand state. (c) The introduction of occluded states, with both gates closed around a bound ion, precludes the possibility of the second gate opening before the first gate has fully closed, which would allow ions to flow down their electrochemical potential gradient at rates several orders of magnitude higher than those at which the pump or transporter can move them against their electrochemical potential gradient. This is because ion movement through pumps or transporters is limited by gating rates, which are much slower than electrodiffusive ion flow.
Owing to these diametrically opposed roles of ion channels and ion pumps, together with perceived mechanistic distinctions, related partly to differences in their ion throughput speeds and partly to the different methodological approaches used to characterize them, the tendency has been to treat them as separate entities. This great divide held sway despite demonstrations that, formally, a pump could be represented as a channel with an ion-binding site between two gates that open and close alternately to allow access from one side of the membrane, and then the other, sequentially (figure 1b; Patlak 1957; Jardetsky 1966; Vidaver 1966; Läuger 1979, 1991). Because ions flow rapidly through open ion channels (approx. 107 ions s−1 is a typical rate), whereas the conformational changes that open and close the gates occur much more slowly (at rates commonly of the order of approx. 102 s−1), this formalism is subject to strict limits. If, anywhere along the reaction trajectory between the two transporter conformations in figure 1b, the second gate began to open before the first gate had fully closed, the resulting conformer would represent an ion channel, through which ions could flow ‘downhill’ some 105-fold faster than the transporter could ever pump them uphill. The implicit assumption, then, in alternating-access transport models of the kind illustrated in figure 1b is that the two gates can never be open at the same time: in the above example, for the transporter to do useful work, the probability of finding both gates open must be much less than 10−5. Nature's solution to this problem, especially in the case of primary pumps (but see also Gouaux 2009) is ‘occluded’ states (Post et al. 1972) in which both gates are closed, entrapping the transported substrate, before the opposite-side gate opens to release it (figure 1c).
2. Na+,K+-ATPase occluded-ion states
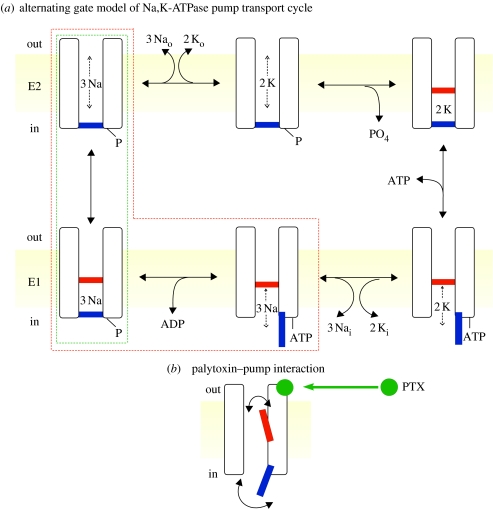
The cycle of conformational changes (Post et al. 1965; Albers 1967) by which the Na+,K+-ATPase pump transports three Na+ ions out across the cell membrane, and then two K+ ions in, can similarly be viewed as that of a channel with two gates (figure 2a). This cycle also includes two occluded states, one enclosing three Na+ ions (figure 2a, bottom left) and the other two K+ ions (top right). In the Na+,K+ pump's E1 conformation, with open cytoplasmic-side gate but closed extracellular-side gate, once three Na+ ions have entered the binding sites the γ-phosphate of ATP is transferred to a conserved aspartate in a reaction that closes the cytoplasmic gate. This occludes the Na+ ions in the E1P phosphorylated state (whence the family name ‘P-type’ ATPases). Relaxation to the E2P state, with open extracellular-side gate but closed cytoplasmic-side gate, prompts release of the Na+ ions and binding of two K+ ions, whereupon the external gate closes, occluding the K+ ions and triggering dephosphorylation of the aspartyl phosphate. In the recent X-ray structure of Na+,K+-ATPase crystallized in complex with phosphate analogue MgF42− (Morth et al. 2007, 2009), the two occluded Rb+ ions (as K+ surrogates) are invisible from either side of the protein, confirming that closure of both gates effectively isolates the binding sites from both cytoplasmic and extracellular milieux.
Figure 2.
(a) Alternating-gate model of the Post–Albers (Post et al. 1965; Albers 1967) transport cycle of the Na+,K+-ATPase pump represented in cartoon form as an ion channel with two gates, an extracellular-side (labelled out) gate (red) and cytoplasmic-side (in) gate (blue), that open alternately, but are never simultaneously open. Also indicated are E2 (upper row; extracellular-side gate may open) and E1 (lower row; cytoplasmic-side gate may open) states. Occluded states, with both gates shut, follow binding of two external K+ (top right) and of three internal Na+ and subsequent phosphorylation (bottom left). ATP acts with low affinity to speed up the opening of the cytoplasmic-side gate and concomitant K+ deocclusion, and acts with high affinity to phosphorylate the pump. The states enclosed by the red dashed box are occupied in the presence of ATP and Na+ ions, but in the absence of K+ ions. The states within the green dashed box are occupied in the presence of ATP and Na+ ions, but in the absence of ADP and K+ ions, and yield voltage-jump-induced charge movements, associated with deocclusion and release to the exterior of the three transported Na+ ions (Holmgren et al. 2000). (b) Cartoon of channel-like Na+,K+ pump with bound palytoxin (PTX; green ball), which allows the pump's two gates sometimes to be open at the same time. (Modified from Artigas & Gadsby 2003.)
The existence of these occluded states implies some form of communication between the gates, so that the second gate never opens before the first gate is closed. But exactly how this communication is achieved, and precisely what constitutes either gate, is not yet known. Crystal structures of a closely related P-type pump, the sarcoplasmic- and endoplasmic-reticulum Ca2+-ATPase (SERCA), in several conformations (e.g. Toyoshima & Nomura 2002; Olesen et al. 2004, 2007; Sorensen et al. 2004; Toyoshima et al. 2004; Jensen et al. 2006), including E1P-like states in which two Ca2+ ions were found occluded (Toyoshima et al. 2000; Sorensen et al. 2004; Toyoshima & Mizutani 2004; Olesen et al. 2007), reveal substantial structural rearrangements between E1 and E2 conformations. The structures show that gating in these pumps, similar to gating in K+ ion channels (e.g. Jiang et al. 2002; Long et al. 2007), involves relative movements of entire domains.
