Abstract
Flow cytometers designed to analyze large particles are enabling new applications in biology and chemistry. Likewise, flow spectroscopy approaches are extending the capabilities of the flow cytometry platform. Here we report on the adaptation of a commercial large particle analyzer to measure fluorescence and Raman spectra of individual particles at high speeds. We modified a Union Biometrica COPAS Plus instrument to allow red excitation and optical fiber-based light collection and spectral analysis using a spectrograph and CCD array detector. These modifications did not compromise the ability of the instrument to resolve different sized particles based on their extinction and time of flight signals. The modified instrument has the sensitivity and spectral resolution to measure the fluorescence and Raman signals from individual particles with signal integration times of 10 usec. The high speed spectral analysis of individual particles in flow will enable new applications in biological and chemical analyses.
Keywords: multiplex analysis, multiparameter, SERS, flow cytometry, large particles
Introduction
In many respects, flow cytometry bears the hallmarks of a mature technology, including multiple vendors of instruments, software and reagents, as well as standards to promote exchangeability of data. In other respects, flow cytometry is a dynamic technology that is undergoing constant evolution to meet the analytical challenges of modern biomedical research. One aspect of this evolution is the adaptation of flow cytometry to measure large particles approaching a millimeter in size. These include whole organisms such as C. elegans(1,2), multicellular structures such as pancreatic islets(3,4), and polymer resins bearing combinatorial libraries(5,6). Custom and commercial instruments(7,8,9,10) have been developed to meet these challenges, and have opened up flow cytometry to new communities of biological researchers. A second area of evolution for flow cytometry is in the area of spectral analysis. The traditional flow cytometric approach to spectral resolution using dichroic mirrors and bandpass filters is generally acceptable for measuring the total intensities of common fluorophores that have broad emission spectra. However, the desire to measure optical features of cells with higher spectral resolution has spurred several recent efforts to collect complete spectra from individual particles in flow(11,12,13). We have been interested in exploiting the relatively high information content of Raman scattering to expand the capabilities for highly multiparameter and multiplexed measurements using flow cytometry(13). Here we report the modification of a commercial large particle flow cytometer to enable the measurement of fluorescence and surface enhanced Raman scattering (SERS) spectra from single large particles in flow.
Methods
Instrumentation
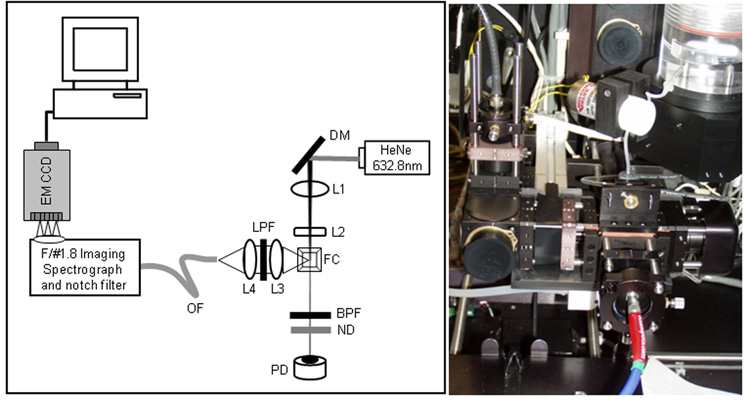
The Large Particle Spectral Flow Cytometer is based on a commercial platform, the Union Biometrica COPAS Plus large particle sorter. The standard COPAS PLUS system measures three channels of fluorescence excited by either blue (488 nm) or green (514 nm) excitation selected from an Argon laser using laser line filters, with detection triggered via a forward angle extinction signal generated as a particle passes through a collinear laser beam from a 670 nm diode laser. To adapt this system to make spectral measurements, we replaced the stock 1mm square flow cuvette with a 500 um square cuvette, and fabricated a custom screw adjustment plate with an aperture to allow us to collect light from the open side of the COPAS flow cuvette. Excitation was provided by a fiber-coupled HeNe laser beam (17 mW at 633 nm) focused through a long focal length spherical lens (f=80mm) and short focal length cylindrical lens (f=6mm) resulting