Abstract
Cyclin-dependent protein kinase 6 (CDK6), in cooperation with cyclin Ds, drives cell cycle progression from G1 to S phase through phosphorylation and subsequent inactivation of Retinoblastoma 1 protein (Rb). Alteration of this pathway results in both non-hematologic and hematologic malignancies, which include a small subset of B-cell lymphoproliferative disorders (BLPDs). We identified 5 cases of BLPD that carried CDK6 chromosomal translocations and characterized their clinical, pathologic, immunophenotypic, and genetic features. Common clinical characteristics included marked neoplastic lymphocytosis, systemic lymphadenopathy, splenomegaly, and bone marrow involvement. Three patients were diagnosed with low-grade B-cell lymphoma and had an indolent clinical course, and 2 patients (one who transformed to large B-cell lymphoma and another who was initially diagnosed with a high-grade B-cell lymphoma) had an aggressive clinical course. Immunophenotypically, the neoplastic B-cells expressed CD5, CDK6, and cytoplasmic Rb in all cases, expressed phospho-RB, p27kip1, and cyclin D2 in most cases, and uniformly lacked expression of all other cyclins. In four cases, the CDK6 translocation partner was kappa immunoglobulin light-chain gene (IGK); and in the fifth case, the CDK6 translocation partner was unknown. These distinct clinicopathologic and cytogenetic features distinguish the CDK6 translocation-associated BLPDs (CDK6-BLPDs) from other mature B-cell lymphomas.
Keywords: cyclin D, cyclin dependent protein kinase (CDK), chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), mantle cell lymphoma (MCL), splenic marginal zone lymphoma (SMZL), and Retinoblastoma protein (Rb)
INTRODUCTION
Cyclin-dependent protein kinases 4 and 6 (CDK4 and CDK6), coupled with cyclin Ds (D1, D2 and D3), drive progression of cell cycle from G1 to S phase by phosphorylating and subsequently inactivating retinoblastoma 1 protein (Rb). 4, 31, 38 Alteration of this pathway by aberrant overexpression of cyclins or CDKs results in malignant transformation best exemplified by the overexpression of cyclin D1 as a result of t(11;14)/IGH-CCND1 in mantle cell lymphoma (MCL).43, 49, 51, 52
Likewise, alteration of the CDK6/cyclin D complex by CDK6 overexpression has been reported in both hematologic 7, 8, 19, 32, 34, 35, 42, 44, 50 and non-hematologic 9 malignancies. In a subset of B-cell lymphoproliferative disorders (BLPDs), overexpression of CDK6 results from juxtaposition of the CDK6 gene locus at 7q21-22 to an immunoglobulin (IG) heavy chain (IGH) gene locus at 14q32, 19, 50 IG kappa light chain (IGK) gene locus at 2p12, 8, 42 IG lambda light chain (IGL) gene locus at 22q11, 19 or to the long arm of chromosome 21 (21q). 8 Nevertheless, due to their rarity, the clinicopathologic features of these CDK6 translocation-associated BLPDs (CDK6-BLPDs) were insufficiently reported. In this study we characterized the clinical, pathologic, immunophenotypic, cytogenetic features, and the potential molecular oncogenic mechanism of CDK6-BLPDs.
MATERIALS AND METHODS
Patient Selection
We first searched the Mayo Clinic cytogenetic database, which contained the conventional karyotyping results of 4,528 cases of mature B-cell lymphomas for specimens with chromosome 7q21-22 abnormalities. To increase the case number, we also searched for the cases that showed chromosomal abnormalities involving both chromosomes 2 and 7 with ambiguous break points detected by conventional cytogenetic studies. We then screened paraffin sections of these specimens for CDK6 rearrangements by interphase fluorescence in situ hybridization (FISH) (see below). In addition, as CDK6 translocations have been previously described in splenic marginal zone lymphomas (SMZL),8 we screened a tissue microarray (TMA) of 54 paraffin-embedded SMZL specimens using the same FISH approach. The Mayo Clinic Institutional Review Board approved this study, and all patients approved of research use of their tissue samples.
FISH Analysis for CDK6 Translocation
Interphase FISH analysis was performed on isolated nuclei from paraffin blocks or directly on tissue microarray sections (listed in Table 1) as previously described. 37, 41, 42 All cases were first screened for the presence of CDK6 translocations using CDK6 break-apart probes (BAP) comprising SpectrumOrange- (BACS: CTB-10G5, RP5-1099C19, CTB-85C5, and RP5-850G1) and SpectrumGreen-labeled (BACS: CTB-104I4, GS1-440B14, and GS1-165I4) DNA probes that hybridize to flanking regions of the CDK6 break point. Cases with positive CDK6 BAP signals were further analyzed using dual-fusion (D-FISH) DNA probes for IGH-CDK6, IGK-CDK6 and IGL-CDK6 (BACS: CTB-10G5, RP5-1099C19, CTB-85C5, RP5-850G1, GS1-119P5, GS1-552A1, CTB-104I4, GS1-440B14, and GS1-165I4), as well as BAP FISH probes for IGH (Vysis Inc., Downers Grove, IL, USA), IGK and IGL. 13 For the isolated nuclei specimens, 100 consecutive qualifying interphase nuclei from different areas of the same slide were examined using scoring criteria and normal cutoffs established from previous validation studies. 13 For the TMA specimens, a minimum of 50 cells were scored per case, with a minimum of 20 abnormal cells were required for that sample to be considered abnormal.42 SpectrumOrange-labeled signals are referred to as red (R), SpectrumGreen-labeled signals as green (G), and SpectrumOrange-SpectrumGreen fusion signals as fusion (F).
Table 1.