3. Na+,K+-ATPase currents versus channel currents
Because an entire conformational cycle is required to transport a handful of ions, the ion throughput rate of pumps is limited by the low speed of the gating rearrangements (up to approx. 100 s−1). Thus, although the Na+,K+-ATPase pumps unequal numbers of Na+ and K+ ions in opposite directions across the cell membrane, and so generates a net current, the current is too small (at most a few atto-amperes) to be measured for a single pump. By contrast, ions can flow through open channels at rates approaching the diffusion limit, and single-channel currents frequently measure in the pico ampere range. But animal cells often contain many millions of Na+,K+-ATPase pumps and, although their transport cycles are not synchronized, their combined steady-state ion transport generates a readily measurable current (e.g. Thomas 1972; Gadsby et al. 1985). Under conditions designed to make the Na+/K+ transport cycle (clockwise, figure 2a) kinetically irreversible, i.e. with saturating levels of intracellular ATP and Na+, and of extracellular K+, the component of cell membrane current generated by the pump remains outwards (net cation extrusion) over a broad (200 mV) range of membrane potentials, regardless of how high the extracellular [Na+] or [K+] is raised (Nakao & Gadsby 1989).
Thus, there is no sign of any of the millions of Na+,K+-ATPase pumps acting as an ion channel (i.e. signalling a failure in communication between the two gates, which allows both to be open at the same time) since then inward current would be expected at high extracellular [Na+] or [K+] and large inside-negative potentials. Even if the Na+/K+ transport cycle is arrested by the withdrawal of all K+, so restricting the pump to conformations that interact with Na+ (red dashed box, figure 2a), the pump-generated component of steady membrane current simply falls to zero, and remains zero at all membrane potentials (Nakao & Gadsby 1986; Gadsby & Nakao 1989). Again, there is no sign of even fleeting open-channel behaviour giving rise to electrodiffusive ion current.
Interestingly, under the even more restrictive conditions of saturating [ATP] and zero [ADP] which limit pumps to phosphorylated states in which the cytoplasmic gate remains shut (green dashed box, figure 2a), sudden jumps of membrane potential do elicit pump-generated currents, but those currents instantaneously rise to a maximum and then rapidly decay back to zero (Nakao & Gadsby 1986; Hilgemann 1994; Holmgren et al. 2000). These pump-mediated currents reach a steady-state value of zero regardless of the membrane potential, and so again rule out open ion channel-like conformations. However, a reasonable interpretation of these transient currents, which relax in three distinct phases (Holmgren et al. 2000), is that they reflect movements of Na+ ions in and out of an ion channel that is closed at one end (e.g. Läuger 1991), i.e. the E2P conformation of the Na+,K+-ATPase pump that normally releases, but that can also rebind, Na+ ions (figure 2a, top left). That single state in the cartoon in reality represents four states, with zero, one, two, or three Na+ ions occupying the binding sites deep within the protein. The three observed relaxations monitor the redistribution among these four states, plus the occluded E1P(Na+)3 state (figure 2a, lower left), that is instigated by a change of membrane potential (Hilgemann 1994; Heyse et al. 1994; Holmgren et al. 2000).
4. Na+,K+-ATPase pump–channels opened by palytoxin
In none of the conditions described so far, with physiological levels of [ATP] and of internal and external [Na+], with or without extracellular K+, was there any sign of a leakage, ion channel-like current through Na+,K+-ATPase pumps. This observation (or, strictly, lack of one) is consistent with the above-posited negligibly small probability of both gates ever being open at the same time in a working ion pump. However, nature has produced a deadly tool that overcomes this stricture. It is called palytoxin, and it is a complex 2.7 kDa molecule (with 63 stereogenic centres) originally extracted from Palythoa zoanthids collected from coral reefs in Hawaii (Tosteson 2000). Palytoxin binds tightly to Na+,K+-ATPase pumps, transforming them into cation channels (figure 2b; Habermann 1989; Scheiner-Bobis et al. 1994; Artigas & Gadsby 2004). Within a few seconds, a saturating concentration of palytoxin (100 nM) converts every one of the thousands of Na+,K+-ATPase pumps in an excised outside-out patch into channels, as evident from the rapid development of inward (negative) current in the presence of an inward electrochemical potential gradient (e.g. figure 3a). By applying three orders of magnitude lower concentrations of palytoxin, and some patience, the transformation of a single pump into a channel can be captured (figure 3c). Quickly washing away free palytoxin as soon as the first pump–channel is observed then allows characterization of that palytoxin-bound pump–channel (figure 3c,d) since, with Na+ solutions in the absence of K+, palytoxin can remain bound to an individual pump for hours (or at least many minutes, depending on the presence of ATP; Artigas & Gadsby 2004). At resting membrane potentials in the negative physiological range, millions of Na+ ions per second flow through an open pump–channel; the channels select relatively poorly among small monovalent cations, and they conduct organic cations as large as N-methyl-d-glucamine (7.5 Å or more wide) only approximately 50-fold more slowly than Na+ ions (Artigas & Gadsby 2004; Reyes & Gadsby 2006).
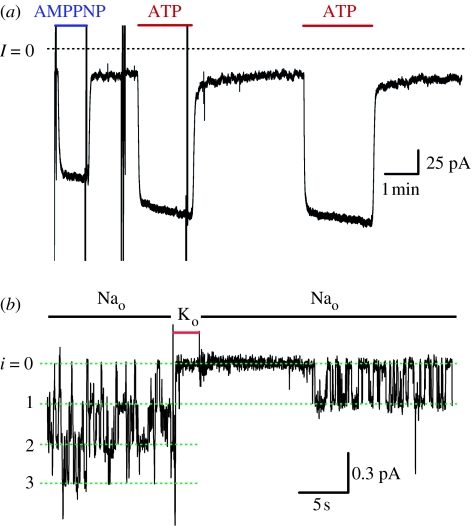
Figure 3.
