Abstract
Astrocytes can exocytotically release the transmitter glutamate. Increased cytosolic Ca2+ concentration is necessary and sufficient in this process. The source of Ca2+ for the Ca2+-dependent exocytotic release of glutamate from astrocytes predominately comes from endoplasmic reticulum (ER) stores with contributions from both inositol 1,4,5 trisphosphate- and ryanodine/caffeine- sensitive stores. An additional source of Ca2+ comes from the extracellular space via store-operated Ca2+ entry due to the depletion of ER stores. Here transient receptor potential canonical type 1 containing channels permit entry of Ca2+ to the cytosol, which can then be transported by the store-specific Ca2+-ATPase to (re)fill ER. Mitochondria can modulate cytosolic Ca2+ levels by affecting two aspects of the cytosolic Ca2+ kinetics in astrocytes. They play a role in immediate sequestration of Ca2+ during the cytosolic Ca2+ increase in stimulated astrocytes as a result of Ca2+ entry into the cytosol from ER stores and/or extracellular space. As cytosolic Ca2+declines due to activity of pumps, such as the smooth ER Ca2+-ATPase, free Ca2+ is slowly released by mitochondria into cytosol. Taken together, the trinity of Ca2+ sources, ER, extracellular space and mitochondria, can vary concentration of cytosolic Ca2+ which in turn can modulate Ca2+- dependent vesicular glutamate release from astrocytes. An understanding of how these Ca2+ sources contribute to glutamate release in (patho)physiology of astrocytes will provide information on astrocytic functions in health and disease and may also open opportunities for medical intervention.
Keywords: astrocytes, glutamate release, exocytosis, Ca2+ ER stores, SOC entry, TRPC1, mitochondria
A developing body of evidence implicates astrocytes as active participants in multi-directional signaling in the central nervous system (CNS) [reviewed in (Haydon and Carmignoto, 2006; Theodosis et al., 2008)]. Astrocytes communicate with neurons (Nedergaard, 1994; Parpura et al., 1994), endothelial cells of the cerebrovasculature (Zonta et al., 2003; Mulligan and MacVicar, 2004), and microglia (Davalos et al., 2005). They appear to be an integral part of neural communication throughout the CNS. For example, in the hippocampus, astrocytes integrate neuronal signals (Perea and Araque, 2005), potentiate transmitter release at single synapses (Perea and Araque, 2007), enhance long-term potentiation (Pascual et al., 2005), and synchronize neuronal activity (Fellin et al., 2004). In the thalamus, astrocytes have been shown to drive neuronal activity as a consequence of astrocytic intrinsic Ca2+ oscillations (Parri et al., 2001). In the supraoptic nucleus of the hypothalamus, morphological plasticity of astrocytes (Hatton, 2004) leading to differential synaptic coverage by astrocytic processes can modulate the activity of the output neurons (Panatier et al., 2006).
The abundance of astrocytes and their proximity to neurons enables their communication with neurons. Early studies indicated the numerical preponderance of glial cells in the CNS. For example, in layers I and IV of the rat cerebral cortex, glial cells consisting of astrocytes and oligodendrocytes, outnumbered neurons 3 to 1 (Bass et al., 1971). As more sophisticated tools for cell counting emerged, it appears, however, that the glia to neuron ratio in human cortex is ~ 1.65:1, while in rodent this ratio is about 0.3:1 (Nedergaard et al., 2003; Sherwood et al., 2006). Astrocytes occupy distinct domains within the CNS, only at the cells’ peripheries do their processes overlap (Bushong et al., 2002; Ogata and Kosaka, 2002). Within their individual domains astrocytes have extensive morphological interactions with neurons. For example, in the cortex of adult mice, one astrocyte may contact 4 to 8 neurons and surround ~300 to 600 neuronal dendrites (Halassa et al., 2007). In the hippocampus, astrocytes are positioned to contact even more synapses. In adult rats, one astrocyte is estimated to contact ~140,000 synapses of CA1 pyramidal cells (Bushong et al., 2002). Classically, the intimate position of astrocytic processes around the neurons and the synapses has been solely attributed to glutamate uptake and regulation of extracellular K+ by astrocytes. To provide these services, astrocytes possess excitatory amino acid transporters (EAAT’s) (Huang and Bergles, 2004; Tzingounis and Wadiche, 2007; Yang and Rothstein, 2009) and K+ inward rectifying (Kir) channels (Sontheimer et al., 1994; Kofuji and Newman, 2009), respectively. However, it is also at synapses where astrocytes have been shown to have modulatory effects on the activities of presynaptic and postsynaptic neurons by astrocytic ability to release a variety of transmitters using many different mechanisms [reviewed in (Malarkey and Parpura, 2009)]. This led to the concept of a functional tripartite synapse (Araque et al., 1999), a topic that has been recently reviewed elsewhere (Ni et al., 2007; Halassa and Haydon, 2009).
In this review we focus on the Ca2+ sources for the exocytotic release of the excitatory transmitter glutamate from astrocytes, which can readily occur under physiological conditions (Parpura and Haydon, 2000). We start with a historical brief on the Ca2+-dependent glutamate release from astrocytes [reviewed in detail in (Montana et al., 2004)]. We then discuss the trinity of Ca2+ sources for this release: intracellular stores, extracellular space and mitochondria. An understanding of how these Ca2+ sources contribute to the glutamate release will provide information on glial functions in heath and disease and may also introduce opportunities for medical intervention.
Ca2+-dependent glutamate release in astrocytes: a historical brief
Astrocytes can couple their inherent intracellular Ca2+ excitability to the exocytotic release of transmitters including but not limited to glutamate (Parpura et al., 1994). Throughout this review we interchangeably use Ca2+ -dependent and exocytotic attributes of glutamate release from astrocytes. Excitation-secretion coupling in astrocytes involves the molecular machinery for vesicular fusion, including the core members of the soluble N-ethyl maleimide-sensitive fusion protein attachment protein receptor (SNARE) complex, as well as proteins responsible for packaging of glutamate inside vesicles, vacuolar type proton ATPase and vesicular glutamate transporters [reviewed in (Montana et al., 2006; Malarkey and Parpura, 2008)].