Clinical and Laboratory Features
| Case | Sex/Age | Maximum Lymphocytosis (109/L) | Neutrophil (109/L) | HGB (g/dL) | Platelet (109/L) | LDH (U/L) | Bone Marrow Involvement (%) | Serum Immunoglobin Heavy Chains/Light Chains | Lymphadenopathy And Lymph Node Biopsied | Splenomegaly And Splenectomy Performed | Large Cell Transformation | Treatment/Alive |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M/66 | 8.8 | 3.1 | 14.6 | 221 | 144 | 30† F | IgM (1.1g/dL) Kappa | No | No | No | No/Yes |
| 2 | M/56 | 31 | 38.9 | 13.3 | 203 | 471 | 30† | Negative | Yes† | Yes†F | Yes* | Yes/No |
| 3 | F/68 | 35 | 2.72 | 8.9 | 45 | 154 | 15† | IgM (trace) IgG (trace) Lambda | Yes† | Yes† F | No | Yes/Yes |
| 4 | M/77 | 37 | 11.78 | 11.5 | 211 | 573 | 80† | Negative | Yes† | Yes† F | Yes | Yes/Yes |
| 5 | F/83 | 30.8 | 2.4 | 10.9 | 116 | 114 | ND | ND | Yes† | Yes† F | No | Yes/Yes |
ND: not done.
High-grade B-cell lymphoma at initial diagnosis.
Tissue specimens were morphologically and immunohistochemically studied.
Tissues were used for FISH studies.
Morphologic and Immunohistochemical Studies
Bone marrow (n=4), spleen (n=4) and lymph node (n=4) specimens used for histologic studies were fixed in B5 or formalin fixatives, embedded in paraffin and stained with hematoxylin and eosin (H&E). Giemsa-Wright stains (GW) were performed on the peripheral blood and bone marrow aspirate smears. We examined the morphologic features of peripheral blood smears, bone marrow aspirates and biopsies, lymph nodes, and spleen tissues by light microscopy. Immunohistochemical studies were performed on the paraffin-embedded bone marrow, lymph node, or spleen specimens in each case using previously described techniques and reagents, 25 including antibodies against CD3 (Novocastra Laboratories, Newcastle Upon Tyne, UK), CD5 (Novocastra), CD10 (Novocastra), CD20 (Dako, Carpinteria, CA, USA), and CD23 (The Binding Site Limited, Birmingham, UK). Pathologic diagnosis was given to each case based on the 2001 WHO classification.23 Additional antibodies employed in this study were CDK6 (LabVision, Fremont, CA, USA), cyclin A (Vision Biosystems, Norwell, MA, USA), cyclin B1 (Vision Biosystems), cyclin D1 (LabVision), cyclin D2 (Cell Signaling Technology, Danvers, MA, USA), cyclin D3 (Vision Biosystems), cyclin E (Vision Biosystems), p27kip1 (Dako), phospho-Rb (Cell Signaling Technology), and Rb (Epitomics, Burlingame, CA). Microscopy images were captured with an Olympus digital camera DP71 (Olympus America Inc., Center Valley, PA, USA), and processed using Adobe Photoshop CS3 software (Adobe Systems Inc., San Jose, CA, USA).
Immunophenotyping by Flow Cytometry
Multicolor flow cytometric immunophenotyping analysis was performed on peripheral blood, spleen, and lymph node specimens according to previously described methods 23 using fluorochrome-conjugated antibodies to the following antigens: CD3, CD5, CD10, CD11c, CD19, CD20, CD22, CD23, CD38, CD45, CD103, and surface kappa and lambda immunoglobulin light chains (sIg). All antibody conjugates were obtained from Becton Dickinson Biosciences (San Jose, CA, USA). Analysis was performed on a FACSCalibur instrument (Becton Dickinson), and the data were processed using CellQuest software (Becton Dickinson).
RESULTS
FISH results
We identified 5 patients with BLPD that carried a CDK6 translocation by interphase FISH analysis using CDK6 BAP probes (Figure 1A, 1B and Table 1). Two specimens (patients 1 and 2) had an abnormal chromosome 7q21-22 and one specimen (patient 3) had abnormalities involving chromosomes 2 and 7, as detected by conventional cytogenetic studies (detailed in Table 3). The remaining 2 specimens (patients 4 and 5) were identified by the SMZL TMA screening. The bone marrow specimen from patient 4 had an abnormal karyotype (Table 3), although it lacked a recognizable 7q21-22 abnormality. Additional FISH studies demonstrated that the CDK6 translocation partner was IGK in 4 cases (patients 1, 2, 3, and 5) (Figure 1C and 1D, Table 3) with intact IGL or IGH. The recipient gene locus of the CDK6 translocation of case 4 was unknown and was not an IG gene since IG gene abnormalities were not detected either by IG BAP or IG-CDK6 D-FISH analyses (data not shown).
Figure 1. Identification of CDK6 translocations by FISH analysis.

A: Isolated nuclei from a normal donor bone marrow aspirate (A) were hybridized using CDK6 BAP probes. The FISH pattern of two fusion signals (2F) indicates the absence of a CDK6 break point. B–D: Isolated nuclei from paraffin-embedded sections of bone marrow biopsy from patient 1 were hybridized with CDK6 BAP probes (B), IGK BAP probes (C), and IGK-CDK6 D-FISH probes (D). Patient 1 had positive CDK6 (1R1G1F) (B) and IGK (1R1G1F) (C) break points. The two CDK6-IGK fusion signals (1R1G2F) in the lower right nucleus (D) indicate CDK6-IGK translocation. The upper left nucleus (D) is negative for the CDK6-IGK translocation (2R2G).
Table 3.
