Abstract
Objective
TDP-43 is deposited as cytoplasmic and intranuclear inclusions in brains of subjects with frontotemporal lobar degeneration with ubiquitinated inclusions (FTLD-U) and amyotrophic lateral sclerosis (ALS). Previous studies reported that abnormal phosphorylation takes place in deposited TDP-43. The aim of this study was to identify the phosphorylation sites and responsible kinases, and to clarify the pathological significance of phosphorylation of TDP-43.
Methods
We generated multiple antibodies specific to phosphorylated TDP-43 by immunizing phosphopeptides of TDP-43, and analyzed FTLD-U and ALS brains by immunohistochemistry, immunoelectron microscopy and immunoblots. Additionally, we performed investigations aimed at identifying the responsible kinases and we assessed the effects of phosphorylation on TDP-43 oligomerization and fibrillization.
Results
We identified multiple phosphorylation sites in carboxyl-terminal regions of deposited TDP-43. Phosphorylation-specific antibodies stained more inclusions than antibodies to ubiquitin and, unlike existing commercially-available anti-TDP-43 antibodies, did not stain normal nuclei. Ultrastructurally, these antibodies labeled abnormal fibers of 15 nm diameter, and on immunoblots recognized hyperphosphorylated TDP-43 at 45 kDa, with additional 22–28 kDa fragments in sarkosyl-insoluble fractions from FTLD-U and ALS brains. The phosphorylated epitopes were generated by casein kinase 1 and 2, and phosphorylation led to increased oligomerization and fibrillization of TDP-43.
Interpretation
These results suggest that phosphorylated TDP-43 is a major component of the inclusions, and that abnormal phosphorylation of TDP-43 is a critical step in the pathogenesis of FTLD-U and ALS. Phosphorylation-specific antibodies will be powerful tools for the investigation of these disorders.
INTRODUCTION
Tau-negative and ubiquitin-positive inclusions (UPIs) that include neuronal cytoplasmic inclusions (NCIs), neuronal intranuclear inclusions (NIIs) and dystrophic neurites (DNs) are the pathological hallmarks of frontotemporal lobar degeneration with ubiquitinated inclusions (FTLD-U) with or without clinical features of motor neuron disease (MND)1. Recently, several genes and chromosomal loci, including the progranulin (PGRN) gene2, 3, valosin-containing protein (VCP) gene4 and an unidentified site at chromosome 9p5, 6, have been reported to be associated with familial FTLD-U. Ubiquitin-positive tau-negative NCIs have also been recognized in patients with the classic type of MND, amyotrophic lateral sclerosis (ALS)7, in which skein-like cytoplasmic inclusions are found in the lower motor neurons of the hypoglossal nucleus and spinal cord8, 9. In both FTLD-U and ALS, understanding why these inclusions form may provide critical clues to the neurodegenerative process‥
Recently, TAR DNA-binding protein of 43 kDa (TDP-43), which functions in regulating transcription and alternative splicing10, 11, was identified as a component of these UPIs12–14. TDP-43 appears to belong to the group of 2 RNA-binding domain (RBD)-Gly RNA-binding proteins, which include the heterogeneous nuclear ribonucleoprotein (hnRNP) family and factors involved in RNA splicing and transport15. TDP-43 binds hnRNP A/B and hnRNP A1 through its C-terminal region, inhibiting pre-mRNA splicing16. Several disorders, including FTLD-U, FTLD-MND and ALS are now referred to as TDP-43 proteinopathies12–14. Immunoblot analysis of the sarkosyl-insoluble fraction extracted from brains of patients afflicted with these disorders shows an abnormal TDP-43 immunoreactive band at 45 kDa. The electric mobility of this band changes after dephosphorylation, suggesting that abnormal phosphorylation takes place in accumulated TDP-4312, 13. However, the phosphorylation sites, responsible kinases and pathological significance of phosphorylation are still unknown.
In this report we demonstrate that multiple antibodies raised against TDP-43 phosphopeptides label UPIs in histological sections from FTLD-U and ALS brains. These antibodies may offer advantages over previous antibodies used to identify these structures as they appear to be more sensitive than anti-ubiquitin antibodies and, unlike commercially-available anti-TDP-43 antibodies, do not stain normal neuronal nuclei. Additionally, these antibodies specifically recognize abnormal TDP-43 species on immunoblots of sarkosyl-insoluble fractions extracted from FTLD-U and ALS brains. Furthermore, we show that the multiple phosphorylation epitopes identified in aggregated TDP-43 are generated by casein kinase-1 (CK1) and that oligomerization or fibril formation of TDP-43 is promoted by phosphorylation with CK1 in vitro. These results suggest that phosphorylated TDP-43 is a critical component of UPIs in FTLD-U and ALS and that phosphorylation of TDP-43 by CK1 may be involved in the accumulation of the protein.
MATERIALS AND METHODS
Materials
Human brain tissue was obtained from the Brain Donation Program at Sun Health Research Institute, Sun City AZ and from Aichi Medical University, Jichi Medical University, National Shimofusa Mental Hospital, and Tokyo Metropolitan Matsuzawa Hospital, Japan. Small blocks of brain tissue were dissected at autopsy and frozen rapidly at −70–80 °C or fixed in 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (pH 7.4) for 2 days. Brain tissue from sporadic FTLD-U, familial FTLD-U with PGRN mutations (mPGRN), sporadic ALS and sporadic FTLD-MND was compared with brain tissue from Alzheimer’s disease (AD) and neurologically normal control subjects. The age, gender, brain regions examined, diagnosis and histopathological subtyping for these cases are given in Table 1. Neuropathological diagnoses of FTLD-U, FTLD-MND, ALS and AD were made in accordance with published guidelines1, 9, 17–19. Although none of the 3 ALS cases had a documented history of dementia, all had immunohistochemical evidence of pathology in the neocortex.
Table 1.
Subjects, brain regions, pathological diagnosis and subtypes examined.
| Case # | Age (yrs) | Sex | Region | Diagnosis | Subtype |
|---|---|---|---|---|---|
| 1 | 67 | M | Hip, T, F | FTLD-U(sporadic) | 1 |
| 2 | 59 | M | Hip, T | FTLD-U(sporadic) | 1 |
| 3 | 68 | F | Hip, T | FTLD-U(sporadic) | 1 |
| 4 | 49 | F | T | FTLD-MND | 2 |
| 5 | 76 | M | Prec | FTLD-MND | 2 |
| 6 | 66 | M | F, SC | ALS | 2 |
| 7 | 70 | M | Prec, SC | ALS | 2 |
| 8 | 69 | M | Prec | ALS | 2 |
| 9 | 53 | M | Hip, T, F | FTLD-U (mRGRN) | 3 |
| 10 | 56 | F | Hip, T, F | FTLD-U (mRGRN) | 3 |
| 11 | 54 | M | Hip, T, F | FTLD-U (mRGRN) | 3 |
| 12 | 68 | F | Hip, T | AD | - |
| 13 | 83 | F | Hip, T | AD | - |
| 14 | 65 | M | Hip, T | Control | - |
| 15 | 72 | M | Hip, T | Control | - |
Hip, hippocampus; T, temporal; F, frontal; SC, spinal cord; Prec, precentral; FTLD-U, frontotemporal lobar degeneration with ubiquitinated inclusions; MND, motor neuron disease; ALS, amyotrophic lateral sclerosis; mPGRN, mutations of progranulin gene; AD, Alzheimer’s disease
Preparation of antibodies
Immunogens consisted of 39 synthetic phosphopeptides representing 36 of the 63 Ser/Thr/Tyr sites in the human TDP-43 molecule. All peptides were conjugated at the amino-terminus by a cysteine linkage to synthetic thyroglobulin using m-maleimidobenzoyl-N-hydroxysuccinimide ester as a coupling reagent20. The rabbit antisera were purified by obtaining flow-through fractions from a Toyopearl AF-Tresyl-650M (TOSOH Co., Tokyo, Japan) or SulfoLink Coupling Gel (Pierce Biotechnology Inc., Rockford, IL) precoated with the nonphosphorylated synthetic peptide. The specificities of the antibodies were verified by ELISA and immunoblot. A phosphorylation-independent rabbit polyclonal antibody to TDP-43 was also produced using a C-terminal peptide of TDP-43 (405–414) as immunogen.