Evidence for Ca2+-dependent release of glutamate from astrocytes was originally demonstrated in experiments where the application of the Ca2+ ionophore ionomycin stimulated the release of glutamate from astrocytes in the presence of external free Ca2+ (2.4 mM). However, this ionophore failed to cause glutamate release when applied to astrocytes that were equilibrated in solution with low external free Ca2+ (24 nM), for 40–60 minutes, thus causing the depletion of internal Ca2+ stores and preventing the Ca2+ entry from the extracellular space (Parpura et al., 1994). These data indicate that Ca2+ is sufficient and necessary to cause glutamate release from astrocytes. Indeed, depleting internal Ca2+ stores by application of thapsigargin, a blocker of the sarco-endoplasmic reticulum Ca2+-ATPase (SERCA), or buffering cytoplasmic Ca2+ with the membrane permeable Ca2+ chelator, 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid acetoxymethyl ester (BAPTA-AM), resulted in reduction of glutamate release (Araque et al., 1998a; Bezzi et al., 1998; Innocenti et al., 2000; Hua et al., 2004; Montana et al., 2004). Furthermore, alternative stimuli that directly increased astrocytic intracellular Ca2+ concentration([Ca2+]i), such as mechanical stimulation (Parpura et al., 1994; Araque et al., 1998a; Araque et al., 1998b; Hua et al., 2004; Montana et al., 2004), photostimulation (Parpura et al., 1994), and photolysis of Ca2+ cages (Araque et al., 1998b; Parpura and Haydon, 2000), all caused release of glutamate.
The trinity of Ca2+ sources
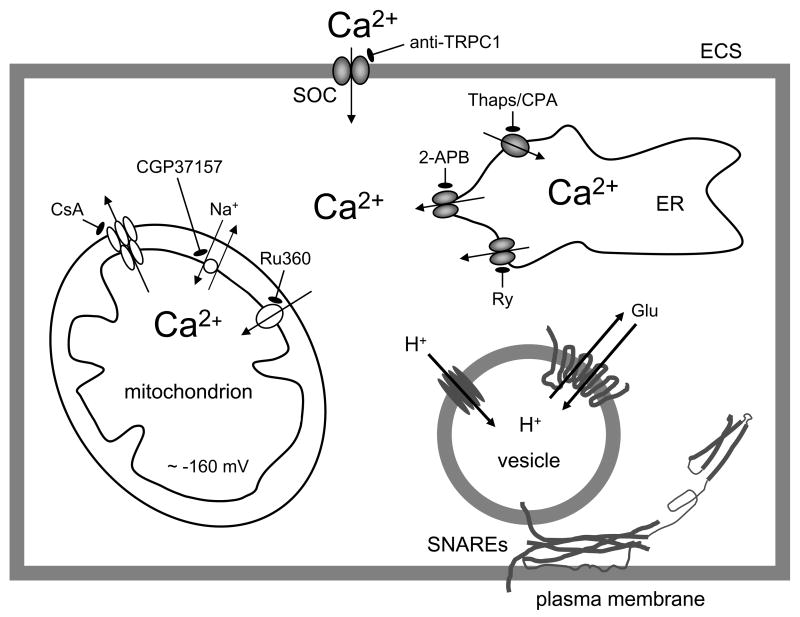
The source of Ca2+ for Ca2+-dependent release of glutamate from astrocytes is tripartite, from (i) internal ER stores, (ii) extracellular space and (iii) mitochondria (Figure 1).
Figure 1.
Tripartite Ca2+cyt handling in Ca2+- dependent vesicular glutamate release from astrocytes. A drawing of sources of Ca2+ for [Ca2+]cyt increase. [Ca2+]cyt can be affected by the endoplasmic reticulum (ER), store-operated Ca2+ (SOC) entry and mitochondria. Ca2+cyt accumulation could be caused by the entry of Ca2+ from the internal stores of the ER that possess IP3 and ryanodine receptors. The pre-incubation of astrocytes with ryanodine (Ry) can block Ca2+ release from ryanodine/caffeine-sensitve ER stores, while diphenylboric acid 2-aminoethyl ester (2-APB), an IP3R antagonist, reduces Ca2+ release from an IP3-senistive stores. Thapsigargin (Thaps) and cyclopiazonic acid (CPA), blockers of store specific Ca2+-ATPase, prevent filling of these stores by Ca2+ and, thus, deplete stores of this ion. Ultimately, the (re)filling of ER Ca2+ stores requires Ca2+ entry from the extracellular space (ECS) through SOC channels, in particular those containing canonical type 1 transient receptor potential (TRPC1) protein. A specific TRPC1 antibody blocks TRPC1 containing SOC channels and reduces Ca2+ entry to the cytosol. A negative membrane potential (about −160 mV) exists across the inner mitochondrial membrane. Mitochondrial Ca2+ uptake is mediated by the uniporter as the electropotential gradient drives Ca2+ into the matrix. Ru360 blocks Ca2+ influx through the uniporter. Free Ca2+ exits the mitochondrial matrix through the Na+/Ca2+ exchanger, and this process can be blocked by CGP37157. Ca2+ accumulation in mitochondria is increased by cyclosporin A (CsA) via preventing formation of the mitochondrial permeability transition pore, which releases Ca2+ and other components of the mitochondrial matrix at high Ca2+ loads. Increase of Ca2+cyt is sufficient and necessary to cause vesicular fusions and release of glutamate, which requires action by proteins of the ternary SNARE complex and their associated proteins (not shown). Additionally, vesicles possess the vacuolar type H+-ATPase which creates the proton gradient used by vesicular glutamate transporters to fill these organelles with glutamate (Glu). Drawing is not to scale.
(i) Inositol 1,4,5 trisphosphate (IP3)- and ryanodine/caffeine-sensitive ER stores
The ER is the major source of intracellular Ca2+, and it appears to be the main determinant of Ca2+ excitability in astrocytes (Verkhratsky, 2006; Deitmer et al., 2008). The concentration of free Ca2+ inside the ER ([Ca2+]ER) ranges from 100 to 800 μM (Burdakov et al., 2005) while the basal concentration of this ion in cytosol ([Ca2+]cyt) is ~100 nM. Ca2+ can be released from the ER through IP3 receptors (IP3R) and Ca2+ -induced Ca2+ release (CICR) by activation of caffeine/ryanodine sensitive receptors (RyR) (Simpson et al., 1998). These two ER receptor/channel types may gate distinct Ca2+ stores (Golovina and Blaustein, 2000), although this notion has not been widely accepted [e.g., (Hua et al., 2004); see below]. The [Ca2+]ER can affect the sensitivity of the RyRs to cytosolic Ca2+ as well as the activity of the SERCA pumps (Burdakov et al., 2005). Thus, at the level of the ER, there is regulation of Ca2+ release and entry.