Summary of Selected Clinical, Pathologic, Immunophenotypic and Genetic Features of the CDK6 Translocation-Positive Cases in This Series and in The Literature
| Studies | Patient | Age | Sex | Key Cytogenetic Results by Conventional Chromosomal Karyotyping |
Gene Locus Involved |
CD5 | CD23 | Leukemic Presentation |
Primary Pathologic Diagnosis |
|---|---|---|---|---|---|---|---|---|---|
| Chen et. al. | 1 | 66 | M | 46,XY,t(2;7)(p11.2;q22) | K | + | − | Yes | Low grade BLPD |
| 2 | 56 | M | 46,XY,del(1)(p34.1p36.1),t(2;7)(p11.2;q 22),add(7)(q32),- 8,add(13) (p11.2),add(16)(q22), -17,+2mar |
K | + | − | Yes | High grade B-cell lymphoma, not further classifiable | |
| 3 | 68 | F | 44–48,XX,add(2)(p13),add(7)(p11.2),-8,add(9)(p22)X2,-13,del(13)(q12q14),-14, -17,-17,-19,+7,13mar |
K | + | − | Yes | SMZL | |
| 4 | 77 | M | 41–44,XY,-3,add(7)(q32),-9,-11,-12,-14,add(16)(q22),-17,-18,der(?)t(12;?) (q13;?),+3- 6mar[cp9]/76–78,XX,-Y-Y,add(7)(q32)X2,-9,-9,-11,-11,-12,-12,-14,14,-15,-15,-16,add(16)(q22)X2,-17,-17,-17,-18,-18,-19,-19,-20,-21,der(?)t(12;?)(q13;?)X2,+6mar[cp2] |
? | + | - | Yes | SMZL | |
| 5 | 83 | F | no evidence of +12 by FISH | K | + | − | Yes | SMZL | |
|
| |||||||||
| Corcoran et al. 8 | 52 | M | 46,XY,t(2;7)(p11;q22) | K | ? | ? | ? | SMZL | |
| 64 | M | 46,XY,t(2;7)(p11;q21.2) | K | ? | ? | ? | SMZL | ||
| 76 | F | 46,XX,t(2;7)(p11;q22),del(7)(q34q36),der(8)t(8;12)(p21;q11) | K | ? | ? | ? | SMZL | ||
| 70 | F | 45,XX,t(7;21)(q22;q22),t(9;17)(p11;q11),der(9)t(9;17)(p11;q11),717 | 21q22 | ? | ? | ? | SMZL | ||
|
| |||||||||
| Brito-Babapulle et al. 7 | 75 | F | 46,XY,t(2;7)(p12;q21-22) | K | + | − | Yes | SMZL | |
|
| |||||||||
| Wahbi et al.50 | 64 | F | t(7;14)(q21;q32) | H | + | + | Yes | CLL/SLL | |
|
| |||||||||
| Hayetteet al.19 | 61 | M | 46,XY,t(7;22)(q22;q11),del(13)(q13q14) | L | + | + | Yes | CLL/SLL | |
| 79 | M | 46,X,-Y,+12, t(7;14)(q21;q32) | H | + | + | Yes | CLL/SLL | ||
| 61 | M | 45,XY,t(2;7)(p11-12;q22),der(8)t(8;17)(p12;p11),-17/47,XY, t(2;7),+5,+12 | NA | + | + | Yes | CLL/SLL | ||
BLPD, B-cell lymphoproliferative disorder; CLL/SLL, chronic lymphocytic leukemia/lymphoma; SMZL, splenic marginal zone lymphoma; K,kappa Ig light chain; L, lambda Ig light chain; H, Ig heavy chain; NA, data not available; ?, unknown. The trisomy 12 is underlined.
Clinical and Laboratory Features
The clinical characteristics are detailed in Table 1. In summary, there were 3 men and 2 women (median age, 68; range, 56–83 years). The 4 patients who presented with lymphadenopathy and splenomegaly (patients 2–5) all had marked neoplastic lymphocytosis (range, 30.8–37 × 109/L) with an absent to negligible M-spike; while the remaining patient (patient 1) presented with a more prominent M-spike (1.1 g/dL), a more modest lymphocytosis (8.8 × 109/L), and absence of lymphadenopathy or splenomegaly. All 4 patients tested had bone marrow involvement.
Patient 1 had no extramedullary involvement, received no treatment, and was lost to follow-up. Three of the remaining 4 patients (patients 3, 4, and 5) had SMZL: patient 3 was treated with rituximab, relapsed in one year, and was lost to follow-up 10 months later; patient 4 transformed to large B-cell lymphoma involving the bone marrow 5 months later, was treated with CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone), and died of disease a year later; and patient 5 was treated with rituximab for 4 weeks and is alive 2 years later. At presentation, patient 2 had a high-grade B-cell lymphoma, involving the right axillary lymph node and a low-grade BLPD involving the peripheral blood and bone marrow. He was initially treated with Vanderbilt protocol, 30 stem cell transplant, and R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone), but relapsed with high-grade B-cell lymphoma involving his left axillary lymph node, right upper lobe of lung, spleen, and thigh soft tissue 9 months later. Despite treatment with R-DHAP (rituximab, dexamethasone, cytarabine, and cisplatin), the patient died of disease 5 months later.
Morphologic Features
All of the available peripheral blood, bone marrow smears, and tissue sections were morphologically examined (listed in Table 1). The neoplastic lymphocytes in the peripheral blood and bone marrow from patient 1 (Figure 2A and 2B) were predominantly small lymphocytes with moderate cytoplasm, irregular nuclear membranes, inconspicuous nucleoli, and condensed chromatin. They did not have recognizable short villi. The patterns of bone marrow infiltration were nodular, peritrabecular (Figure 2C and 2D), and interstitial. There was no overt intrasinusoidal distribution, which was confirmed by CD20 and p27kip1 immunohistochemical stains (Figure 2E and 2F). The lymph nodes and spleen from patient 2 (Figure 4A) showed sheets of neoplastic intermediate to large cells with high-grade cytologic features. The spleens from patients 3 (Figure 2G–I and 4F), 4, and 5 showed nodular and focally diffuse distribution of small lymphocytes with abundant cytoplasm and slightly irregular nuclei involving both white and red pulp. The white pulp showed a distinct zonal distribution with central small nonclefted lymphocytes and peripheral small monocytoid lymphocytes. None of the tissues (listed in Table 1) showed prominent proliferation centers, a distinct morphologic feature in chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL). The primary pathologic diagnoses are listed in Table 3.
Figure 2. Morphologic features of the peripheral blood and bone marrow from a CDK6-BLPD.