in an elliptical beam approximately 40 um high by 400 um wide. The COPAS system employs an extinction measurement in the forward angle direction to estimate particle size, and we inserted a 0.8 OD ND filter before the detector to attenuate the light from the HeNe laser. A light collection assembly composed of high numerical aperture aspherical lenses was constructed to collect light from the open side of the flow cell perpendicular to the excitation beam and focus it into an optical fiber (Figure 1). Optical cage system components (Linos) were used to position a 21 mm aspheric lens to collect and collimate light from the particle in the flow cell through a 640 long pass filter and an identical 21 mm lens was used to focus the collected light into a 600 um ID multimode fiber (NA = 0.32, ThorLabs). The optical fiber is coupled to a Kaiser f/1.8 HoloSpec imaging spectrograph, which disperses the collected light and images it onto a back-illuminated EMCCD detector (Newton, Andor) with 16 um pixels configured in a 200×1600 pixel array. The spectrograph was calibrated using an Hg-Ne calibration lamp (Newport). For some experiments, the light from the optical fiber was focused onto a photomultiplier tube (Hamamatsu R3896) through a 640 nm long pass filter (Chroma), and the PMT output converted to voltage and processed by the COPAS data system. The detector is triggered by the extinction signal from the COPAS, and the CCD integration time was matched to the extinction pulse widths (typically 10 usec). The pixels were binned on chip to a single vertical pixel and 100 horizontal pixels (16 × binning). Sheath and sample delivery are provided by pressure-driven flow. Sheath and sample volumetric flow rates were approximately 60 and 2 ml/min, respectively resulting in linear particle flow rates of ~4 m/sec and particle transit times of ~10 usec or longer through the 40 um high laser beam, depending on the size of the particle.
Figure 1. Large Particle Spectral Flow Cytometer.
Schematic (right) and photo (left) of the spectral detection leg constructed on the open face of the COPAS flow cell. DM: dichroic mirror; L1: 80 mm spherical lens; L2: 6 mm cylindrical lens; FC: flow cell; L3, L4: 21 mm aspherical lenses; OF: optical fiber; ND: 0.8 OD neutral density filter.
Nano- and micro-particles
Biotinylated, silica encapsulated nanoparticle aggregates of silver colloids with adsorbed Raman scatterers were prepared as described(13,14). The bulk SERS spectra of the biotinylated nanoparticles were measured using a cuvette holder (Ocean Optics) with the same HeNe laser, spectrograph, and CCD used for the flow cytometry measurements. Polymer microspheres (polystyrene/divinylbenzene (~1×105, 55 um diameter, Spherotech) were preswelled in MeOH, for one hour followed by staining with oxazine170 perchlorate (2–10 uM, Aldrich) in MeOH for 1 hour, followed by dilution in PBS/0.02% Tween20) and extensive washing by several rounds of centrifugation and resuspension. To prepare surface-labeled labeled microspheres, the same polymer microparticles (~1×105) were washed twice in PBS and incubated with 100 ug of avidin (Sigma) in a total volume of 1 ml, and tumbled in the cold for 4 hours. The beads were washed twice before labeling of aliquots with biotin-coated SERS nanoparticles (NPs).
Data analysis
Spectral data, typically spectra from 1000 events per sample, was converted to ASCI text format using the Andor Solis software. Spectra from individual particles were parsed, and saved in both ASCI and Excel2007 formats. Flow cytometry data (five parameters consisting of pulse area for three channels of fluorescence plus extinction area and pulse width (time of flight) were saved as text. Flow cytometry data were analyzed using FCS Express (de Novo Software) and spectral data were analyzed using Excel and SigmaPlot (Jandel Scientific).