The initial, but indirect indication that ER stores play role in Ca2+-dependent release of glutamate from astrocytes came from experiments in which the neuroligand bradykinin which caused cytosolic Ca2+ elevation in astrocytes also induced glutamate release from these glial cells (Parpura et al., 1994). Bradykinin binds to G-protein coupled plasma membrane receptors which activate the IP3 pathway, and cause release of Ca2+ from the ER (Cholewinski et al., 1988; Stephens et al., 1993). In addition to bradykinin, other ligands including ATP can induce Ca2+ release from the ER (Peuchen et al., 1996) through the IP3 pathway (Kastritsis et al., 1992). Indeed, ATP stimulation of purinergic 2 receptors caused an increase in astrocytic cytosolic Ca2+ which coincided with an increase in glutamate release from cultured astrocytes to the extracellular space (Jeremic et al., 2001). This ATP action was greatly reduced when astrocytes were pre-incubated with thapsigargin, implicating the role of ER stores in providing cytosolic Ca2+ for exocytotic glutamate release.
Hua et al. (2004) demonstrated that both IP3- sensitive and ryanodine/caffeine-sensitive stores play a role in Ca2+ -dependent mechanically-induced glutamate release from astrocytes. The Ca2+ source for this exocytotic release is predominately from internal stores, as indicated by reduction of mechanically-induced glutamate release in the presence of thapsigargin. To test whether IP3- sensitive internal stores mediate Ca2+-dependent glutamate release from astrocytes, these cells were bathed in diphenylboric acid 2-aminoethyl ester (2-APB) solution, a cell-permeant IP3R antagonist. This agent greatly reduced exocytotic glutamate release. Next, the role of ryanodine/caffeine-sensitive ER stores was assessed by incubating astrocytes with ryanodine, which at concentrations used (10 μM) blocked the release of Ca2+ from the ryanodine/caffeine- sensitive stores. Ryanodine also attenuated mechanically -induced glutamate release. Furthermore, the sustained presence of caffeine that depleted ryanodine/caffeine stores, also reduced mechanically -induced glutamate release. It should be noted, however, that the functionality of ryanodine receptors in astrocytes is still debated, since the lack of their activity in astrocytes in situ had been reported (Beck et al., 2004). Nonetheless, when astrocytes in culture were pre-treated with the combination of 2-APB with ryanodine or caffeine, there was no additive effect on reduction of mechanically-induced glutamate release when compared to treatments with one pharmacological agent only. Since all pharmacological agents used in this study showed cytosolic Ca2+ changes that were in good agreement with glutamate release, these data suggested that exocytotic glutamate release requires the co-activation of both IP3- and ryanodine/caffeine-sensitive internal Ca2+ stores and that these stores are operating jointly (Figure 1).
(ii) Extracellular space
Astrocytes utilize extracellular Ca2+ as well as ER Ca2+ for exocytotic glutamate release (Figure 1). This is evidenced by the reduction of Ca2+-dependent glutamate release from astrocytes not only in the presence of thapsigargin, but also in the presence of extracellular Cd2+, a blocker of Ca2+ entry from the extracellular space (Hua et al., 2004). It should be noted that remaining glutamate release in stimulated astrocytes pre-treated with thapsigargin or Cd2+ were ~30% and 55% of control, respectively, indicating that the internal stores are the predominant source of Ca2+ for exocytotic release.
Ca2+ entry across the astrocytic plasma membrane involves a variety of channels [reviewed in (Verkhratsky et al., 1998); see below]. Ultimately, the (re)filling of ER Ca2+ stores requires Ca2+ entry from the extracellular space through so called store-operated Ca2+ (SOC) channels (Takemura and Putney, 1989; Golovina, 2005). Canonical transient receptor potential (TRPC) channels, which have been implicated in SOC entry, have been found in astrocytes where they play a role in the regulation of Ca2+ homeostasis (Pizzo et al., 2001; Grimaldi et al., 2003; Golovina, 2005). TRPC1 functionally contributes to Ca2+-dependent glutamate release from astrocytes. Malarkey et al. (2008) detected the presence of TRPC1 in cultured and freshly-isolated astrocytes, along with two other isoforms with which TRPC1 is known to form heteromultimeric channels, TRPC4 and TRPC5 (Strubing et al., 2001; Hofmann et al., 2002; Strubing et al., 2003). It should be noted that TRPC1 does not commonly form homomeric channels (Strubing et al., 2003). When looking at the function of these proteins, Malarkey et al. (2008) measured the extent of SOC entry in cultured astrocytes. Astrocytic internal Ca2+ stores were depleted by applying cyclopiazonic acid (CPA), which blocks SERCA and (re)filling the stores. Concomitantly, the entry of external Ca2+ was prevented by bathing the astrocytes in a Ca2+ free external saline solution. This depletion of Ca2+ from internal stores led to opening of SOC channels, which was evident by the increased cytosolic Ca2+ when the bathing solution was changed to external solution containing Ca2+. This increase was pharmacologically confirmed to be due to Ca2+ entry through SOC channels using La3+, Gd3+ and MRS-1845, compounds known to inhibit SOC channels (Harper et al., 2003; Rychkov and Barritt, 2007).
In experiments using a similar SOC entry assay to the one described above, Golovina (2005) demonstrated significant reduction of SOC entry in cultured astrocytes after an antisense knockdown of the TRPC1 gene. To address whether the acute block of TRPC1 affects SOC entry, Malarkey et al. (2008) used an antibody against TRPC1 that was designed to bind to the pore forming region of TRPC1 and block the functioning of the channel (Wang et al., 1999). This antibody caused a significant decrease in the measured amount of SOC entry in astrocytes. Such immunological interference with TRPC1 channels also showed that the SOC entry through TRPC1 plays a role in mechanically-induced cytosolic Ca2+ elevations and the consequent Ca2+-dependent release of glutamate from astrocytes (Figure 1). The reduction of glutamate release in astrocytes pretreated with antibody was ~ 39% of control, thus at similar level to that seen in astrocytes treated with thapsigargin (Hua et al., 2004).