Palytoxin (PTX) transforms Na+,K+-ATPase pumps into channels, one Na+,K+-ATPase pump at a time. (a,b) Macroscopic currents induced by the application of 100 nM PTX to outside-out patches excised from guinea-pig ventricular myocytes, bathed in approximately 160 mM Na+ solutions, and held at −40 mV, (a) with 5 mM MgATP or (b) with no ATP present in the internal solution; note very different current scales in (a) versus (b). (c,d) Representative palytoxin-induced single-channel recordings from outside-out myocyte patches, held at −70 mV and bathed in approximately 160 mM Na+ solutions; dashed lines marked C and O indicate closed and open current levels, respectively. (c) A patch with 5 mM MgATP in the pipette was exposed to 50 pM PTX, which was quickly removed (at second arrow) once the channel opened; long open bursts characterized the gating behaviour of the channel, which remained active for approximately 2 hours. (d) Trace from a patch without pipette ATP, showing gating behaviour shortly after the removal of unbound PTX: open bursts were of shorter duration than seen in the presence of MgATP, and longer closed periods were more frequent. (Adapted from Artigas & Gadsby 2002.)
A palytoxin-bound pump–channel does not stay open all the time, but opens and closes as long as palytoxin remains bound, and only finally closes once palytoxin dissociates. However, the fraction of time a palytoxin-bound pump–channel spends open (called the open probability) is some five- to sixfold greater in the presence of cytoplasmic ATP (open probability >0.9) than in its absence (open probability<0.2), largely because without nucleotide the channel closes relatively soon after opening (compare figure 3c with d). In similarly sized outside-out patches containing thousands of pumps, this difference is evident as a several-fold smaller, and slower, activation of pump–channel current on adding palytoxin in the absence of pipette ATP (compare figure 3a with b). If inside-out patches are used instead, with palytoxin in the pipette already bound to Na+,K+-ATPase pumps, a measurable current attributable to palytoxin-bound pump–channels flows in the absence of nucleotide (figure 4a) and that current is promptly increased five- to sixfold upon exposure to 1 mM ATP, or even to 1 mM AMPPNP, a poorly hydrolysable ATP analogue. Indeed, ATP, AMPPNP or ADP all cause a comparable increase in palytoxin-bound pump–channel current, all with half-maximal effects above 10 μM (Artigas & Gadsby 2003, 2004). Those effects correspond to the known ability of these nucleotides to act on the Na+,K+-ATPase with relatively low apparent affinity to facilitate release to the cytoplasm of occluded K+ ions (Simons 1975; Glynn & Richards 1982; Forbush 1987), consistent with an increase in the probability of the cytoplasmic-side gate being open, as cartooned in figure 2a (lower right).
Figure 4.
Gating of palytoxin-bound pump–channels by the pump's physiological ligands. (a) Augmentation of macroscopic palytoxin-bound pump–channel current by applications of 1 mM AMPPNP or 1 mM ATP, as indicated, to an inside-out patch from a HEK 293 cell, with 100 nM PTX dissolved in the Na solution inside the pipette. Holding potential, −10 mV; vertical deflections reflect 100 ms step changes in voltage. (b) Single palytoxin-bound pump–channel currents in outside-out patch from a guinea-pig ventricular myocyte, with Na internal solution without ATP. 100 pM palytoxin was applied for approximately 30 s, 1 min before the beginning of the trace. Holding potential, −50 mV. The 150 mM Na+ in the external solution was replaced by 150 mM K+ for the 2 s period indicated by the red line; the trace during those 2 s has been corrected for an instantaneous 1.8 pA change of seal current, also seen before palytoxin application.
In the unmodified Na+,K+-ATPase pump, external K+ normally promotes closure of the extracellular-side gate, leading to K+-ion occlusion (Post et al. 1972; Beaugé & Glynn 1979). Correspondingly, in outside-out patches, replacement of all external Na+ with K+ rapidly shuts palytoxin-bound pump–channels (figure 4b; ATP was absent from the pipette in this experiment). Because at least one of those pump–channels reopened following restoration of external Na+, although neither external solution any longer contained palytoxin, external K+ ions must be able to reversibly close palytoxin-bound pump–channels, presumably by acting on the extracellular-side gate (Artigas & Gadsby 2003). The reduced number of active pump–channels after the brief exposure to external K+ reflects the lowered affinity for palytoxin binding to K+-closed pump–channels, and hence dissociation of palytoxin from some of the channels, so reverting them to pumps; rebinding of palytoxin, applied again in the external Na+ solution, can then once more convert the pumps into pump–channels (Artigas & Gadsby 2003, 2004).
This reversibility of palytoxin's effect on Na+,K+-ATPase pumps, and the ability of cytoplasmic nucleotide and external K+ ions, the pump's physiological ligands, to modulate the open probability of pump–channels while palytoxin remains bound, suggests that the physical action of palytoxin is rather subtle. Far from destroying one or both of the pump's gates, palytoxin appears to leave them intact and responsive to physiological signals while disrupting the communication between them, allowing both gates to sometimes be open simultaneously, so effectively transforming a pump into a channel. Of course, the consequence is anything but subtle, the up to 106-fold gain of function in terms of dissipative cation flow meaning that transformation of even a single Na+,K+-ATPase pump can be enough to overwhelm and kill an entire cell!
5. Palytoxin as a tool for peering into Na+,K+-ATPase pumps
If palytoxin indeed interferes with the coupling of the two gates, but does not otherwise structurally disfigure the pump, then it represents a powerful tool with which to probe the pathway along which the transported Na+ and K+ ions are translocated. Borrowing methods used successfully in studies of ion channels, accessibility of single introduced target cysteines to small, variously charged, hydrophilic, sulphydryl-specific methanethiosulphonate (MTS) reagents can be assessed by recording the consequences of cysteine modification (i.e. covalent attachment of a specific adduct; Karlin & Akabas 1998) on palytoxin-bound pump–channel current.
To avoid signals from native Na+,K+-ATPase pumps in the cells chosen to express the cysteine-substituted pumps, point mutations are introduced in those pumps to make them resistant to the specific Na+,K+-ATPase pump inhibitor ouabain (Price et al. 1990; Canessa et al. 1992); enough ouabain can then be added to all solutions to silence the endogenous pumps while sparing the mutants. As Na+,K+-ATPase pumps contain some 23 native cysteines, it is also necessary to identify and remove any of them that are found reactive in the electrophysiological assay. Fortunately, in native Xenopus laevis Na+,K+-ATPase pumps, only the cysteine at position 113 in the first (NH2-terminal) transmembrane segment (TM1) of the α1 subunit reacts with MTS reagents applied from the extracellular surface (Guennoun & Horisberger 2000). We find that Xenopus Na+,K+-ATPase pumps comprising Xenopus β3 subunits with either Xenopus C113Y (cf. Canessa et al. 1992; Artigas & Gadsby 2006) or C113S–Q120D–N131R (cf. Guennoun & Horisberger 2000; Reyes & Gadsby 2006) α1 subunits, after expression in Xenopus oocytes, are resistant to both ouabain and MTS reagents.