Immunohistochemistry
Following cryoprotection in 15% sucrose in 0.01 M phosphate-buffered saline (PBS) (pH 7.4), PFA-fixed tissue blocks were cut on a freezing microtome at 30 µm thickness. The free floating sections were immunostained with an anti-ubiquitin antibody (DF2, a gift from Dr. Mori, 1:200)21, a commercially-obtained phosphorylation-independent anti-TDP-43 antibody (10782-1-AP, ProteinTech Group Inc, Chicago, IL; 1:2000) and a panel of phosphorylation-dependent anti-TDP-43 antibodies including pS409/410, using methods previously described13. Double-label immunofluorescence was performed using Fluorescein isothiocianate (FITC)- and tetramethylrhodamine isothiocyanate (TRITC)-conjugated secondary antibodies; sections were examined with a confocal laser microscope (LSM5 PASCAL; Carl Zeiss MicroImaging gmbh, Jena, Germany).
Immunoelectron microscopy
Tissue blocks of ALS lumber spinal cord were fixed in PFA and embedded in LR White Resin (London Resin, Reading, UK). Ultrathin sections were incubated with pS409/410 (1:1000), and the immunoreaction products were probed using colloidal gold particles (1:10 dilution; BBinternational, Cardiff, UK) according to a standard immunogold-based post-embedding electron microscopic procedure22.
Immunoblotting
Sarkosyl-insoluble, urea-soluble fractions were extracted from the frontal and the temporal regions of control, FTLD-U and ALS brains as previously described23. The samples before (−) and after (+) the treatment with lambda protein phosphatase (λPPase) were loaded on 10% SDS-PAGE. Proteins in the gel were then electrotransferred onto a polyvinylidene difluoride membrane (Millipore Corp., Bedford, MA). After blocking with 3% gelatin in Tris-buffered saline (20 mM Tris-HCl, pH 7.5, 500 mM NaCl), membranes were incubated overnight with the primary antibodies. Following incubation with an appropriate biotinylated secondary antibody, labeling was detected as previously described13, 23.
In vitro phosphorylation and fibrillization of TDP-43
Human TDP-43 cDNA were subcloned into pRK172 expression vectors and transformed into E coli BL21(DE3). For in vitro phosphorylation, crude extracts from E coli that expressed human TDP-43 were used to prepare partially purified TDP-43 using heparin-Toyopearl column chromatography and elution with 0.5 M NaCl. The elutes were phosphorylated with CK1 (10,000units/ml, New England Biolabs, Beverly, MA), casein kinase-2 (CK2) (10,000units/ml, New England Biolabs) or glycogen synthase kinase-3β (GSK3β) (10,000units/ml, New England Biolabs) at 30°C for 14hrs. To study fibrillization, partially purified TDP-43 aliquots were incubated in 30 mM Tris-HCl at pH 7.5 containing 4mM magnesium chloride, 2 mM ATP, with or without CK1 (10,000units/ml) at 30°C for 3 days. A few drops of reaction solution was then applied to a carbon-coated copper grid, and allowed to air dry. The grid was placed on a drop of blocking solution (10 mg/ml bovine serum albumin, BSA,in PBS) for 10 minutes, and then placed on a drop of primary antibody (pS409/410, 1:200) for 2 hr at room temperature. After washing with 10 mg/ml BSA in PBS, the grid was placed on a drop of the secondary antibody conjugated to 10 nm colloidal gold particles (Sigma, 1:50) for 1 hr at room temperature. Finally, after another round of washing, the grid was negatively stained with 2% sodium phosphotungstate, and examined with the electron microscopy (JEM-1230, JEOL LTD., Japan).
RESULTS
Multiple sites within TDP-43 are abnormally phosphorylated in FTLD-U and ALS
There are multiple potential phosphorylation sites within human TDP-43, including 41 Serine (Ser), 15 Threonine (Thr) and 7 Tyrosine (Tyr) residues. In order to identify the critical phosphorylation sites of TDP-43, we raised antibodies against 39 different synthetic phosphopeptides, representing 36 out of 63 candidate phosphorylation sites (Table 2). The major strategy was to choose Ser and Thr residues that cover known protein kinase consensus phosphorylation motifs, including R-X-pSer/Thr for protein kinase A (PKA), pSer/Thr-X-X-Ser/Thr for CK1, pSer/Thr-X-X-E/D for CK2, pSer/Thr-X-X-X-Ser for GSK3 and CK1. Additionally, Ser/Thr residues in C-terminal region of TDP-43 were chosen because they are analogous to abnormal phosphorylation sites found in tau or alpha-synuclein.
Table 2.
Antigen peptides for immunization of rabbits
| Site | Antigen peptide | |
|---|---|---|
| 1 | PT8 | EYIRVT(p)EDENDEC |
| 2 | PS20 | PIEIPS(p)EDDGTC |
| 3 | pS29 | GTVLLS(p)TVTAC |
| 4 | pT88 | CNYPKDNKRKMDET(p)D |
| 5 | pS91 | CDETDAS(p)SAVKVKR |
| 6 | pS92 | CDETDASS(p)AVKVKR |
| 7 | PS91/92 | DETDAS(p)S(p)AVKVC |
| 8 | pT103 | CKRAVQKT(p)SDLIVLG |
| 9 | pS104 | CKRAVQKTS(p)DLIVLG |
| 10 | pT116 | PWKTT(p)EQDLKEC |
| 11 | pT141 | KKDLKT(p)GHSKGC |
| 12 | pT153 | CGFVRFT(p)EYETQVK |
| 13 | pS180 | CKLPNS(p)KQSQDE |
| 14 | pS183 | CPNSKQS(p)QDEPLR |
| 15 | pS190 | CKQSQDEPLRS(p)RK |
| 16 | pT199 | CT(p)EDMTEDLE |
| 17 | pT203 | RCTEDMT(p)EDELR |
| 18 | pT233 | CRAFADVT(p)FADDQ |
| 19 | pS254 | IIKGIS(p)VKISC |
| 20 | pS258 | CISVHIS(p)NAEPK |
| 21 | pS266 | CPKHNS(p)NRQLER |
| 22 | PS273 | CRQLERS(p)GRFGGN |
| 23 | pS292 | CGFGNS(p)RGGGA |
| 24 | PS305 | CNNQGS(p)NMGGG |
| 25 | PS317 | CFGAFS(p)INPAM |
| 26 | PS332 | CAALQS(p)SWGMM |
| 27 | PS350 | CQSGPS(p)GNNQN |
| 28 | pS375 | GNNSYS(p)GSNSGC |
| 29 | PS379 | CSGSNS(p)GAAIG |
| 30 | PS389 | CGWGSAS(p)NAGS |
| 31 | PS393 | CASNAGS(p)GSGF |
| 32 | PS395 | CAGSGS(p)GFNGG |
| 33 | pS403 | CGFNGGFGS(p)SMD |
| 34 | pS404 | CNGGFGSS(p)MDSK |
| 35 | pS403/404 | CNGGFGS(p)S(p)MDSK |
| 36 | pS407 | CGSSMDS(p)KSSGW |
| 37 | PS409 | CMDSKS(p)SGWGM |
| 38 | PS410 | CMDSKSS(p)GWGM |
| 39 | PS409/410 | CMDSKS(p)S(p)GWGM |
Of the generated antibodies, pS379, pS403/404, pS409, pS410 and pS409/410 intensely immunostained the UPIs in FTLD-U and ALS and demonstrated, on immunoblots of sarkosyl-insoluble fractions extracted from brains of the FTLD-U and ALS cases, an abnormal band of 45 kDa but not the 43 kDa band corresponding to normal TDP-43. The results suggest that these sites are phosphorylated in the abnormal aggregates of TDP-43 present in FTLD-U and ALS.
