Abstract
Proactive priming before the next pandemic could induce immune memory responses to novel influenza antigens. In an open-label study, we analyzed B cell memory and antibody responses of 54 adults who received 2 7.5-μg doses of MF59-adjuvanted A/Vietnam/1194/2004 clade 1 (H5N1) vaccine. Twenty-four subjects had been previously primed with MF59-adjuvanted or plain clade 0-like A/duck/Singapore/1997 (H5N3) vaccine during 1999–2001. The prevaccination frequency of circulating memory B cells reactive to A/Vietnam/1194/2004 was low in both primed and unprimed individuals. However, at day 21 after boosting, MF59-adjuvanted primed subjects displayed a higher frequency of H5N1-specific memory B cells than plain-primed or unprimed subjects. The immune memory was rapidly mobilized by a single vaccine administration and resulted in high titers of neutralizing antibodies to antigenically diverse clade 0, 1, and 2 H5N1 viruses already at day 7. In general, postvaccination antibody titers were significantly higher in primed subjects than in unprimed subjects. Subjects primed with MF59-adjuvanted vaccine responded significantly better than those primed with plain vaccine, most notably in early induction and duration of cross-reacting antibody responses. After 6 months, high titers of cross-reactive antibody remained detectable among MF59-primed subjects. We conclude that distant priming with clade 0-like H5N3 induces a pool of cross-reactive memory B cells that can be boosted rapidly years afterward by a mismatched MF59-adjuvanted vaccine to generate high titers of cross-reactive neutralizing antibodies rapidly. These results suggest that pre-pandemic vaccination strategies should be considered.
Avian influenza (H5N1) was first associated with human disease in 1997 (1). Since its re-emergence in 2003, antigenically distinct H5N1 viruses have become widely dispersed among birds and have caused more than 400 human infections (2, 3). Clade 0 H5N1 viruses were responsible for the 1997 Hong Kong outbreaks but have not been isolated since then. Clade 1 H5N1 viruses predominated in the Indochina peninsula before 2007, whereas H5N1 viruses from Indonesia, Central Asia, Europe, and Africa are clustered in a divergent clade 2 group with geographically distinct sublineages and are responsible for most current human infections.
Rapid deployment of vaccine is critical to ameliorating the impact of the next pandemic, and effective vaccination strategies against H5N1 are an urgent priority (4). To avoid predicted shortfalls in vaccine supply during the first pandemic waves, stockpiling and/or proactive pre-pandemic use of vaccine has been suggested (5). However, the antigenic diversity and future evolution of H5N1 viruses pose uncertainties about strain selection for a stockpiled or pre-pandemic vaccine, because preparations from current isolates may be suboptimally matched to a future pandemic virus. Therefore, induction of immune memory and cross-clade neutralizing antibodies are essential components of a pre-pandemic vaccine strategy.
Inactivated subvirion vaccines prepared from H5 strains are poorly immunogenic (6–8). The addition of oil-in-water emulsion adjuvant enhances immunogenicity, but 2 doses generally are needed in susceptible subjects (8–13). This requirement may be logistically challenging at the time of pandemic declaration, even if stockpiled vaccines are available. Proactive pre-pandemic priming could induce long-lasting immune memory and allow a single booster vaccine to induce protection when needed.
We administered 7.5 μg of MF59-adjuvanted surface-antigen clade 1 H5N1 vaccine to unprimed subjects and subjects who had been immunized at least 6 years earlier with MF59-adjuvanted or non-adjuvanted clade 0 A/duck/Singapore/1997 (H5N3) vaccine (8–11). Preliminary findings indicated that cross-reactive hemagglutination-inhibition (HI) responses were induced following the booster vaccination (14). Here, we describe safety and immunogenicity data and show that earlier priming induced a pool of memory B cells that rapidly expanded after a single booster dose, resulting in neutralizing antibodies to antigenically diverse wild-type H5N1 viruses. Our results support a prime-boost strategy with MF59-adjuvanted vaccines to protect against all current human H5N1 isolates.
Results
Vaccine Was Well Tolerated.
The presence of local and systemic adverse reactions was collected during the first 7 days following either vaccination. Most reactions were self limiting, and 70% of all reported symptoms were graded as mild. There was no indication that the frequency or severity of reactions was greater after the second vaccine dose than after the first (shown in SI Text and Fig. S1). The frequency of reactions did not differ significantly among vaccination groups (P > 0.05). No serious vaccine-related adverse events were recorded.
Frequency of H5N1-Specific Memory B Cells Is Higher in MF59-Primed Subjects.