It is obvious by now that astrocytes are unlike neurons in which the predominant source of Ca2+ for exocytotic release at synaptic terminals is extracellular, mediated via Ca2+ entry though voltage-gated Ca2+ channels (VGCCs). However, VGCCs were found in astrocytes (Latour et al., 2003; Mulligan and MacVicar, 2004). Since the driving force for the for Ca2+ entry into astrocytes is similar to that in neurons with the extracellular Ca2+ concentration ([Ca2+]ext) ~2 mM being four orders greater than [Ca2+]cyt, the inability to detect the activity of VGCCs in hippocampal astrocytes in situ may implicate the low level of expression and/or low plasma membrane density of VGCCs (Carmignoto et al., 1998). Although the role of VGCCs in glutamate release from astrocytes is unclear at present, there is some evidence in support of their involvement in this process. Using freshly prepared slices from ventrobasal thalamus, Parri et al. (2001) showed that astrocytes in situ display intrinsic [Ca2+]cyt oscillations. These oscillations were not driven by neuronal activity since they could not be blocked by tetrodotoxin. Astrocytes in situ displaying spontaneous [Ca2+]cyt oscillations could cause N-methy-D-aspartate (NMDA) receptor-mediated neuronal excitability. Thus astrocytic spontaneous [Ca2+]cyt oscillations lead to glutamate release from these glial cells. Although oscillations where driven by Ca2+ released from intracellular stores since they were blocked by SERCA blockers thapsigargin and CPA, they were also inhibited to the similar extent by the dihydropyridine (DHP) antagonist nifedipine (Parri et al., 2001). This demonstrates the involvement of DHP-sensitive L-type VGCCs in astrocytic cytosolic Ca2+ dynamics. Conversely, the DHP agonist BayK8644 increased the number of astrocytes displaying cytosolic Ca2+ oscillations (Parri and Crunelli, 2003). Additionally, increasing the driving force for Ca2+ entry by increasing [Ca2+]ext (to 5 mM) increased the number of spontaneously active astrocytes and the number of cytosolic Ca2+ transient increases displayed by individual astrocytes (Parri and Crunelli, 2003). Even though the effect of VGCC manipulations on glutamate release from astrocytes of ventrobasal thalamus has not been assessed, it is tempting to speculate that these channels may be a conduit for Ca2+ supply for glutamate release from astrocytes (Figure 1).
Ionotropic neurotransmitter receptors represent an additional entry of Ca2+ in astrocytes [reviewed in (Verkhratsky, 2009)]. The role of ligand-gated ionotropic receptors in exocytotic glutamate release from astrocytes is provisional at the moment, however. Given that astrocytes can respond to glutamatergic and cholinergic synaptic inputs by increases in [Ca2+]cyt (Dani et al., 1992; Porter and McCarthy, 1996; Araque et al., 2002), we briefly discuss nicotinic acetylcholine receptors, and glutamatergic, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and NMDA, receptors on astrocytes. Recent findings indicate that astrocytes express functional nicotinic acetylcholine receptors, particularly those with α7 subunits (Sharma and Vijayaraghavan, 2001). Activation of these receptors on astrocytes can lead to [Ca2+]cyt increases, which require CICR and the activation of ryanodine/caffeine-sensitve internal stores. The dominant glutamatergic ionotropic receptors on astrocytes are AMPA receptors, which are found in astroglial cells throughout the brain (Verkhratsky and Steinhauser, 2000; Seifert and Steinhauser, 2001; Aguirre et al., 2004). Astrocytes in hippocampus express GluR2 subunit and thus display low Ca2+ permeability through their AMPA channels (Seifert et al., 2003). Hence, a challenge of hippocampal astrocytes with AMPA does not cause glutamate release (Bezzi et al., 1998). However, some types of astrocytes do not express the GluR2 subunit. For example, Bergmann glia, an astrocytic cell found in the cerebellum, expresses AMPA receptors that are Ca2+ permeable. These cells ensheathe parallel fiber-Purkinje cell synapses (Grosche et al., 1999). There, electrical stimulation of parallel fibers to release glutamate caused rapid and transient [Ca2+]cyt elevations within Bergmann glial processes (Grosche et al., 1999). Functional astrocytic NMDA receptors were only recently detected in cortical astrocytes (Lalo et al., 2006). Interestingly, astrocytic NMDA channels grossly differ from neuronal since they do not show a Mg2+ block at resting membrane potentials (around −80 mV). Whether activation of these, and other ionotropic receptors expressed by astrocytes leading to increases in [Ca2+]cyt would also lead to Ca2+-dependent glutamate release is unknown at the moment.
Another Ca2+ entry point that needs further investigation is at plasma membrane Na+/Ca2+ exchangers. While they are known to extrude Ca2+ during high cytosolic loads of this cation in conjuction with the plasma membrane Ca2+ -ATPase (PMCA), several reports have shown that the exchanger works in reverse to increase [Ca2+]cyt (Goldman et al., 1994; Benz et al., 2004; Rojas et al., 2007). Hence, release of homeocysteic acid (HCA) from cultured astrocytes can be Ca2+-dependent and mediated by activation of glutamate receptors (Benz et al., 2004). It appears that the agonist stimulation led to intracellular Na+ load through ionotropic glutamate receptors, which then activated Na+/Ca2+ exchangers to extrude Na+ from astrocytes while importing Ca2+ to the cytosol, resulting in a rise of [Ca2+]cyt. This cascade of events was inferred from the observation that agonist-induced HCA release was blocked in the presence of benzamil, a Na+/Ca2+ exchanger blocker. While some have argued that the reverse mode of this exchanger only occurs at pathophysiological conditions, it is worth investigating what this exchanger contributes to physiological Ca2+ entry and consequential exocytoctic glutamate release from astrocytes.
(ii) Mitochondria
In astrocytes, mitochondria form filamentous reticula, and are in perpetual fusion and fission as they are in other cell types (Westermann, 2002). Mitochondria congregate perinuclearly, but can be located at the cell periphery including processes (Collins et al., 2002). To achieve translocation, mitochondria travel on microtubules and actin filaments. While the actin cytoskeleton dictates the distribution of mitochondria, it appears that the intermediate filament vimentin may be involved as well (Tang et al., 2008). Interestingly, in neurons mitochondria display activity-dependent motility at the synapses (Li et al., 2004; Chang et al., 2006; Mironov, 2006).
Mitochondria are efficient Ca2+ sinks during periods when rapid increases in [Ca2+]cyt occur in a cell. They possess a mitochondrial Ca2+ uniporter (MCU) that can transport Ca2+ into the mitochondrial matrix powered by a negative membrane potential (between −140 mV and −180 mV) across the inner mitochondrial membrane generated by the electron transport chain. In contrast to the SERCA and PMCA which have higher affinities for Ca2+ than the MCU, Ca2+ is taken up by MCU only at [Ca2+]cyt greater than ~ 0.5 μM (Miyata et al., 1991; Simpson and Russell, 1998) and dominates Ca2+ transport at ~10 μM and above (Alonso et al., 2006). While such high concentrations are normally not reached in the bulk cytosol, they exist at high Ca2+ loci known as microdomains (Schneggenburger and Neher, 2005; Oheim et al., 2006). Indeed, Rizzuto et al. (1998) showed that mitochondria are juxtaposed to IP3 channels of the ER and are exposed to higher concentrations of Ca2+ than what would exist at the bulk cytosol. Indeed, Ca2+ released from the IP3-sensitve intracellular stores in mucosal mast cells caused local [Ca2+]cyt increases of over 16 μM (Csordas et al., 2006). Thus, it seems reasonable to assume that similar localized [Ca2+]i increases would occur in astrocytes.