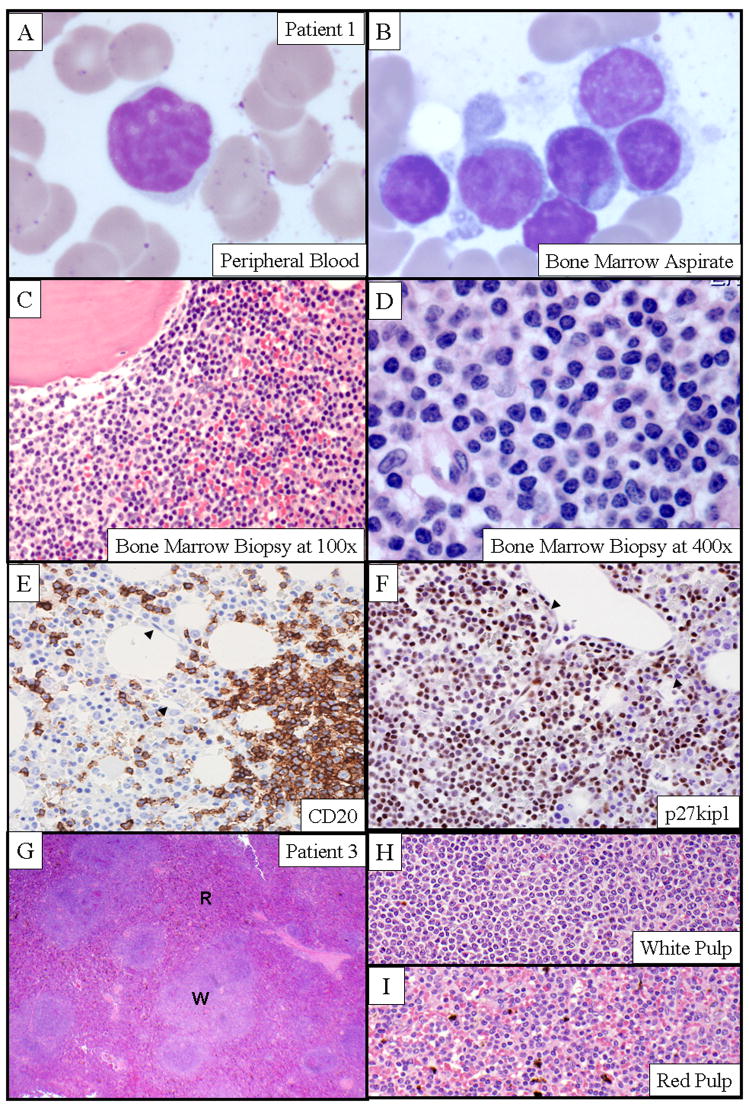
A–B: The morphologic features of circulating neoplastic lymphocytes in peripheral blood (A) and bone marrow aspirate (B) (patient 1) (GW stain, x600); C–D: Bone marrow biopsy (patient 1) at a lower magnification (H&E stain, x100) (C) and higher magnification (H&E stain, x400) (D) showed the peritrabecular and interstitial infiltration by the morphologically distinct small lymphocytes. E–F: The absence of intrasinusoidal distribution of the neoplastic B-cells was demonstrated by immunohistochemical stains on bone marrow biopsy sections (patient 1) with antibodies to CD20 (E) and p27kip1 (F). Note the intact bone marrow sinuses (arrowhead). G–I: H&E stained sections of the involved spleen (patient 3) showed morphologic features that were most consistent with SMZL. Micrographic images of a lower (x40) (G) and a higher magnification (x400) of the involved white pulp (H) and red pulp (I) demonstrated a zonal distribution of the neoplastic B-cells. “W” indicates the white pulp; “R”, the red pulp.
Figure 4. Expression of CDK6 and other cell cycle-related genes.

Paraffin H&E and immunohistochemical stains of the spleens from patient 2 (A–E) and 3 (F–J) using antibodies directed against CDK6 (B and G), cyclin D2 (C and H), phospho-Rb (D and I), and p27kip1 (E and J) were performed. Both involved spleens showed cytoplasmic and nuclear staining for CDK6 (B and G) and nuclear staining for cyclin D2 (C and H), phospho-Rb (D and I), and p27kip1 (E and J).
Immunophenotypic Features
The results of the immunophenotypic studies by flow cytometry are exemplified in Figure 3 (patients 2 and 5) and summarized in Table 2 and 3. The circulating neoplastic B-lymphocytes (CD19+, bright CD20+, CD22+, and bright monotypic sIg+) coexpressed CD5 (except for patient 4), but were negative for CD10, CD23, and CD103. The patterns of CD5 expression ranged from bright and uniform (Figure 3, patient 2) to dim and partial (Figure 3, patient 5). Focal CD5 expression by the neoplastic cells from patient 4’s spleen was confirmed by immunohistochemical studies (Figure 3, patient 4). Additional immunohistochemical studies demonstrated that the neoplastic cells from all cases expressed CDK6 with a spectrum of weak (patients 1, 3, and 5; Figure 4G) to moderate-strong expression (patients 2 and 4; Figure 4B). Cytoplasmic Rb was detected in all specimens. Phospho-RB and Cyclin D2 were detected in 4 of 5 cases (patients 2–5; Figure 4C, 4D, 4H and 4I). Except for patient 4, the neoplastic cells from the other 4 patients all expressed p27kip1 (Figure 2F, 4E and 4J). Immunohistochemical stains for cyclin A, B1, D1, D3, and E were negative.
Figure 3. Immunophenotypic features of CDK6-BLPD.

A–D and E–H: Flow cytometric studies of the circulating neoplastic lymphocytes in peripheral blood from patients 2 and 5 showed distinct populations of kappa immunoglobulin light-chain restricted B-cells that coexpressed bright or dim CD5, respectively. I–L: The micrographic images of the immunohistochemical studies of the involved spleen from patient 4 (x400) showed partial/dim CD5 coexpression (L) by the neoplastic CD20-positive B-cells (J). I and J: Paraffin H&E and CD3 immunohistochemical stains of the spleen section.
Table 2.