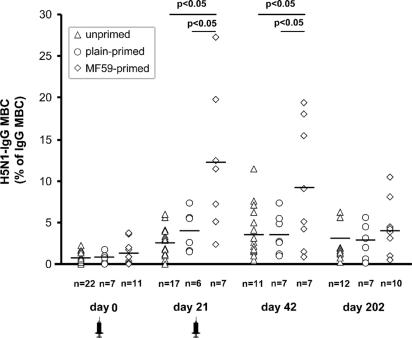
Fig. 1 shows that the prevaccination frequency of H5N1-IgG memory B cells (H5N1-IgG MBC) was slightly higher in MF59-primed subjects than in plain-primed or unprimed subjects (mean frequency [95% CI]: 1.2% [0.27%–2.13%]; 0.75% [0.11%–1.39%]; and 0.69 [0.43%–0.95%], respectively). By day 21, the frequency of H5N1-IgG MBC increased in all groups, with the highest frequency observed in MF59-primed subjects (mean [95%CI]: 12% [4.16%–20%]; 3.55% [1.22%–5.88%], and 2.44 [1.55%–3.34%] in MF59-primed, plain-primed, and unprimed subjects, respectively). At day 42, the frequency of H5N1-IgG MBC did not increase further in either primed group, but a small increase was observed in unprimed subjects (mean [95% CI]: 9.23% [2.96%–15%], 3.62% [1.45%–5.79%], and 3.59% [2.03%–5.15%] in MF59-primed, plain-primed, and unprimed subjects, respectively). At day 202 after vaccination, the frequency of H5N1-IgG MBC remained above prevaccination levels in all groups (mean [95% CI]: 4.07% [1.78%–6.35%], 2.91% [0.98%–4.84%], and 3.04% [1.38%–4.7%] in MF59-primed, plain-primed, and unprimed subjects, respectively).
Fig. 1.
The frequency of H5N1-IgG memory B cells (H5N1-IgG MBC) before and after vaccination in individual subjects from unprimed (open triangles), plain-primed (open circles), and MF59 primed groups (open diamonds). Mean values area indicated by horizontal lines. P-values for significant differences were obtained by 1-factor ANOVA. Syringe indicates vaccine administration.
MF59-Primed Subjects Have Highest Antibody Responses to the NIBRG-14 Vaccine Strain.
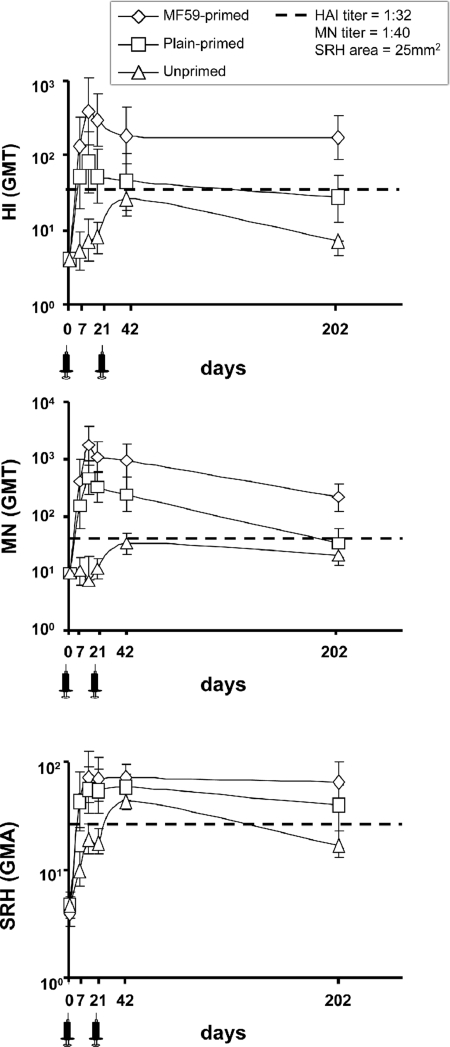
Fig. 2 shows the geometric mean titers (GMT) of antibody to NIBRG-14 by HI, neutralization (MN), and single radial hemolysis (SRH) before and after vaccination. Prevaccination antibody was undetected, except in 4 subjects who had SRH above detection limits but negative HI and MN titers, the reasons for which are unknown. By each assay, on each postvaccination visit, antibody titers among primed subjects were in general significantly higher than those among unprimed (all P ≤ 0.003, except on day 42 by HI). From day 14 onwards, antibody titers by HI and MN in MF59-primed subjects were higher than in plain-primed subjects (all P < 0.05). Among all participants, the highest responses were on day 14 in MF59-primed subjects, with GMT of 1:378 by HI, 1:1754 by MN, and 73 mm2 by SRH, compared with 1:79, 1:473, and 56 mm2, respectively, in plain-primed subjects and 1:7, 1:12 and 19 mm2, respectively, in unprimed subjects. The second vaccine dose increased titers between day 21 and 42 among unprimed subjects but had no effect in either of the primed groups. No relation between the number or dosage of previously administered H5N3 vaccine and postvaccination titers was observed in primed subjects.
Fig. 2.
Antibody responses following administration of MF59-adjuvanted NIBRG-14 vaccine. Geometric mean titers (GMT) of antibody and geometric mean areas (GMA) from unprimed (open triangles), plain-primed (open circles), and MF59-primed groups (open diamonds) by hemagglutinin-inhibition (HI) (Upper), neutralization (MN) (Middle), and single radial (SRH) hemolysis (Lower) are shown. Dotted lines indicate titers of HI 1:32; MN 1:40; and SRH 25 mm2. Syringe indicates vaccine administration.
Antibody Responses to Clade 1 and 2 Vaccine Strains Meet the Criteria of the European Union Committee for Medicinal Products for Human Use.