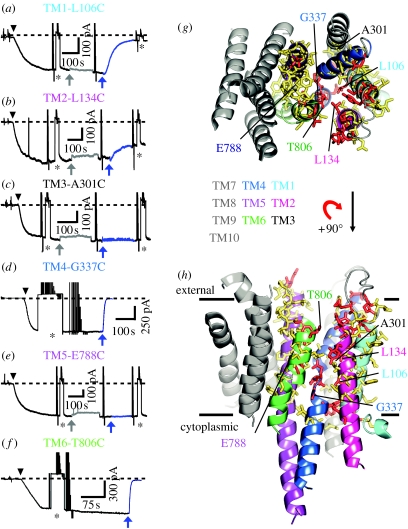
Application of MTS reagents of similar size, but positively (MTSET+, 2-trimethylammonium-ethyl-methanethiosulphonate) or negatively (MTSES−, 2-sulphonato-ethyl-methanethiosulphonate) charged or neutral (MTSACE, 2-aminocarbonyl-ethyl-methanethiosulphonate), to outside-out patches of membrane excised from oocytes expressing the mutant pumps that contained a single target cysteine introduced somewhere in the TM domain (figure 5h) yielded one of four kinds of results. For some target cysteines, palytoxin-bound pump–channel current was unaffected by MTSET+ (e.g. at all tested positions in TM3 or TM5: figure 5c,e,g,h), and in most of those cases it is likely that MTSET+ failed to react with the cysteine because it was inaccessible. For some other target cysteines expected to occupy relatively superficial locations, pump–channel current was decreased by positively charged MTSET+, but increased by negatively charged MTSES− and not changed by neutral MTSACE; this response is characteristic of positions (such as those in a wide vestibule) close enough to the ion pathway entrance to electrostatically influence the local concentration of current-carrying cation there, but located in a large enough space for the approximately 6Å×8Å neutral adduct of MTSACE not to sterically impede Na+-ion flow through the channels (e.g. at positions 131 in the TM1–TM2 external loop, 321 near the outer end of TM4 and 805 near the top of TM6: Artigas & Gadsby 2006; Reyes & Gadsby 2006). For a third class of target cysteines, pump–channel current was decreased not only by MTSET+ but also by MTSES− and MTSACE; this is the response expected of cysteines in a region of the pathway narrow enough for the MTS reagent adduct to impede Na+-ion flow regardless of any electrostatic influence of its charge (e.g. at position 806 atop TM6: figure 5f–h; Reyes & Gadsby 2006). The deepest cysteine targets made up the fourth class, for which pump–channel current was markedly decreased by MTSET+ and somewhat decreased by MTSACE but did not respond to MTSES−; subsequent reaction with MTSET+ after application of MTSES−confirmed that these cysteines had not reacted with MTSES− (e.g. at position 337 just below the cation-binding residues on TM4, or at nearby position 106 on TM1: figure 5a,d,g,h; Reyes & Gadsby 2006; Takeuchi et al. 2008).
Figure 5.
Characteristics of the ion pathway through the palytoxin-bound Na+,K+-ATPase as revealed by a combination of cysteine scanning and homology modelling. (a–f) Effects of MTSET+ on current through palytoxin-bound Na+,K+ pump–channels with single target cysteines introduced at representative positions in TM1 (a, L106), TM2 (b, L134), TM3 (c, A301), TM4 (d, G337), TM5 (e, E788) or TM6 (f, T806). Application of 50 nM palytoxin (black arrowheads) generated inward (negative) current, Ipalytoxin (dashed line marks zero total membrane current) in outside-out patches exposed to symmetrical Na+ concentrations. Temporary substitution (asterisk) of less permeant tetramethylammonium (TMA+) for external Na+ monitored patch integrity. Application of 10 mM dithiothreitol (grey arrows, grey traces) caused a small, reversible, poorly understood current decrease. Then 1 mM MTSET+ (blue arrows, blue traces) was applied until the current became steady. (g, h) MTSET+-reactive (red sticks; Ipalytoxin altered >10% by 1 mM MTSET+) and non-responsive (yellow sticks) residues were mapped onto a homology model of the Na+,K+-ATPase transmembrane domain based on the SERCA E2·BeF3− structure, shown viewed from (g) the extracellular surface or (h) the membrane plane. Representative residues featured in (a–f) are labelled and helices are coloured pale blue (TM1), magenta (TM2), dark grey (TM3), blue (TM4), purple (TM5), green (TM6) and the rest (TM7–TM10) grey. Reaction rate constants for MTSET+ decreased from 104 M−1 s−1 or more for superficial positions to 10 M−1 s−1 or more for deep positions. (Modified from Takeuchi et al. 2008.)
The failure of MTSES− to modify cysteines introduced at positions deeper than the cation-binding sites, located about two-thirds of the way across the membrane from the external side, can be attributed to an electrostatic cation versus anion selectivity filter formed by some of the acidic side chains of residues contributing to those binding sites (Reyes & Gadsby 2006). To investigate this, cysteines were substituted one at a time for five residues with oxygen-containing side chains that were expected from mutagenesis (Nielsen et al. 1998), homology modelling (Ogawa & Toyoshima 2002; Rakowski & Sagar 2003) and X-ray crystallography (cf. Toyoshima et al. 2000; Toyoshima & Nomura 2002; Morth et al. 2007) to help coordinate pumped cations within binding sites I and II. The consequences for cation/anion selectivity were then assessed before and after attempts to further modify each side chain by deposition of a positively charged adduct from MTSET+. Cysteine substitution for any of three residues expected to contribute to site I, N785 or E788 in TM5, or D817 in TM6, was found not to affect the perfect cation selectivity of palytoxin-bound pump–channels, and none of those cysteines reacted with 1 mM MTSET+. By contrast, cysteine replacement of site II residues E336 in the unwound part of TM4, or of D813 in TM6, substantially weakened cation/anion selectivity, which was further grossly impaired (D813C) or even reversed (E336C) after reaction of the cysteine with MTSET+ (Reyes & Gadsby 2006). The finding that every MTSET+-modified cysteine modelled to lie deeper than these binding-site residues was unable to react with negatively charged MTSES− suggests that there is only a single pathway for ions through palytoxin-bound pump–channels, and that it contains a cation-selective filter contributed by cation-coordinating acidic residues of binding site II (Reyes & Gadsby 2006; Takeuchi et al. 2008).