Immunohistochemical characterization of phosphorylated TDP-43
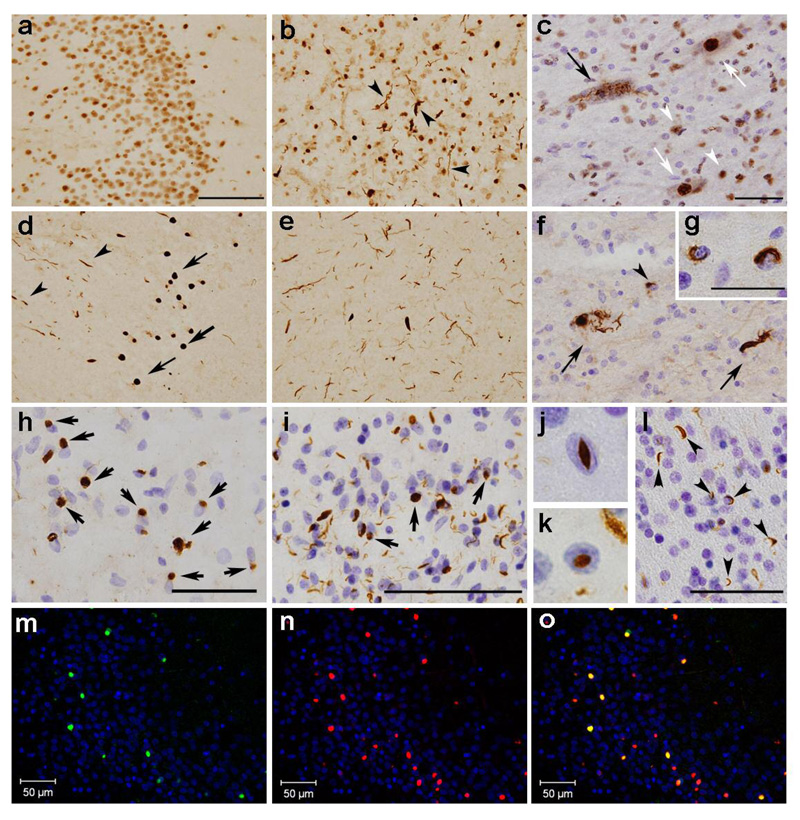
Immunohistochemical staining showed that five of the phosphorylation-specific anti-TDP-43 antibodies identified UPIs in both FTLD-U and ALS brains. Of the phosphorylation-specific antibodies, the immunoreactivity of pS409/410 was particularly robust (Fig. 1). In comparison, the commercially-obtained phosphorylation-independent anti-TDP-43 antibody labeled NCIs, DNs and neuronal nuclei in the dentate gyrus (Fig. 1a) and the temporal cortex (Fig. 1b) of the sporadic FTLD-U cases, and skein-like inclusions and neuronal nuclei in the spinal cord of ALS cases (Fig. 1c). It was particularly difficult to distinguish the staining of NCIs from that of neuronal nuclei in the dentate gyrus of the sporadic FTLD-U cases (Fig. 1a). By contrast, NCIs and DNs were unambiguously identified by the phosphorylation-specific antibody pS409/410, with no nuclear staining (Fig. 1d, e). Similarly, pS409/410 clearly labeled skein-like inclusions (Fig. 1f) and glial inclusions (Fig. 1g) in the spinal cord, and NCIs in the frontal and precentral cortices of the FTLD-MND and ALS cases (Fig. 1h). Glial inclusions in the frontal and precentral regions of the FTLD-MND and ALS cases were also immunopositive (data not shown). In the cases of familial FTLD-U with PGRN mutations, pS409/410 intensely stained NCIs, DNs, and NIIs in the cerebral cortex (Fig. 1i–k) and abundant positive structures in the cerebral white matter (Fig. 1l). The results of double immunofluorescence staining showed that pS409/410 identified more NCIs than the ubiquitin antibody (Fig. 1m–o).
Fig. 1.
Immunohistochemical comparison of FTLD-U and ALS brains using the phosphorylation independent anti-TDP-43 antibody (ProteinTech) (a–c) and the phosphorylation dependent anti-TDP-43 antibody (pS409/410) (d–l), in the dentate gyrus (a, d) and temporal cortex (b, e) of the sporadic FTLD-U cases, in the lumbar spinal cord (c, f, and g) and the frontal cortex (h) of the ALS cases and in the the frontal cortex (i, j, and k) and the frontal white matter (l) of the familial FTLD-U cases with PGRN mutations. a: Since most of the nuclei of dentate gyrus granular neurons are immunopositive with the phosphorylation-independent antibody, it is difficult to identify NCIs. b: TDP-43 positive DNs are recognizable (arrowheads) in addition to the nuclei. c: The black arrow indicates a cell with skein-like inclusions. White arrows and arrowheads indicate the normal nuclei of anterior horn cells and glial nuclei, respectively. Photomicrographs d ~ f illustrate the corresponding areas to a ~ c, respectively. Note the absence of nuclear staining in d ~ g with the phosphorylation-dependent antibody pS409/410. d: NCIs (arrows) and DNs (arrowheads) are clearly seen. e: More abundant DNs are seen than in b. f: Arrows indicate skein-like inclusions; the arrowheads indicate glial inclusions. The insert (g) shows glial inclusions at a higher magnification. h: NCIs in the frontal cortices of the ALS case are immunopositive. In the cases with PGRN mutations, pS409/410 clearly stains NCIs (arrows), DNs (i) and NIIs (j and k) in the superficial cortical layers, and abundant immunopositive structures in the white matter (arrowheads in l), with no nuclear staining. The sections are counterstained with hematoxylin to reveal nuclei in c, f, g, h, i, j, k, and l. Calibration bars a (also for b, d and e) and i indicate 100 µm; c (also for f), h and l indicate 50 µm; g denotes 25 µm; j (also for k) is 10 µm. m–o: Anti-ubiquitin (DF2) and pS409/410 double-label immunofluorescence histochemistry of the dentate gyrus in the FTLD-U case. Only some of the pS409/410 positive NCIs are also ubiquitin positive. m DF2; n pS409/410; g merge. The cell nuclei are stained with TO-PRO-3 (Invitrogen, Tokyo, Japan), producing a blue color.