Results and Discussion
COPS Plus Modifcations
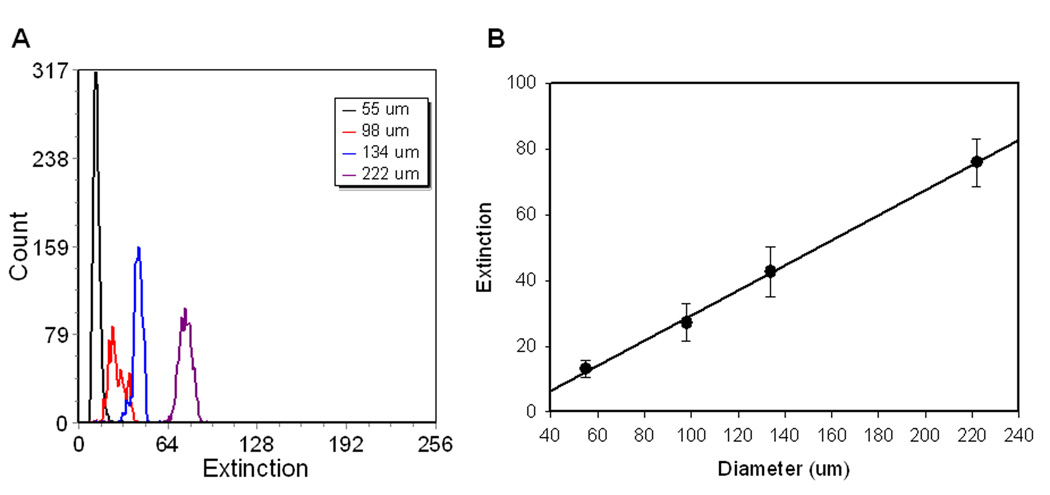
One of the applications envisioned for Raman flow cytometry involves the preparation and screening of combinatorial libraries prepared on encoded polymeric beads(15,16). For this reason we chose the Union Biometrica COPAS large particle sorter as a platform for the implementation of Raman detection capabilities. The standard COPAS features a wide inner dimension (500 um or 1000 um) square cuvette in which sample is hydrodynamically focused by pressure-driven sheath flow. An argon ion laser provides 488 nm or 514 nm (~10mW) excitation (selected by line pass filters). Dichroic mirrors and bandpass filters select green, orange, and red fluorescence for detection on PMTs. A red diode laser (670 nm, ~1 mW) provides a co-linear illumination beam for the estimation of particle size by measurement of extinction on a photodiode detector placed in the forward direction. As described above, a new detection leg (Figure 1) was constructed on the open face of the COPAS flow cell, opposite the fluorescence detection path. A red laser (633 nm) was used for both the extinction measurement and excitation of fluorescence or SERS. The extinction measurement retained its sensitivity to particle diameter (Figure 2) and this signal was used to trigger the detection of spectra from individual particles.
Figure 2. Particle size resolution by extinction.
A) One parameter histograms of extinction for particles of 55 um, 98 um, 134 um, and 222 um diameter measured with the Raman Flow Cytometer. B) Dependence of the extinction signal on particle diameter. Error bars are the standard deviation of the distributions.
Fluorescence spectral measurements
To assess the performance of EMCCD-based detection relative to a conventional PMT measurement, we analyzed polymer beads stained with a red fluorescent dye, oxazine170 (Figure 3A). Presented in Figure 3B is the average spectrum of 250 beads, plus and minus one standard deviation. The leading edge of the spectrum is truncated by the notch and bandpass filters in the spectral detection path, but the majority of the fluorescence emission is collected. Comparison of fluorescence intensity frequency distributions between beads measured with the spectrograph and CCD (Figure 3C) and beads measured with long pass filters and a PMT (Figure 3D) showed improved resolution of stained beads from background autofluorescence for spectral-based detection, likely due to the improved quantum efficiency of this detector (~90–95%) compared to the PMT (~10–15%) in this wavelength range.