Immunophenotypic Features
| Patient | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Tissue type | Bone marrow | Spleen and lymph node | Spleen | Spleen | Spleen |
| CD5* | + | + | + | −/+** | + |
| CD23* | − | − | − | − | − |
| sIg light chain* | Kappa | Kappa | Lambda | Lambda | Kappa |
| Cyclin A | − | − | − | − | − |
| Cyclin B | − | − | − | − | − |
| Cyclin D1 | − | − | − | − | − |
| Cyclin D2 | (−) | ++ | + | +/− | + |
| Cyclin D3 | − | − | − | − | − |
| Cyclin E | − | − | − | − | − |
| CDK6 | (+/−) | ++ | +/− | + | +/− |
| RB | + | ++ | ++ | ++ | ++ |
| Phospho-RB | (+/−) | + | + | + | + |
| P27kip1 | + | + | + | − | + |
By flow cytometry studies on the peripheral blood samples.
Negative by flow cytometry studies on the peripheral blood sample but focally positive by immunohistochemistry study on paraffin sections of spleen.
(): The immunohistochemical stains were suboptimal for decalcified bone marrow biopsy specimen.
+/− : Weakly or focally expressed.
+ : Moderately expressed in more than 10% of the neoplastic B-cells.
++ : Strongly expressed.
sIg: Surface immunoglobulin.
DISCUSSION
Alteration of the cell cycle by CDK6 overexpression has been demonstrated in T-cell lymphoblastic lymphoma, 44 peripheral T-cell lymphomas, 32 and a small group of BLPDs. 7, 8, 19, 34, 35, 42, 50 In this study, we identified and characterized the clinical and pathological features of 5 cases of BLPD with a chromosomal translocation involving CDK6 on chromosome 7q21–22.
The frequency of CDK6 gene translocations in BLPDs seems low. We identified only 5 cases of CDK6-BLPD: 3 out of 4,528 cases by conventional cytogenetics (0.07%), and 2 cases from a SMZL TMA containing 54 patient specimens (4%). Although this low prevalence is consistent with previous studies, 7, 8, 19, 34, 35, 42, 50 it may not be accurate. The initial conventional cytogenetic database screening, employed in the present study and other reported series, 7, 8, 19, 34, 35, 42, 50 might not be comprehensive, and potentially could be biased for selecting aggressive BLPDs. Since the conventional karyotyping is generally more sensitive for detecting cytogenetic abnormalities of mitotically active and clinically aggressive BLPDs than those of low-grade BLPDs with low proliferation indices. By screening cases with karyotypes containing ambiguous chromosome 2 (IGK) and 7 abnormalities by CDK6 BAP FISH analysis, we were able to identify one additional case with a CDK6 translocation. Anticipating an extremely low yield, we did not further screen the specimens with ambiguous abnormal chromosome 7 and 14 (IGH) or chromosome 7 and 22 (IGL). In addition, potential institutional bias of our cytogenetic database and disease selection bias of the SMZL TMA could also influence the screening for CDK6-BLPDs.
CDK6-BLPDs have distinct clinicopathologic characteristics. All patients in this study and others (Table 3) are elderly and typically present with marked and persistent lymphocytosis as their initial clinical manifestation. The circulating neoplastic lymphocytes are usually small B-cells that morphologically resemble monocytoid B-cells. Lymphadenopathy, splenomegaly, and bone marrow involvement are very common. In all cases in the present series, B-cells expressed CD5 and were negative for CD23 and cyclin D1. The clinical courses in this series vary. Patients 1, 3, and 5 had an indolent clinical course, which is consistent with other reported series.8, 19, 50 The other 2 patients (patients 2 and 4) either presented with or progressed to a higher grade lymphoma, which could be explained by their more complex chromosomal abnormalities (Table 3) or additional molecular abnormalities.
CDK6-BLPDs clinically and pathologically overlap with other BLPD/mature B-cell lymphomas, such as CLL/SLL. Some patients in our study were initially diagnosed with CLL/SLL due to the marked lymphocytosis. However, CDK6-BLPDs lacked characteristic features of CLL/SLL, such as condensed chromatin and smudge cells by cytology, proliferation centers by histologic examination on bone marrow, lymph node and spleen specimens, and the characteristic constellation of dim expression of monotypic surface immunoglobulin and CD20 coupled with coexpression of CD5 and CD23 by immunophenotyping. Other CDK6 translocation-positive cases reported in the literature have been diagnosed as CLL/SLL, but it is not clear whether they had classic characteristic features of CLL/SLL or could have in fact been CLL/SLL variants. Trisomy 12, which is common in CLL/SLL and other BLPDs, was present in 2 of the 4 reported CDK6 translocation-positive CLL/SLL cases (Table 3), 19, 50 and absent in all cases in our study. Interestingly, although IGK is the most common CDK6 translocation partner overall, IGH-CDK6 and IGL-CDK6 translocations are more common in cases diagnosed as CDK6 translocation-positive CLL/SLL (Table 3).19, 50
The CDK6-BLPDs share some clinical and pathologic features with SMZL, especially SMZL with villous lymphocytes. 2, 3, 17, 22, 23, 36, 42, 48 Three patients in our series were diagnosed with SMZL based on the cytology of the neoplastic cells and the histologic features of the involved spleens. However, these cases differed from typical SMZL cases in several ways. First, most patients presented with generalized lymphadenopathy as well as splenomegaly. Second, in all cases the neoplastic peripheral blood and bone marrow lymphocytes lacked overt short villi. Third, the bone marrow biopsies showed no intrasinusoidal neoplastic involvement, a frequent feature of SMZL.10, 26 Finally, all cases with karyotypes in both our series and the literature lacked gains of the long arm of chromosomes 3 and 12, or loss of the long arm of chromosome 7, each of which is present in approximately 20% of SMZL.1, 12, 21, 42 They also lacked the morphologic features of a recently described small subset of SMZL with red pulp distribution and distinct basophilic villous lymphocytes. 47 In the absence of splenomegaly, case 1 was a diagnostic challenge and the possibility of early involvement by SMZL could not be excluded.