It should be noted that as conditions in a cell are not homogenous i.e. ion concentration, mitochondrial Ca2+ handling is not homogenous either. In T lymphocytes, subsets of mitochondria modulates the entry of Ca2+ through the SOC channels by translocation (Hoth et al., 1997; Quintana et al., 2007). This is a likely function of some mitochondria in astrocytes as well. Kolikova et al. (2006) demonstrated using total internal reflection fluorescence microscopy that at least a subset of mitochondria become trapped near the plasma membrane when cultured astrocytes are challenged with glutamate or ATP. Indeed, numerous studies of various cells types report that different subsets of mitochondria take up Ca2+ depending whether stimulated cells released ER Ca2+ or whether they allowed Ca2+ entry from the extracellular space (Simpson and Russell, 1996; Collins et al., 2001).
As sinks to trap free Ca2+ released from the ER, mitochondria are positioned to affect transient Ca2+ increases required for vesicle fusion, and exocytosis in secretory cells. Reyes and Parpura (2008) demonstrated that mitochondria can modulate [Ca2+]cyt and glutamate release in cortical astrocytes challenged with mechanical stimuli. Taking a pharmacological approach, we showed in parallel experiments that blocking the MCU with ruthenium 360 (Ru60) increased the cytosolic Ca2+ accumulation, and glutamate release in cortical astrocytes. In contrast to Ru360 treatment, decreasing mitochondrial Ca2+ efflux by blocking the mitochondrial Na+/Ca2+ exchanger with CGP37157, or increasing mitochondrial Ca2+ load by inhibiting formation of the mitochondrial permeability transition pore with cyclosporin A, decreased cytosolic Ca2+ accumulation, and glutamate release in cortical astrocytes. Hence, these data suggest that the transient increase in [Ca2+]cyt is correlative to the amount of glutamate released in cortical astrocytes, and that mitochondria have the capacity to modulate the degree of this release. Taken together, astrocytic mitochondria may be active modulators of Ca2+-dependent glutamate release from astrocytes. They could act as temporary holding compartments of excess Ca2+ at the fine astrocytic processes surrounding the tripartite synapse, in a similar manner as has previously been shown in neuronal terminals at the neuromuscular junction (Tang and Zucker, 1997).
Concluding Remarks
The purpose of this review was to summarize the Ca2+ sources for exocytotic glutamate release from astrocytes. There are three major Ca2+ sources that contribute to this process: (i) the predominant source from internal ER stores, (ii) the SOC entry from the extracellular space and (iii) mitochondria, as outlined in Figure 1. There are several issues that remain to be resolved. As indicated earlier, the activation of ionotropic receptors expressed by astrocytes can lead to increases in [Ca2+]cyt. Whether this excitation is coupled to the Ca2+-dependent exocytotic glutamate release from these cells awaits further experimentation. Furthermore, it will be necessary to determine whether the same Ca2+ sources operate under physiological and pathological conditions or whether there are specific Ca2+ sources operating under particular conditions. For example, acute oxidative stress in astrocytes causes influx of extracellular Ca2+ through L-type VGCCs (Bond and Greenfield, 2007). Whether this Ca2+ source plays a role in exocytotic glutamate release from astrocytes and whether such release occur in various neurodegenerative diseases causatively linked to oxidative stress remains to be assessed. Alternatively, all possible Ca2+ sources could operate together in astrocytes at all times, but each contributing a different portion to the total amount of glutamate being released. It appears that addressing these questions in cell culture systems depends on application of sufficient human time and effort, since many of the specific reagents and methods, some of which are described earlier, for teasing apart the contributions from individual Ca2+ sources have already been developed. Although such an approach would require a labor intensive effort of information collection and analysis, it is a pre-requisite before taking experiments out of culture dishes and rigorously testing hypotheses on freely-moving animals and humans.
Acknowledgments
We thank Randy F. Stout, Jr. for comments on previous versions of this manuscript. This work is supported by a grant from the National Institute of Mental Health (MH 069791 to VP) and the National Institute of Heath Neuroscience Training Program in Neurobiology of Cognition and Cognitive Disorders at University of Alabama, Birmingham (to RCR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguirre AA, Chittajallu R, Belachew S, Gallo V. NG2-expressing cells in the subventricular zone are type C-like cells and contribute to interneuron generation in the postnatal hippocampus. J Cell Biol. 2004;165:575–589. doi: 10.1083/jcb.200311141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso MT, Villalobos C, Chamero P, Alvarez J, Garcia-Sancho J. Calcium microdomains in mitochondria and nucleus. Cell Calcium. 2006;40:513–525. doi: 10.1016/j.ceca.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Glutamate-dependent astrocyte modulation of synaptic transmission between cultured hippocampal neurons. Eur J Neurosci. 1998a;10:2129–2142. doi: 10.1046/j.1460-9568.1998.00221.x. [DOI] [PubMed] [Google Scholar]
- Araque A, Sanzgiri RP, Parpura V, Haydon PG. Calcium elevation in astrocytes causes an NMDA receptor-dependent increase in the frequency of miniature synaptic currents in cultured hippocampal neurons. J Neurosci. 1998b;18:6822–6829. doi: 10.1523/JNEUROSCI.18-17-06822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- Araque A, Martin ED, Perea G, Arellano JI, Buno W. Synaptically released acetylcholine evokes Ca2+ elevations in astrocytes in hippocampal slices. J Neurosci. 2002;22:2443–2450. doi: 10.1523/JNEUROSCI.22-07-02443.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass NH, Hess HH, Pope A, Thalheimer C. Quantitative cytoarchitectonic distribution of neurons, glia, and DNa in rat cerebral cortex. J Comp Neurol. 1971;143:481–490. doi: 10.1002/cne.901430405. [DOI] [PubMed] [Google Scholar]