Table 1 shows mean geometric increase in antibody titers and in seroconversion and seroprotection rates to NIBRG-14, and, for HI and SRH, their relation to the European Union Committee for Medicinal Products for Human Use (CHMP) licensing criteria for inter-pandemic vaccines. On each postvaccination visit, the highest responses occurred among MF59-primed subjects. From day 14 onwards, there was a significantly greater response by HI and MN among MF59-primed than among plain-primed subjects. MF59-primed subjects fulfilled 3 of 3 CHMP criteria by HI and SRH by day 7 after 1 booster dose. Plain-primed subjects fulfilled 2 criteria by HI and 3 criteria by SRH at day 7. Unprimed subjects fulfilled 2 of 3 criteria by SRH at days 14 and 21 after 1 dose and 3 of 3 criteria by SRH after 2 doses.
Table 1.
Responses to NIBRG-14 after booster vaccination
| Group | Mean Geometric Increase in Antibody (Ratio to Day 0, 95% CI) |
Seroconversion Rate (%, 95% CI) |
Seroprotection Rate (%, 95% CI) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Unprimed N = 30 | MF59 N = 12 | Plain N = 12 | Unprimed N = 30 | MF59 N = 12 | Plain N = 12 | Unprimed N = 30 | MF59 N = 12 | Plain N = 12 | |
| Hemagglutination-inhibition (HI) | |||||||||
| Day 7 | 1.3 (0.7–2.3) | 32* (13–81) | 13* (4.8–34) | 8% (1–25) | 90%* (55–100) | 56%* (21–86) | 8% (1–25) | 90%* (55–100) | 56% (21–86) |
| Day 14 | 1.8 (1–3.4) | 95* (33–267) | 20* (7.8–50) | 14% (3–35) | 100%* (63–100) | 60%* (26–88) | 14% (3–35) | 100%* (63–100) | 60% (26–88) |
| Day 21 | 2 (1.2–3.3) | 72* (32–160) | 13* (5.9–29) | 23% (10–42) | 100%* (74–100) | 58%* (28–85) | 23% (10–42) | 100%* (74–100) | 58% (28–85) |
| Day 42 | 6.6* (3.8–12) | 45* (19–109) | 11* (4.6–26) | 52%* (33–71) | 92%* (62–100) | 58%* (28–85) | 52% (33–71) | 92%* (62–100) | 58% (28–85) |
| Day 202 | 1.8 (1.2–2.9) | 43* (22–83) | 6.6* (3.3–13) | 8% (1–25) | 92%* (62–100) | 64%* (31–89) | 8% (1–25) | 92%* (62–100) | 64% (31–89) |
| Single Radial Hemolysis (SRH) | |||||||||
| Day 7 | 2.2* (1.4–3.2) | 11* (5.9–21) | 8.7* (4.4–17) | 23% (9–44) | 90%* (55–100) | 67%* (30–93) | 27% (12–48) | 90%* (55–100) | 78%* (40–97) |
| Day 14 | 3.9* (2.6–5.8) | 18* (9.3–36) | 11* (6.2–21) | 55%* (32–76) | 100%* (63–100) | 80%* (44–97) | 59% (36–79) | 100%* (63–100 | 90%* (55–100) |
| Day 21 | 3.9* (2.7–5.4) | 18* (10–30) | 11* (6.7–20) | 50%* (1–69) | 100%* (74–100) | 83%* (52–98) | 53% (34–72) | 100%* (74–100) | 92%* (62–100) |
| Day 42 | 9.7* (7.5–12) | 18* (12–27) | 12* (8.4–18) | 90%* (73–98) | 100%* (74–100) | 92%* (62–100) | 93%* (77–99) | 100%* (74–1000 | 100%* (74–1000 |
| Day 202 | 3.8* (2.8–5.1) | 17* (11–26) | 8.3* (5.2–13) | 50%* (30–70) | 100%* (74–100) | 82%* (48–98) | 54% (33–73) | 100%* (74–100) | 91%* (59–100) |
| Microneutralization (MN) | |||||||||
| Day 7 | 1.1 (0.6–1.9) | 41 (17–99) | 15 (5.9–38) | 4% (0.1–20) | 90% (55–100) | 56% (21–86) | 4% (0.1–20) | 90% (55–100) | 56% (21–86) |
| Day 14 | 1.2 (0.8–2) | 175 (80–384) | 47 (23–95) | 9% (1–29) | 100% (63–100) | 100% (69–100) | 9% (1–29) | 100% (63–100) | 100% (69–100) |
| Day 21 | 1.2 (0.8–1.8) | 107 (57–199) | 32 (17–60) | 10% (2–27) | 100% (74–100) | 92% (62–100) | 10% (2–27) | 100% (74–100) | 92% (62–1000 |
| Day 42 | 3.3 (2.2–5.1) | 94 (48–185) | 24 (12–47) | 55% (36–74) | 100% (74–100) | 83% (52–98) | 55% (36–74) | 100% (74–100) | 83% (52–980 |
| Day 202 | 2.1 (1.4–3.0) | 22 (12–38) | 3.4 (1.9–6) | 12% (2–30) | 92% (62–100) | 45% (17–77) | 12% (2–30) | 92% (62–100) | 45% (17–77) |
Seroconversion for HI and MN is defined as a 4-fold or greater increase in titer, for SRH as a 50% or greater increase in area. Seroprotection for HI is defined as 1:32 or greater, seroprotection for SRH is defined as 25 mm2 or greater; seroprotection for MN is defined as 1:40 or greater. CHMP criteria for HI and SRH (18–60 yrs) are mean geometric increase > 2.5; seroconversion rate > 40%; seroprotection rate > 70%. There are no MN criteria. Figures marked by asterisks fulfill criteria for inter-pandemic vaccines.
