Abstract
Substructures of the prefrontal cortex (PFC) and the medial-temporal lobe are critical for associating objects presented over time. Previous studies showing frontal and medial-temporal involvement in associative encoding have not addressed the response specificity of these regions to different aspects of the task, which include instructions to associate and binding of stimuli. This study used a novel paradigm to temporally separate these two components of the task by sequential presentation of individual images with or without associative instruction; fMRI was used to investigate the temporal involvement of the PFC and the parahippocampal cortex in encoding each component. Although both regions showed an enhanced response to the second stimulus of a pair, only the PFC had increased activation during the delay preceding a stimulus when associative instruction was given. These findings present new evidence that prefrontal and medial-temporal regions provide distinct temporal contributions during associative memory formation.
INTRODUCTION
Animal lesion models and studies involving patients with selective damage to structures of the medial-temporal lobe (MTL) have demonstrated critical involvement of this brain region in the encoding and retrieval of long-term declarative memory, the memory for facts and events (Squire, 1992). Multiple studies have demonstrated particular involvement of the parahippocampal cortex (PHC) in successful memory formation (Eichenbaum, Yonelinas, & Ranganath, 2007; Murray & Ranganath, 2007; Gold et al., 2006; Kirwan & Stark, 2004; Davachi, Mitchell, & Wagner, 2003; Davachi & Wagner, 2002; Brewer, Zhao, Desmond, Glover, & Gabrieli, 1998; Henke, Buck, Weber, & Wieser, 1997). Neuroimaging and neuropsychological studies of patients with damage to the prefrontal cortex (PFC) have also suggested the contribution of the PFC to the encoding of long-term memory (LTM) (Blumenfeld & Ranganath, 2007; Murray & Ranganath, 2007; Sperling et al., 2003; Brewer et al., 1998; Wagner et al., 1998). Although imaging studies have commonly reported PFC and PHC activity in lockstep during associative encoding, the hypothesis of the present study was that activity in these two regions is dissociable, with PFC activity preceding PHC activity, supporting a mechanism for top–down modulation of MTL structures involved in associative encoding.
Anatomical studies using anterograde and retrograde tracing techniques in monkeys (Goldman-Rakic, Selemon, & Schwartz, 1984) and imaging methods combining functional magnetic resonance imaging (fMRI) and diffusion tensor imaging (DTI) in humans (Takahashi, Ohki, & Kim, 2007) examined the connectivity between the PFC and the PHC. Both studies reported direct and indirect anatomical connections between subregions of the PFC and PHC. Recently, Gazzaley and D'Esposito (2007) examined the process of top–down modulation from the PFC to the visual association cortex and the PHC during scene-selective processing. The top–down influence of prefrontal activity upon parahippocampal activity is consistent with the anatomical connectivity between these brain regions.
A recent fMRI study examined PFC activity using an associative memory paradigm in which two sequentially presented words were associated when the presentation of the second word was accompanied by a relational question and not associated when accompanied by an item-specific question (Murray & Ranganath, 2007). Greater activation of the left PHC, the dorsolateral pre-frontal cortex (DLPFC), and the ventrolateral prefrontal cortex (VLPFC) was observed during the encoding of the second presented word in relational trials compared to item-specific trials. As associative instructions were presented concurrently with the second word, increased activation could only be examined at that time point, and functional specificity of PFC and MTL activity could not be addressed. The present study, however, was designed to pursue this question of the particular involvement of these two brain regions in processes recruited for associative encoding.
In nonhuman primates, multiunit recording data suggest that PFC neurons play a role in associating temporally separate stimuli and show delay-period increases in activity (Deco, Ledberg, Almeida, & Fuster, 2005; Fuster, Bodner, & Kroger, 2000). Fuster et al. (2000) conducted extracellular recordings from bilateral regions of the dorsolateral frontal cortex while monkeys performed an audiovisual memory task. As monkeys learned the low tone–green and high tone–red associations, cells in this region showed the same relationship of firing to low and high tones as to green and red colors, respectively, with maintained activity during the delay between tones and associated colors. The presence of delay-period activity in medial-temporal regions, however, has been more controversial. Some studies report rarely seen increases in delay-period activity in medial-temporal regions such as the PHC in monkeys (Vidyasagar, Salzmann, & Creutzfeldt, 1991) or the hippocampus in rats early in the delay period (Hampson & Deadwyler, 2003), whereas other studies in monkeys report the presence of delay-period activity in medial-temporal regions (Young, Otto, Fox, & Eichenbaum, 1997; Cahusac, Miyashita, & Rolls, 1989; Watanabe & Niki, 1985). Despite the disagreement in the literature regarding MTL activity during the delay period in associative tasks, there is strong electrophysiological evidence for PFC activity during the delay period in rats and monkeys.
The present study further examines the involvement of the PFC and the PHC in the encoding of associative memory compared to single-item memory. Rapid event-related fMRI was used to identify the temporal involvement of the PFC and the PHC in encoding sequentially presented images with varying interstimulus intervals (ISIs). A plus-sign presented during some ISIs instructed participants to associate the image preceding and following the plus-sign as a pair. The timing separation between the plus-sign (instructing the subject to pair the previous image with the upcoming image) and the presentation of the second image (at which point the images can be associated) allowed temporal investigation of PFC and PHC involvement in associative memory encoding. After the scan, participants completed a recognition test examining associative and single-item memory. Based on previous findings, the hypotheses were that the PFC and the PHC would show greater activation during the encoding of paired versus unpaired images. Prefrontal activity was expected to precede parahippocampal activity supporting top–down influence on the PHC.
METHODS
Participants
Thirteen healthy volunteers (mean age = 23.69, 3 men) recruited from the University of California, San Diego (UCSD) community and the surrounding area were enrolled in this study. Participants gave informed consent approved by the UCSD Institutional Review Board and had normal or corrected-to-normal vision. Twelve additional volunteers (mean age = 25.08, 6 men) were recruited for a behavioral pilot task.
Stimuli
Stimuli included 256 color images of common objects which were presented individually while the participant was in the scanner. A plus-sign appeared between some of the stimuli. An additional 40 novel stimuli were used during the recognition test following the scan. Images were acquired from Rossion and Pourtois (2004) color Snodgrass images (www.nefy.ucl.ac.be/facecatlab/stimuli. htm) and Hemera object library (Hemera Technologies).