- Beck A, Nieden RZ, Schneider HP, Deitmer JW. Calcium release from intracellular stores in rodent astrocytes and neurons in situ. Cell Calcium. 2004;35:47–58. doi: 10.1016/s0143-4160(03)00171-4. [DOI] [PubMed] [Google Scholar]
- Benz B, Grima G, Do KQ. Glutamate-induced homocysteic acid release from astrocytes: possible implication in glia-neuron signaling. Neuroscience. 2004;124:377–386. doi: 10.1016/j.neuroscience.2003.08.067. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998;391:281–285. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- Bond CE, Greenfield SA. Multiple cascade effects of oxidative stress on astroglia. Glia. 2007;55:1348–1361. doi: 10.1002/glia.20547. [DOI] [PubMed] [Google Scholar]
- Burdakov D, Petersen OH, Verkhratsky A. Intraluminal calcium as a primary regulator of endoplasmic reticulum function. Cell Calcium. 2005;38:303–310. doi: 10.1016/j.ceca.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Bushong EA, Martone ME, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci. 2002;22:183–192. doi: 10.1523/JNEUROSCI.22-01-00183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmignoto G, Pasti L, Pozzan T. On the role of voltage-dependent calcium channels in calcium signaling of astrocytes in situ. J Neurosci. 1998;18:4637–4645. doi: 10.1523/JNEUROSCI.18-12-04637.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang DT, Honick AS, Reynolds IJ. Mitochondrial trafficking to synapses in cultured primary cortical neurons. J Neurosci. 2006;26:7035–7045. doi: 10.1523/JNEUROSCI.1012-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholewinski AJ, Hanley MR, Wilkin GP. A phosphoinositide-linked peptide response in astrocytes: evidence for regional heterogeneity. Neurochem Res. 1988;13:389–394. doi: 10.1007/BF00972490. [DOI] [PubMed] [Google Scholar]
- Collins TJ, Lipp P, Berridge MJ, Bootman MD. Mitochondrial Ca(2+) uptake depends on the spatial and temporal profile of cytosolic Ca(2+) signals. J Biol Chem. 2001;276:26411–26420. doi: 10.1074/jbc.M101101200. [DOI] [PubMed] [Google Scholar]
- Collins TJ, Berridge MJ, Lipp P, Bootman MD. Mitochondria are morphologically and functionally heterogeneous within cells. Embo J. 2002;21:1616–1627. doi: 10.1093/emboj/21.7.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csordas G, Renken C, Varnai P, Walter L, Weaver D, Buttle KF, Balla T, Mannella CA, Hajnoczky G. Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol. 2006;174:915–921. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JW, Chernjavsky A, Smith SJ. Neuronal activity triggers calcium waves in hippocampal astrocyte networks. Neuron. 1992;8:429–440. doi: 10.1016/0896-6273(92)90271-e. [DOI] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- Deitmer JW, Singaravelu K, Lohr C. Calcium ion signaling in astrocytes. In: Parpura V, Haydon PG, editors. Astrocytes in (patho)physiology of the Nervous System. Boston, MA: Springer; 2008. [Google Scholar]
- Fellin T, Pascual O, Gobbo S, Pozzan T, Haydon PG, Carmignoto G. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron. 2004;43:729–743. doi: 10.1016/j.neuron.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Goldman WF, Yarowsky PJ, Juhaszova M, Krueger BK, Blaustein MP. Sodium/calcium exchange in rat cortical astrocytes. J Neurosci. 1994;14:5834–5843. doi: 10.1523/JNEUROSCI.14-10-05834.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golovina VA. Visualization of localized store-operated calcium entry in mouse astrocytes. Close proximity to the endoplasmic reticulum. J Physiol. 2005;564:737–749. doi: 10.1113/jphysiol.2005.085035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golovina VA, Blaustein MP. Unloading and refilling of two classes of spatially resolved endoplasmic reticulum Ca(2+) stores in astrocytes. Glia. 2000;31:15–28. doi: 10.1002/(sici)1098-1136(200007)31:1<15::aid-glia20>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Grimaldi M, Maratos M, Verma A. Transient receptor potential channel activation causes a novel form of [Ca2+]I oscillations and is not involved in capacitative Ca2+ entry in glial cells. J Neurosci. 2003;23:4737–4745. doi: 10.1523/JNEUROSCI.23-11-04737.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosche J, Matyash V, Moller T, Verkhratsky A, Reichenbach A, Kettenmann H. Microdomains for neuron-glia interaction: parallel fiber signaling to Bergmann glial cells. Nat Neurosci. 1999;2:139–143. doi: 10.1038/5692. [DOI] [PubMed] [Google Scholar]
- Halassa MM, Haydon PG. The tripartite synapse. In: Parpura V, Haydon PG, editors. Astrocytes in (patho)physiology of the nervous system. Boston, MA: Springer; 2009. In Press. [Google Scholar]
- Halassa MM, Fellin T, Takano H, Dong JH, Haydon PG. Synaptic islands defined by the territory of a single astrocyte. J Neurosci. 2007;27:6473–6477. doi: 10.1523/JNEUROSCI.1419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JL, Camerini-Otero CS, Li AH, Kim SA, Jacobson KA, Daly JW. Dihydropyridines as inhibitors of capacitative calcium entry in leukemic HL-60 cells. Biochem Pharmacol. 2003;65:329–338. doi: 10.1016/s0006-2952(02)01488-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton GI. Dynamic neuronal-glial interactions: an overview 20 years later. Peptides. 2004;25:403–411. doi: 10.1016/j.peptides.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- Hofmann T, Schaefer M, Schultz G, Gudermann T. Subunit composition of mammalian transient receptor potential channels in living cells. Proc Natl Acad Sci U S A. 2002;99:7461–7466. doi: 10.1073/pnas.102596199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoth M, Fanger CM, Lewis RS. Mitochondrial regulation of store-operated calcium signaling in T lymphocytes. J Cell Biol. 1997;137:633–648. doi: 10.1083/jcb.137.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X, Malarkey EB, Sunjara V, Rosenwald SE, Li WH, Parpura V. Ca2+-dependent glutamate release involves two classes of endoplasmic reticulum Ca2+ stores in astrocytes. J Neurosci Res. 2004;76:86–97. doi: 10.1002/jnr.20061. [DOI] [PubMed] [Google Scholar]