Antibody responses to a heterologous clade 2.2 A/turkey/Turkey/1/2005/NIBRG-23 vaccine strain were evaluated also (SI Text and Table S1). On each postvaccination visit, the highest response occurred among MF59-primed subjects. MF59-primed subjects fulfilled 3 of 3 CHMP criteria by HI and SRH by day 7. Plain-primed subjects fulfilled 2 criteria by HI and 3 criteria by SRH by day 7. Unprimed subjects fulfilled 2 of 3 criteria by SRH at days 14 and 21 after 1 dose and 3 of 3 criteria by SRH after 2 doses.
MF59-Primed Subjects Generate Cross-Reactive Antibody.
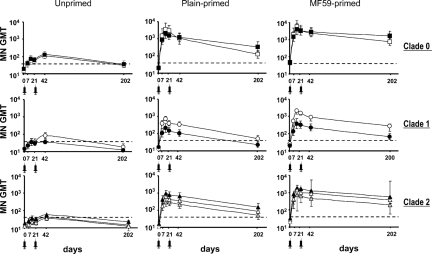
Fig. 3 and Table 2 show MN antibody against wild-type H5N1 viruses and the original priming A/duck/Singapore/1997 antigen. There were significantly higher prevaccination antibody titers to clade 0 A/duck/Singapore/97 (P < 0.001) and A/Hong Kong/156/97 (P < 0.001) viruses in MF59-primed subjects than in plain-primed or unprimed subjects.
Fig. 3.
Neutralizing responses to wild-type H5N1 and priming clade 0 H5N3 viruses following MF59-adjuvanted NIBRG-14 vaccine. Mean geometric mean titers (MN GMT) of antibody from unprimed (Left), plain-primed (Middle), and MF59-primed (Right) groups. Virus strains are clade 0: A/duck/Singapore/97 (filled sqaures), A/Hong Kong/156/97 (open circles); clade 1: A/Vietnam/1194/2004 (open circles), A/Cambodia/R0405050/2007(filled circles); clade 2.3.4: A/Anhui/1/2005(gray triangle); clade 2.1.3 A/Indonesia/5/2005 (open triangles); and clade 2.2: A/Turkey/15/2006 (black triangle). Dotted line shows titer of 1:40. Syringe indicates vaccine administration.
Table 2.
Neutralizing responses to wild-type H5 viruses after booster vaccination
| Vaccine Group Antigen | Virus Clade | Geometric Mean Titer of Antibody (95% CI) |
Seroconversions (≥4-Fold Rise from Day 0) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Day of Postvaccination Visit |
Day of Postvaccination Visit |
||||||||
| 7 | 14 | 42 | 202 | 7 | 14 | 42 | 202 | ||
| Unprimed | |||||||||
| A/duck/Sing/97 | 0 | 37 (22–63) | 58 (33–101) | 106 (71–157) | 31 (22–43) | 27% (12–48) | 41% (21–64) | 62% (42–79) | 15% (4–35)) |
| A/HK/156/97 | 0 | 42 (28–62) | 72 (46–113) | 128 (87–189) | 36 (24–55) | 23% (9–44) | 45% (24–68) | 55% (36–74) | 35% (17–56 |
| A/VN/1194/04 | 1 | 19 (13–28) | 32 (21–47) | 53 (37–75) | 12 (9–17) | 12% (2–30) | 23% (8–45) | 48% (29–67) | 4% (0.1–20) |
| A/Camb/2007 | 1 | 22 (16–32) | 35 (23–53) | 35 (26–48) | 12 (9–16) | 8% (1–25) | 18% (5–40) | 24% (10–44) | 4% (0.1–20) |
| A/Indo/5/05 | 2.1.3 | 24 (18–34) | 33 (22–48) | 41 (30–57) | 15 (11–22) | 0% (0–13) | 23% (8–45) | 31% (15–51) | 8% (1–25) |
| A/ty/Ty/15/06 | 2.2 | 25 (19–35) | 35 (26–46) | 57 (42–77) | 22 (15–31) | 0% (0–13) | 23% (8–45) | 48% (29–67) | 4% (0.1–20) |
| A/Anhui/1/05 | 2.3.4 | 15 (10–22) | 21 (15–30) | 36 (25–50) | 12 (9–17) | 0% (0–13) | 9% (1–29) | 34% (18–54) | 4% (0.1–20) |
| MF59-primed | |||||||||
| A/duck/Sing/97 | 0 | 2033 (867–4769) | 5914 (2334–14989) | 2470 (1335–4568) | 736 (442–1224) | 100% (69–100) | 100% (63–100) | 100% (74–100) | 83% (52–98) |
| A/HK/156/97 | 0 | 1506 (801–2832) | 4150 (1958–8798) | 2878 (1569–5281) | 1684 (911–3113) | 90% (55–100) | 100% (63–100) | 100% (74–100) | 100% (74–100) |
| A/VN/1194/04 | 1 | 291 (156–544) | 828 (433–1580) | 500 (291–859) | 226 (140–365) | 90% (55–100) | 100% (63–100) | 100% (74–100) | 92% (62–100) |
| A/Camb/2007 | 1 | 139 (79–247) | 381 (194–747) | 230 (143–368) | 67 (45–100) | 90% (55–100) | 100% (63–100) | 100% (74–100) | 58% (28–85) |
| A/Indo/5/05 | 2.1.3 | 378 (224–640) | 749 (397–1414) | 464 (280–772) | 195 (115–332) | 90% (55–100) | 88% (47–100) | 92% (62–100) | 75% (43–95) |
| A/ty/Ty/15/06 | 2.2 | 799 (483–1320) | 1809 (1119–2924) | 1266 (787–2038) | 568 (341–946) | 100% (69–100) | 100% (63–100) | 100% (74–100) | 100% (74–100) |