Experimental Procedure
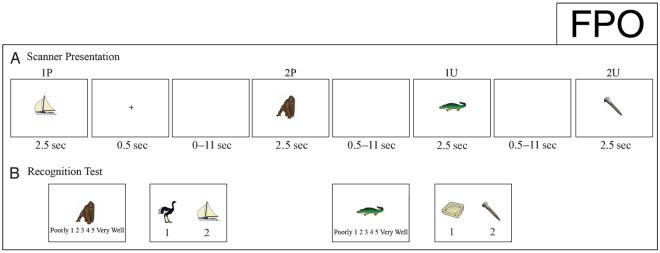
While in the scanner, participants were presented with individual images (each remaining on the screen for 2.5 sec) followed by jittered ISIs ranging from 0.5 to 11 sec (Figure 1A). Jitter was calculated to optimize the design (Dale, 1999; Dale & Buckner, 1997). Immediately following some of the images, a plus-sign appeared in the center of the screen for 0.5 sec. Participants were asked to remember the presented images and, if an image was followed by a plus-sign, to associate the image with the subsequent image as a pair. Participants were given a button box and were asked to press one button if the image represented a living object and the other button if the image represented a nonliving object. Image stimuli were presented in a series of four runs, each lasting 362 sec and containing 64 images. Over all four runs, 130 images were included in associated pairs and 126 images were unpaired. The presentation of stimuli varied pseudorandomly between paired and unpaired stimuli. For analysis purposes, but unannounced to the participants, paired and unpaired items were presented sequentially in multiples of two. For simplicity, stimuli preceding a plus-sign will be denoted as “1P,” and the stimuli following the plus-sign as “2P.” After a “2P” stimulus, the next image could be a “1P” (which would then be followed by a plus-sign and a “2P”), or the next image could be an individual unpaired stimulus, denoted “1U” for unpaired. “1U” was always followed by “2U”. This terminology is used in Figure 1 and throughout the analysis.
Figure 1.
Experimental design. (A) Schematic depiction of the scanner presentation of two paired and two unpaired stimuli. For the first 0.5 sec of a 0.5- to 11-sec ISI, the associative memory instruction of a plus-sign is present between two images that should be paired (1P and 2P) and is not present between two images that should remain unpaired (1U and 2U). (B) Schematic depiction of the recognition test conducted following the scan. Participants were asked if they remember seeing the image in the scanner (“poorly” if they think it is a novel item; “very well” if they remember seeing the item). If the image was presented in the scanner, participants were then shown a second screen with two choice images; they were asked to report which image (1 or 2) was the associated pair if the target image was paired or to report if the target image was unpaired (3).
Following the scan, participants completed a recognition test (Figure 1B). Participants were shown an image and were asked to rate how well they remembered seeing that image during the scanner presentation, 1 being “Poorly” and 5 being “Very Well.” This question was asked for each of the 256 images that the participant was shown while in the scanner plus 40 additional novel images. After rating each image, participants were shown two additional images, labeled “1” and “2,” and were instructed to identify the pair of the originally presented image or to identify the original image as unpaired (option labeled “3”). If the original image was novel, this question was skipped all-together and the next recognition image was presented. The postscan recognition test lasted approximately 30 min.
fMRI Parameters
Participants were scanned using a 3-T GE scanner at the Keck Center for Functional MRI at the University of California, San Diego. Functional images were acquired using a gradient-echo, echo-planar, T2*-weighted pulse sequence (repetition time = 1.5 sec; one shot per repetition; echo time = 30; flip angle = 90°; bandwidth = 31.25 MHz). Twenty-two slices covering the brain were obtained perpendicular to the long axis of the hippo-campus with 4 × 4 × 7 mm voxels. T1-weighted structural scans were acquired in the same plane as the functional scans and of the same voxel size. Structural images were also acquired using high resolution T1-weighted (1 × 1 × 1 mm) magnetization-prepared rapid gradient-echo sequence.
Data Analysis
Data from each run were reconstructed using the AFNI (Cox, 1996) suite of programs. Slices were aligned temporally and then coregistered using a three-dimensional image alignment algorithm. A threshold mask of the functional data was used to eliminate voxels outside the brain. A series of functional images from separate runs were corrected for motion and concatenated. Two general linear models were constructed using multiple regression analysis. Each model included six motion regressors obtained from the registration process and additional task-related regressors in which impulse responses were modeled from the data for each of the stimulus conditions. The first general linear model included regressors for 1P, 2P, 1U, 2U condition correct and incorrect responses. The second general linear model included regressors for paired trials (1P and 2P with an ISI of 3.5 sec) and unpaired trials (1U and 2U with an ISI of 3.5 sec) (Daselaar et al., 2007; Schluppeck, Curtis, Glimcher, & Heeger, 2006). An ISI of 3.5 sec was selected because it was the most frequent jitter interval and allowed sufficient measurements for analysis. In addition, parameter estimates for all delay periods between two paired images with remembered associative properties were analyzed relative to all delay periods between two unpaired images using repeated measures ANOVA.
Only paired and individual unpaired images correctly identified during the postscan recognition test were included in the analysis of fMRI data. The hemodynamic response function was derived from the fMRI data using signal deconvolution and a defined time window following stimulus onset (AFNI Software; Cox, 1996). This time window was from 0 to 15 sec for single stimulus events, and 0 to 21 sec for two-stimulus trials with 3.5-sec ISIs. Standard landmarks were defined manually on the anatomical scans. Data from the anatomical and functional scans were then transformed into Talairach and Tournoux (1998) space by AFNI using nearest-neighbor interpolation. No spatial smoothing was performed. The areas under the hemodynamic response function for the following conditions were examined using voxelwise t tests (two-tailed) carried out across all 13 participants: (1) 2P versus 2U, (2) trials with two paired images with an ISI of 3.5 sec (with a plus-sign present for the first 0.5 sec of the ISI) versus trials with two unpaired images with an ISI of 3.5 sec. Given the reduced number of trials with an ISI of 3.5 sec, all trials were included in this analysis. A voxelwise threshold of p < .01 was used to identify significant regional activity. Analyses were restricted to clusters containing at least four voxels connected by face surfaces, yielding a significance value of p < .01 when corrected for multiple comparisons across the whole brain. These clusters were used to create impulse–response plots displaying the temporal characteristics of the activation.