Based on morphological aspects, TDP-43 proteinopathies have been classified into 4 subtypes24. Type 1 is characterized by DNs with few NCIs and no NIIs, Type 2 has numerous NCIs with few DNs and no NIIs, Type 3 has numerous NCIs and DNs and an occasional NIIs and Type 4 has numerous NIIs and DNs with few NCIs. Type 4 is specific for familial FTLD-U with mutations of VCP gene. In the present study, using the commercial anti-TDP-43 antibody, all of the sporadic FTLD-U cases showed type 1 pathology, all FTLD-MND and ALS cases showed type 2 pathology, and all FTLD-U with mPGRN showed type 3 pathology, in agreement with previous reports 24, 25.
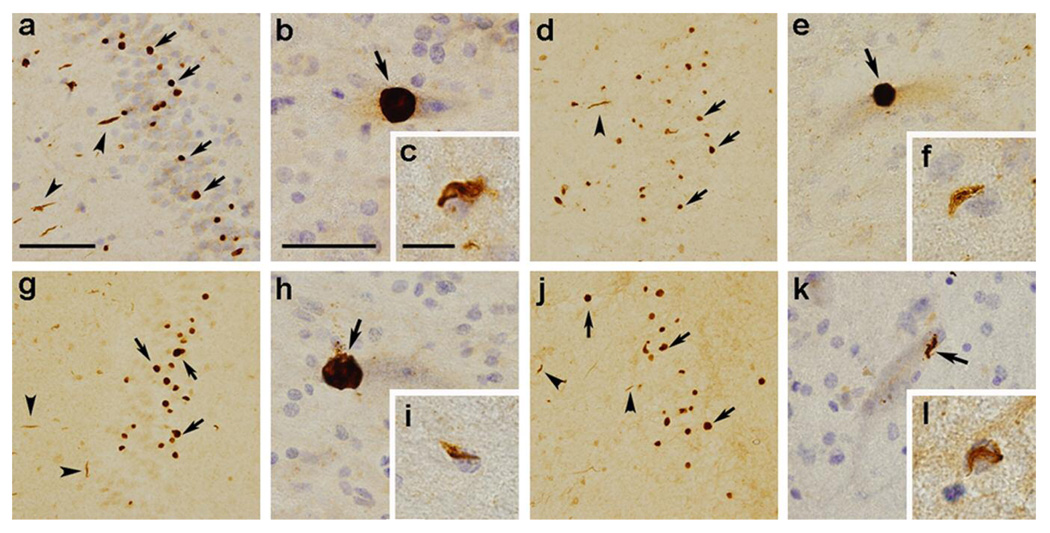
The staining patterns obtained with our phosphorylation-dependent antibodies were very similar to those seen with the commercial phosphorylation-independent antibody, suggesting that all of the inclusion types previously described contain phosphorylated TDP-43. Similar staining patterns were obtained using pS379 (Fig. 2a–c), pS403/404 (Fig. 2d–f), pS409 (Fig. 2g–i) and pS410 (Fig. 2j–l). Preabsorption of the antibodies with phosphopeptide immunogens abolished the labeling of these structures (data not shown).
Fig. 2.
Immunohistochemistry of FTLD-U brains and ALS spinal cords using the phosphorylation-dependent anti-TDP-43 antibodies specific for pS379 (a–c), pS403/404 (d–f), pS409 (g–i), and pS410 (j–l). These antibodies recognize NCIs (arrows in a, d, g, and j) and DNs (arrowheads in a, d, g, and j) in the dentate gyrus of the sporadic FTLD-U cases and motoneuronal round inclusions (arrow in b, e, and h), skein-like inclusion (arrow in k) and glial inclusions (c, f, i, and l) in the lumber spinal cord of the ALS cases. Note the absence of nuclear staining. The sections are counterstained with hematoxylin to reveal nuclei in a, b, c, e, f, h, i, k, and l. Bars a (also for d, g, and j) denote 100 µm; b (also for e, h, and k) is 25 µm; c (also for f, i, and l) is 12.5 µm.
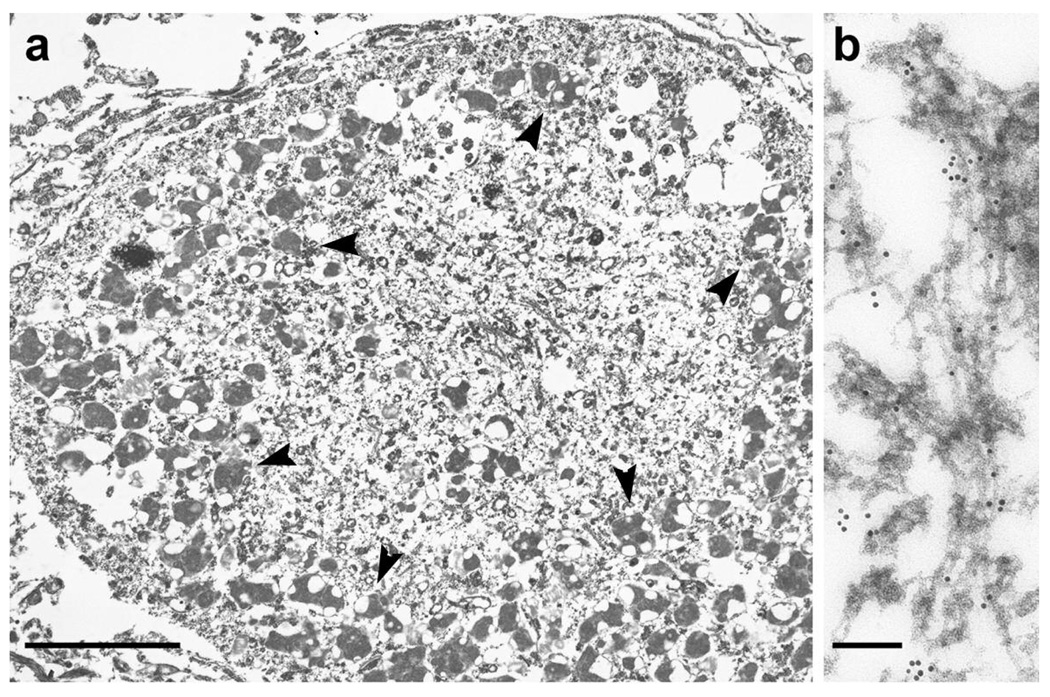
Immunoelectron microscopic examination of the spinal cord motoneuron inclusions of an ALS patient with the pS409/410 antibody showed immunopositive abnormal fibers of 15 nm diameter (Fig. 3a, b).
Fig. 3.
a: A low power immunoelectron micrograph of a phosphorylated TDP-43-positive motoneuronal inclusion in the spinal cord of an ALS patient. The irregularly shaped structure surrounded by lipofuscins (arrowheads) is the inclusion. b: At higher magnification, abnormal filaments of 15 nm diameter are immunopositive. Immunoreaction with pS409/410, probed with immunogold particles (diameter 10 nm), appears as black dots. Bars indicate, for a 5 µm; b 500 nm.