| A/Anhui/1/05 | 2.3.4 | 416 (217–796) | 1462 (819–2612) | 856 (519–1413) | 379 (231–662) | 90% (55–100) | 100% (63–100) | 100% (74–100) | 92% (62–100) |
| Plain-primed | |||||||||
| A/duck/Sing/97 | 0 | 1544 (628–3792) | 3541 (1541–8136) | 1093 (591–2022) | 126 (74–215) | 100% (66–100) | 100% (69–100) | 100% (74–100) | 64% (31–89) |
| A/HK/156/97 | 0 | 913 (469–1776) | 2024 (1033–3963) | 1203 (656–2208) | 343 (181–652) | 100% (66–100) | 100% (69–100) | 100% (74–100) | 100% (74–100) |
| A/VN/1194/04 | 1 | 187 (97–362) | 371 (208–662) | 211 (123–363) | 39 (23–64) | 67% (30–93) | 90% (55–100) | 83% (52–98) | 45% (17–77) |
| A/Camb/2007 | 1 | 113 (62–207) | 211 (116–386) | 105 (65–168) | 23 (15–35) | 67% (30–93) | 90% (55–100) | 58% (28–85) | 18% (2–52) |
| A/Indo/5/05 | 2.1.3 | 177 (102–308) | 290 (164–511) | 218 (131–363) | 51 (30–89) | 67% (30–93)) | 90% (55–100) | 100% (74–100) | 55% (23–83) |
| A/ty/Ty/15/06 | 2.2 | 412 (243–700) | 939 (611–1443) | 654 (407–1053) | 150 (88–256) | 89% (52–100 | 100% (69–100) | 100% (74–100) | 82% (48–98) |
| A/Anhui/1/05 | 2.3.4 | 179 (90–356) | 646 (385–1086) | 368 (223–608) | 87 (52–146) | 89% (52–100) | 100% (69–100) | 100% (74–100) | 82% (48–98) |
Virus strains: A/duck/Singapore/97, A/Hong Kong/156/97, A/Vietnam/1194/2004, A/Cambodia/R0405050/2007, A/Indonesia/5/2005, A/Turkey/15/2006 and A/Anhui/1/2005.
On each postvaccination visit, MN antibody to all antigens displayed similar kinetics in both primed groups. However, significantly higher titers were seen in primed subjects than in unprimed subjects (MF59-primed, P < 0.001; plain-primed, P = 0.0011; all antigens on all visits except A/Cambodia/2007 on day 202). MF59-primed subjects displayed higher MN antibody titers than plain-primed subjects from day 21 onwards (P < 0.005; all antigens except A/HK/156/97 were significantly higher by day 42, and A/ty/Turkey/1/2005 were significantly higher on days 14, 21, and 202). Early cross-reactive MN responses show that by day 7 after the first booster 90% of MF59-primed subjects and 44% of plain-primed subjects had titers of 1:80 or more to all viruses tested. By day 14, 100% of MF59-primed subjects and 90% of plain-primed subjects had titers of 1:80 or more to all viruses tested (SI Text, Fig. S2).
On each postvaccine visit, there were higher seroconversion rates (≥ 4-fold rise in titers) to each antigen among primed subjects than among unprimed subjects. The proportion of unprimed subjects seroconverting after 2 doses at day 42 ranged from 24% to 62% in unprimed subjects, from 58% to 100% in plain-primed subjects, and from 92% to 100% in MF59-primed subjects.
Protective Antibody Persists 6 Months After Boosting in MF59-Primed Subjects.
Fig. 2 and Table 1 show that by day 202, antibody to NIBRG-14 had declined in all groups. However, rate of decline was slower in MF59-primed subjects, and titers remained significantly higher than in unprimed (P < 0.0001) or plain-primed (P < 0.0001) subjects. All 3 CHMP criteria remained fulfilled by HI and SRH at day 202 in the MF59-primed group. Fig. 3 and Table 2 show that at day 202 seroconversion rates (by MN) and GMT in MF59-primed subjects generally were higher than in unprimed or plain-primed subjects.
Antibody Responses Correlate with the B-Cell Response.
Regression analysis was applied to assess the correlation between the frequency of pre- and postvaccination H5N1-MBC and MN titers (SI Text, Fig. S3). No correlation was found between the prevaccination frequency of H5N1-IgG MBC and MN titers to any antigen (data not shown). Significant linear correlations (P < 0.001) were observed between the frequency of H5N1-MBC at day 21 and postvaccination titers to A/Vietnam/1194/2004 (Fig. S3), and all other study viruses (data not shown).