6. Mapping the ion pathway through the Na+,K+-ATPase pump
Systematic analysis, by cysteine scanning from TM1 to TM6 (the four remaining helical segments, TM7–TM10, have yet to be examined), shows that there is indeed an unbroken pathway of MTSET+-reactive positions (red sticks in figure 5g,h) through palytoxin-bound pump–channels, from one side of the membrane to the other, that courses between TM1, TM2, TM4 and TM6, and passes through site II (Takeuchi et al. 2008). Non-responsive positions (yellow sticks in figure 5g,h) surround the reactive ones, suggesting that the scan was complete and that there is no other pathway for cation flow through palytoxin-bound pump–channels. That conclusion receives further support from the near abolition of Na+ current through pump–channels upon modification by MTSET+ of cysteines at various positions along the identified pathway, such as 106 in TM1 (figure 5a), 337 in TM4 (figure 5d) or 806 in TM6 (figure 5f).
A difficulty in accurately depicting this pathway is that the sole X-ray crystal structure of a Na+,K+-ATPase pump is of the occluded conformation, E2·MgF42− Na+,K+-ATPase, containing two trapped Rb+ ions, which mimics the conformation immediately following hydrolysis of the aspartyl-phosphate bond of the E2P state (Morth et al. 2007, 2009). In this E2·MgF42− Na+,K+-ATPase both gates are shut, whereas in palytoxin-bound Na+,K+-ATPase pump–channels both gates can evidently be open at the same time. A crystal structure of palytoxin-bound Na+,K+-ATPase is eagerly awaited, but in the meantime it seems safest to display the cysteine-scanning data on a homology model of the Na+,K+-ATPase (figure 5g,h) based on the structure of SERCA Ca2+-ATPase, homologous to the α subunit of the Na+,K+-ATPase pump, in a BeF3−-trapped E2P-like state, in which the extra-cytoplasmic-side gate is wide open although the cytoplasmic-side gate is firmly closed (Olesen et al. 2007). Not surprisingly, the pathway data obtained in the palytoxin-opened Na+,K+-ATPase map reasonably well onto the homology model from the extracellular surface down to the cation-binding sites, while the form of the cytoplasmic access pathway remains less certain.
One surprise from the scanning data was the lack of response to MTSET+ of cysteines in 20 contiguous positions (I778–I797) along TM5. This was unexpected because TM5 residues equivalent to S784, N785 and E788 appear to help coordinate Rb+ at site I in the occluded E2·MgF42− Na+,K+-ATPase pump, when both gates are shut (Morth et al. 2007), and in the open SERCA E2·BeF3− structure the E788-equivalent TM5 residue looks as though it ought to be accessible from the extra-cytoplasmic side (Olesen et al. 2007). It is just possible that some of these TM5 sites did react with MTSET+ but without modifying current, although this seems unlikely given that MTSET+ reaction at many nearby positions substantially altered pump–channel current (figure 5g,h). We cannot rule out that distortion of the Na+,K+-ATPase ion pathway by palytoxin made TM5 residues inaccessible, but this also seems unlikely for a number of reasons. First, palytoxin action can be readily reversed, and repeated, on the same population of Na+,K+ pumps (Artigas & Gadsby 2003). Second, as already described above (figures 3 and 4), the gates to the ion pathway through palytoxin-bound pump–channels still respond to the Na+,K+ pumps’ physiological ligands (Artigas & Gadsby 2003). Third, positions as deep as the pathway narrowing (e.g. position 806 in TM6) are accessible to MTSET+ without palytoxin (Takeuchi et al. 2008). Fourth, blockers of access channels to ion-binding sites in unmodified Na+,K+-ATPase pumps similarly impede cation movement in palytoxin-bound pumps (Harmel & Apell 2006). Fifth, MTSET+-accessible positions in palytoxin-bound pump–channels map reasonably onto unmodified pump structures (figure 5g,h; Guennoun & Horisberger 2002; Horisberger et al. 2004; Reyes & Gadsby 2006; Takeuchi et al. 2008) and include sites expected to interact with transported ions (Nielsen et al. 1998; Ogawa & Toyoshima 2002; Einholm et al. 2005, 2007; Morth et al. 2007). So we conclude that TM5 positions within site I do not lie on the principal ion pathway mapped out by reactivity with MTSET+ because they occupy a cavity that imposes unfavourable geometry for MTSET+ reaction. This is consistent with little apparent influence of the side-chain charge of site-I residue E778 in TM5 on cation selectivity of Na+,K+-ATPase pump–channels (Reyes & Gadsby 2006). It is also consistent with the finding that, in contrast to MTSET+, a smaller reagent MTSMT+(1-trimethylammonium-methyl-methanethiosulphonate) can react with E788C, but only very slowly (Takeuchi et al. 2008); similarly small MTSEA+ (cf. Guennoun & Horisberger 2000) is a less reliable reporter of accessibility as it is membrane permeant and slowly reacts with C113Y Xenopus Na+,K+-ATPase pumps lacking introduced target cysteines (Takeuchi et al. 2008).