RESULTS
Behavioral Pilot Task
A behavioral pilot task was conducted to evaluate whether an instructional cue can effectively manipulate episodic associative memory for items presented sequentially and to ensure that incidental associations are not being made between proximally presented unpaired images. Following the encoding task, which was the same as was used for the imaging study, participants completed a postscan recognition task similar to that used in the current experiment, except for that they were asked which of two images was presented closest in time to the image they just saw during the previous item memory question. Participants identified the item presented adjacent in time when no associative cue had been presented at a low rate (60 ± 4%), significantly below their performance in identifying the item presented adjacent in time when an associative cue had been presented (81 ± 6%; p < .001, t = 6.623).
Behavioral Analysis
Eighty-two percent (±3%) of paired stimuli was recognized with a high degree of confidence (subject response of 4 or 5), and for those recognized items, the correct associated pair was identified at a rate of 71% (±4%). Unpaired items were recognized with a high degree of confidence at a rate of 73% (±4%). Data for correctly identified paired and unpaired items were included in the fMRI analysis. Subjects incorrectly identified novel images as recognized at a rate of 11% (±3%).
fMRI Analysis
Based on previous studies that have found activation in the MTL structures as well as in regions of the PFC during the encoding of associated items (Dickerson et al., 2007; Murray & Ranganath, 2007; Tendolkar et al., 2007; Gold et al., 2006; Staresina & Davachi, 2006; Law et al., 2005; Meltzer & Constable, 2005; Prince, Daselaar, & Cabeza, 2005; Pihlajamaki et al., 2003; Sperling et al., 2003; Yonelinas, Hopfinger, Buonocore, Kroll, & Baynes, 2001; Dolan & Fletcher, 1997; Henke et al., 1997; Rombouts et al., 1997), analysis was focused on these brain regions. For the present study, parahippocampal regions were predicted to show greater activation during the encoding of 2P stimuli (items paired with the preceding stimulus) than during the encoding of 2U stimuli (items not paired with the preceding stimulus and which only follow unpaired stimuli). Prefrontal regions, specifically the DLPFC and the VLPFC, were also predicted to show increased activation during the encoding of 2P stimuli.
Activation during the viewing of 2P stimuli was compared to activation during the viewing of 2U stimuli. This contrast between processing 2P versus 2U stimuli revealed left PHC activation (p < .01; Figure 2A, B). The impulse–response curve for 2U indicated PHC activity during single-item encoding as well as during associative encoding; however, the activity in this region was greater during the encoding of 2P stimuli (Figure 2C).
Figure 2.
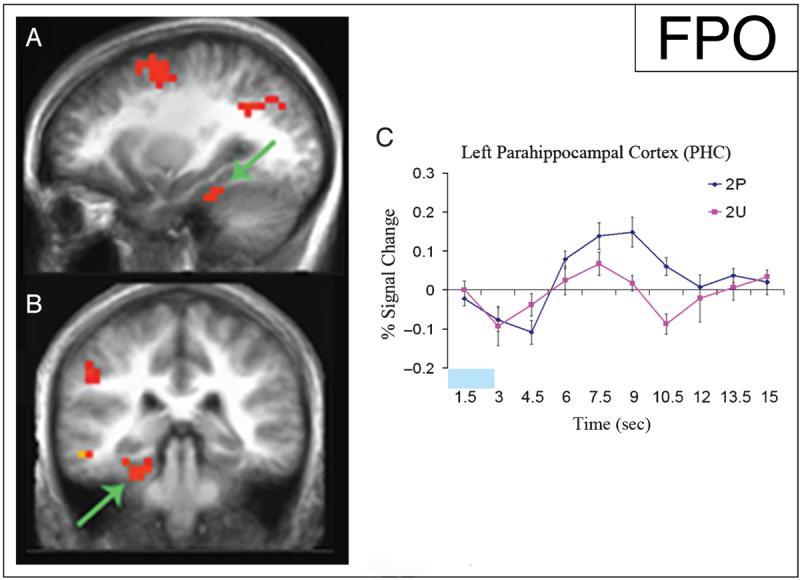
Increased activity in the left PHC during the encoding of the second paired (2P) stimuli versus the second unpaired (2U) stimuli. Statistical activation maps illustrating greater activation (p < .01) during the encoding of 2P versus 2U stimuli are superimposed on sagittal (A) and coronal (B) slices of mean anatomical scan images across all 13 subjects; arrows indicate the left PHC cluster used for time-course analysis. (C) Time course of activity in the left PHC beginning with the onset of 2P stimuli (blue) and 2U stimuli (pink) demonstrates activity during item encoding, with increased activity during associative encoding. The time of stimulus presentation is represented by the light blue block. The y-axis represents percent signal change, the x-axis is time in seconds (sec), and the error bars represent the standard error of the mean.
Previous studies reported that the DLPFC (Brodmann's area 9, 46) is active during encoding of individual items (Staresina & Davachi, 2006; Brewer et al., 1998), and such activity is further increased by associative memory conditions (Murray & Ranganath, 2007). Consistent with these findings, greater activation of the DLPFC during encoding of 2P stimuli relative to the encoding of 2U stimuli was observed (p < .01; Figure 3A, B). Similar to PHC involvement, the DLPFC was active during the encoding of all remembered images; however, the activation was greatly enhanced during associative encoding of 2P (Figure 3C). Activity in the VLPFC was also analyzed for this contrast between the encoding of 2P stimuli and 2U stimuli. Similar to activity in the DLPFC, there was an increase in activation in the VLPFC (Brodmann's area 44, 45, 47) during the encoding of 2P stimuli (p < .01; Figure 3A, B). However, the VLPFC did not show significant activity for 2U stimuli (p > .05). Examination of the time course of activity in the VLPFC also showed a small response for 2U stimuli that did not reach significance (Figure 3D). A complete list of regions of activation for this contrast is listed in Table 1.
Figure 3.