Discussion
Mathematical modeling of influenza transmission suggests that vaccination, if capable of inducing protective responses within 2 weeks of the onset of a pandemic outbreak, could limit the impact of the pandemic (4, 15). Consequently, rapid deployment of effective vaccine is a critical feature of planning and remains an urgent public health priority. Although oil-in-water emulsions (8, 13) and whole-virus vaccines (12) enhance immunogenicity of pandemic vaccines, 2 doses are required to complete primary immunization and fulfill CHMP or Food and Drug Administration licensing criteria (16, 17). Because the production of a pandemic-specific vaccine cannot begin until the emergent strain is characterized, the rapid use of well-matched vaccine will be challenging. Proactive pre-pandemic priming may be more flexible and may reduce delays in vaccine manufacture and administration (5).
To evaluate the potential of a pre-pandemic vaccination strategy, we analyzed the frequency of antigen-specific B-cell memory and antibody responses in subjects primed with either plain or MF59-adjuvanted clade 0 H5N3 vaccine and subsequently boosted at least 6 years later with a heterologous clade 1 vaccine. Our findings indicate that priming subjects with H5 antigen induces immune memory that can be mobilized rapidly by the single administration of a distinct H5 vaccine. Seven days after administration of the booster, 90% of MF59-primed subjects exhibited high titers of neutralizing antibodies to diverse H5N1 viruses, as well as to the original H5 priming strains. As expected, unprimed subjects required 2 vaccine doses to achieve acceptable responses (6–8, 11–13). We found no evidence that primed subjects preferentially responded to the original clade 0 priming antigens, because antibodies to different H5 virus clades displayed similar kinetics, reducing concerns that have been raised about original antigenic sin (18).
Our results demonstrate that memory B cells induced by primary vaccination with MF59-adjuvanted vaccine, although circulating at frequency comparable to that seen in plain-primed and unprimed individuals, expand more rapidly upon booster immunization, generating potent neutralizing antibody responses as well as new memory cells. In addition to the greater B-cell response, antibody kinetics after the booster vaccination also showed a slower decay in MF59-primed subjects than in plain-primed subjects. We also observed significantly higher prevaccination antibody titers to clade 0 viruses in MF59-primed subjects, suggesting that prolonged responses are achieved when immunizing with MF59-adjuvanted vaccine.
The original priming trials evaluating A/duck/Singapore/97 vaccines during 1999–2001 found that 2 doses of non-adjuvanted vaccine achieved poor seroconversion rates, failing CHMP criteria (8). Although poorly immunogenic at priming, the non-adjuvanted H5N3 vaccine induced long-lasting cross-reactive memory that could be boosted by a single dose of MF59-adjuvanted heterologous vaccine at least 6 years later, contrasting with a report examining distant prime-boost immunization in which recipients primed with a poorly immunogenic non-adjuvanted baculovirus-expressed recombinant clade 0 H5 vaccine were boosted by high-dose (90 μg) non-adjuvanted clade 1 subvirion vaccine (19). Only 24% of primed subjects seroconverted to both homologous and heterologous H5N1 viruses tested, in comparison with the high seroconversion rates to diverse viruses observed within 7 to 14 days of a 7.5-μg MF59-adjuvanted booster in this study. Our results add to observations that oil-in-water emulsions such as MF59 allow antigen dose sparing, induce broad and potent effector antibody responses, and generate long-lasting boostable memory B cells with broad reactivity to heterologous antigens (8–11,13).
Limitations include the age range, number of subjects and variability of the priming vaccine schedule. Interpretation of pandemic vaccine trials is complicated by the poor reproducibility of MN assays and the lack of recognized correlates of protection against avian influenza, requiring evaluation against criteria used for seasonal vaccine assessment (16). Three independent serological assays produced consistent findings in our study. Because our study did not have the power to detect differences in the number or antigenic content of priming vaccine, appropriately powered studies to evaluate priming schedules are needed.
We previously demonstrated that boosting of cross-reactive antibody can be achieved by MF59-adjuvanted homologous re-vaccination within 18 months of primary immunization (9). We extend this observation to clade 0/1 prime-boosting after at least 6 years. Because clade 0 viruses no longer circulate, it is important to assess prime-boosting with clade 1/2 or future emergent strains. A pre-pandemic vaccine could be stockpiled for distribution at a time of imminent threat to induce cross-protective responses while waiting for pandemic vaccine. Proactive priming could be performed today to induce memory responses for a future event. The booster could be either a stockpiled homologous or, more likely, a heterologous vaccine prepared from a future variant. Longevity of memory is important, because boosting may occur many years after priming. Evaluation of optimal intervals is underway to give authorities much-needed flexibility. Population priming must be balanced with the safety of mass immunization, and a reasonable approach may to implement an initial vaccination strategy among critical workers (e.g., health care staff) (5).
Preparedness plans show that vaccination is critical for controlling the next pandemic. Some authorities have invested in H5N1 vaccine stockpiles, but these resources are small in comparison to global demand. The use of stockpiled vaccine is challenged by the need for 2 doses and secondary manufacturing constraints (e.g., filling capacity). MF59, a proprietary adjuvant, was licensed in seasonal influenza vaccines in 1997, and more than 30 million doses have been administered safely so far. Our findings suggest that consideration could be given to advance priming to induce memory responses that enable cross-reactive antibodies to be generated rapidly after infection with the pandemic virus or by a single low-dose vaccination when required at the onset of the next pandemic.