Immunoblot analysis of phosphorylated TDP-43
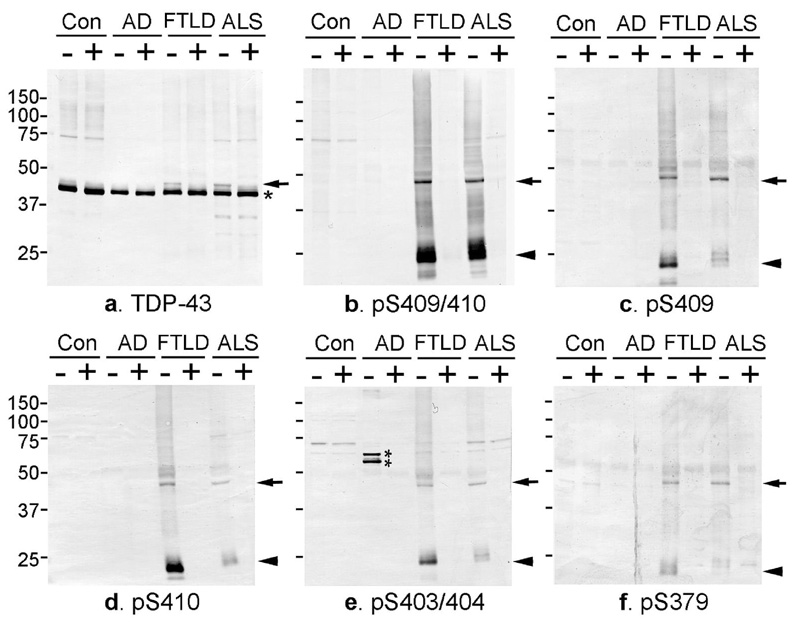
Immunoblot analyses of sarkosyl-insoluble fractions extracted from the brains of control, AD, FTLD-U and ALS cases with the phosphorylation-independent TDP-43 antibody (ProteinTech) revealed a band of 43 kDa in all cases and an additional 45 kDa band that was present only in FTLD-U and ALS cases, as previously described12, 13 (Fig. 4a). The phosphorylation-dependent antibodies specific for pS409/410 (Fig. 4b), pS409 (Fig. 4c), pS410 (Fig. 4d), pS403/404 (Fig. 4e), and pS379 (Fig. 4f) did not recognize the normal 43 kDa band, showing a single band at ~45 kDa, several smaller fragments at ~25 kDa and indistinct smears in FTLD-U and ALS cases but not in control and AD cases (Fig. 4b–f). The intensity of the ~25 kDa fragments tended to be greater than that of the 45 kDa band in FTLD-U (Fig. 4b, c, d and e) and in ALS (Fig. 4b, d). As for the immunohistochemical findings, the antibody to pS409/410 showed the most intense labeling (Fig. 4b). All of the immunoreactive bands were completely abolished by dephosphorylation, which was performed with lambda protein phosphatase (8,000units/ml, New England Biolabs) at 30°C for 2hrs.
Fig. 4.
a: Immunoblot analyses of sarkosyl-insoluble, urea-soluble fractions from control, AD, FTD (FTLD-U) and ALS brains with phosphorylation-independent anti-TDP-43 antibody (ProteinTech) (a) and phosphorylation-dependent anti-TDP-43 antibodies specific for pS409/410 (b), pS409 (c), pS410 (d), pS403/404 (e), and pS379 (f) before (−) and after (+) the treatment with lambda protein phosphatase (λPPase). a. With the phosphorylation-independent antibody, a positive band of 43 kDa is commonly seen in all cases (asterisk), while an additional band of 45 kDa is observed only in FTD and ALS (arrow), the labeling of which is abolished after dephosphorylation. b–f. The phosphorylation-dependent antibodies specifically label the ~45 kDa band (arrow) and the ~25 kDa fragment (arrowhead) as well as a smear, only in FTD and ALS. These immunoreactivities are abolished after dephosphorylation. Normal 43 kDa TDP-43 in control and diseased brains is not stained by these phosphorylation-dependent antibodies. The two bands recognized by the antibody specific for pS403/404 in AD (double asterisk in e) disappear after dephosphorylation, suggesting a cross reaction of the antibody to other phosphorylated proteins.
Immunoblot distinction between clinicopathological subtypes of TDP-43
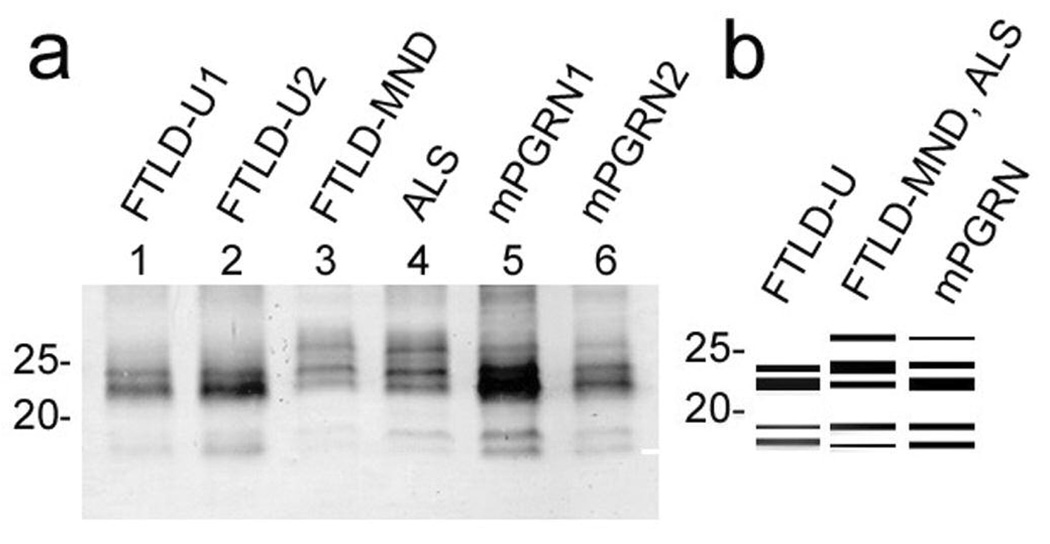
To investigate the biochemical basis of the different TDP-43 clinicopathological subtypes, we have carefully compared the results of immunoblots of the sarkosyl-insoluble, urea-soluble fractions from cerebral cortex of sporadic FTLD-U, FTLD-MND, ALS and mPGRN cases. The results showed that the band patterns of the 18~26 kDa fragments differed between clinicopathological subtypes (Fig. 5a, b). Sporadic FTLD-U cases showed two major bands at 23 and 24 kDa and two minor bands at 18 and 19 kDa, while FTLD-MND and ALS cases showed three major bands at 23, 24 and 26kDa and two minor bands at 18 and 19 kDa. A 23 kDa band is the most intense in sporadic FTLD-U, while a 24 kDa band is the most intense in FTLD-MND and ALS. Furthermore, the band pattern of mPGRN cases was not distinctive but intermediate between FTLD-U, FTLD-MND and ALS cases.
Fig. 5.
A relationship between the clinicopathological subtypes of TDP-43 proteinopathies and the band pattern of the C-terminal fragments of phosphorylated TDP-43. a: Immunoblots of the sarkosyl-insoluble, urea-soluble fractions from sporadic FTLD-U, FTLD-MND, ALS and mPGRN cases with the pS409/410 antibody. The samples are loaded on 15% polyacrylamide gel. Sporadic FTLD-U cases (lanes 1, 2) show a band pattern with two major bands at 23 and 24 kDa and two minor bands at 18 and 19 kDa. A band of 24 kDa is weaker than that of 23 kDa, and a 19 kDa band is weaker than an 18 kDa band. FTLD-MND (lane 3) and ALS (lane 4) cases show a pattern with three major bands at 23, 24 and 26kDa and two minor bands at 18 and 19kDa. A 24 kDa band is the most intense, and an 18 kDa band is weaker than a 19 kDa band. mPGRN (lanes 5, 6) cases show three major bands at 23, 24 and 26kDa and two minor bands at 18 and 19kDa. A 23 kDa band is the most intense, and a band of 18 kDa and that of 19 kDa show similar intensity. The band pattern of mPGRN cases is therefore a composite of that seen in FTLD-U, FTLD-MND and ALS. b: Schematic diagram of the band pattern of the C-terminal fragments of phosphorylated TDP-43.