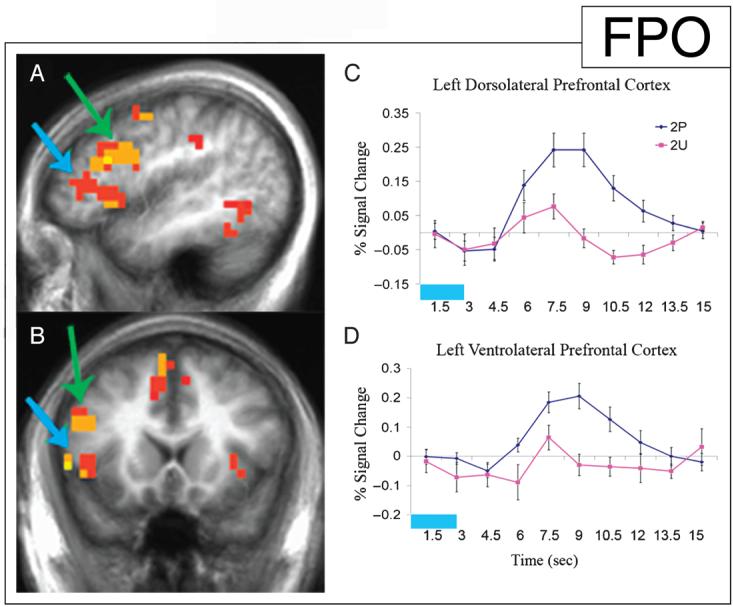
Increased activity in the left DLPFC and the left VLPFC during the encoding of the second paired (2P) stimuli versus the second unpaired (2U) stimuli. Statistical activation maps illustrating greater activation (p < .01) during the encoding of 2P versus 2U stimuli are superimposed on sagittal (A) and coronal (B) slices of the mean anatomical scan images across all 13 subjects; arrows indicate left DLPFC (green) and left VLPFC (blue) clusters used for time-course analysis. (C) Time course of activity in the left DLPFC beginning with the onset of 2P stimuli (blue) and 2U stimuli (pink) demonstrating activity during item encoding, with increased activity during associative encoding. The time of stimulus presentation is represented by the light blue block. (D) Time course of activity in the left VLPFC for the same comparison demonstrates activity only during associative encoding, with no significant response during the encoding of individual items.
Table 1.
Significantly Active Brain Regions for Paired Stimuli versus Unpaired Stimuli (2P vs. 2U)
| # Volume | x | y | z | t | |
|---|---|---|---|---|---|
| L DLPFC (BA 9/46) | 3904 | −46 | 14.4 | 25.5 | 5.54 |
| L VLPFC (BA 45) | 2816 | −46.6 | 22.1 | 5 | 5.49 |
| L Superior frontal (BA 6) | 2624 | −4.5 | 11.3 | 52.4 | 6.65 |
| L Middle frontal (BA 6) | 2176 | −25.6 | 0.2 | 51.6 | 7.18 |
| L Angular (BA 39) | 1344 | −28.6 | −60.3 | 32.2 | 4.60 |
| L Parahippocampal (BA 36) | 896 | −26.3 | −35.3 | −14.8 | 5.55 |
| L Middle occipital (BA 19) | 640 | −48.9 | −57.9 | −3 | 4.12 |
| R Cingulate (BA 31) | 640 | 26.4 | −48.8 | 25.1 | 4.11 |
| L Middle temporal (BA 21) | 576 | −53 | −30.8 | −6.1 | 5.93 |
| R Cerebellum | 512 | 29.7 | −51.7 | −27.9 | 5.12 |
| R Supramarginal (BA 40) | 512 | 51.1 | −47.3 | 33.4 | −3.95 |
| L Inferior temporal (BA 20) | 448 | −50.3 | −51.7 | −13.6 | 4.90 |
| L Inferior parietal (BA 40) | 448 | −48.5 | −32.1 | 35.5 | 5.55 |
| R Insula (BA 47) | 384 | 31.4 | 17.3 | 2.5 | 4.03 |
| L Inferior parietal (BA 40) | 320 | −42 | −49.6 | 45.9 | 3.68 |
| L Caudate | 256 | −14 | 10.2 | 4.1 | 3.25 |
| R Supramarginal (BA 40) | 256 | 54 | −51.1 | 20.2 | −4.18 |
| L Supramarginal (BA 40) | 256 | −41 | −43 | 35.2 | 3.92 |
| L Precentral (BA 6) | 256 | −46 | −0.7 | 48.8 | 4.74 |
Analyses were also performed comparing activity during the encoding of trials with two paired images versus trials with two unpaired images, allowing for the examination of activity differences during the ISI. The time course of activity was examined beginning with the presentation of the first paired (1P) or unpaired (1U) image followed by a 3.5-sec ISI and the presentation of the second image (i.e., 2P or 2U, respectively). Although ISIs varied between 0.5 and 11 sec due to jitter, trials with 3.5-sec ISIs were used for this comparison. The same functional regions of interest as were previously discussed were also predicted to be important in this contrast.
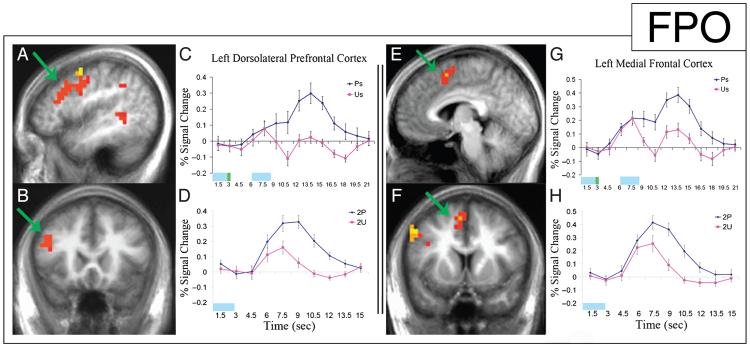
The comparison between the encoding of two paired stimuli and two unpaired stimuli showed increased activity in two frontal regions, the left DLPFC (Figure 4A, B) and the left medial frontal cortex (Figure 4E, F) during the paired trials (p < .01). The time course of activity in the left DLPFC showed a similar response for 1P and 1U (4.5–7.5 sec). In the unpaired trial, the activation decreased during the ISI and then increased during the presentation of 2U with a time course similar to that of 1U. In contrast, the paired trial showed sustained DLPFC activity throughout the ISI and rising further with the onset of 2P (Figure 4C). The time course of activity during paired and unpaired trials diverged at 7.5 sec, corresponding to the instruction to associate the 1P stimulus with the following stimulus. The larger activation during the encoding of 2P relative to that of 2U, seen in Figure 4C, was the result of increased size of response in addition to the increase in baseline (revealed when all jittered ISI trials are analyzed with separate covariates for paired and unpaired ISIs modeled as sustained responses; Figure 4D).