The CDK6-BLPDs also share some features with cyclin D1-negative MCLs that are potentially caused by alternative translocations involving cyclin D2 or D3 and an immunoglobulin gene locus. 15, 16, 20, 53 For example, both CDK6-BLPD and MCL may express CD5 and overexpress cyclinD2, as shown in this series and in literature. 15, 16, 20, 53 However, the clinical presentation and median survival of CDK6-BLPDs of the present series and others are generally more favorable than most MCLs, including the cyclin D1-negative variant. Histologically, none of the tissues examined in the present series showed the usual features of MCL, such as singly placed epithelioid macrophages or hyalinized blood vessels. P27kip1 expression, mostly undetectable in MCLs, 40 was present in all but one of our CDK6-BLPD cases. Lastly, most of the reported CCND2 or CCND3 translocation-associated MCL involved an immunoglobulin locus. In the present series, except for the involved CDK6/IGK, all other IG loci were intact.
The oncogenic mechanism of altered CDK6 protein expression is through its interaction with a cyclin D and subsequent phosphorylation of Rb. 4, 31, 38 The concomitant expression of CDK6 and cyclin D2 in 4 cases implicates cyclin D2 as the potential activator of CDK6. Since cyclin D2 overexpression has been observed in other BLPDs, 11, 46 the definitive evidence for CDK6-cyclin D2 interaction relies on additional biochemical studies. The positive Rb phosphorylation in all 5 cases supports Rb pathway being the downstream route of CDK6, similar to that of cyclin D1. However, the overexpression of CDK6 may not have the same oncogenic potency as that of cyclin D1. First, MCLs are clinically more aggressive than most CDK6-BLPDs. Second, both of our patients with higher grade lymphomas had complex karyotypes, suggesting that the CDK6 abnormality by itself may not be sufficient to cause an aggressive clinical course. Finally, p27kip1, a potent CDK inhibitor 6, 18 that is usually suppressed in MCL, 27, 39, 40 is expressed in most CDK6- BLPDs and may thus offset the downstream effects of the CDK6-cyclin D complex. Moreover, the single case that showed absent p27kip1 expression (patient 4) subsequently transformed to large B-cell lymphoma.
With the advent of target therapy, knowing the molecular nature of a hematologic malignancy becomes relevant. For example, small molecules such as PD0332991, which has a profoundly inhibitory effect on CDK4/CDK6-cyclin D-mediated Rb phosphorylation, 29 could potentially benefit the patients with CDK6-BLPDs.
Acknowledgments
Supported in part by grant CA97274 from the National Institutes of Health, Bethesda, Maryland, USA.
We thank Anne E. Wiktor for her technical support on initial cytogenetics database search.
References
- 1.Andersen CL, Gruszka-Westwood A, Atkinson S, et al. Recurrent genomic imbalances in B-cell splenic marginal-zone lymphoma revealed by comparative genomic hybridization. Cancer Genet Cytogenet. 2005;156:122–128. doi: 10.1016/j.cancergencyto.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 2.Bassan R, Amaru R, Rambaldi A, et al. The natural history of monoclonal villous lymphocytosis: a chronic lymphoproliferative disorder of CD11c+ B cells. Leuk Lymphoma. 1996;21:181–183. doi: 10.3109/10428199609067598. [DOI] [PubMed] [Google Scholar]
- 3.Bassan R, Neonato MG, Abbate M, et al. Monoclonal lymphocytosis with villous lymphocytes: a chronic lymphoproliferative disease of CD11c+ B-cells. Leukemia. 1991;5:799–806. [PubMed] [Google Scholar]
- 4.Bates S, Parry D, Bonetta L, et al. Absence of cyclin D/cdk complexes in cells lacking functional retinoblastoma protein. Oncogene. 1994;9:1633–1640. [PubMed] [Google Scholar]
- 5.Bell ND, King JA, Kusyk C, et al. CD5 negative diffuse mantle cell lymphoma with splenomegaly and bone marrow involvement. South Med J. 1998;91:584–587. doi: 10.1097/00007611-199806000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Blanchard DA, Affredou MT, Vazquez A. Modulation of the p27kip1 cyclin-dependent kinase inhibitor expression during IL-4-mediated human B cell activation. J Immunol. 1997;158:3054–3061. [PubMed] [Google Scholar]
- 7.Brito-Babapulle V, Gruszka-Westwood AM, Platt G, et al. Translocation t(2;7)(p12;q21-22) with dysregulation of the CDK6 gene mapping to 7q21-22 in a non-Hodgkin’s lymphoma with leukemia. Haematologica. 2002;87:357–362. [PubMed] [Google Scholar]
- 8.Corcoran MM, Mould SJ, Orchard JA, et al. Dysregulation of cyclin dependent kinase 6 expression in splenic marginal zone lymphoma through chromosome 7q translocations. Oncogene. 1999;18:6271–6277. doi: 10.1038/sj.onc.1203033. [DOI] [PubMed] [Google Scholar]
- 9.Costello JF, Plass C, Arap W, et al. Cyclin-dependent kinase 6 (CDK6) amplification in human gliomas identified using two-dimensional separation of genomic DNA. Cancer Res. 1997;57:1250–1254. [PubMed] [Google Scholar]
- 10.Costes V, Duchayne E, Taib J, et al. Intrasinusoidal bone marrow infiltration: a common growth pattern for different lymphoma subtypes. Br J Haematol. 2002;119:916–922. doi: 10.1046/j.1365-2141.2002.03934.x. [DOI] [PubMed] [Google Scholar]
- 11.Delmer A, Ajchenbaum-Cymbalista F, Tang R, et al. Overexpression of cyclin D2 in chronic B-cell malignancies. Blood. 1995;85:2870–2876. [PubMed] [Google Scholar]