The phosphorylation epitopes are generated by casein kinase 1 (CK1)
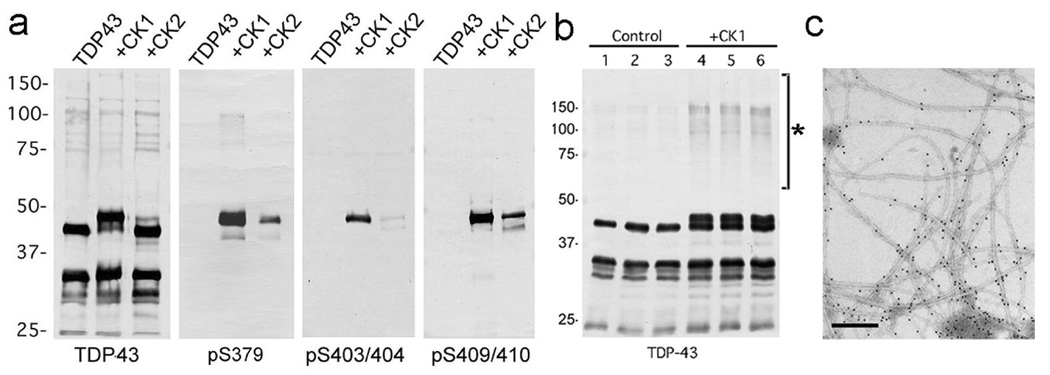
To investigate the kinase responsible for the abnormal phosphorylation of TDP-43, recombinant TDP-43 was treated in vitro with CK1, CK2 and GSK3β. Immunoblot analyses of the recombinant TDP-43 revealed that phosphorylation by CK1 caused a reduction in gel mobility of TDP-43 to ~45kDa and strong immunoreactivity to the phosphorylation-specific antibodies (Fig 5a). TDP-43 phosphorylated by CK2 was only weakly immunoreactive for these antibodies (Fig 5a) and that phosphorylated by GSK3β was negative (data not shown). Kinase activity capable of generating the ~45 kDa TDP-43 with PS409/410 epitopes was also detected in crude rat brain extracted with a high concentration (10–20 mM) of MgCl2 (data not shown). This kinase activity was not inhibited by the CK2 inhibitor heparin, suggesting that CK1 may be the major kinase in brain extract. Interestingly, increased levels of SDS-stable TDP-43 oligomers were observed after phosphorylation by CK1 (Fig 5b). Furthermore, based on immunoelectron microscopic analysis, recombinant TDP-43 phosphorylated by CK1 formed abundant filaments when applied on a carbon coated copper grid (Fig 5c), while nonphosphorylated recombinant TDP-43 formed few filaments (data not shown).
DISCUSSION
We show here that antibodies generated to multiple TDP-43 phosphorylation sites stain the pathological structures in FTLD-U and ALS. These structures include NCIs, NIIs and DNs in the cerebral cortex and hippocampus as well as skein-like inclusions, round inclusions and glial inclusions in the spinal cord. The phosphorylation-dependent antibodies stain these structures more extensively than an anti-ubiquitin antibody and do not stain normal neuronal nuclei. Furthermore, on immunoelectron microscopy, the phosphorylation-dependent antibodies label abnormal filaments in the motoneuronal inclusion of the ALS case although these findings may not be the same as for other types of cytoplasmic and intranuclear inclusions26. Immunoblot analysis of sarkosyl-insoluble fractions from FTLD-U and ALS brains shows that these antibodies specifically stain abnormal TDP-43 species. These findings are therefore analogous to previous discoveries of phosphorylation-specific epitopes for tau and alpha-synuclein in tauopathies and alpha-synucleinopathies27–29.
At least 5 sites on TDP-43 are phosphorylated (Ser 379, Ser 403/404, Ser 409/410) in subjects with FTLD-U and ALS. These results suggest that abnormal phosphorylation takes place mainly near the carboxyl (C)-terminal region of TDP-43. This again is similar to tauopathies and synucleinopathies27, 28, where multiple Ser residues in the C-terminal region, including Ser422 in tau and C-terminal Ser129 in alpha-synuclein, are abnormally phosphorylated. It has been established that hyperphosphorylated tau and alpha-synuclein represent the earliest detectable molecular change in the brain in these neurodegenerative diseases29, 30. Thus, the results of the present study suggest that abnormally phosphorylated TDP-43 is a critical component of UPIs in FTLD-U and ALS.
There is a close relationship between the pathological subtypes of TDP-43 proteinopathy and the immunoblot pattern of C-terminal fragments of phosphorylated TDP-43. These findings confirm and extend the previous reports by Sampathu et al.31 and Neumann et al.12 that showed C-terminal fragment composition varied between cases with type 1 and type 2 pathology. Furthermore, we have shown that cases with type 3 pathology have a band pattern that is mixed or intermediate. These results parallel our earlier findings of differing C-terminal tau fragments in progressive supranuclear palsy and corticobasal degeneration, despite identical composition of tau isoforms32. Taken together, these results suggest that elucidating the mechanism of C-terminal fragment origination may shed light on the pathogenesis of several neurodegenerative disorders involving TDP-43 proteinopathy and tauopathy.
These phosphorylation-specific antibodies are a new and powerful tool for the investigation of TDP-43 proteinopathies. Since phosphorylation-dependent antibodies to TDP-43 react only with abnormally deposited TDP-43, they offer advantages over existing commercially available antibodies for the pathological diagnosis and subtyping of TDP-43 proteinopathies. Additionally, and again in analogy with tauopathies, these antibodies may be useful for detecting abnormal TDP-43 in biological fluids such as cerebrospinal fluid33.
The results suggest that CK1 is involved in the abnormal phosphorylation and accumulation of TDP-43. In the present study, the treatment of recombinant TDP-43 by CK1 generates the same phosphorylation epitopes that are recognized by phosphorylation-dependent antibodies. Additionally, phosphorylation at these epitopes facilitates filament formation. In comparison, several protein kinases have been reported to be responsible for phosphorylating tau and alpha-synuclein. They include, for tau phosphorylation34–37, GSK3β, cyclin-dependent kinase 5, mitogen activated protein kinase, and MAP/microtubule affinity-regulating kinase, and for alpha-synuclein phosphorylation38–40, CK1, CK2 and G-protein-coupled receptor kinase 5.
The pathological significance of phosphorylation of TDP-43 is not clear. It is well known that protein phosphorylation plays an important role in regulating transcription and pre-mRNA splicing. Several splicing factors including hnRNPs, small nuclear ribonucleoproteins (snRNPs), and serine/arginine-rich protein family are known to be phosphorylated in vivo. Various kinases including CK1 have been implicated in phosphorylating these factors41–43. Phosphorylation of these factors modulates protein-protein and protein-RNA interactions and affects their subcellular localization and physiological functions41. For instance, Habelhah et al. showed that phosphorylation of hnRNP-K by extracellular-signal-regulated kinase results in its cytoplasmic accumulation and also inhibits mRNA translation44. Guil et al. reported that stress-induced activation of the MAP-kinase kinase3/6-p38 pathway causes hyperphosphorylation and cytoplasmic accumulation of hnRNP A1, affecting alternative splicing regulation45. Thus, phosphorylation of TDP-43 may lead to its cytoplasmic accumulation and influence various physiological functions. At present, however, it is unclear whether TDP-43 is physiologically phosphorylated in brain. Although in HeLa cells, Ser91 and Ser92 of TDP-43 were reported to be phosphorylated46, the antibody specific to pS91/pS92 we made in this study did not stain any structures in normal brains (data not shown). Despite the normal nuclear location of TDP-43, none of the our five phosphorylation-dependent antibodies stained normal nuclei, suggesting that phosphorylation of these sites is a disease-specific phenomenon.