Figure 3. Fluorescence spectral measurements.
A) Fluorescence spectra of oxazine170. B) Average spectra of ~200 stained microspheres (black lines), +/− 1 SD (grey lines). C) Frequency histograms of integrated emission from CCD-based spectral measurements of fluorescent (open bars) and blank (filled bars) beads. D. Frequency histograms from PMT-based measurements of fluorescent (open) and blank (filled) beads.
Measurement of SERS signals from individual microspheres
To test our ability to measure Raman spectra, we labeled avidin-coated microspheres (55 um) with biotinylated SERS nanoparticles that we have previously described. Presented in Figure 4 are representative spectra from individual microspheres labeled with Ag-oxazine170 nanoparticles and Ag-rhodamine800 nanoparticles, along with the average spectra of >100 microspheres, demonstrating our ability to resolve these two spectrally-distinct tags. The staining of these particles is more heterogeneous (CV of than the relatively uniformly stained fluorescent microspheres, a reflection of as yet unoptimized reagents and staining conditions. The integration times for these measurements were more than ten times faster compared to our previously reported measurements on a custom Raman flow cytometer(13), and demonstrate our ability to make these measurements at speeds comparable to conventional flow cytometry measurements. The overall analysis rate, however, is still determined by the readout speed of the CCD detector, and is limited to 100–200 events per second.
Figure 4. Raman spectral measurements.
Representative spectra from single beads (10 usec integration times) stained with Ag-oxazine170 (A) and Ag-rhodamine800 (B) SERS nanoparticles and the respective average spectra (C, D) from 100–200 individual bead measurements.
Conclusions
The analysis of whole organisms(1,2), assemblies of cells(3,4), and solid phase chemistry resins(5,6) represent emerging applications for flow cytometry and are driving instrument developments. Spectral measurements of fluorescence and Raman labels are also providing new possibilities for multiplexed and multiparameter flow cytometry. In this paper, we describe the modification of a commercial large particle analyzer to enable spectral measurements of fluorescence and Raman labels. The relatively open architecture of the COPAS Plus allowed us to construct a new detection leg opposite the existing PMT-based fluorescence detection leg of the instrument, and the same forward angle extinction measurement that triggers the PMT-based detection was used to trigger data acquisition by a high sensitivity CCD detector. This configuration allows us to acquire complete spectral information from individual particles as they flow through the probe volume. For beads labeled with the red-excited fluorophore Oxazine170, the combination of spectrograph and CCD-based detection provided superior signal to noise compared to PMT-based detection. The spectral resolution of the system is sufficient to allow beads labeled with different SERS nanoparticles to be distinguished based on their Raman spectra. We expect that the addition of spectral analysis capabilities to fluorescence flow cytometers will enable new approaches to biological and chemical analysis.