What does this mapped pathway through Na+,K+-ATPase pump–channels tell us? We already knew that the channels are wide enough to conduct N-methyl-d-glucamine ions (Artigas & Gadsby 2004). So it should not be surprising that the approximately 6 Å wide and approximately 12 Å long MTS reagents MTSET+, MTSES− and MTSACE can pass through palytoxin-bound Na+,K+-ATPase pump–channels (MTSES− only as far as the cation selectivity filter) and modify side chains of cysteines introduced throughout the full width of the membrane. Additional information consistent with a relatively wide pathway comes from measurements of the Na+ flux ratio exponent (Ussing 1949; Hodgkin & Keynes 1955) for palytoxin-bound Na+,K+-ATPase pump–channels that yielded a value of approximately 1.0 (Rakowski et al. 2007). The same study also showed that bi-ionic reversal potentials of those channels were independent of ion concentration over a 10-fold range (Rakowski et al. 2007). Both findings suggest that if Na+ ions flow in a queue along the principal pathway (the pathway that we now know passes through site II), then that queue is usually likely to be only one Na+ ion long. However, fluorescence measurements indicate that, on average, a palytoxin-bound pump–channel is occupied by two Na+ ions (Harmel & Apell 2006). These findings can all be reconciled if a second Na+ ion occupies site I, which lies off the main pathway (as described above) but linked to it via a side connection that must be narrow enough to preclude reactivity with MTSET+ of cysteine targets in site I. Interestingly, in SERCA, X-ray crystal structures show two Ca2+ ions bound side by side in sites I and II (Toyoshima et al. 2000; Sorensen et al. 2004; Toyoshima & Mizutani 2004; Olesen et al. 2007), and binding of the second Ca2+ ion at site II is known to lock in the Ca2+ ion at site I (Zhang et al. 2000) before the two Ca2+ ions are released sequentially (Inesi 1987). So the results from SERCA are also consistent with the picture developed here for the Na+,K+-ATPase pump: that is, of transported ions negotiating a single common pathway from the cytoplasm to the ion-binding sites in E1 states, and from those sites to the extra-cytoplasmic space (reticulum lumen in the case of SERCA) during release in the E2P state. The snapshot of an ion pathway right through the Na+,K+-ATPase pump afforded by studies with palytoxin therefore appears to offer a general structural basis for understanding cation translocation in P-type pumps.
Acknowledgements
Our research reported here was supported by NIH grants HL36873 and HL49907 to D.C.G., and a fellowship from the Vicente Trust (to P.A.); N.R. is presently a Jane Coffin Fund Fellow. We thank Paola Vergani and Peter Hoff for their work on figure 1, and Nazim Fataliev for molecular biological support. cDNAs encoding Xenopus α1 and β3 Na+,K+-ATPase subunits were a generous gift from our late colleague Bob Rakowski, to whom we dedicate this paper.
Footnotes
One contribution of 16 to a Discussion Meeting Issue ‘Membrane transport in flux: the ambiguous interface between channels and pumps’.
References
- Albers R.W. Biochemical aspects of active transport. Annu. Rev. Biochem. 1967;36:727–756. doi: 10.1146/annurev.bi.36.070167.003455. doi:10.1146/annurev.bi.36.070167.003455 [DOI] [PubMed] [Google Scholar]
- Artigas P., Gadsby D.C. Ion channel-like properties of the Na+/K+-pump. Ann. NY Acad. Sci. 2002;976:31–40. doi: 10.1111/j.1749-6632.2002.tb04711.x. [DOI] [PubMed] [Google Scholar]
- Artigas P., Gadsby D.C. Na+/K+-pump ligands modulate gating of palytoxin-induced ion channels. Proc. Natl Acad. Sci. USA. 2003;100:501–505. doi: 10.1073/pnas.0135849100. doi:10.1073/pnas.0135849100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artigas P., Gadsby D.C. Large diameter of palytoxin-induced Na/K pump-channels and modulation of palytoxin interaction by Na/K pump ligands. J. Gen. Physiol. 2004;123:357–376. doi: 10.1085/jgp.200308964. doi:10.1085/jgp.200308964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artigas P., Gadsby D. Oubain affinity determining residues lie close to the Na/K pump ion pathway. Proc. Natl Acad. Sci. USA. 2006;103:12 613–12 618. doi: 10.1073/pnas.0602720103. doi:10.1073/pnas.0602720103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaugé L.A., Glynn I.M. Occlusion of K ions in the unphosphorylated sodium pump. Nature. 1979;280:510–512. doi: 10.1038/280510a0. doi:10.1038/280510a0 [DOI] [PubMed] [Google Scholar]
- Canessa C.M., Horisberger J.D., Louvard D., Rossier B.C. Mutation of a cysteine in the first transmembrane segment of Na,K-ATPase alpha subunit confers ouabain resistance. EMBO J. 1992;11:1681–1687. doi: 10.1002/j.1460-2075.1992.tb05218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einholm A.P., Toustrup-Jensen M., Andersen J.P., Vilsen B. Mutation of Gly-94 in transmembrane segment M1 of Na+,K+-ATPase interferes with Na+ and K+ binding in E2P conformation. Proc. Natl Acad. Sci. USA. 2005;102:11 254–11 259. doi: 10.1073/pnas.0501201102. doi:10.1073/pnas.0501201102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einholm A.P., Andersen J.P., Vilsen B. Importance of Leu99 in transmembrane segment M1 of the Na+,K+-ATPase in the binding and occlusion of K+ J. Biol. Chem. 2007;282:23 854–23 866. doi: 10.1074/jbc.M702259200. doi:10.1074/jbc.M702259200 [DOI] [PubMed] [Google Scholar]
- Forbush B. Rapid release of 42K and 86Rb from an occluded state of the Na,K-pump in the presence of ATP or ADP. J. Biol. Chem. 1987;262:11 104–11 115. [PubMed] [Google Scholar]