Our in vitro studies suggest that phosphorylation of TDP-43 facilitates the formation of SDS-stable oligomers and filaments of TDP-43. These abnormal structures may be neurotoxic, as suggested previously for tauopathies and alpha-synucleinopathies30. Thus, abnormal phosphorylation of TDP-43 may be pathological through either a loss-of-function or a toxic gain-of-function, or both, leading to the characteristic neuronal degeneration and clinical syndromes.
Fig. 6.
a: Immunoblot analyses of recombinant TDP-43 phosphorylated in vitro. The crude extract from E coli that expressed human TDP-43 is treated with CK1 and CK2 at 30°C for 14hrs, and probed with a phosphorylation-independent antibody against a C-terminal peptide of TDP-43 (405–414), and with phosphorylation-dependent antibodies pS379, pS403/404 and pS409/410. Phosphorylation by CK1 causes the mobility shift to ~45kDa and induction of intense immunoreactivity to the phosphorylation-dependent antibodies. b: Immunoblot analyses of recombinant TDP-43 phosphorylated by CK1. The recombinant TDP-43, which is partially purified by heparin-Toyopearl column chromatography, is incubated with (lanes 4~6) or without (lanes 1~3) CK1 in the presence of ATP at 37°C for 14 hrs, and probed with the phosphorylation-independent TDP-43 antibody (ProteinTech). Results in three independent, representative experiments are shown. Note the SDS-stable TDP-43 oligomers at ~100 ~ 200 kDa (asterisk) are detected after phosphorylation by CK1. c: Positive immunolabeling by pS409/410 of filaments assembled from recombinant TDP-43 phosphorylated by CK1 (10 nm colloidal gold). Bar denotes 200nm.
Acknowledgements
We thank Mses. H. Kondo and Y. Izumiyama for their excellent technical assistance. This research was supported by a Grant-in-Aid for Scientific Research on Priority Areas – Research on Pathomechanisms of Brain Disorders (to M.H.), a Grant-in-Aid for Scientific Research (B) (to M.H.), Grants-in-Aid for Scientific Research (C) (to T.A.& T.N.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan. The Brain Donation Program at Sun Health Research Institute is supported by the National Institute on Aging (P30 AG19610 Arizona Alzheimer’s Disease Core Center, the Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011 and 05–901) and the Prescott Family Initiative of the Michael J. Fox Foundation for Parkinson’s Research.
REFERENCES
- 1.Mackenzie IRA, Feldman HH. Ubiquitin immunohistochemistry suggests classic motor neuron disease, motor neuron disease with dementia, and frontotemporal dementia of the motor neuron disease type represent a clinicopathological spectrum. J Neuropathol Exp Neurol. 2005;64:730–739. doi: 10.1097/01.jnen.0000174335.27708.0a. [DOI] [PubMed] [Google Scholar]
- 2.Baker M, Mackenzie IR, Pickering-Brown SM, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–919. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]
- 3.Cruts M, Gijselinck I, van der Zee J, et al. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature. 2006;442:920–924. doi: 10.1038/nature05017. [DOI] [PubMed] [Google Scholar]
- 4.Watts GDJ, Wymer J, Kovach MJ, et al. Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat Genet. 2004;36:377–381. doi: 10.1038/ng1332. [DOI] [PubMed] [Google Scholar]
- 5.Morita M, Al-Chalabi A, Anderson PM, et al. A locus on chromosome 9p confers susceptibility to ALS and frontotemporal dementia. Neurology. 2006;66:839–844. doi: 10.1212/01.wnl.0000200048.53766.b4. [DOI] [PubMed] [Google Scholar]
- 6.Vance C, Al-Chalabi A, Ruddy D, et al. Familial amyotrophic lateral sclerosis with frontotemporal dementia is linked to a locus on chromosome 9p13.2–21.3. Brain. 2006;129:868–875. doi: 10.1093/brain/awl030. [DOI] [PubMed] [Google Scholar]
- 7.Leigh PN, Anderton BH, Dodson A, et al. Ubiquitin deposits in anterior horn cells in motor neurone disease. Neurosci Lett. 1988;93:197–203. doi: 10.1016/0304-3940(88)90081-x. [DOI] [PubMed] [Google Scholar]
- 8.Lowe J, Lennox G, Jefferson D, et al. A filamentous inclusion body within anterior horn neurones in motor neurone disease defined by immunocytochemical localization of ubiquitin. Neurosci Lett. 1988;94:203–210. doi: 10.1016/0304-3940(88)90296-0. [DOI] [PubMed] [Google Scholar]
- 9.Piao YS, Wakabayashi K, Kakita A, et al. Neuropathology with clinical correlations of sporadic amyotrophic lateral sclerosis: 102 autopsy cases examined between 1962 and 2000. Brain Pathol. 2003;13:10–22. doi: 10.1111/j.1750-3639.2003.tb00002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ou SH, Wu F, Harrich D, et al. Cloning and characterization of a novel cellular protein, TDP-43, that binds to human immunodeficiency virus type 1 TAR DNA sequence motifs. J Virol. 1995;69:3584–3596. doi: 10.1128/jvi.69.6.3584-3596.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buratti E, Dork T, Zuccato E, et al. Nuclear factor TDP-43 and SR proteins promote in vitro and in vivo CFTR exon 9 skipping. EMBO J. 2001;20:1774–1784. doi: 10.1093/emboj/20.7.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neumann M, Sampathu DM, Kwong LK, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 13.Arai T, Hasegawa M, Akiyama H, et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351:602–611. doi: 10.1016/j.bbrc.2006.10.093. [DOI] [PubMed] [Google Scholar]
- 14.Davidson Y, Kelley T, Mackenzie IRA, et al. Ubiquitinated pathological lesions in frontotemporal lobar degeneration contain the TAR DNA-binding protein, TDP-43. Acta Neuropathol (Berl) 2007;113:521–533. doi: 10.1007/s00401-006-0189-y. [DOI] [PubMed] [Google Scholar]
- 15.Wang H-Y, Wang I-F, Bose J, Shen C-KJ. Structural diversity and functional implications of the eukaryotic TDP gene family. Genomics. 2004:130–139. doi: 10.1016/s0888-7543(03)00214-3. [DOI] [PubMed] [Google Scholar]
- 16.Buratti E, Brindisi A, Giombi M, et al. TDP-43 binds heterogeneous nuclear ribonucleoprotein A/B through its C-terminal tail. J Biol Chem. 2005;280:37572–37584. doi: 10.1074/jbc.M505557200. [DOI] [PubMed] [Google Scholar]
- 17.McKhann GM, Albert MS, Grossman M, et al. Clinical and pathological diagnosis of frontotemporal dementia: report of the work group on frontotemporal dementia and Pick's disease. Arch Neurol. 2001;58:1803–1809. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- 18.Dickson DW, Josephs KA, Amardor-Ortiz C. TDP-43 in differential diagnosis of motor neuron disorders. Acta Neuropathol (Berl) 2007;114:71–79. doi: 10.1007/s00401-007-0234-5. [DOI] [PubMed] [Google Scholar]