Figure 4.
Initiation of activity increase in the left DLPFC and the left medial frontal cortex at onset of associative memory instruction. Statistical activation maps illustrating greater activation (p < .01) during the encoding of two paired images (with a 3.5-sec ISI) versus two unpaired images (with a 3.5-sec ISI) are superimposed on sagittal (A, E) and coronal (B, F) slices of mean anatomical scan images across all 13 subjects; arrows indicate the left DLPFC (A, B) and left medial frontal (E, F) clusters used for time-course analysis. (C, G) Time courses of activity in the left DLPFC (C) and the left medial frontal cortex (G) beginning with the onset of the first image of two paired images (blue) and the first image of two unpaired images (pink) demonstrate divergence at the onset of associative instruction. The time of stimulus presentation is represented by the light blue block, and the time of associative instruction presentation is represented by the green block. (D, H) Time courses of activity in the left DLPFC (D) and the left medial frontal cortex (H) during the presentation of 2P (blue) and 2U (pink) stimuli illustrate the enhanced response to the second stimulus in the associated condition. The time of stimulus presentation is represented by the light blue block.
The left medial frontal cortex showed a similar increase in activity following the plus-sign in the paired trials (Figure 4E, F). Much like the left DLPFC, the response curve for the left medial frontal cortex showed a matched response for 1P and 1U, with divergence occurring at the instruction to associate and a further increase in response during the presentation of 2P (Figure 4G). Figure 4H illustrates the larger left medial frontal response during the encoding of 2P than during 2U (for a complete list of regions of activation for this contrast, see Table 2).
Table 2.
Significantly Active Brain Regions for Two Paired Stimuli (3.5-sec ISI) versus Two Unpaired Stimuli (3.5-sec ISI)
| # Volume | x | y | z | t | |
|---|---|---|---|---|---|
| L DLPFC (BA 9) | 4480 | −47.3 | 7.5 | 35.3 | 5.93 |
| L Angular (BA 39) | 3584 | −32.6 | −56.4 | 35.5 | 5.82 |
| L Medial frontal (BA 6) | 1280 | −5.4 | 5.3 | 52.5 | 6.71 |
| L Middle temporal (BA 21) | 704 | −47 | −46.6 | 5.8 | 4.50 |
| L Fusiform (BA 37) | 512 | −37.4 | −43.3 | −8.6 | 4.05 |
| R Cuneus (BA 19) | 512 | 19 | −83.9 | 30.7 | −3.79 |
| R Superior temporal (BA 39) | 512 | 32.1 | −52.7 | 31.6 | 5.18 |
| R Cerebellum | 384 | 1 | −38.1 | −14.8 | 3.91 |
| L Middle frontal (BA 6) | 384 | −27.3 | −7.1 | 46.2 | 3.80 |
| L Middle temporal (BA 39) | 320 | −39.5 | −50.2 | 10.9 | 5.39 |
| L Superior frontal (BA 10) | 320 | −31.6 | 48.8 | 17.7 | 3.68 |
No significant clusters were identified in the PHC using the comparison of the above subset of trials containing two paired or two unpaired stimuli with 3.5-sec ISIs (p > .05). In addition, when ISIs spanning all delay periods were analyzed, the left DLPFC showed a significant increase in activity during the delay period between two paired images (p < .05, t = 2.195). However, there was no significant difference in activity during the delay periods between paired images and between un-paired images in the left PHC (p = .51, t = 0.673). An interaction analysis between these two delay-period conditions for each brain region showed a significant Region by Condition interaction (p < .05).
DISCUSSION
The present study is the first to examine temporal contributions of the PFC and the PHC in associative memory encoding by separating the associative instruction from the time at which binding may occur. Activity in the PFC and the PHC was analyzed while subjects were instructed to encode sequentially presented stimuli as paired or as separate items. Contrasts between the encoding of 2P and 2U stimuli and the encoding of paired and unpaired trials with a 3.5-sec ISI were examined. The left PHC and DLPFC were active for all correctly encoded stimuli, with increased activity during 2P encoding versus 2U encoding. In contrast, the left VLPFC was significantly active during 2P, but not during 2U encoding.
Declarative Memory Encoding with Associative Instruction
In the present study, participants were instructed only to associate two stimuli when a plus-sign intervened; all other stimuli were to be remembered as single items. Although it is possible that associations can develop between sequentially presented images with or without associative instruction, episodic associative memory was improved by the presence of the cue. In addition, subsequent recognition of individual stimuli was improved by the presence of the cue (paired items recognized at a rate of 82%, and unpaired items recognized at a rate of 73%; p < .01). Thus, the instruction to associate modulates episodic memory performance along with its enhancement of brain activity.
Using sequential presentation of single images, semantic information was balanced across stimuli. However, the instruction to associate may engage verbal processes when nameable stimuli are used. It is possible that using nonverbal stimuli could result in different patterns or degrees of left frontal lobe activation. The left lateralization reported in the present study with nameable stimuli is, on one hand, similar to that reported in other encoding studies using verbal stimuli (Blumenfeld & Ranganath, 2007; Murray & Ranganath, 2007; Sperling et al., 2003; Wagner et al., 1998). On the other hand, the data are also in agreement with the revised Hemispheric Encoding/Retrieval Asymmetry model (Habib, Nyberg, & Tulving, 2003), which would predict left-sided activation for encoding regardless of stimulus type. The paradigm presented here could be adapted to address such questions through the use of nonverbal stimuli.
Increased PHC Activity during Associative Encoding
The involvement of particular MTL substructures in various aspects of long-term memory is debated in the literature (Eichenbaum et al., 2007). The present study showed PHC activity during encoding of individual images and pairs of associated images; however, this region showed selectivity through an increased response during associative encoding relative to individual-item encoding. These results complement other studies demonstrating PHC involvement in item encoding with enhanced activity during associative encoding (Murray & Ranganath, 2007; Kirwan & Stark, 2004). Using a different paradigm where three words were presented concurrently under instructions to repeat the words throughout the trial or to order the words according to their desirability, different patterns of brain activity were reported (Davachi & Wagner, 2002). Bilateral hippocampus was active for both encoding tasks, whereas the right entorhinal and bilateral parahippocampal gyri were more active during the repetition task. Although only the reordering task is described as using relational processing, both tasks could involve associative encoding. The cognitive strategies adopted to perform each type of task, however, will differ. The current study, which requires the association of two nameable visual stimuli, involves a cognitive strategy that is perhaps more similar to the repetition condition than to the reorder condition of the previous study (Davachi & Wagner, 2002). Therefore, the presence of parahippocampal activity in both the present study and in the repetition task in the previous study could reflect a common strategy.