- Huang YH, Bergles DE. Glutamate transporters bring competition to the synapse. Curr Opin Neurobiol. 2004;14:346–352. doi: 10.1016/j.conb.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Innocenti B, Parpura V, Haydon PG. Imaging extracellular waves of glutamate during calcium signaling in cultured astrocytes. J Neurosci. 2000;20:1800–1808. doi: 10.1523/JNEUROSCI.20-05-01800.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeremic A, Jeftinija K, Stevanovic J, Glavaski A, Jeftinija S. ATP stimulates calcium-dependent glutamate release from cultured astrocytes. J Neurochem. 2001;77:664–675. doi: 10.1046/j.1471-4159.2001.00272.x. [DOI] [PubMed] [Google Scholar]
- Kastritsis CH, Salm AK, McCarthy K. Stimulation of the P2Y purinergic receptor on type 1 astroglia results in inositol phosphate formation and calcium mobilization. J Neurochem. 1992;58:1277–1284. doi: 10.1111/j.1471-4159.1992.tb11339.x. [DOI] [PubMed] [Google Scholar]
- Kofuji P, Newman EA. Regulation of potassium by glial cells in the central nervous system. In: Parpura V, Haydon PG, editors. Astrocytes in (patho)physiology of the nervous system. Boston, MA: Springer; 2009. In Press. [Google Scholar]
- Kolikova J, Afzalov R, Giniatullina A, Surin A, Giniatullin R, Khiroug L. Calcium-dependent trapping of mitochondria near plasma membrane in stimulated astrocytes. Brain Cell Biol. 2006;35:75–86. doi: 10.1007/s11068-006-9000-1. [DOI] [PubMed] [Google Scholar]
- Lalo U, Pankratov Y, Kirchhoff F, North RA, Verkhratsky A. NMDA receptors mediate neuron-to-glia signaling in mouse cortical astrocytes. J Neurosci. 2006;26:2673–2683. doi: 10.1523/JNEUROSCI.4689-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latour I, Hamid J, Beedle AM, Zamponi GW, Macvicar BA. Expression of voltage-gated Ca2+ channel subtypes in cultured astrocytes. Glia. 2003;41:347–353. doi: 10.1002/glia.10162. [DOI] [PubMed] [Google Scholar]
- Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Malarkey EB, Parpura V. Mechanisms of glutamate release from astrocytes. Neurochem Int. 2008;52:142–154. doi: 10.1016/j.neuint.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malarkey EB, Parpura V. Mechanisms of transmitter release from astrocytes. In: Parpura V, Haydon PG, editors. Astrocytes in (patho)physiology of the nervous system. Boston, MA: Springer; 2009. pp. 301–350. [Google Scholar]
- Malarkey EB, Ni Y, Parpura V. Ca2+ entry through TRPC1 channels contributes to intracellular Ca2+ dynamics and consequent glutamate release from rat astrocytes. Glia. 2008 doi: 10.1002/glia.20656. [DOI] [PubMed] [Google Scholar]
- Mironov SL. Spontaneous and evoked neuronal activities regulate movements of single neuronal mitochondria. Synapse. 2006;59:403–411. doi: 10.1002/syn.20256. [DOI] [PubMed] [Google Scholar]
- Miyata H, Silverman HS, Sollott SJ, Lakatta EG, Stern MD, Hansford RG. Measurement of mitochondrial free Ca2+ concentration in living single rat cardiac myocytes. Am J Physiol. 1991;261:H1123–1134. doi: 10.1152/ajpheart.1991.261.4.H1123. [DOI] [PubMed] [Google Scholar]
- Montana V, Ni Y, Sunjara V, Hua X, Parpura V. Vesicular glutamate transporter-dependent glutamate release from astrocytes. J Neurosci. 2004;24:2633–2642. doi: 10.1523/JNEUROSCI.3770-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montana V, Malarkey EB, Verderio C, Matteoli M, Parpura V. Vesicular transmitter release from astrocytes. Glia. 2006;54:700–715. doi: 10.1002/glia.20367. [DOI] [PubMed] [Google Scholar]
- Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431:195–199. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- Nedergaard M. Direct signaling from astrocytes to neurons in cultures of mammalian brain cells. Science. 1994;263:1768–1771. doi: 10.1126/science.8134839. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 2003;26:523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Ni Y, Malarkey EB, Parpura V. Vesicular release of glutamate mediates bidirectional signaling between astrocytes and neurons. J Neurochem. 2007;103:1273–1284. doi: 10.1111/j.1471-4159.2007.04864.x. [DOI] [PubMed] [Google Scholar]
- Ogata K, Kosaka T. Structural and quantitative analysis of astrocytes in the mouse hippocampus. Neuroscience. 2002;113:221–233. doi: 10.1016/s0306-4522(02)00041-6. [DOI] [PubMed] [Google Scholar]
- Oheim M, Kirchhoff F, Stuhmer W. Calcium microdomains in regulated exocytosis. Cell Calcium. 2006;40:423–439. doi: 10.1016/j.ceca.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Panatier A, Theodosis DT, Mothet JP, Touquet B, Pollegioni L, Poulain DA, Oliet SH. Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell. 2006;125:775–784. doi: 10.1016/j.cell.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Parpura V, Haydon PG. Physiological astrocytic calcium levels stimulate glutamate release to modulate adjacent neurons. Proc Natl Acad Sci U S A. 2000;97:8629–8634. doi: 10.1073/pnas.97.15.8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- Parri HR, Crunelli V. The role of Ca2+ in the generation of spontaneous astrocytic Ca2+ oscillations. Neuroscience. 2003;120:979–992. doi: 10.1016/s0306-4522(03)00379-8. [DOI] [PubMed] [Google Scholar]
- Parri HR, Gould TM, Crunelli V. Spontaneous astrocytic Ca2+ oscillations in situ drive NMDAR-mediated neuronal excitation. Nat Neurosci. 2001;4:803–812. doi: 10.1038/90507. [DOI] [PubMed] [Google Scholar]
- Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- Perea G, Araque A. Properties of synaptically evoked astrocyte calcium signal reveal synaptic information processing by astrocytes. J Neurosci. 2005;25:2192–2203. doi: 10.1523/JNEUROSCI.3965-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea G, Araque A. Astrocytes potentiate transmitter release at single hippocampal synapses. Science. 2007;317:1083–1086. doi: 10.1126/science.1144640. [DOI] [PubMed] [Google Scholar]
- Peuchen S, Clark JB, Duchen MR. Mechanisms of intracellular calcium regulation in adult astrocytes. Neuroscience. 1996;71:871–883. doi: 10.1016/0306-4522(95)00515-3. [DOI] [PubMed] [Google Scholar]
- Pizzo P, Burgo A, Pozzan T, Fasolato C. Role of capacitative calcium entry on glutamate-induced calcium influx in type-I rat cortical astrocytes. J Neurochem. 2001;79:98–109. doi: 10.1046/j.1471-4159.2001.00539.x. [DOI] [PubMed] [Google Scholar]