- Gadsby D.C., Kimura J., Noma A. Noma voltage dependence of Na/K pump current in isolated heart cells. Nature. 1985;315:63–65. doi: 10.1038/315063a0. doi:10.1038/315063a0 [DOI] [PubMed] [Google Scholar]
- Gadsby D.C., Nakao M. Steady-state current–voltage relationship of the Na/K pump in guinea-pig ventricular myocytes. J. Gen. Physiol. 1989;94:511–537. doi: 10.1085/jgp.94.3.511. doi:10.1085/jgp.94.3.511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I.M., Richards D.E. Occlusion of rubidium ions by the sodium–potassium pump: its implications for the mechanism of potassium transport. J. Physiol. 1982;330:17–43. doi: 10.1113/jphysiol.1982.sp014326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouaux E. The molecular logic of sodium-coupled neurotransmitter transporters. Phil. Trans. R. Soc. B. 2009;364:149–154. doi: 10.1098/rstb.2008.0181. doi:10.1098/rstb.2008.0181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guennoun S., Horisberger J.D. Structure of the 5th transmembrane segment of the Na,K-ATPase α subunit: a cysteine-scanning mutagenesis study. FEBS Lett. 2000;482:144–148. doi: 10.1016/s0014-5793(00)02050-0. doi:10.1016/S0014-5793(00)02050-0 [DOI] [PubMed] [Google Scholar]
- Guennoun S., Horisberger J.D. Cysteine-scanning mutagenesis study of the sixth transmembrane segment of the Na,K-ATPase α subunit. FEBS Lett. 2002;513:277–281. doi: 10.1016/s0014-5793(02)02323-2. doi:10.1016/S0014-5793(02)02323-2 [DOI] [PubMed] [Google Scholar]
- Harmel N., Apell H.J. Palytoxin-induced effects on partial reactions of the Na,K-ATPase. J. Gen. Physiol. 2006;128:103–118. doi: 10.1085/jgp.200609505. doi:10.1085/jgp.200609505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermann E. Palytoxin acts through Na+K+ ATPase. Toxicon. 1989;27:1171–1187. doi: 10.1016/0041-0101(89)90026-3. doi:10.1016/0041-0101(89)90026-3 [DOI] [PubMed] [Google Scholar]
- Heyse S., Wuddel I., Apell H.J., Stürmer W. Partial reactions of the Na,K-ATPase: determination of rate constants. J. Gen. Physiol. 1994;104:197–240. doi: 10.1085/jgp.104.2.197. doi:10.1085/jgp.104.2.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgemann D.W. Channel-like function of the Na,K pump probed at microsecond resolution in giant membrane patches. Science. 1994;263:1429–1432. doi: 10.1126/science.8128223. doi:10.1126/science.8128223 [DOI] [PubMed] [Google Scholar]
- Hodgkin A.L., Keynes R.D. The potassium permeability of a giant nerve fibre. J. Physiol. 1955;128:61–88. doi: 10.1113/jphysiol.1955.sp005291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren M., Wagg J., Bezanilla F., Rakowski R.F., De Weer P., Gadsby D.C. Three distinct and sequential steps in the release of sodium ions by the Na+/K+ ATPase. Nature. 2000;403:898–901. doi: 10.1038/35002599. doi:10.1038/35002599 [DOI] [PubMed] [Google Scholar]
- Horisberger J.D., Kharoubi-Hess S., Guennoun S., Michielin O. The fourth transmembrane segment of the Na,K-ATPase α subunit: a systematic mutagenesis study. J. Biol. Chem. 2004;279:29 542–29 550. doi: 10.1074/jbc.M400585200. doi:10.1074/jbc.M400585200 [DOI] [PubMed] [Google Scholar]
- Inesi G. Sequential mechanism of calcium binding and translocation in sarcoplasmic reticulum adenosine triphosphatase. J. Biol. Chem. 1987;262:16 338–16 342. [PubMed] [Google Scholar]
- Jardetzky O. Simple allosteric model for membrane pumps. Nature. 1966;211:969–970. doi: 10.1038/211969a0. doi:10.1038/211969a0 [DOI] [PubMed] [Google Scholar]
- Jensen A.M., Sørensen T.L., Olesen C., Møller J.V., Nissen P. Modulatory and catalytic modes of ATP binding by the calcium pump. EMBO J. 2006;25:2305–2314. doi: 10.1038/sj.emboj.7601135. doi:10.1038/sj.emboj.7601135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Lee A., Chen J., Cadene M., Chait B.T., MacKinnon R. Crystal structure and mechanism of a calcium-gated potassium channel. Nature. 2002;417:515–522. doi: 10.1038/417515a. doi:10.1038/417515a [DOI] [PubMed] [Google Scholar]
- Karlin A., Akabas M.H. Substituted-cysteine accessibility method. Methods Enzymol. 1998;293:123–145. doi: 10.1016/s0076-6879(98)93011-7. doi:10.1016/S0076-6879(98)93011-7 [DOI] [PubMed] [Google Scholar]
- Läuger P. A channel mechanism for electrogenic ion pumps. Biochim. Biophys. Acta. 1979;552:143–161. doi: 10.1016/0005-2736(79)90253-0. doi:10.1016/0005-2736(79)90253-0 [DOI] [PubMed] [Google Scholar]
- Läuger P. Sinauer; Sunderland, MA: 1991. Electrogenic ion pumps. [Google Scholar]
- Long S.B., Tao X., Campbell E.B., MacKinnon R. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature. 2007;450:376–382. doi: 10.1038/nature06265. doi:10.1038/nature06265 [DOI] [PubMed] [Google Scholar]
- Morth J.P., Pedersen B.P., Toustrup-Jensen M.S., Sørensen T.L., Petersen J., Andersen J.P., Vilsen B., Nissen P. Crystal structure of the sodium–potassium pump. Nature. 2007;450:1043–1049. doi: 10.1038/nature06419. doi:10.1038/nature06419 [DOI] [PubMed] [Google Scholar]
- Morth J.P., Poulsen H., Toustrup-Jensen M.S., Andersen J.P., Vilsen B., Nissen P. The structure of the Na+,K+-ATPase and mapping of isoform differences and disease-related mutations. Phil. Trans. R. Soc. B. 2009;364:217–227. doi: 10.1098/rstb.2008.0201. doi:10.1098/rstb.2008.0201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao M., Gadsby D.C. Voltage dependence of Na translocation by the Na/K pump. Nature. 1986;323:628–630. doi: 10.1038/323628a0. doi:10.1038/323628a0 [DOI] [PubMed] [Google Scholar]