Materials and Methods
Study Volunteers.
Using clinical trial records, we contacted subjects who had received A/duck/Singapore/97 (H5N3) vaccine in randomized trials during 1999 and 2001 (8–11). In these studies, subjects received 2 doses of MF59-adjuvanted or non-adjuvanted vaccine containing 7.5, 15, or 30 μg H5 hemagglutinin, 21 days apart. Some subjects received a third dose 16 months later (9). Twenty-four subjects who had received previous H5N3 vaccine agreed to participate (SI Appendix and Table S2). Each had received 2 or 3 doses (containing 7.5, 15, or 30 μg H5 hemagglutinin) of either non-adjuvanted (“plain-primed”) or MF59-adjuvanted (“MF59-primed”) H5N3 vaccine. Of the 12 plain-primed recipients, 7 subjects received 2 doses, and 5 received 3 doses. Of the 12 MF59-primed recipients, 5 subjects received 2 doses, and 7 received 3 doses. Unprimed subjects who had not received H5N3 vaccine were enrolled. Demographic characteristics were similar, except there were more Asians among unprimed subjects. Four additional subjects were laboratory staff who received MF59-adjuvanted H5N3 vaccine outside controlled trials. Because the quality of vaccine storage could not be guaranteed, their results were included in safety analyses but were excluded from immunogenicity analyses (Fig. S4).
Vaccination.
This open-label study was done from May to December, 2007 at the Leicester Royal Infirmary, United Kingdom. (ClinicalTrials.gov, NCT00478816). All subjects received 2 doses, 21 days apart, of 7.5 μg of H5 hemagglutinin in MF59-adjuvanted vaccine by i.m. injection into the deltoid of the nondominant arm (days 0 and 21). MF59-adjuvanted surface-antigen vaccine containing 7.5 μg of hemagglutinin derived from A/Vietnam/1194/2004/NIBRG-14 virus was used. The United Kingdom Medicines and Human Products Regulatory Agency, an independent ethics committee, and University Hospitals Leicester approved the study. Eligibility criteria, reactogenicity data collection, and vaccine details are described in the SI Text.
Measurement of Antibody Responses.
Antibodies by MN (20) and horse HI (21) were measured at the Health Protection Agency, United Kingdom, with NIBRG-14 and NIBRG-23 viruses. Antibodies by MN were measured at the Centers for Disease Control and Prevention, Atlanta with wild-type A/Hong Kong/156/97, A/Vietnam/1194/2004, clade 1 variant A/Cambodia/R04050550/2007, A/Indonesia/5/2005, A/Turkey/15/2006, and A/Anhui/1/2005 (H5N1) strains and A/duck/Singapore/1997 (H5N3) in enhanced BSL3+. For MN, sera were tested at an initial dilution of 1:20, and those that were negative were assigned a titer of 1:10. For HI, sera were tested at an initial dilution of 1:8, and those that were negative were assigned a titer of 1:4. SRH (22) was performed at the University of Siena, Italy, with NIBRG-14 and NIBRG-23 viruses. All sera were tested separately and in duplicate, and the geometric mean value was used for analysis.
Enumeration of H5N1-Specific Memory B Cells.
Frequencies of MBC were determined by the ELISA-coupled limiting dilution assay (23, 24; details in SI Text) and expressed as percentages of the total IgG-secreting cell precursors measured.
Statistics.
Group sizes were not based on sample or power calculations. Descriptive statistics were used to summarize demographic, immunogenicity, and safety variables (detailed in SI Text).
Supplementary Material
Acknowledgments.
We thank the trial participants, University Hospitals of Leicester R&D, and colleagues from the Ministries of Health of Vietnam, Turkey, China, and Indonesia for providing viruses used in this study. J.D.V. received financial support from the Oak Ridge Institute of Science and Education, Oak Ridge, TN. The study was supported by Novartis Vaccines and Diagnostics. Investigators from Novartis Vaccines were involved in the study design, data collection, analysis, and writing of the report.
Footnotes
Conflict of interest statement: The sponsor is an employee of Novartis Vaccines, Siena, Italy. A.B., M.B., V.B., F.C., G.D.G., G.G., C.M., and M.P. are employees of Novartis Vaccines and Diagnostics. K. Hoschler, E.M., K.N., and I.S. have received support from vaccine producers including Novartis Vaccines for scientific research, speaker's fees, and attendance at scientific meetings. The Health Protection Agency has received funding for vaccine immunogenicity trials from manufacturers including Novartis Vaccines.
This article contains supporting information online at www.pnas.org/cgi/content/full/0903181106/DCSupplemental.
References
- 1.Yuen KY, et al. Clinical features and rapid viral diagnosis of human disease associated with influenza H5N1 virus. Lancet. 1998;351:467–471. doi: 10.1016/s0140-6736(98)01182-9. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Epidemic and pandemic alert response. [Accessed February 1, 2009];Avian influenza. Available at www.who.int/csr/disease/avian_influenza/en/index.html.