- 12.Dierlamm J, Rosenberg C, Stul M, et al. Characteristic pattern of chromosomal gains and losses in marginal zone B cell lymphoma detected by comparative genomic hybridization. Leukemia. 1997;11:747–758. doi: 10.1038/sj.leu.2400635. [DOI] [PubMed] [Google Scholar]
- 13.Einerson RR, Law ME, Blair HE, et al. Novel FISH probes designed to detect IGK-MYC and IGL-MYC rearrangements in B-cell lineage malignancy identify a new breakpoint cluster region designated BVR2. Leukemia. 2006;20:1790–1799. doi: 10.1038/sj.leu.2404340. [DOI] [PubMed] [Google Scholar]
- 14.Espinet B, Sole F, Pedro C, et al. Clonal proliferation of cyclin D1-positive mantle lymphocytes in an asymptomatic patient: an early-stage event in the development or an indolent form of a mantle cell lymphoma? Hum Pathol. 2005;36:1232–1237. doi: 10.1016/j.humpath.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 15.Fu K, Weisenburger DD, Greiner TC, et al. Cyclin D1-negative mantle cell lymphoma: a clinicopathologic study based on gene expression profiling. Blood. 2005;106:4315–4321. doi: 10.1182/blood-2005-04-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gesk S, Klapper W, Martin-Subero JI, et al. A chromosomal translocation in cyclin D1-negative/cyclin D2-positive mantle cell lymphoma fuses the CCND2 gene to the IGK locus. Blood. 2006;108:1109–1110. doi: 10.1182/blood-2006-01-0015. [DOI] [PubMed] [Google Scholar]
- 17.Gimeno E, Salido M, Sole F, et al. CD5 negative and CD5 positive splenic marginal B-cell lymphomas have differential cytogenetic patterns. Leuk Res. 2005;29:981–982. doi: 10.1016/j.leukres.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Guan KL, Jenkins CW, Li Y, et al. Growth suppression by p18, a p16INK4/MTS1- and p14INK4B/MTS2-related CDK6 inhibitor, correlates with wild-type pRb function. Genes Dev. 1994;8:2939–2952. doi: 10.1101/gad.8.24.2939. [DOI] [PubMed] [Google Scholar]
- 19.Hayette S, Tigaud I, Callet-Bauchu E, et al. In B-cell chronic lymphocytic leukemias, 7q21 translocations lead to overexpression of the CDK6 gene. Blood. 2003;102:1549–1550. doi: 10.1182/blood-2003-04-1220. [DOI] [PubMed] [Google Scholar]
- 20.Herens C, Lambert F, Quintanilla-Martinez L, et al. Cyclin D1-negative mantle cell lymphoma with cryptic t(12;14)(p13;q32) and cyclin D2 overexpression. Blood. 2008;111:1745–1746. doi: 10.1182/blood-2007-10-120824. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez JM, Garcia JL, Gutierrez NC, et al. Novel genomic imbalances in B-cell splenic marginal zone lymphomas revealed by comparative genomic hybridization and cytogenetics. Am J Pathol. 2001;158:1843–1850. doi: 10.1016/S0002-9440(10)64140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isaacson PG, Matutes E, Burke M, et al. The histopathology of splenic lymphoma with villous lymphocytes. Blood. 1994;84:3828–3834. [PubMed] [Google Scholar]
- 23.Jaffe ES, Harris NL, Stein H, et al. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues: WHO Classification of Tumours. Lyons, France: IARC Press; 2001. [Google Scholar]
- 24.Kaptain S, Zukerberg LR, Ferry JA. Bcl-1/cyclinD1+ CD5-mantle cell lymphoma. Mod Pathol. 1998;1998:133a. [Google Scholar]
- 25.Kurtin PJ, Hobday KS, Ziesmer S, et al. Demonstration of distinct antigenic profiles of small B-cell lymphomas by paraffin section immunohistochemistry. Am J Clin Pathol. 1999;112:319–329. doi: 10.1093/ajcp/112.3.319. [DOI] [PubMed] [Google Scholar]
- 26.Labouyrie E, Marit G, Vial JP, et al. Intrasinusoidal bone marrow involvement by splenic lymphoma with villous lymphocytes: a helpful immunohistologic feature. Mod Pathol. 1997;10:1015–1020. [PubMed] [Google Scholar]
- 27.Letestu R, Ugo V, Valensi F, et al. Prognostic impact of p27KIP1 expression in cyclin D1 positive lymphoproliferative disorders. Leukemia. 2004;18:953–961. doi: 10.1038/sj.leu.2403337. [DOI] [PubMed] [Google Scholar]
- 28.Martin ML, Marques ML, Garcia A, et al. [Cytogenetic alterations found by chromosome analysis and FISH technique in two patients with variant chronic lymphocytic leukemia] Sangre (Barc) 1995;40:425–429. [PubMed] [Google Scholar]
- 29.Marzec M, Kasprzycka M, Lai R, et al. Mantle cell lymphoma cells express predominantly cyclin D1a isoform and are highly sensitive to selective inhibition of CDK4 kinase activity. Blood. 2006;108:1744–1750. doi: 10.1182/blood-2006-04-016634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McMaster ML, Greer JP, Wolff SN, et al. Results of treatment with high intensity, brief duration chemotherapy in poor prognosis non-Hodgkin’s lymphoma. Cancer. 1991;68:233–241. doi: 10.1002/1097-0142(19910715)68:2<233::aid-cncr2820680203>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 31.Meyerson M, Harlow E. Identification of G1 kinase activity for cdk6, a novel cyclin D partner. Mol Cell Biol. 1994;14:2077–2086. doi: 10.1128/mcb.14.3.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagel S, Leich E, Quentmeier H, et al. Amplification at 7q22 targets cyclin-dependent kinase 6 in T-cell lymphoma. Leukemia. 2007 doi: 10.1038/sj.leu.2405028. [DOI] [PubMed] [Google Scholar]
- 33.Nodit L, Bahler DW, Jacobs SA, et al. Indolent mantle cell lymphoma with nodal involvement and mutated immunoglobulin heavy chain genes. Hum Pathol. 2003;34:1030–1034. doi: 10.1053/s0046-8177(03)00410-6. [DOI] [PubMed] [Google Scholar]