PFC Activity and Dissociation of Substructures
Results from the current study showed increased DLPFC and VLPFC activity during the encoding of stimuli under associative conditions and revealed that enhancement of DLPFC activity begins at associative memory instruction. The noted further increase in DLPFC and VLPFC activity during 2P stimuli agrees with the present literature. The DLPFC is also active during the encoding of unpaired stimuli, whereas the VLPFC does not significantly respond to unpaired stimuli. This dissociation differs from previous results examining regional specificity within the PFC.
Previous studies have examined dissociations between regions of the PFC in relational and item-specific memory encoding (Murray & Ranganath, 2007; Blumenfeld & Ranganath, 2006). In an fMRI study using pairs of sequentially presented words, the second word was accompanied by a question prompting the participant to (1) relate the two words together (“relational trial”) or (2) semantically evaluate the second word (“item-specific trial”) (Murray & Ranganath, 2007). A dorsal–ventral dissociation was reported in lateral PFC activation. Both regions showed increased activation for encoding relational words versus item-specific words. VLPFC activity also predicted both successful relational and item-specific encoding, whereas DLPFC activity only predicted successful relational encoding. An earlier study examined the function of the DLPFC in long-term memory formation using a paradigm where three words were presented with the instruction either to rehearse the words or to reorder them according to the weight of the object (Blumenfeld & Ranganath, 2006). Based on results showing increased DLPFC activity during the encoding of reorder trials relative to rehearse trials and for the encoding of reorder trials where words were subsequently remembered, this study concluded that the DLPFC is involved in encoding organizational information. There are, however, several key differences between the current study and previous studies examining subregional contributions of the PFC to long-term memory.
The purpose of the present study was to examine the contributions of the PFC and the PHC in the encoding of pairs of associated images versus the encoding of un-paired images. Differences in activation between remembered compared to forgotten images were not the focus of the current study, and will be a topic of future investigation. Only correctly encoded images, as determined by the recognition task, were included in the analysis. A design optimized to examine subsequent memory-related activity might reveal different results. For example, activity seen in the DLPFC for the encoding of unpaired images might not differ based upon subsequent memory performance. Such results would then support previous findings of DLPFC activity predicting successful associative, and not individual-item, encoding.
Results from the current study show that the VLPFC does not significantly respond to the encoding of subsequently remembered unpaired images. These observations appear to differ from those of previous studies, which report VLPFC involvement in successful encoding of relational and item-specific memory (Murray & Ranganath, 2007) and memory for word rehearsal and reordered words (Blumenfeld & Ranganath, 2006). However, small differences in VLPFC cluster location may be relevant. The location of DLPFC activity (BA 46, 9) in the present study is very similar to the location of DLPFC activity in the previous studies, but the peak location of VLPFC activity (BA 45) is more anterior in the present study. One study separated the VLPFC into two different clusters, the anterior VLPFC (BA 47, 45), with a location similar to the current study, and the posterior VLPFC (6, 44), and although both clusters were predictive of subsequent memory for reorder trials, only the posterior cluster was predictive of subsequent memory for rehearse trials (Blumenfeld & Ranganath, 2006). Another study that also reports VLPFC activation predictive of subsequent memory for item-specific trials also describes a VLPFC cluster that appears more posterior than the VLPFC cluster in the present study (Murray & Ranganath, 2007).
The points discussed earlier in the discussion concerning the differences between three-word reordering/rehearsal paradigms and the present paradigm regarding activation in the PHC are also relevant when discussing dissociations in PFC activity. Rehearsing and reordering words may each involve associative memory, with reordering implementing additional working memory components. Rehearsal could establish a phonological association, whereas reordering may create visual and spatial associations. Although both types of trials may involve associative memory formation, each may utilize different organizational strategies resulting in differential VLPFC activity. In contrast, the DLPFC has been shown to be involved in task switching (Loose, Kaufmann, Tucha, Auer, & Lange, 2006; Vanderhasselt, De Raedt, Baeken, Leyman, & D'haenen, 2006; Smith, Taylor, Brammer, & Rubia, 2004; Sylvester et al., 2003). The above studies examining associative memory formation, as well as the present study, require a switch in task as instructed by a cue, which may contribute to the overlapping activity of the DLPFC despite the differences in study design.
Top–Down Influence of the PFC on PHC Activity
The sequential presentation of stimuli and an intervening plus-sign allowed for temporal separation of the neural activity related to (1) instructions to associate and (2) presentation of the second stimulus required to form the association. Following the plus-sign, the left DLPFC and the medial frontal cortex showed a sustained increase in activation relative to ISIs without a plus-sign (during which, activity in these regions returned to baseline; Figure 4C, G). Left PHC activity was not significantly different during the ISIs in paired and unpaired conditions (p = .51). These results suggest that the left DLPFC and the left medial frontal cortex are involved in maintaining 1P in working memory to create the association once 2P is presented.
When 2P is presented, increased activity is observed in the left DLPFC, in the medial frontal cortex, and in the PHC compared to the response to 2U. The left VLPFC is also active during the encoding of 2P, but does not show a significant response to 2U (Figure 3D). These results suggest that the left DLPFC, VLPFC, medial frontal cortex, and PHC are involved in associating the two paired stimuli. The left DLPFC and the medial frontal cortex also show increased activity in the paired trials starting at the plus-sign and continuing through the ISI (blank screen) and 2P, whereas the left PHC and the VLPFC show increased activity beginning at the presentation of 2P. The dynamics of encoding activation across the DLPFC/medial frontal cortex and the PHC/VLPFC demonstrate the temporal characteristics of functional interaction between these regions in associative encoding.