- 19.Newell KL, Hyman BT, Growdon JH, Hedley-Whyte ET. Application of the National Institute on Aging (NIA)-Reagan Institute criteria for the neuropathological diagnosis of Alzheimer disease. J Neuropathol Exp Neurol. 1999;58:1147–1155. doi: 10.1097/00005072-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Kitagawa T, Aikawa T. Enzyme coupled immunoassay of insulin using a novel coupling reagent. J Biochem (Tokyo) 1976;79:233–236. doi: 10.1093/oxfordjournals.jbchem.a131053. [DOI] [PubMed] [Google Scholar]
- 21.Mori H, Kondo J, Ihara Y. Ubiquitin is a component of paired helical filaments in Alzheimer's disease. Science. 1987;235:1641–1644. doi: 10.1126/science.3029875. [DOI] [PubMed] [Google Scholar]
- 22.Arima K, Mizutani T, Alim MA, et al. NACP/alpha-synuclein and tau constitute two distinctive subsets of filaments in the same neuronal inclusions in brains from a family of parkinsonism and dementia with Lewy bodies: double-immunolabeling fluorescence and electron microscopic studies. Acta Neuropathol (Berl) 2000;100:115–121. doi: 10.1007/s004010050002. [DOI] [PubMed] [Google Scholar]
- 23.Hasegawa M, Arai T, Akiyama H, et al. TDP-43 is deposited in the Guam parkinsonism-dementia complex brains. Brain. 2007;130:1386–1394. doi: 10.1093/brain/awm065. [DOI] [PubMed] [Google Scholar]
- 24.Cairns NJ, Bigio EH, Mackenzie IR, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol (Berl) 2007;114:5–22. doi: 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Snowden J, Neary D, Mann D. Frontotemporal lobar degeneration: clinical and pathological relationships. Acta Neuropathol (Berl) 2007;114:31–38. doi: 10.1007/s00401-007-0236-3. [DOI] [PubMed] [Google Scholar]
- 26.Amador-Ortiz C, Lin WL, Ahmed Z, et al. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer's disease. Ann Neurol. 2007;61:435–445. doi: 10.1002/ana.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hasegawa M, Jakes R, Crowther RA, et al. Characterization of mAb AP422, a novel phosphorylation-dependent monoclonal antibody against tau protein. FEBS Lett. 1996;384:25–30. doi: 10.1016/0014-5793(96)00271-2. [DOI] [PubMed] [Google Scholar]
- 28.Fujiwara H, Hasegawa M, Dohmae N, et al. a-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4:160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- 29.Goedert M, Spillantini MG, Davies SW. Filamentous nerve cell inclusions in neurodegenerative diseases. Curr Opin Neurobiol. 1998;8:619–632. doi: 10.1016/s0959-4388(98)80090-1. [DOI] [PubMed] [Google Scholar]
- 30.Goedert M. The significance of tau and alpha-synuclein inclusions in neurodegenerative diseases. Curr Opin Genet Dev. 2001;11:343–351. doi: 10.1016/s0959-437x(00)00200-8. [DOI] [PubMed] [Google Scholar]
- 31.Sampathu DM, Neumann M, Kwong LK, et al. Pathological heterogeneity of frontotemporal lobar degeneration with ubiquitin-positive inclusions delineated by ubiquitin immunohistochemistry and novel monoclonal antibodies. Am J Pathol. 2006;169:1343–1352. doi: 10.2353/ajpath.2006.060438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arai T, Ikeda K, Akiyama H, et al. Identification of amino-terminally cleaved tau fragments that distinguish progressive supranuclear palsy from corticobasal degeneration. Ann Neurol. 2004;55:72–79. doi: 10.1002/ana.10793. [DOI] [PubMed] [Google Scholar]
- 33.Ishiguro K, Ohno H, Arai H, et al. Phosphorylated tau in human cerebrospinal fluid is a diagnostic marker for Alzheimer's disease. Neurosci Lett. 1999;270:91–94. doi: 10.1016/s0304-3940(99)00476-0. [DOI] [PubMed] [Google Scholar]
- 34.Ishiguro K, Takamatsu M, Tomizawa K, et al. Tau protein kinase I converts normal tau protein into A68-like component of paired helical filaments. J Biol Chem. 1992;267:10897–10901. [PubMed] [Google Scholar]
- 35.Baumann K, Mandelkow EM, Biernat J, et al. Abnormal Alzheimer-like phosphorylation of tau-protein by cyclin-dependent kinases cdk2 and cdk5. FEBS Lett. 1993;336:417–424. doi: 10.1016/0014-5793(93)80849-p. [DOI] [PubMed] [Google Scholar]
- 36.Drewes G, Lichtenberg-Kraag B, Döring F, et al. Mitogen activated protein (MAP) kinase transforms tau protein into an Alzheimer-like state. EMBO J. 1992;11:2131–2138. doi: 10.1002/j.1460-2075.1992.tb05272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drewes G, Ebneth A, Preuss U, et al. MARK, a novel family of protein kinases that phosphorylate microtubule-associated proteins and trigger microtubule disruption. Cell. 1997;89:297–308. doi: 10.1016/s0092-8674(00)80208-1. [DOI] [PubMed] [Google Scholar]
- 38.Ishii A, Nonaka T, Taniguchi S, et al. Casein kinase 2 is the major enzyme in brain that phosphorylates Ser129 of human alpha-synuclein: Implication for alpha-synucleinopathies. FEBS Lett. 2007;581:4711–4717. doi: 10.1016/j.febslet.2007.08.067. [DOI] [PubMed] [Google Scholar]
- 39.Arawaka S, Wada M, Goto S, et al. The role of G-protein-coupled receptor kinase 5 in pathogenesis of sporadic Parkinson's disease. J Neurosci. 2006;26:9227–9238. doi: 10.1523/JNEUROSCI.0341-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pronin AN, Morris AJ, Surguchov A, Benovic JL. Synucleins are a novel class of substrates for G protein-coupled receptor kinases. J Biol Chem. 2000;275:26515–26522. doi: 10.1074/jbc.M003542200. [DOI] [PubMed] [Google Scholar]
- 41.Soret J, Tazi J. Phosphorylation-dependent control of the pre-mRNA splicing machinery. Prog Mol Subcell Biol. 2003;31:89–126. doi: 10.1007/978-3-662-09728-1_4. [DOI] [PubMed] [Google Scholar]
- 42.Gross SD, Loijens JC, Anderson RA. The casein kinase Ialpha isoform is both physically positioned and functionally competent to regulate multiple events of mRNA metabolism. J Cell Sci. 1999;112:2647–2656. doi: 10.1242/jcs.112.16.2647. [DOI] [PubMed] [Google Scholar]
- 43.Mayrand SH, Dwen P, Pederson T. Serine/threonine phosphorylation regulates binding of C hnRNP proteins to pre-mRNA. Proc Natl Acad Sci USA. 1993;90:7764–7768. doi: 10.1073/pnas.90.16.7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Habelhah H, Shah K, Huang L, et al. ERK phosphorylation drives cytoplasmic accumulation of hnRNP-K and inhibition of mRNA translation. Nat Cell Biol. 2001;3:325–330. doi: 10.1038/35060131. [DOI] [PubMed] [Google Scholar]
- 45.van der Houven van Oordt W, Diaz-Meco MT, Lozano J, et al. The MKK(3/6)-p38-signaling cascade alters the subcellular distribution of hnRNP A1 and modulates alternative splicing regulation. J Cell Biol. 2000;149:307–316. doi: 10.1083/jcb.149.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olsen JV, Blagoev B, Gnad F, et al. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell. 2006;127:635–648. doi: 10.1016/j.cell.2006.09.026. [DOI] [PubMed] [Google Scholar]