- Porter JT, McCarthy KD. Hippocampal astrocytes in situ respond to glutamate released from synaptic terminals. J Neurosci. 1996;16:5073–5081. doi: 10.1523/JNEUROSCI.16-16-05073.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana A, Schwindling C, Wenning AS, Becherer U, Rettig J, Schwarz EC, Hoth M. T cell activation requires mitochondrial translocation to the immunological synapse. Proc Natl Acad Sci U S A. 2007;104:14418–14423. doi: 10.1073/pnas.0703126104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes RC, Parpura V. Mitochondria modulate Ca2+-dependent glutamate release from rat cortical astrocytes. J Neurosci. 2008;28:9682–9691. doi: 10.1523/JNEUROSCI.3484-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- Rojas H, Colina C, Ramos M, Benaim G, Jaffe EH, Caputo C, DiPolo R. Na+ entry via glutamate transporter activates the reverse Na+/Ca2+ exchange and triggers Ca(i)2+-induced Ca2+ release in rat cerebellar Type-1 astrocytes. J Neurochem. 2007;100:1188–1202. doi: 10.1111/j.1471-4159.2006.04303.x. [DOI] [PubMed] [Google Scholar]
- Rychkov G, Barritt GJ. TRPC1 Ca(2+)-permeable channels in animal cells. Handb Exp Pharmacol. 2007:23–52. doi: 10.1007/978-3-540-34891-7_2. [DOI] [PubMed] [Google Scholar]
- Schneggenburger R, Neher E. Presynaptic calcium and control of vesicle fusion. Curr Opin Neurobiol. 2005;15:266–274. doi: 10.1016/j.conb.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Seifert G, Steinhauser C. Ionotropic glutamate receptors in astrocytes. Prog Brain Res. 2001;132:287–299. doi: 10.1016/S0079-6123(01)32083-6. [DOI] [PubMed] [Google Scholar]
- Seifert G, Weber M, Schramm J, Steinhauser C. Changes in splice variant expression and subunit assembly of AMPA receptors during maturation of hippocampal astrocytes. Mol Cell Neurosci. 2003;22:248–258. doi: 10.1016/s1044-7431(03)00039-3. [DOI] [PubMed] [Google Scholar]
- Sharma G, Vijayaraghavan S. Nicotinic cholinergic signaling in hippocampal astrocytes involves calcium-induced calcium release from intracellular stores. Proc Natl Acad Sci U S A. 2001;98:4148–4153. doi: 10.1073/pnas.071540198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood CC, Stimpson CD, Raghanti MA, Wildman DE, Uddin M, Grossman LI, Goodman M, Redmond JC, Bonar CJ, Erwin JM, Hof PR. Evolution of increased glia-neuron ratios in the human frontal cortex. Proc Natl Acad Sci U S A. 2006;103:13606–13611. doi: 10.1073/pnas.0605843103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson PB, Russell JT. Mitochondria support inositol 1,4,5-trisphosphate-mediated Ca2+ waves in cultured oligodendrocytes. J Biol Chem. 1996;271:33493–33501. doi: 10.1074/jbc.271.52.33493. [DOI] [PubMed] [Google Scholar]
- Simpson PB, Russell JT. Role of mitochondrial Ca2+ regulation in neuronal and glial cell signalling. Brain Res Brain Res Rev. 1998;26:72–81. doi: 10.1016/s0165-0173(97)00056-8. [DOI] [PubMed] [Google Scholar]
- Simpson PB, Holtzclaw LA, Langley DB, Russell JT. Characterization of ryanodine receptors in oligodendrocytes, type 2 astrocytes, and O-2A progenitors. J Neurosci Res. 1998;52:468–482. doi: 10.1002/(SICI)1097-4547(19980515)52:4<468::AID-JNR11>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Sontheimer H, Fernandez-Marques E, Ullrich N, Pappas CA, Waxman SG. Astrocyte Na+ channels are required for maintenance of Na+/K(+)-ATPase activity. J Neurosci. 1994;14:2464–2475. doi: 10.1523/JNEUROSCI.14-05-02464.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens GJ, Cholewinski AJ, Wilkin GP, Djamgoz MB. Calcium-mobilizing and electrophysiological effects of bradykinin on cortical astrocyte subtypes in culture. Glia. 1993;9:269–279. doi: 10.1002/glia.440090405. [DOI] [PubMed] [Google Scholar]
- Strubing C, Krapivinsky G, Krapivinsky L, Clapham DE. TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron. 2001;29:645–655. doi: 10.1016/s0896-6273(01)00240-9. [DOI] [PubMed] [Google Scholar]
- Strubing C, Krapivinsky G, Krapivinsky L, Clapham DE. Formation of novel TRPC channels by complex subunit interactions in embryonic brain. J Biol Chem. 2003;278:39014–39019. doi: 10.1074/jbc.M306705200. [DOI] [PubMed] [Google Scholar]
- Takemura H, Putney JW., Jr Capacitative calcium entry in parotid acinar cells. Biochem J. 1989;258:409–412. doi: 10.1042/bj2580409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang HL, Lung HL, Wu KC, Le AH, Tang HM, Fung MC. Vimentin supports mitochondrial morphology and organization. Biochem J. 2008;410:141–146. doi: 10.1042/BJ20071072. [DOI] [PubMed] [Google Scholar]
- Tang Y, Zucker RS. Mitochondrial involvement in post-tetanic potentiation of synaptic transmission. Neuron. 1997;18:483–491. doi: 10.1016/s0896-6273(00)81248-9. [DOI] [PubMed] [Google Scholar]
- Theodosis DT, Poulain DA, Oliet SH. Activity-dependent structural and functional plasticity of astrocyte-neuron interactions. Physiol Rev. 2008;88:983–1008. doi: 10.1152/physrev.00036.2007. [DOI] [PubMed] [Google Scholar]
- Tzingounis AV, Wadiche JI. Glutamate transporters: confining runaway excitation by shaping synaptic transmission. Nat Rev Neurosci. 2007;8:935–947. doi: 10.1038/nrn2274. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A. Glial calcium signaling in physiology and pathophysiology. Acta Pharmacol Sin. 2006;27:773–780. doi: 10.1111/j.1745-7254.2006.00396.x. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A. Neurotransmitter receptors in astrocytes. In: Parpura V, Haydon PG, editors. Astrocytes in (patho)physiology of the nervous system. Boston, MA: Springer; 2009. In Press. [Google Scholar]
- Verkhratsky A, Steinhauser C. Ion channels in glial cells. Brain Res Brain Res Rev. 2000;32:380–412. doi: 10.1016/s0165-0173(99)00093-4. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Orkand RK, Kettenmann H. Glial calcium: homeostasis and signaling function. Physiol Rev. 1998;78:99–141. doi: 10.1152/physrev.1998.78.1.99. [DOI] [PubMed] [Google Scholar]
- Wang W, O’Connell B, Dykeman R, Sakai T, Delporte C, Swaim W, Zhu X, Birnbaumer L, Ambudkar IS. Cloning of Trp1beta isoform from rat brain: immunodetection and localization of the endogenous Trp1 protein. Am J Physiol. 1999;276:C969–979. doi: 10.1152/ajpcell.1999.276.4.C969. [DOI] [PubMed] [Google Scholar]
- Westermann B. Merging mitochondria matters: cellular role and molecular machinery of mitochondrial fusion. EMBO Rep. 2002;3:527–531. doi: 10.1093/embo-reports/kvf113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Rothstein JD. Specialized neurotransmitter transporters in astrocytes. In: Parpura V, Haydon PG, editors. Astrocytes in (patho)physiology of the nervous system. Boston, MA: Springer; 2009. In Press. [Google Scholar]
- Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]