Increases in PFC activity during the delay period under associative instruction supports results from electrophysiology studies using nonhuman primates (Deco et al., 2005; Fuster et al., 2000). Fuster et al. (2000) reported PFC neuronal activity in the delay period during the association of tones and colors. Similarly, the present study shows increased PFC activity in the delay period during the association of two visual stimuli using human functional imaging (Figure 4). Electrophysiological evidence of MTL activity in rats and nonhuman primates during the delay period is less consistent, with some studies reporting the presence of MTL activity (Young et al., 1997; Cahusac et al., 1989; Watanabe & Niki, 1985) and others reporting very rare MTL activity (Hampson & Deadwyler, 2003; Vidyasagar et al., 1991). Such discrepancies in MTL delay-period activity may be the result of subtle differences in tasks. Nevertheless, two of the studies using delayed nonmatch-to-sample in rats also reported divergent results. Further study is required to examine the circumstances in which sustained MTL delay-period activity may be present in rats, monkeys, and humans. In the current study, no significant increase in MTL activity was observed during the delay period between paired stimuli.
Previous studies have shown direct and indirect anatomical connections between the PFC and the PHC using anterograde and retrograde tracing techniques in rhesus monkeys (Goldman-Rakic et al., 1984) and using DTI and fMRI in humans (Takahashi et al., 2007). Furthermore, top–down modulation from the PFC to the PHC has been examined using human imaging techniques, including fMRI, electroencephalography (EEG), and transcranial magnetic stimulation (TMS). Gazzaley and D'Esposito (2007) employed a visual working memory task for scenes with constant sensory input for all conditions to control bottom–up processing and to isolate top–down mechanisms of enhancement and suppression. Event-related fMRI and EEG measured enhanced activity relative to passive baseline in scene-specific visual association areas (parahippocampal/lingual gyrus) when subjects were told to remember scenes and to ignore faces. When opposite instructions were given, these regions showed suppressed activity. This effect demonstrated top–down modulation of the PHC. Further research is examining whether the PFC is critical for modulating PHC activity; preliminary results using repetitive TMS to disrupt PFC activity and studies using working memory tasks that challenge PFC function suggest that disrupted PFC activity results in deficits in top–down suppression (Gazzaley & D'Esposito, 2007).
The current study shows that PFC and PHC responses to a visual stimulus change depending upon the presence or absence of preceding associative instruction. Enhanced prefrontal activity at the presentation of associative instruction and the resulting enhancement of PFC and PHC activity during the following stimulus correspond to improved subsequent memory for that item as well as for the association. These findings reveal that frontal and medial-temporal regions subserve different functions in maintaining and binding visual stimuli into long-term associative memory.
Acknowledgments
This work was supported by the National Institute of Neurological Disorders and Stroke K23 NS050305 and the University of California, San Diego Departments of Neurosciences and Radiology.
REFERENCES
- Blumenfeld RS, Ranganath C. Dorsolateral prefrontal cortex promotes long-term memory formation through its role in working memory organization. Journal of Neuroscience. 2006;26:916–925. doi: 10.1523/JNEUROSCI.2353-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld RS, Ranganath C. Prefrontal cortex and long-term memory encoding: An integrative review of findings from neuropsychology and neuroimaging. Neuroscientist. 2007;13:280–291. doi: 10.1177/1073858407299290. [DOI] [PubMed] [Google Scholar]
- Brewer JB, Zhao Z, Desmond JE, Glover GH, Gabrieli JD. Making memories: Brain activity that predicts how well visual experience will be remembered. Science. 1998;281:1185–1187. doi: 10.1126/science.281.5380.1185. [DOI] [PubMed] [Google Scholar]
- Cahusac PMB, Miyashita Y, Rolls ET. Responses of hippocampal formation neurons in the monkey related to delayed spatial response and object-place memory tasks. Behavioral Brain Research. 1989;33:299–240. doi: 10.1016/s0166-4328(89)80118-4. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Human Brain Mapping. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Buckner RL. Selective averaging of rapidly presented individual trials using fMRI. Human Brain Mapping. 1997;5:329–340. doi: 10.1002/(SICI)1097-0193(1997)5:5<329::AID-HBM1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Rice HJ, Greenberg DL, Cabeza R, LaBar KS, Rubin DC. The spatiotemporal dynamics of autobiographical memory: Neural correlates of recall, emotional intensity, and reliving. Cerebral Cortex. 2007;18:217–229. doi: 10.1093/cercor/bhm048. [DOI] [PubMed] [Google Scholar]
- Davachi L, Mitchell JP, Wagner AD. Multiple routes to memory: Distinct medial temporal lobe processes build item and source memories. Proceedings of the National Academy of Sciences, U.S.A. 2003;100:2157–2162. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L, Wagner AD. Hippocampal contributions to episodic encoding: Insights from relational and item-based learning. Journal of Neurophysiology. 2002;88:982–990. doi: 10.1152/jn.2002.88.2.982. [DOI] [PubMed] [Google Scholar]
- Deco G, Ledberg A, Almeida R, Fuster J. Neural dynamics of cross-modal and cross-temporal associations. Experimental Brain Research. 2005;166:325–336. doi: 10.1007/s00221-005-2374-y. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Miller SL, Greve DN, Dale AM, Albert MS, Schacter DL, et al. Prefrontal–hippocampal–fusiform activity during encoding predicts intraindividual differences in free recall ability: An event-related functional–anatomic MRI study. Hippocampus. 2007;17:1060–1070. doi: 10.1002/hipo.20338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan RJ, Fletcher PC. Dissociating prefrontal and hippocampal function in episodic memory encoding. Nature. 1997;388:582–585. doi: 10.1038/41561. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annual Review of Neuroscience. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM, Bodner M, Kroger JK. Cross-modal and cross-temporal association in neurons of frontal cortex. Nature. 2000;405:347–351. doi: 10.1038/35012613. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, D'Esposito M. Top–down modulation and normal aging. Annals of the New York Academy of Sciences. 2007;1097:67–83. doi: 10.1196/annals.1379.010. [DOI] [PubMed] [Google Scholar]