Acknowledgements
Grant Sponsor: NIH; Grant number R01 EB003824
LITERATURE CITED
- 1.Couillault C, Pujol N, Reboul J, Sabatier L, Guichou JF, Kohara Y, Ewbank JJ. tlr-independent control of innate immunity in caenorhabditis elegans by the tir domain adaptor protein tir-1, an ortholog of human sarm. Nat Immunol. 2004;5:488–494. doi: 10.1038/ni1060. [DOI] [PubMed] [Google Scholar]
- 2.Hertweck M, Baumeister R. automated assays to study longevity in c. elegans. Mech Ageing Dev. 2005;126:139–145. doi: 10.1016/j.mad.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez LA, Hatch EW, Armann B, Odorico JS, Hullett DA, Sollinger HW, Hanson MS. validation of large particle flow cytometry for the analysis and sorting of intact pancreatic islets. Transplantation. 2005;80:729–737. doi: 10.1097/01.tp.0000179105.95770.cd. [DOI] [PubMed] [Google Scholar]
- 4.Tjernberg J, Ekdahl KN, Lambris JD, Korsgren O, Nilsson B. acute antibody-mediated complement activation mediates lysis of pancreatic islets cells and may cause tissue loss in clinical islet transplantation. Transplantation. 2008;85:1193–1199. doi: 10.1097/TP.0b013e31816b22f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dixon S, Ziebart KT, He Z, Jeddeloh M, Yoo CL, Wang X, Lehman A, Lam KS, Toney MD, Kurth MJ. aminodeoxychorismate synthase inhibitors from one-bead one-compound combinatorial libraries: "staged" inhibitor design. J Med Chem. 2006;49:7413–7426. doi: 10.1021/jm0609869. [DOI] [PubMed] [Google Scholar]
- 6.Tornoe CW, Sanderson SJ, Mottram JC, Coombs GH, Meldal M. combinatorial library of peptidotriazoles: identification of [1,2,3]-triazole inhibitors against a recombinant leishmania mexicana cysteine protease. Journal of Combinatorial Chemistry. 2004;6:312–324. doi: 10.1021/cc020085v. [DOI] [PubMed] [Google Scholar]
- 7.Freyer JP, Wilder ME, Jett JH. viable sorting of intact multicellular spheroids by flow cytometry. Cytometry. 1987;8:427–436. doi: 10.1002/cyto.990080413. [DOI] [PubMed] [Google Scholar]
- 8.Harkins KR, Galbraith DW. factors governing the flow cytometric analysis and sorting of large biological particles. Cytometry. 1987;8:60–70. doi: 10.1002/cyto.990080110. [DOI] [PubMed] [Google Scholar]
- 9.Jett JH, Alexander RG. droplet sorting of large particles. Cytometry. 1985;6:484–486. doi: 10.1002/cyto.990060514. [DOI] [PubMed] [Google Scholar]
- 10.Rohde CB, Zeng F, Gonzalez-Rubio R, Angel M, Yanik MF. microfluidic system for on-chip high-throughput whole-animal sorting and screening at subcellular resolution. Proc Natl Acad Sci U S A. 2007;104:13891–13895. doi: 10.1073/pnas.0706513104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goddard G, Martin JC, Naivar M, Goodwin PM, Graves SW, Habbersett R, Nolan JP, Jett JH. single particle high resolution spectral analysis flow cytometry. Cytometry A. 2006;69A:842–851. doi: 10.1002/cyto.a.20320. [DOI] [PubMed] [Google Scholar]
- 12.Robinson JP, Rajwa B, Gergori G, Jones J, Patsekin V. multispectral cytometry of single bio-particles using a 32 channel pmt. Proc SPIE-Int Soc Opt Eng. 2005;5692:359–365. [Google Scholar]
- 13.Watson DA, Brown LO, Gaskill DF, Naivar M, Graves SW, Doorn SK, Nolan JP. a flow cytometer for the measurement of raman spectra. Cytometry A. 2008;73A:119–128. doi: 10.1002/cyto.a.20520. [DOI] [PubMed] [Google Scholar]
- 14.Brown LO, Doorn SK. a controlled and reproducible pathway to dye-tagged, encapsulated silver nanoparticles as substrates for sers multiplexing. Langmuir. 2008;24:2277–2280. doi: 10.1021/la703853e. [DOI] [PubMed] [Google Scholar]
- 15.Fenniri H, Terreau O, Chun S, Oh SJ, Finney WF, Morris MD. classification of spectroscopically encoded resins by raman mapping and infrared hyperspectral imaging. J Comb Chem. 2006;8:192–198. doi: 10.1021/cc050128i. [DOI] [PubMed] [Google Scholar]
- 16.Fenniri H, Chun S, Terreau O, Bravo-Vasquez JP. preparation and infrared/raman classification of 630 spectroscopically encoded styrene copolymers. J Comb Chem. 2008;10:31–36. doi: 10.1021/cc7001292. [DOI] [PubMed] [Google Scholar]