- 3.Chen H, et al. Establishment of multiple sublineages of H5N1 influenza virus in Asia. Proc Natl Acad Sci USA. 2006;103:2845–2850. doi: 10.1073/pnas.0511120103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferguson N, et al. Strategies for mitigating an influenza pandemic. Nature. 2006;442:448–452. doi: 10.1038/nature04795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jennings LC, Monto AS, Chan PK, Szucs T, Nicholson KG. Stockpiling influenza vaccines: A cornerstone of pandemic plans. The Lancet Infectious Diseases. 2008;8:650–658. doi: 10.1016/S1473-3099(08)70232-9. [DOI] [PubMed] [Google Scholar]
- 6.Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated influenza A (H5N1) vaccine. N Engl J Med. 2006;354:1343–1351. doi: 10.1056/NEJMoa055778. [DOI] [PubMed] [Google Scholar]
- 7.Bresson JL, et al. Safety and immunogenicity of an inactivated split virion A/Vietnam/1194/2004 (H5N1) vaccine. Lancet. 2006;367:1657–1664. doi: 10.1016/S0140-6736(06)68656-X. [DOI] [PubMed] [Google Scholar]
- 8.Nicholson KG, et al. Safety and immunogenicity of non-adjuvanted and MF59-adjuvanted A/Singapore/97 (H5N3) vaccine: A randomised trial of vaccine against influenza H5N1. Lancet. 2001;357:1937–1943. doi: 10.1016/S0140-6736(00)05066-2. [DOI] [PubMed] [Google Scholar]
- 9.Stephenson I, et al. Boosting immunity to influenza H5N1 with MF59-adjuvanted H5N3 vaccine in a primed population. Vaccine. 2003;21:1687–1693. doi: 10.1016/s0264-410x(02)00632-1. [DOI] [PubMed] [Google Scholar]
- 10.Stephenson I, et al. Cross reactivity to highly pathogenic influenza H5N1 viruses after vaccination with non-adjuvanted and MF59-adjuvanted A/duck/Singapore/97 vaccine. J Infect Dis. 2005;191:1210–1215. doi: 10.1086/428948. [DOI] [PubMed] [Google Scholar]
- 11.Stephenson I, et al. Phase I evaluation of trivalent inactivated intranasal vaccine with non-toxigenic E coli enterotoxin and biovector as mucosal adjuvants in healthy volunteers. J Virol. 2006;80:4962–4972. doi: 10.1128/JVI.80.10.4962-4970.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin J, et al. Safety and immunogenicity of an inactivated adjuvanted whole virus influenza (H5N1) vaccine: Phase I randomised controlled trial. Lancet. 2006;368:991–997. doi: 10.1016/S0140-6736(06)69294-5. [DOI] [PubMed] [Google Scholar]
- 13.Leroux-Roels I, et al. Antigen sparing and cross-reactive immunity with an adjuvanted rH5N1 prototype pandemic influenza vaccine. Lancet. 2007;370:580–589. doi: 10.1016/S0140-6736(07)61297-5. [DOI] [PubMed] [Google Scholar]
- 14.Stephenson I, et al. Antigenically distinct MF59-adjuvanted vaccine to boost immunity to H5N1. New England Journal of Medicine. 2008;359:1631–1633. doi: 10.1056/NEJMc0805274. [DOI] [PubMed] [Google Scholar]
- 15.Department of Health. [Accessed February 1, 2009];United Kingdom. Available at www.dh.gov.uk/en/Publichealth/Flu/PandemicFlu/index.htm.
- 16.Committee for Human Medicinal Products. Note for guidance on harmonisation of requirements for influenza vaccines, CPMP/BWP/214/96 Circulaire no. 96- 0661:1–22. 1997 Available at www.emea.europa.eu/pdfs/human/bwp/021496en.pdf.
- 17.US Food and Drug Administration. [Accessed February 1, 2009];Draft guidance on clinical data needed to support the licensure of pandemic vaccines. Available at www.fda.gov/cber/gdln/psanfluvac.pdf.
- 18.Haaheim LR. Original antigenic sin. A confounding issue? Dev Biol. 2003;115:49–53. [PubMed] [Google Scholar]
- 19.Goji NA, Nolan C, Hill H, Treanor J. Immune responses to a single dose of inactivated A/Vietnam/1203/2004 (H5N1) vaccine after priming with an antigenic variant. J Infect Dis. 2008;198:635–641. doi: 10.1086/590916. [DOI] [PubMed] [Google Scholar]
- 20.Rowe T, et al. Detection of antibody to influenza H5N1 in human sera by using a combination of serological assays. J Clin Microbiol. 1999;37:937–943. doi: 10.1128/jcm.37.4.937-943.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stephenson I, Wood JM, Nicholson KG, Zambon M. Sialic acid receptor specificity on erythrocytes affects detection of antibody to avian hemagglutinin. J Med Virol. 2003;70:391–388. doi: 10.1002/jmv.10408. [DOI] [PubMed] [Google Scholar]
- 22.Wood JM, Melzack D, Newman RW, Major D. In: Proceedings of Options for the Control of Influenza IV. Osterhaus A, Cox N, Hampson A, editors. New York: Excerpta Medica; pp. 461–466. [Google Scholar]
- 23.Amanna IJ, Slifka MK. Quantitation of rare memory B cell populations by two independent and complementary methods. J Immunol Methods. 2006;317:175–185. doi: 10.1016/j.jim.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grazia G, et al. Adjuvanted H5N1 vaccine induces early CD4 T cell response which predicts long term persistence of protective antibody levels. Proc Natl Acad Sci USA. 2009;106:3877–3882. doi: 10.1073/pnas.0813390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.