- 34.Oscier DG, Gardiner A, Mould S. Structural abnormalities of chromosome 7q in chronic lymphoproliferative disorders. Cancer Genet Cytogenet. 1996;92:24–27. doi: 10.1016/s0165-4608(96)00025-8. [DOI] [PubMed] [Google Scholar]
- 35.Oscier DG, Matutes E, Gardiner A, et al. Cytogenetic studies in splenic lymphoma with villous lymphocytes. Br J Haematol. 1993;85:487–491. doi: 10.1111/j.1365-2141.1993.tb03337.x. [DOI] [PubMed] [Google Scholar]
- 36.Parry-Jones N, Matutes E, Gruszka-Westwood AM, et al. Prognostic features of splenic lymphoma with villous lymphocytes: a report on 129 patients. Br J Haematol. 2003;120:759–764. doi: 10.1046/j.1365-2141.2003.04165.x. [DOI] [PubMed] [Google Scholar]
- 37.Paternoster SF, Brockman SR, McClure RF, et al. A new method to extract nuclei from paraffin-embedded tissue to study lymphomas using interphase fluorescence in situ hybridization. Am J Pathol. 2002;160:1967–1972. doi: 10.1016/S0002-9440(10)61146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peters G. The D-type cyclins and their role in tumorigenesis. J Cell Sci Suppl. 1994;18:89–96. doi: 10.1242/jcs.1994.supplement_18.13. [DOI] [PubMed] [Google Scholar]
- 39.Quintanilla-Martinez L, Davies-Hill T, Fend F, et al. Sequestration of p27Kip1 protein by cyclin D1 in typical and blastic variants of mantle cell lymphoma (MCL): implications for pathogenesis. Blood. 2003;101:3181–3187. doi: 10.1182/blood-2002-01-0263. [DOI] [PubMed] [Google Scholar]
- 40.Quintanilla-Martinez L, Thieblemont C, Fend F, et al. Mantle cell lymphomas lack expression of p27Kip1, a cyclin-dependent kinase inhibitor. Am J Pathol. 1998;153:175–182. doi: 10.1016/S0002-9440(10)65558-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Remstein ED, Dogan A, Einerson RR, et al. The incidence and anatomic site specificity of chromosomal translocations in primary extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) in North America. Am J Surg Pathol. 2006;30:1546–1553. doi: 10.1097/01.pas.0000213275.60962.2a. [DOI] [PubMed] [Google Scholar]
- 42.Remstein ED, Law M, Mollejo M, et al. The prevalence of IG translocations and 7q32 deletions in splenic marginal zone lymphoma. Leukemia. 2007 doi: 10.1038/sj.leu.2405027. [DOI] [PubMed] [Google Scholar]
- 43.Rosenberg CL, Wong E, Petty EM, et al. PRAD1, a candidate BCL1 oncogene: mapping and expression in centrocytic lymphoma. Proc Natl Acad Sci U S A. 1991;88:9638–9642. doi: 10.1073/pnas.88.21.9638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Santiago F, Clark E, Chong S, et al. Transcriptional up-regulation of the cyclin D2 gene and acquisition of new cyclin-dependent kinase partners in human T-cell leukemia virus type 1-infected cells. J Virol. 1999;73:9917–9927. doi: 10.1128/jvi.73.12.9917-9927.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swerdlow SH, Zukerberg LR, Yang WI, et al. The morphologic spectrum of non-Hodgkin’s lymphomas with BCL1/cyclin D1 gene rearrangements. Am J Surg Pathol. 1996;20:627–640. doi: 10.1097/00000478-199605000-00009. [DOI] [PubMed] [Google Scholar]
- 46.Teramoto N, Pokrovskaja K, Szekely L, et al. Expression of cyclin D2 and D3 in lymphoid lesions. Int J Cancer. 1999;81:543–550. doi: 10.1002/(sici)1097-0215(19990517)81:4<543::aid-ijc7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 47.Traverse-Glehen A, Baseggio L, Bauchu EC, et al. Splenic red pulp lymphoma with numerous basophilic villous lymphocytes: a distinct clinicopathologic and molecular entity? Blood. 2008;111:2253–2260. doi: 10.1182/blood-2007-07-098848. [DOI] [PubMed] [Google Scholar]
- 48.Troussard X, Cornet E. Outline for writing an article for current treatment options in oncology: splenic lymphoma with villous lymphocytes. Curr Treat Options Oncol. 2007;8:97–108. doi: 10.1007/s11864-007-0015-3. [DOI] [PubMed] [Google Scholar]
- 49.Vandenberghe E, De Wolf-Peeters C, van den Oord J, et al. Translocation (11;14): a cytogenetic anomaly associated with B-cell lymphomas of non-follicle centre cell lineage. J Pathol. 1991;163:13–18. doi: 10.1002/path.1711630104. [DOI] [PubMed] [Google Scholar]
- 50.Wahbi K, Hayette S, Callanan M, et al. Involvement of a human endogenous retroviral sequence (THE-7) in a t(7;14)(q21;q32) chromosomal translocation associated with a B cell chronic lymphocytic leukemia. Leukemia. 1997;11:1214–1219. doi: 10.1038/sj.leu.2400716. [DOI] [PubMed] [Google Scholar]
- 51.Williams ME, Swerdlow SH, Rosenberg CL, et al. Chromosome 11 translocation breakpoints at the PRAD1/cyclin D1 gene locus in centrocytic lymphoma. Leukemia. 1993;7:241–245. [PubMed] [Google Scholar]
- 52.Williams ME, Westermann CD, Swerdlow SH. Genotypic characterization of centrocytic lymphoma: frequent rearrangement of the chromosome 11 bcl-1 locus. Blood. 1990;76:1387–1391. [PubMed] [Google Scholar]
- 53.Wlodarska I, Dierickx D, Vanhentenrijk V, et al. Translocations targeting CCND2, CCND3, and MYCN do occur in t(11;14)-negative mantle cell lymphomas. Blood. 2008;111:5683–5690. doi: 10.1182/blood-2007-10-118794. [DOI] [PubMed] [Google Scholar]
- 54.Yatabe Y, Suzuki R, Matsuno Y, et al. Morphological spectrum of cyclin D1-positive mantle cell lymphoma: study of 168 cases. Pathol Int. 2001;51:747–761. doi: 10.1046/j.1440-1827.2001.01277.x. [DOI] [PubMed] [Google Scholar]