- Gold JJ, Smith CN, Bayley PJ, Shrager Y, Brewer JB, Stark CEL, et al. Item memory, source memory, and the medial temporal lobe: Concordant findings from fMRI and memory-impaired patients. Proceedings of the National Academy of Sciences, U.S.A. 2006;103:9351–9356. doi: 10.1073/pnas.0602716103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Selemon LD, Schwartz ML. Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience. 1984;12:719–743. doi: 10.1016/0306-4522(84)90166-0. [DOI] [PubMed] [Google Scholar]
- Habib R, Nyberg L, Tulving E. Hemispheric asymmetries of memory: The HERA model revisited. Trends in Cognitive Sciences. 2003;7:241–245. doi: 10.1016/s1364-6613(03)00110-4. [DOI] [PubMed] [Google Scholar]
- Hampson RE, Deadwyler SA. Temporal firing characteristics and the strategic role of subicular neurons in short-term memory. Hippocampus. 2003;13:529–541. doi: 10.1002/hipo.10119. [DOI] [PubMed] [Google Scholar]
- Henke K, Buck A, Weber B, Wieser HG. Human hippocampus establishes associations in memory. Hippocampus. 1997;7:249–256. doi: 10.1002/(SICI)1098-1063(1997)7:3<249::AID-HIPO1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Kirwan CB, Stark CE. Medial temporal lobe activation during encoding and retrieval of novel face–name pairs. Hippocampus. 2004;14:919–930. doi: 10.1002/hipo.20014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law JR, Flanery MA, Wirth S, Yanike M, Smith AC, Frank LM, et al. Functional magnetic resonance imaging activity during the gradual acquisition and expression of paired-associate memory. Journal of Neuroscience. 2005;25:5720–5729. doi: 10.1523/JNEUROSCI.4935-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loose R, Kaufmann C, Tucha O, Auer DP, Lange KW. Neural networks of response shifting: Influence of task speed and stimulus material. Brain Research. 2006;1090:146–155. doi: 10.1016/j.brainres.2006.03.039. [DOI] [PubMed] [Google Scholar]
- Meltzer JA, Constable RT. Activation of human hippocampal formation reflects success in both encoding and cued recall of paired associates. Neuroimage. 2005;24:384–397. doi: 10.1016/j.neuroimage.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Murray LJ, Ranganath C. The dorsolateral prefrontal cortex contributes to successful relational memory encoding. Journal of Neuroscience. 2007;27:5515–5522. doi: 10.1523/JNEUROSCI.0406-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihlajamaki MH, Tanila H, Hanninen T, Kononen M, Mikkonen M, Jalkanen V, et al. Encoding of novel picture pairs activates the perirhinal cortex: An fMRI study. Hippocampus. 2003;13:67–80. doi: 10.1002/hipo.10049. [DOI] [PubMed] [Google Scholar]
- Prince SE, Daselaar SM, Cabeza R. Neural correlates of relational memory: Successful encoding and retrieval of semantic and perceptual associations. Journal of Neuroscience. 2005;25:1203–1210. doi: 10.1523/JNEUROSCI.2540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombouts SA, Machielsen WC, Witter MP, Barkhof F, Lindeboom J, Scheltens P. Visual association encoding activates the medial temporal lobe: A functional magnetic resonance imaging study. Hippocampus. 1997;7:594–601. doi: 10.1002/(SICI)1098-1063(1997)7:6<594::AID-HIPO2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Rossion B, Pourtois G. Revisiting Snodgrass and Vanderwart's object pictorial set: The role of surface detail in basic-level object recognition. Perception. 2004;33:217–236. doi: 10.1068/p5117. [DOI] [PubMed] [Google Scholar]
- Schluppeck D, Curtis CE, Glimcher PW, Heeger DJ. Sustained activity in topographic areas of human posterior parietal cortex during memory-guided saccades. Journal of Neuroscience. 2006;26:5098–5108. doi: 10.1523/JNEUROSCI.5330-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AB, Taylor E, Brammer M, Rubia K. Neural correlates of switching set as measured in fast, event-related functional magnetic resonance imaging. Human Brain Mapping. 2004;21:247–256. doi: 10.1002/hbm.20007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R, Chua E, Cocchiarella A, Rand-Giovannetti E, Poldrack R, Schacter DL, et al. Putting names to faces: Successful encoding of associative memories activates the anterior hippocampal formation. Neuroimage. 2003;20:1400–1410. doi: 10.1016/S1053-8119(03)00391-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychological Review. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. Differential encoding mechanisms for subsequent associative recognition and free recall. Journal of Neuroscience. 2006;26:9162–9172. doi: 10.1523/JNEUROSCI.2877-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester CY, Wagner TD, Lacey SC, Hernandez L, Nichols TE, Smith EE, et al. Switching attention and resolving interference: fMRI measures of executive functions. Neuropsychologia. 2003;41:357–370. doi: 10.1016/s0028-3932(02)00167-7. [DOI] [PubMed] [Google Scholar]
- Takahashi E, Ohki K, Kim DS. Diffusion tensor studies dissociated two fronto-temporal pathways in the human memory system. Neuroimage. 2007;34:827–838. doi: 10.1016/j.neuroimage.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. A co-planar stereotaxic atlas of the human brain. Thieme; New York: 1998. [Google Scholar]
- Tendolkar I, Arnold J, Petersson KM, Weis S, Brockhaus-Dumke A, van Eijndhoven P, et al. Probing the neural correlates of associative memory formation: A parametrically analyzed event-related functional MRI study. Brain Research. 2007;1142:159–168. doi: 10.1016/j.brainres.2007.01.040. [DOI] [PubMed] [Google Scholar]
- Vanderhasselt MA, De Raedt R, Baeken C, Leyman L, D'haenen H. The influence of rTMS over the right dorsolateral prefrontal cortex on intentional set switching. Experimental Brain Research. 2006;172:561–565. doi: 10.1007/s00221-006-0540-5. [DOI] [PubMed] [Google Scholar]
- Vidyasagar TR, Salzmann E, Creutzfeldt OD. Unit activity in the hippocampus and the parahippocampal temporobasal association cortex related to memory and complex behaviour in the awake monkey. Brain Research. 1991;544:269–278. doi: 10.1016/0006-8993(91)90064-3. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, et al. Building memories: Remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Niki H. Hippocampal unit activity and delayed response in the monkey. Brain Research. 1985;325:241–254. doi: 10.1016/0006-8993(85)90320-8. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Hopfinger JB, Buonocore MH, Kroll NE, Baynes K. Hippocampal, parahippocampal and occipital–temporal contributions to associative and item recognition memory: An fMRI study. NeuroReport. 2001;12:359–363. doi: 10.1097/00001756-200102120-00035. [DOI] [PubMed] [Google Scholar]
- Young BJ, Otto T, Fox GD, Eichenbaum H. Memory representation within the parahippocampal region. Journal of Neuroscience. 1997;17:5183–5195. doi: 10.1523/JNEUROSCI.17-13-05183.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]