- Nakao M., Gadsby D.C. [Na] and [K] dependence of the Na/K pump current–voltage relationship in guinea-pig ventricular myocytes. J. Gen. Physiol. 1989;94:539–565. doi: 10.1085/jgp.94.3.539. doi:10.1085/jgp.94.3.539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J.M., Pedersen P.A., Karlish S.J., Jorgensen P.L. Importance of intramembrane carboxylic acids for occlusion of K+ ions at equilibrium in renal Na,K-ATPase. Biochemistry. 1998;37:1961–1968. doi: 10.1021/bi972524q. doi:10.1021/bi972524q [DOI] [PubMed] [Google Scholar]
- Ogawa H., Toyoshima C. Homology modeling of the cation binding sites of Na+K+-ATPase. Proc. Natl Acad. Sci. USA. 2002;99:15 977–15 982. doi: 10.1073/pnas.202622299. doi:10.1073/pnas.202622299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen C., Sørensen T.L., Nielsen R.C., Møller J.V., Nissen P. Dephosphorylation of the calcium pump coupled to counterion occlusion. Science. 2004;306:2251–2255. doi: 10.1126/science.1106289. doi:10.1126/science.1106289 [DOI] [PubMed] [Google Scholar]
- Olesen C., Picard M., Winther A.M., Gyrup C., Morth J.P., Oxvig C., Møller J.V., Nissen P. The structural basis of calcium transport by the calcium pump. Nature. 2007;450:1036–1042. doi: 10.1038/nature06418. doi:10.1038/nature06418 [DOI] [PubMed] [Google Scholar]
- Patlak C.S. Contributions to the theory of active transport. II. The gate type non-carrier mechanism and generalizations concerning tracer flow, efficiency, and measurement of energy expenditure. Bull. Math. Biophys. 1957;19:209–235. doi:10.1007/BF02477764 [Google Scholar]
- Post R.L., Hegyvary C., Kume S. Activation by adenosine triphosphate in the phosphyrylation kinetics of sodium and potassium ion transport adenosine triphosphatase. J. Biol. Chem. 1972;247:6350–6540. [PubMed] [Google Scholar]
- Post R.L., Sen A.K., Rosenthal A.S. A phosphorylated intermediate in adenosine triphosphate-dependent sodium and potassium transport across kidney membranes. J. Biol. Chem. 1965;240:1437–1445. [PubMed] [Google Scholar]
- Price E.M., Rice D.A., Lingrel J.B. Structure-function studies of Na,K-ATPase: site-directed mutagenesis of the border residues from the H1-H2 extracellular domain of the a subunit. J. Biol. Chem. 1990;265:6638–6641. [PubMed] [Google Scholar]
- Rakowski R.F., Sagar S. Found: Na(+) and K(+) binding sites of the sodium pump. News Physiol. Sci. 2003;18:164–168. doi: 10.1152/nips.01441.2003. [DOI] [PubMed] [Google Scholar]
- Rakowski R.F., Artigas P., Palma F., Holmgren M., De Weer P., Gadsby D.C. Sodium flux ratio in Na/K pump-channels opened by palytoxin. J. Gen. Physiol. 2007;130:41–54. doi: 10.1085/jgp.200709770. doi:10.1085/jgp.200709770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes N., Gadsby D.C. Ion permeation through the Na+,K+-ATPase. Nature. 2006;443:470–474. doi: 10.1038/nature05129. doi:10.1038/nature05129 [DOI] [PubMed] [Google Scholar]
- Scheiner-Bobis G., Meyer zu Heringdorf D., Christ M., Habermann E. Palytoxin induces K+ efflux from yeast cells expressing the mammalian sodium pump. Mol. Pharmacol. 1994;45:1132–1136. [PubMed] [Google Scholar]
- Simons T.J.B. The interaction of ATP-analogues possessing a blocked gamma-phosphate group with the sodium pump in human red cells. J. Physiol. 1975;244:731–739. doi: 10.1113/jphysiol.1975.sp010822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen T.L.M., Møller J.V., Nissen P. Phosphoryl transfer and calcium ion occlusion in the calcium pump. Science. 2004;304:1672–1675. doi: 10.1126/science.1099366. doi:10.1126/science.1099366 [DOI] [PubMed] [Google Scholar]
- Takeuchi A., Reyes N., Artigas P., Gadsby D.C. The ion pathway through the opened Na+,K+-ATPase pump. Nature. 2008;456:413–416. doi: 10.1038/nature07350. doi:10.1038/nature07350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R.C. Electrogenic sodium pump in nerve and muscle cells. Physiol. Rev. 1972;52:563–594. doi: 10.1152/physrev.1972.52.3.563. [DOI] [PubMed] [Google Scholar]
- Tosteson M.T. Mechanism of action, pharmacology and toxicology. In: Botana L.M., editor. Seafood and freshwater toxins pharmacology, physiology, and detection. Marcel Dekker; New York, NY: 2000. pp. 549–566. [Google Scholar]
- Toyoshima C., Mizutani T. Crystal structure of the calcium pump with a bound ATP analogue. Nature. 2004;430:529–535. doi: 10.1038/nature02680. doi:10.1038/nature02680 [DOI] [PubMed] [Google Scholar]
- Toyoshima C., Nakasako M., Nomura H., Ogawa H. Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 Å resolution. Nature. 2000;405:647–655. doi: 10.1038/35015017. doi:10.1038/35015017 [DOI] [PubMed] [Google Scholar]
- Toyoshima C., Nomura H. Structural changes in the calcium pump accompanying the dissociation of calcium. Nature. 2002;418:605–611. doi: 10.1038/nature00944. doi:10.1038/nature00944 [DOI] [PubMed] [Google Scholar]
- Toyoshima C., Nomura H., Tsuda T. Lumenal gating mechanism revealed in calcium pump crystal structures with phosphate analogues. Nature. 2004;432:361–368. doi: 10.1038/nature02981. doi:10.1038/nature02981 [DOI] [PubMed] [Google Scholar]
- Ussing H.H. The distinction by means of tracers between active transport and diffusion. The transfer of iodide across the isolated frog skin. Acta Physiol. Scand. 1949;19:43–56. [Google Scholar]
- Vidaver G.A. Inhibition of parallel flux and augmentation of counter flux shown by transport models not involving a mobile carrier. J. Theor. Biol. 1966;10:301–306. doi: 10.1016/0022-5193(66)90128-7. doi:10.1016/0022-5193(66)90128-7 [DOI] [PubMed] [Google Scholar]
- Zhang Z., Lewis D., Strock C., Inesi G. Detailed characterization of the cooperative mechanism of Ca2+ binding and catalytic activation in the Ca2+ transport (SERCA) ATPase. Biochemistry. 2000;39:8758–8767. doi: 10.1021/bi000185m. doi:10.1021/bi000185m [DOI] [PubMed] [Google Scholar]